INTRODUCTION
Cap Analysis Gene Expression (CAGE) is a method that identifies the 5' ends of transcripts, allowing the discovery of new promoters and the quantification of gene activity. Combining together promoter location and their expression levels, the CAGE data is essential for annotation-agnostic studies of regulatory gene networks. CAGE originally requires large input amounts of RNA not obtainable from highly refined samples such as tissue microdissections and subcellular fractions. Therefore, we developed nanoCAGE to capture the 5′ ends of transcripts from as little as 10 ng of total RNA. To take advantage of the capacity of current sequencers to produce longer reads (50~100 bp), we abandoned the enzymatic cleavage step that was limiting the informative length of the reads to ~25 bp, and now prepare cap-selected cDNAs ready for direct sequencing of their 5′ end, optionally mate-paired with the 3′ end, that informs about downstream sequences. This protocol describes how to prepare nanoCAGE libraries starting from 50 ng of total RNA for sequencing on an Illumina GA II platform.
RELATED INFORMATION
This revised (Table 1) and simplified nanoCAGE protocol was derived from methodologies originally described by Plessy et al (2010). To enable the preparation of libraries from nanogram levels of RNA, nanoCAGE amplifies the 5′ cDNAs by PCR. For samples where ~50 µg of RNA is available, the original CAGE method (Kodzius et al., 2006) may be preferable as it is free from PCR bias. CAGE libraries can be used for different types of analysis (Carninci, 2010), for instance promoter discovery (in particular when the available transcript annotation for a tissue or a species is rudimentary), differential expression analysis (Valen et al., 2009), inference of transcription factor binding site (Vitezic et al., 2010) or gene networks (Suzuki et al., 2009). For samples where enough RNA is available, we recommend the preparation of technical replicates if the analysis is centered on the use of the expression levels. The nanoCAGE protocol uses the template switching method—based on the reverse-transcription of the mRNA's cap—to enrich for 5′ ends (Chenchik et al., 1998) instead of the cap trapper method (Carninci et al., 1997; Carninci et al., 1996), and uses the semi suppressive PCR approach (Plessy et al, 2010), essential to minimize short PCR artifacts. An overview of the procedure is provided in Figure 1. Libraries prepared with this nanoCAGE protocol can be paired-end sequenced as CAGEscan libraries (Plessy et al, 2010), that link transcript 5′ ends to downstream regions assembled from the mated 3′ ends.
Table 1.
Comprehensive testing of different parameters of nanoCAGE method.
| Library name |
Reverse Transcriptase (RT) |
PCR extension time1(sec) |
RT Temp (°C) |
RT additive2 |
RT Time (min) |
RT cool Temp (°C)3 |
Total tag count4 |
Unique Tag Count5 |
rRNA6 (%) |
Redundanc y7 |
Semi- suppressive PCR cycles8 |
Semi- suppressive PCR conc9 (ng/µl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J53-GA | PrimeScript | 120 | 50 | Betaine | 30 | No | 1572426 | 181750 | 23.23 | 8.65 | 25 | 16.34 |
| J53-GA | PrimeScript | 120 | 50 | S/T | 30 | NO | 2322991 | 359674 | 29.35 | 6.46 | 24 | 15.49 |
| J53-GA | PrimeScript | 120 | 50 | water | 30 | No | 723881 | 108291 | 27.09 | 6.68 | 25 | 12.47 |
| J53-GA | PrimeScript | 120 | 60 | Betaine | 30 | No | 1098557 | 40599 | 5.13 | 27.06 | 32 | 13.08 |
| J53-GA | PrimeScript | 120 | 60 | S/T | 30 | No | 1917298 | 83125 | 4.27 | 23.07 | 33 | 14.22 |
| J53-GA | PrimeScript | 120 | 60 | water | 30 | No | 2150491 | 68244 | 5.74 | 31.51 | 33 | 11.73 |
| J53-GA | PrimeScript | 30 | 50 | Betaine | 30 | No | 1130096 | 112900 | 19.64 | 10.01 | 28 | 9.25 |
| J53-GA | PrimeScript | 30 | 50 | S/T | 30 | No | 2359863 | 202012 | 26.46 | 11.68 | 28 | 8.17 |
| J53-GA | PrimeScript | 30 | 50 | water | 30 | No | 1631842 | 125415 | 24.89 | 13.01 | 30 | 8.85 |
| J53-GA | PrimeScript | 30 | 60 | Betaine | 30 | No | 665230 | 48412 | 5.09 | 13.74 | 32 | 9.28 |
| J53-GA | PrimeScript | 30 | 60 | S/T | 30 | No | 1407092 | 34991 | 4.93 | 40.21 | 37 | 7.64 |
| J53-GA | PrimeScript | 30 | 60 | water | 30 | No | 607120 | 20445 | 8.39 | 29.7 | 37 | 9.35 |
| J54-GA | SuperScriptIII | 120 | 50 | Betaine | 30 | No | 250499 | 103388 | 16.86 | 2.42 | 23 | 28.8 |
| J54-GA | SuperscriptIII | 120 | 50 | S/T | 30 | No | 1224346 | 215729 | 9.77 | 5.68 | 23 | 26.01 |
| J54-GA | SuperscriptIII | 120 | 50 | water | 30 | No | 955875 | 300447 | 20.45 | 3.18 | 24 | 33.99 |
| J54-GA | SuperscriptIII | 120 | 60 | Betaine | 30 | No | 927392 | 119713 | 12.76 | 7.75 | 26 | 20.3 |
| J54-GA | SuperscriptIII | 120 | 60 | S/T | 30 | No | 205397 | 43212 | 16.31 | 4.75 | 27 | 20.42 |
| J54-GA | SuperscriptIII | 120 | 60 | water | 30 | No | 1159443 | 109583 | 10.19 | 10.58 | 27 | 17.89 |
| J54-GA | SuperscriptIII | 30 | 50 | Betaine | 30 | No | 574609 | 158085 | 15.67 | 3.63 | 28 | 32.93 |
| J54-GA | SuperscriptIII | 30 | 50 | S/T | 30 | No | 859521 | 244124 | 11.17 | 3.52 | 27 | 14.27 |
| J54-GA | SuperscriptIII | 30 | 50 | water | 30 | No | 508443 | 155528 | 22.29 | 3.27 | 28 | 13.11 |
| J54-GA | SuperScriptIII | 30 | 60 | Betaine | 30 | No | 924509 | 68005 | 3.12 | 13.59 | 34 | 13.46 |
| J54-GA | SuperScriptIII | 30 | 60 | S/T | 30 | No | 881722 | 119491 | 6.39 | 7.38 | 34 | 16.9 |
| J54-GA | SuperScriptIII | 30 | 60 | water | 30 | No | 1842304 | 99336 | 3.12 | 18.55 | 33 | 21.13 |
| J55-GA | SuperScriptIII | 120 | 50 | Betaine | 30 | No | 1210696 | 240266 | 14.36 | 5.04 | 25 | 26.78 |
| J55-GA | SuperScriptIII | 120 | 60 | S/T | 30 | No | 2263452 | 443357 | 12.67 | 5.11 | 25 | 29.55 |
| J56-GA | SuperScriptIII | 120 | 50 | water | 30 | No | 1678459 | 363513 | 20.77 | 4.62 | 24 | 22.81 |
| J55-GA | SuperScriptIII | 120 | 60 | Betaine | 30 | No | 1617617 | 126028 | 5.52 | 12.84 | 30 | 16.12 |
| J55-GA | SuperScriptIII | 120 | 60 | S/T | 30 | No | 549364 | 42617 | 3.28 | 12.89 | 30 | 14.5 |
| J55-GA | SuperScriptIII | 120 | 60 | water | 30 | No | 431085 | 33658 | 7.8 | 12.81 | 30 | 14.96 |
| J55-GA | SuperScriptIII | 30 | 50 | Betaine | 30 | No | 768911 | 182116 | 14.81 | 4.22 | 28 | 13.69 |
| J55-GA | SuperScriptIII | 30 | 50 | S/T | 30 | No | 811168 | 238256 | 18.97 | 3.4 | 28 | 15.01 |
| J55-GA | SuperScriptIII | 30 | 50 | water | 30 | No | 2034725 | 324021 | 18.14 | 6.28 | 30 | 15.84 |
| J55-GA | SuperScriptIII | 30 | 60 | Betaine | 30 | No | 1474825 | 87977 | 3.38 | 16.76 | 36 | 10.76 |
| J55-GA | SuperScriptIII | 30 | 60 | S/T | 30 | No | 783407 | 47124 | 4.63 | 16.62 | 36 | 12.53 |
| J55-GA | SuperScriptIII | 30 | 60 | water | 30 | No | 1066288 | 57003 | 4.34 | 18.71 | 36 | 8.27 |
| J61-GA | SuperScriptIII | 120 | 40 | Betaine | 30 | No | 1509574 | 489529 | 21.48 | 3.08 | 25 | 51 |
| J61-GA | SuperScriptIII | 120 | 40 | S/T | 30 | No | 1241620 | 311979 | 23.19 | 3.98 | 25 | 29.08 |
| J61-GA | SuperScriptIII | 120 | 40 | water | 30 | No | 1660969 | 499814 | 26.79 | 3.32 | 25 | 58.91 |
| J61-GA | SuperScriptIII | 120 | 45 | Betaine | 30 | No | 2314533 | 588948 | 31.62 | 3.93 | 25 | 36.68 |
| J61-GA | SuperScriptIII | 120 | 45 | S/T | 30 | No | 1194233 | 340681 | 22.45 | 3.51 | 25 | 36.03 |
| J61-GA | SuperScriptIII | 120 | 45 | water | 30 | No | 1366263 | 332132 | 17.32 | 4.11 | 25 | 28.62 |
| J61-GA | SuperScriptIII | 120 | 55 | Betaine | 30 | No | 1282996 | 181602 | 24.66 | 7.06 | 27 | 27.52 |
| J61-GA | SuperScriptIII | 120 | 55 | S/T | 30 | No | 896055 | 227808 | 23.12 | 3.93 | 24 | 17.3 |
| J61-GA | SuperScriptIII | 120 | 55 | water | 30 | No | 1336866 | 262873 | 22.77 | 5.09 | 27 | 28.33 |
| J61-GA | SuperScriptIII | 30 | 40 | Betaine | 30 | No | 940078 | 301506 | 22.53 | 3.12 | 28 | 22.36 |
| J61-GA | SuperScriptIII | 30 | 40 | S/T | 30 | No | 1294643 | 443824 | 21.7 | 2.92 | 28 | 27.6 |
| J61-GA | SuperScriptIII | 30 | 40 | water | 30 | No | 1843239 | 473334 | 27.17 | 3.89 | 28 | 32.47 |
| J61-GA | SuperScriptIII | 30 | 45 | Betaine | 30 | No | 743362 | 191280 | 22.1 | 3.89 | 30 | 18.71 |
| J61-GA | SuperScriptIII | 30 | 45 | S/T | 30 | No | 951527 | 257689 | 22.14 | 3.69 | 30 | 22.99 |
| J61-GA | SuperScriptIII | 30 | 45 | water | 30 | No | 695530 | 226251 | 16.97 | 3.07 | 30 | 27.02 |
| J61-GA | SuperScriptIII | 30 | 55 | Betaine | 30 | No | 973648 | 159747 | 30.95 | 6.09 | 32 | 17.87 |
| J61-GA | SuperScriptIII | 30 | 55 | S/T | 30 | No | 1201971 | 208873 | 32 | 5.75 | 30 | 19.96 |
| J61-GA | SuperScriptIII | 30 | 55 | water | 30 | No | 1410042 | 165453 | 32.18 | 8.52 | 30 | 17.52 |
| J62-GA | SuperScriptIII | 120 | 40 | Betaine | 30 | No | 94717 | 67342 | 15.42 | 1.41 | 28 | 51.91 |
| J62-GA | SuperScriptIII | 120 | 40 | S/T | 30 | No | 143954 | 91610 | 8.71 | 1.57 | 26 | 75.86 |
| J62-GA | SuperScriptIII | 120 | 40 | water | 30 | No | 177528 | 117916 | 15.15 | 1.51 | 25 | 42.51 |
| J62-GA | SuperScriptIII | 120 | 45 | Betaine | 30 | No | 190529 | 99655 | 12.64 | 1.91 | 25 | 37.96 |
| J62-GA | SuperScriptIII | 120 | 45 | S/T | 30 | No | 48803 | 30511 | 3.6 | 1.6 | 22 | 31.54 |
| J62-GA | SuperScriptIII | 120 | 45 | water | 30 | No | 188310 | 121086 | 23.04 | 1.56 | 25 | 53.77 |
| J62-GA | SuperScriptIII | 120 | 55 | Betaine | 30 | No | 157084 | 72413 | 2.71 | 2.17 | 27 | 11.16 |
| J62-GA | SuperScriptIII | 120 | 55 | S/T | 30 | No | 15840 | 10994 | 2.5 | 1.44 | 27 | 16.92 |
| J62-GA | SuperScriptIII | 120 | 55 | water | 30 | No | 78471 | 47392 | 1.38 | 1.66 | 27 | 15.22 |
| J62-GA | SuperScriptIII | 30 | 40 | Betaine | 30 | No | 273149 | 188191 | 10.14 | 1.45 | 25 | 23.89 |
| J62-GA | SuperScriptIII | 30 | 40 | S/T | 30 | No | 249603 | 186982 | 9.45 | 1.33 | 25 | 18.95 |
| J62-GA | SuperScriptIII | 30 | 40 | water | 30 | No | 318088 | 245244 | 13.19 | 1.3 | 25 | 28.14 |
| J62-GA | SuperScriptIII | 30 | 45 | Betaine | 30 | No | 124887 | 74796 | 7.21 | 1.67 | 25 | 16.8 |
| J62-GA | SuperScriptIII | 30 | 45 | S/T | 30 | No | 94417 | 69055 | 13.34 | 1.37 | 25 | 19.86 |
| J62-GA | SuperScriptIII | 30 | 45 | water | 30 | No | 34632 | 24388 | 3.91 | 1.42 | 25 | 18.91 |
| J62-GA | SuperScriptIII | 30 | 55 | Betaine | 30 | No | 395583 | 132449 | 5.75 | 2.99 | 34 | 16.49 |
| J62-GA | SuperScriptIII | 30 | 55 | S/T | 30 | No | 137211 | 65220 | 16.68 | 2.1 | 30 | 17.46 |
| J62-GA | SuperScriptIII | 30 | 55 | water | 30 | No | 96976 | 25855 | 3.48 | 3.75 | 30 | 11.43 |
| J63-GA | PrimeScript | 120 | 40 | Betaine | 30 | No | 1120560 | 516165 | 8 | 2.17 | 22 | 27.87 |
| J63-GA | PrimeScript | 120 | 40 | S/T | 30 | No | 929189 | 442893 | 11.74 | 2.1 | 22 | 25.11 |
| J63-GA | PrimeScript | 120 | 40 | water | 30 | No | 1305601 | 601415 | 10.01 | 2.17 | 22 | 28.33 |
| J63-GA | PrimeScript | 120 | 45 | Betaine | 30 | No | 1368533 | 580968 | 17.64 | 2.36 | 22 | 18.73 |
| J63-GA | PrimeScript | 120 | 45 | S/T | 30 | No | 649456 | 318800 | 15.27 | 2.04 | 22 | 20.76 |
| J63-GA | PrimeScript | 120 | 45 | water | 30 | No | 739001 | 326532 | 18.53 | 2.26 | 22 | 19.22 |
| J63-GA | PrimeScript | 120 | 55 | Betaine | 30 | No | 1003632 | 173383 | 22.31 | 5.79 | 29 | 25.95 |
| J63-GA | PrimeScript | 120 | 55 | S/T | 30 | No | 1217507 | 303138 | 17.41 | 4.02 | 30 | 42.3 |
| J63-GA | PrimeScript | 120 | 55 | water | 30 | No | 1987403 | 401085 | 17.62 | 4.96 | 30 | 25.45 |
| J63-GA | PrimeScript | 30 | 40 | Betaine | 30 | No | 873112 | 428106 | 16.26 | 2.04 | 27 | 22.09 |
| J63-GA | PrimeScript | 30 | 40 | S/T | 30 | No | 930354 | 443523 | 17.4 | 2.1 | 27 | 20.09 |
| J63-GA | PrimeScript | 30 | 40 | water | 30 | No | 848335 | 426379 | 18.12 | 1.99 | 27 | 30.32 |
| J63-GA | PrimeScript | 30 | 45 | Betaine | 30 | No | 574837 | 269437 | 21.96 | 2.13 | 28 | 26.98 |
| J63-GA | PrimeScript | 30 | 45 | S/T | 30 | No | 881363 | 370124 | 20.19 | 2.38 | 28 | 23.45 |
| J63-GA | PrimeScript | 30 | 45 | water | 30 | No | 312482 | 151889 | 13.84 | 2.06 | 28 | 17.7 |
| J63-GA | PrimeScript | 30 | 55 | Betaine | 30 | No | 1170345 | 200170 | 12.99 | 5.85 | 38 | 42.86 |
| J63-GA | PrimeScript | 30 | 55 | S/T | 30 | N0 | 586387 | 127215 | 34.81 | 4.61 | 30 | 14.94 |
| J63-GA | PrimeScript | 30 | 55 | water | 30 | No | 965347 | 119385 | 9.25 | 8.09 | 40 | 37.31 |
| J64-GA | SuperScriptIII | 120 | 50 | Betaine | 30 | 30 | 722783 | 263787 | 28.9 | 2.74 | 25 | 55.85 |
| J64-GA | SSIII | 120 | 50 | Betaine | 30 | 25 | 834995 | 286939 | 24.78 | 2.91 | 25 | 45.05 |
| J64-GA | SSIII | 120 | 50 | Betaine | 30 | 20 | 1396843 | 319729 | 43.93 | 3.57 | 25 | 46.16 |
| J64-GA | SSIII | 120 | 50 | Betaine | 15 | No | 1400473 | 347451 | 37.41 | 4.03 | 27 | 51.23 |
| J64-GA | SSIII | 120 | 50 | Betaine | 45 | No | 110261 | 320173 | 48.44 | 3.44 | 24 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 60 | No | 776672 | 266856 | 39.35 | 2.91 | 24 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 30 | 30 | 917523 | 355315 | 26.38 | 2.58 | 25 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 30 | 25 | 908191 | 371519 | 33.06 | 2.44 | 25 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 30 | 20 | 787615 | 320633 | 27.46 | 2.46 | 25 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 15 | No | 987514 | 348994 | 22.04 | 2.83 | 25 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 45 | N0 | 1387713 | 484912 | 33.68 | 2.86 | 25 | |
| J64-GA | SSIII | 120 | 50 | Betaine | 60 | No | 857554 | 331455 | 39.52 | 2.57 | 21 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 30 | 30 | 1017950 | 274698 | 34.21 | 3.71 | 28 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 30 | 25 | 907559 | 219400 | 47.38 | 4.14 | 27 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 30 | 20 | 705686 | 190585 | 31.01 | 3.7 | 28 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 15 | No | 1285912 | 270461 | 42.59 | 4.75 | 28 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 45 | N0 | 1143364 | 259958 | 54.18 | 4.4 | 23 | |
| J64-GA | PrimeScript | 120 | 50 | Betaine | 60 | No | 1191776 | 196585 | 27.24 | 6.06 | 26 | |
Simple quality indicators for nanoCAGE libraries made with 50 ng of Hep G2 total RN, using different reverse-transcriptases and reaction conditions. that gave the best result with SuperScript III and PrimeScript. The libraries were sequenced on a Genome Analyzer II (Illummina).
Extension time of the semi suppressive PCR and the PCR to adapt linker to Illumina. Short incubation times were tested to reduce the size of the amplified cDNAs, since the Illumina GA II does not efficiently sequence cDNAs longer 1 kb in mixtures.
RT additive: S/T, Sorbitol/Trehalose; water, Nuclease-free water.
After RT reaction, the cDNAs were incubated for 5 min at the indicated temperatures before final cooling on ice/water mixture (see step 2 in the Method section). No: the cool down treatment was not applied to these combinations.
Number of tags remaining after removing the artifacts, primer-dimer etc. from the raw sequences.
Number of unique tags remaining after removing the artifacts, primer-dimer etc. from the raw sequences.
Percentage of total tags mapped to ribosomal RNA.
Total number of tags divided by the number of unique tags.
Number of PCR cycles required to amplify the purified cDNAs by semi-suppressive PCR (see step 4 in the Method section).
Concentration of the semi-suppressive PCR products after purification (see step 11 in the Method section).
Figure 1.
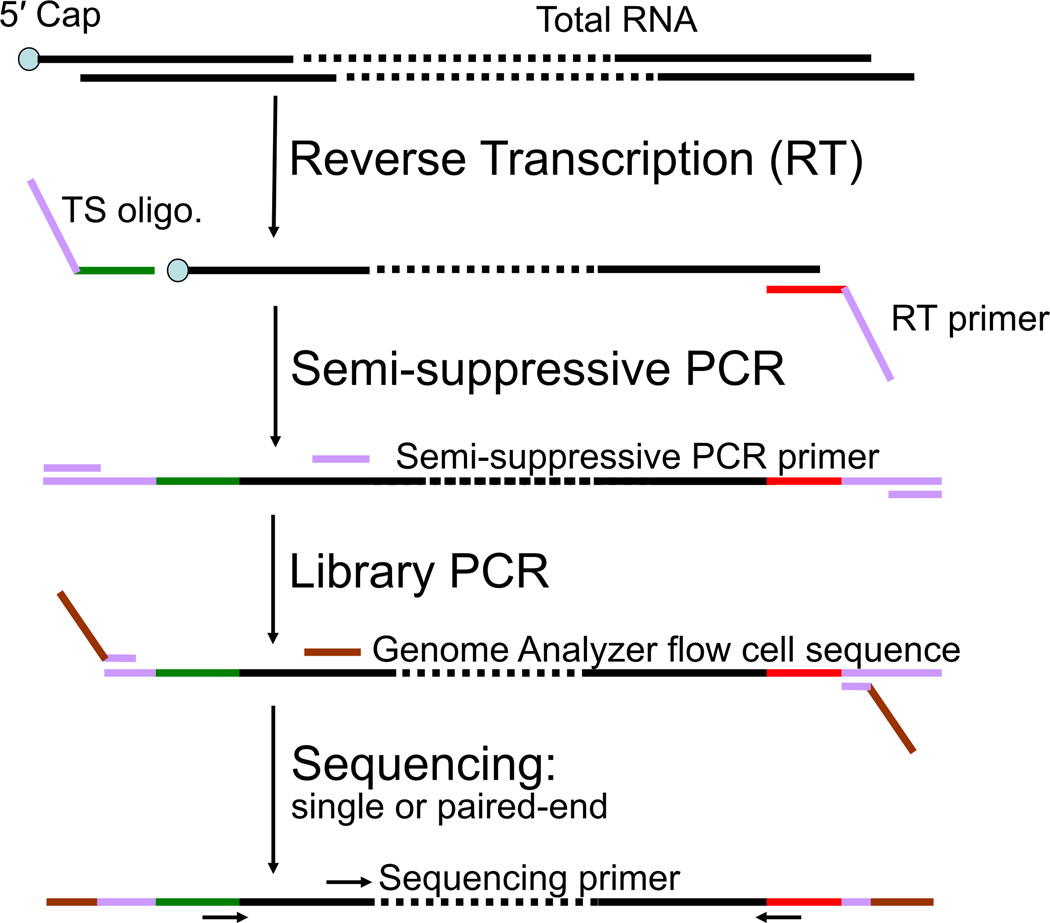
Flowchart of nanoCAGE protocol. Briefly, the template switching (TS) oligonucleotide is added to the first-strand cDNA synthesis reaction along with the reverse transcription (RT) primer. The three guanosine ribonucleosides at the 3′ end of the template switching primer hybridize to cytidine deoxynucleosides added to the 3′ end of the newly synthesized cDNA strand by the reverse transcriptase in a cap-dependent manner (Hirzmann et al., 1993). After hybridization, the reverse transcriptase will extend the cDNA strand using the template switching oligonucleotide as a template. Hence, the cDNA originating from a capped RNA will have at its 3′ end sequence reverse-complementary to the sequence of the template switching oligonucleotide. These sequences are needed for the synthesis and amplification of the second cDNA strand by semi suppressive PCR, that minimizes the amplification of shorter artifacts like primer dimers or aberrant cDNAs template-switched from a RT primer or reverse-transcribed from a TS oligonucleotide. End-sequences required for sequencing the cDNAs on the Genome Analyzer II are introduced by PCR (“Library PCR”), and the cDNAs are then sequenced as single or paired ends.
MATERIALS
Reagents
Acetic acid
Agarose Gel (1%) [R]
Agencourt AMPure XP (Beckman Coulter, Inc. Cat #A63881)
Agencourt RNAClean XP (Beckman Coulter, Inc. Cat #A63987)
Betaine (e.g., Wako Pure Chemical Industries Ltd. Cat #023-10862)
Chelex 100 (e.g., Sigma-Aldrich, Cat # C7901)
dNTP Mixture (2.5 mM, TaKaRa Bio Inc. Cat #4030)
D-Sorbitol (e.g., Wako Pure Chemical Industries Ltd., Cat #198-03755)
D(+) Trehalose dihydrate (e.g., Sigma-Aldrich, Cat #90208)
EDTA (0.5 M, pH 8.0) [R]
Ethanol (70 %)
Ethidium bromide (10 mg/ml) [!]
Ex Taq Hot Start Version (TaKaRa Bio Inc. Cat #RR006A) FastRuler
DNA Ladders Middle Range (Fermentas, Cat # SM1113) High
Sensitivity DNA Kit (Agilent Biotechnologies, Cat #5067-4626) DNA gel loading dye (6×, e.g., Fermentas, Cat #R0621)
NaOH [!]
Nuclease-free water (e.g., Invitrogen Corp, Cat #10977-015)
Reverse Transcriptase (PrimeScript, TaKaRa Bio Inc. Cat #2680A)
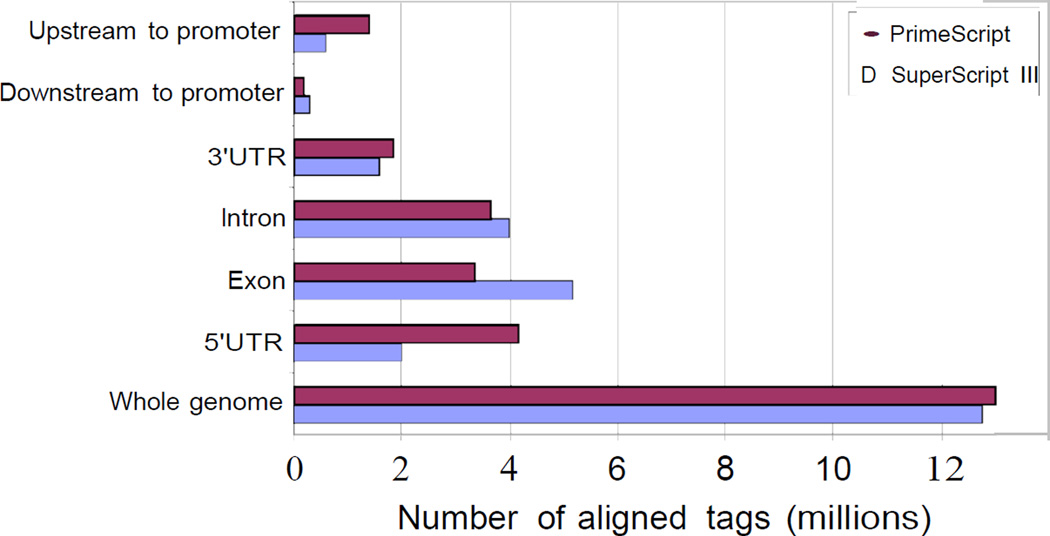
We strongly recommend to use PrimeScript for reverse transcription as we tested this protocol for different commercially available reverse transcriptases and found that PrimeScript was the best for nanoCAGE library preparation (Figure 2).
Figure 2.
Selection of reverse transcriptase for nanoCAGE library preparation. Among the tested reverse transcriptases, we observed that SuperScript III (SSIII) and PrimeScript (PS) give a higher yield of nanoCAGE cDNAs (data not shown). The differences consist in template switching properties and specificity at a given temperature and additive. SuperScript III worked best at high temperature (50 °C) in presence of sorbitol and trehalose while PrimeScript worked best at lower temperature (40 °C) in presence of betaine. We evaluated the libraries according to the fraction of aligned reads corresponding to the rRNA (SSIII: 9.77 %; PS: 8 %) and redundancy (SSIII: 5.68; PS: 2.17), defined by the total number of aligned reads divided by the number of unique aligned reads. At their best condition, SSIII and PS were then further compared after alignment of the reads to the RefSeq gene models. As indicated in the figure, libraries prepared with PrimeScript contain approximately twice more reads aligning to promoter regions as libraries prepared using SuperScripIII.
RNase Zap (Ambion, Cat #9780)
Samples (Total RNA)
Appropriate method should be used for the extraction and purification of total RNA. We have successfully used Trizol LS to extract and 'PureLink RNA Micro Kit’ (Invitrogen, Cat #12183-016) for purification of total RNA for nanoCAGE library preparation. The eluent used for the RNA should be nuclease-free water or an elution buffer provided by the RNA extraction kit. Purified total RNA (> 50 ng) should be tested on an Agilent Bioanalyzer or a NanoDrop spectrophotometer. We recommend total RNA samples with a RIN value ≥ 7. Store total RNA samples for nanoCAGE library preparation at −80 °C until used.
This protocol is optimized for 50 ng of total RNA. For best result, we therefore recommend to use at least 50 ng of total RNA for nanoCAGE library preparation. However, total RNA ranging from 10 ng to 1,250 ng can also be used. Prepare total RNA sample as 50 ng/µl solution or a different concentration ranging from 10–1,250 ng/µl. When few hundreds of nanograms of RNA are available, we recommend making technical replicates and to keep a backup.
Sorbitol/Trehalose (3.3 M / 0.66 M) Stock Solution [R]
SYBR Premix Ex Taq (Perfect Real Time; TaKaRa Bio Inc. Cat #RR041A)
TAE (50×) Buffer [R]
TWEEN 20 (Sigma-Aldrich, Cat #P9416)
Oligonucleotides
Template Switching DNA/RNA oligonucleotide, desalted (Integrated DNA Technologies): 5′-TAGTCGAACTGAAGGTCTCCAGCA(rG)(rG)(rG)-3′
All the bases are deoxynucleotides except (rG) which are guanosine ribonucleotides. For multiplexing libraries in single sequencing lanes, a sequence identifier (“barcode”) can be inserted 5′ of the riboguanosines.
The three riboguanosines are essential and can not be replaced by desoxyriboguanosines. We tested pure deoxyribo-oligonucleotides for template-switching and could amplify some libraries, but the tags obtained did not identify promoters.
Reverse-transcription primer, desalted (Invitrogen): 5′-TAGTCGAACTGAAGGTCTCCGAACCGCTCTTCCGATCTNNNNNN-3′
Forward second-strand PCR, desalted (Invitrogen): 5′-TAGTCGAACTGAAGGTCTCCAGC-3′
Reverse second-strand PCR, desalted (Invitrogen): 5′-TGACGTCGTCTAGTCGAACTGAAGGTCTCCGAACC-3′
Library PCR, forward, desalted (Invitrogen): 5′-AATGATACGGCGACCACCGAGATCTACACTAGTCGAACTGAAGG-3′
Library PCR, reverse, desalted (Invitrogen): 5′-CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGCTG AACCGCTCTTCCGATCT-3′
Sequencing primer, forward, desalted (Invitrogen): 5′-TAGTCGAACTGAAGGT CTCCAGCA-3′
Sequencing primer, reverse, desalted (Invitrogen): 5′-CGGTCTCGGCATTCCT GCTGAACCGCTCTTCCGATCT
Equipment
Adhesive PCR Seal (e.g., 4-titude Units B&C UK, Cat #4ti-0500)
Agilent 2100 Bioanalyzer (Agilent Technologies, Cat # G2928B)
Beaker (200 ml)
Centrifuges
Benchtop Centrifuge
For 96-well plate (e.g., Allegra X-12R, Beckman Coulter, Inc., Cat #ALX03L23)
Centrifugal Concentrator (TOMY Digital Biology Co., Ltd., Cat #35041048)
Genome Analyzer IIx (Illumina)
Electrophoresis unit (e.g., Mupid α; Advance Co. Ltd, Cat #200314)
Gel maker set (e.g., Advance Co. Ltd, Cat #GM-HR)
Gloves
Ice and ice box
Imaging System (e.g., Bio Doc-It; UVP, Upland, CA, USA, Cat # S/N 011007-003 P/N 95-0393-03)
Magnetic stand (Dynal) for bead separation (Beckman)
Micro Balance (e.g., HL 3000; HANSEN Medical Inc., Cat #5221657)
Microcentrifuge tubes, 1.5-ml, and 0.6 ml (e.g., Low retention Tube, ASIST, Cat # AMT.150LC, and AMT.060LC)
Micropipettes
Milli-Q Advantage A10 Water System (Millipore, Cat #Z00Q0V0WW)
NanoDrop 1000 spectrophotometer (Thermo Fisher Inc., Cat #S09NND360)
PCR tube, 0.2 ml (e.g., Fast Gene)
Pipette Tips (e.g., Fast Gene Multifit Pipette Tips; Sorenson BioScience)
Sealing applicator (e.g., 3M, Cat #PA-1)
Step One Plus Real-Time PCR System (Applied Biosystems, Cat #272000116)
Thermocycler
Vortex mixer
µltraAmp PCR plates (e.g., Fast plates 96, Nippon Genetics Co. Ltd., Cat #38801)
METHOD
We strongly recommend that the beginning investigator practices making control libraries to check cDNA synthesis by real-time PCR, to visualize semisuppressive PCR smear on gel and to check Bioanalyzer profile of the prepared libraries with some available total RNA before investigating invaluable samples.
Day 1
-
1
Before beginning, assemble all equipments and solutions (30 min).
-
2First Strand cDNA synthesis by template switching and random priming (1 h 30 min).Perform all procedures in a RNase free conditions on a workbench dedicated only to RNA work.
- Prepare a Primer/Sorbitol/Trehalose solution by mixing 8 µl Sorbitol/Trehalose (3.3 M / 0.66 M) stock solution, 1 µl of 100 µM Reverse-transcription primer, 1 µl of 1 mM Template Switching DNA/RNA oligonucleotide.As Sorbitol-Trehalose solution is viscous, mix it very carefully by pipetting at least 10 times.
- Aliquot 1 µl of Primer/Sorbitol/Trehalose solution into separate 0.2 ml siliconized PCR tubes for the samples and a negative control.
- Add 1 µl total RNA (50 ng) into the sample tubes and 1 µl nuclease-free water into the negative control tube and mix the solution several times for complete homogenization.If the total RNA is in a larger volume, the mixture can be reduced to 2 µl by centrifugal evaporation at room temperature (to avoid RNA degradation). The presence of Sorbitol/Trehalose will slow evaporation as its concentration increases, which reduces the risk of accidentally drying the RNA.
- Incubate the solution at 65 °C for 10 min in a thermocycler.
- Prepare 8 µl of the reverse-transcription reaction mixture per reaction by mixing 2 µl 5 × PrimeScript buffer, 2.5 µl 2.5 mM dNTP, 1 µl 0.1 M DTT, 1.5 µl 5 M betaine and 1 µl 200 U/µl PrimeScript.
- Keep an ice box ready with an ice-water bath.
- At the end of incubation, pause the PCR machine at 22 °C and snap cool the samples and negative control on ice-water bath for 2 min.RNAs must not refold, and cooling is much faster in an ice-water bath, compared to putting the tubes on ice.
- Bring up samples and negative control to 22 °C in the thermocycler.
- Add 8 µl of reverse-transcription mixture (step 1.v) to the samples and the negative control.
- Incubate for 10 min at 22 °C, 30 min at 40 °C and 15 min at 75 °C in the thermocycler.
- Snap cool on ice-water bath immediately after finishing reaction for 2 min.
After this step, the total RNA is converted to first strand cDNAs. cDNA synthesis is done by random priming and the 5′ ends are captured through template switching. We do not recommend checking the reverse-transcription at this step as it will reduce the amount of cDNAs for PCR amplification and therefore increase the number of cycles needed.We do not recommend the use of oligo-dT reverse-transcription primers, as they will preferentially amplify the short transcripts that originate from 3′ promoters (see Carninci et al., 2006, figure 4 B&C). -
3
Purification of first-strand cDNAs by Agencourt RNAClean XP kit (30 min).
Follow the manufacturer's instructions, using pipette mixing, washing with 100 µl 70 % ethanol and eluting with 40 µl nuclease-free water.Before eluting, remove as much washing s olution as possible, and do not let the beads dry, as it may reduce the recovery.This purification will remove smaller artifacts and primer dimers from the cDNAs which cause difficulties in determining the number of PCR cycles required for semi-suppressive PCR by real-time PCR. Again, we do not recommend checking the purification at this step. -
4Determination of PCR cycle number required for second strand cDNA synthesis by quantitative real time PCR (3 h).
- Perform real time PCR in triplicate for the samples and the negative control.
- Prepare 8.5 µl of real time PCR mixture containing 5 µl 2× SYBR Premix Ex, 0.1 µl 10 µM Forward second-strand PCR Primer, 0.1 µl 10 µM Reverse second-strand PCR Primer, 0.2 µl 50× ROX Reference dye II and 3.1 µl nuclease-free water.
- Prepare one µltraAmp PCR plate and add 8.5 µl of real time PCR mixture per well.
- Add 1.5 µl purified cDNA or negative control to master mix.
- Seal the plate with adhesive PCR Seal and complete tight sealing with sealing applicator.
- Centrifuge briefly if there is any splash of the solution above the mixture.
- Start the quantitative real-time PCR (Step One Plus Real-Time PCR System) according to the manufacturer’s instructions. Use ‘Comparative Ct (ΔΔCt)’ for quantification. Employ the following PCR conditions: 95 °C for 1 min, (95 °C 15 s, 65 °C 10 s, 68 °C 2 min) × 40 cycles, hold at 4 °C.
Second strand cDNAs are synthesized and amplified by semi-suppressive PCR at this step. Quantitative real-time PCR performed in small scale to determine the number of cycles required for large scale synthesis. This is important for two reasons: to keep the PCR cycle number as low as possible to reduce bias and to suppress the synthesis of smaller artifacts. -
5Interpretation of the quantitative real time PCR data and determination of number of PCR cycles required (optimum cycle number) for second strand cDNA synthesis (30 min).
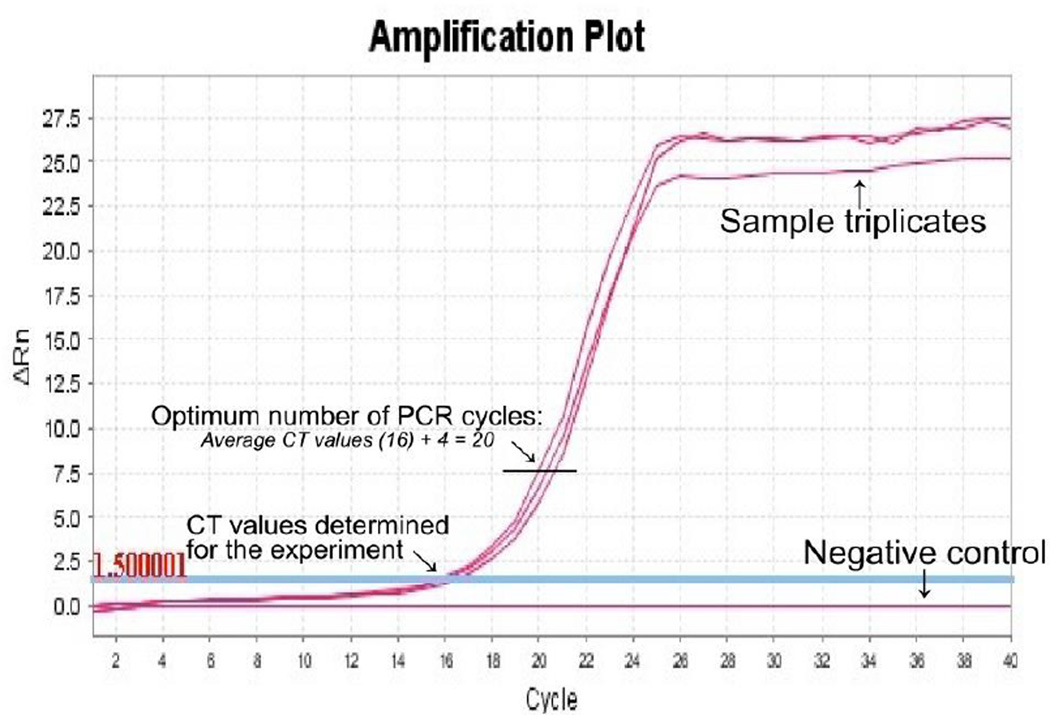
- Collect the ‘Cycle threshold’ (CT) values for the sample and negative control reactions after real-time PCR (Figure 3). Calculate the average CT values for the samples and the negative control.Average CT value for purified cDNA is usually ~16–20 depending on the source of RNA.
- Determine the optimal PCR cycle number by adding 4 to the average CT value.The optimum cycle number should be lower than 25 for the samples and very low or no signal for the negative control.
-
6Second-strand cDNA synthesis and amplification by semi-suppressive PCR (2 h 30 min)
- Perform the second strand cDNA synthesis in large scale: 2 × 100 µl (two tubes) per purified samples and 100 µl for the purified negative control.
- Prepare 85 µl of semi-suppressive PCR mixture per reaction by mixing 10 µl 10× Ex Taq Buffer, 8 µl dNTP Mixture (2.5 mM each), 1 µl 10 µM Forward second-strand PCR Primer, 1 µl 10 µM Reverse second-strand PCR Primer, 0.5 µl TaKaRa Ex Taq HS (5 U/µl) and 64.5 µl nuclease-free water.The concentration of PCR primers is lower than usual, to strengthen the suppressive effect (the competition between primer annealing and self-annealing is the key mechanism of PCR suppression, see Chenchik et al., 1998).
- Add 85 µl of semi-suppressive PCR mixture to each PCR tube.
- Add 15 µl purified cDNA or negative control to each PCR tube.
- Start PCR with the following PCR cycles: 95 °C for 1 min, (95 °C 15 s, 65 °C 10 s, 68 °C 2 min) × n (optimum PCR cycle no.) cycles, hold at 4 °C.The optimum cycle number is usually ~20–24 cycles depending on the source of RNA.
Second strand cDNAs i.e., the nanoCAGE cDNA library synthesis is completed at this step. -
7
PCR tubes may be left overnight in the PCR machine. Store the remaining (5.5 µl) purified cDNAs in the −20 °C refrigerator. Clean up the working bench.
Figure 3.
An example for the determination of the optimum number of PCR cycles for the synthesis of second strand cDNAs by semi-suppressive PCR. A cycle threshold (CT) value is determined for the experiment done by quantitative real-time PCR. The CT value is automatically defined by the qPCR software as the number of cycles required for the fluorescent signal to cross the threshold i.e., to exceeds background level. The optimum cycle number for large scale synthesis of the second strand cDNAs can be determined by adding 4 cycles to the detected CT.
Day 2
-
8
Before beginning, assemble all equipments and solutions (30 min).
-
9Purification of the amplified cDNAs with the Agencourt AMPure XP kit (30 min).PCR and purification should be done at different places using different equipments to avoid cross-contamination.Do not purify the negative control. It is required for later confirmation by electrophoresis that the primer-dimers and traces of the short PCR artifacts were eliminated from the amplified cDNAs.Pool the PCR solutions from two tubes into one. Follow the manufacturer's instructions, using pipette mixing, washing with 600 µl 70 % ethanol and eluting with 30 µl nuclease-free water.Before eluting, remove as much washing solution as possible, and do not let the beads dry, as it may reduce the recovery.
-
10Confirmation of PCR amplification and purification by 1% Agarose gel electrophoresis (30 min).
- Aliquot 2 µl of purified nanoCAGE cDNAs, mix them with 3 µl nuclease-free water and 1 µl 6× loading dye.
- Aliquot 5 µl of non purified negative control, mix with 1 µl 6× loading dye.
- Load all the mixtures and 4 µl of DNA marker in a 1% agarose gel.
- Run the electrophoresis until the separation of markers from 100 bp to 2 kb range. This is typically obtained in 15–30 minutes for agarose minigels.
- Visualize the gel under UV. See Figure 4 for a typical example.
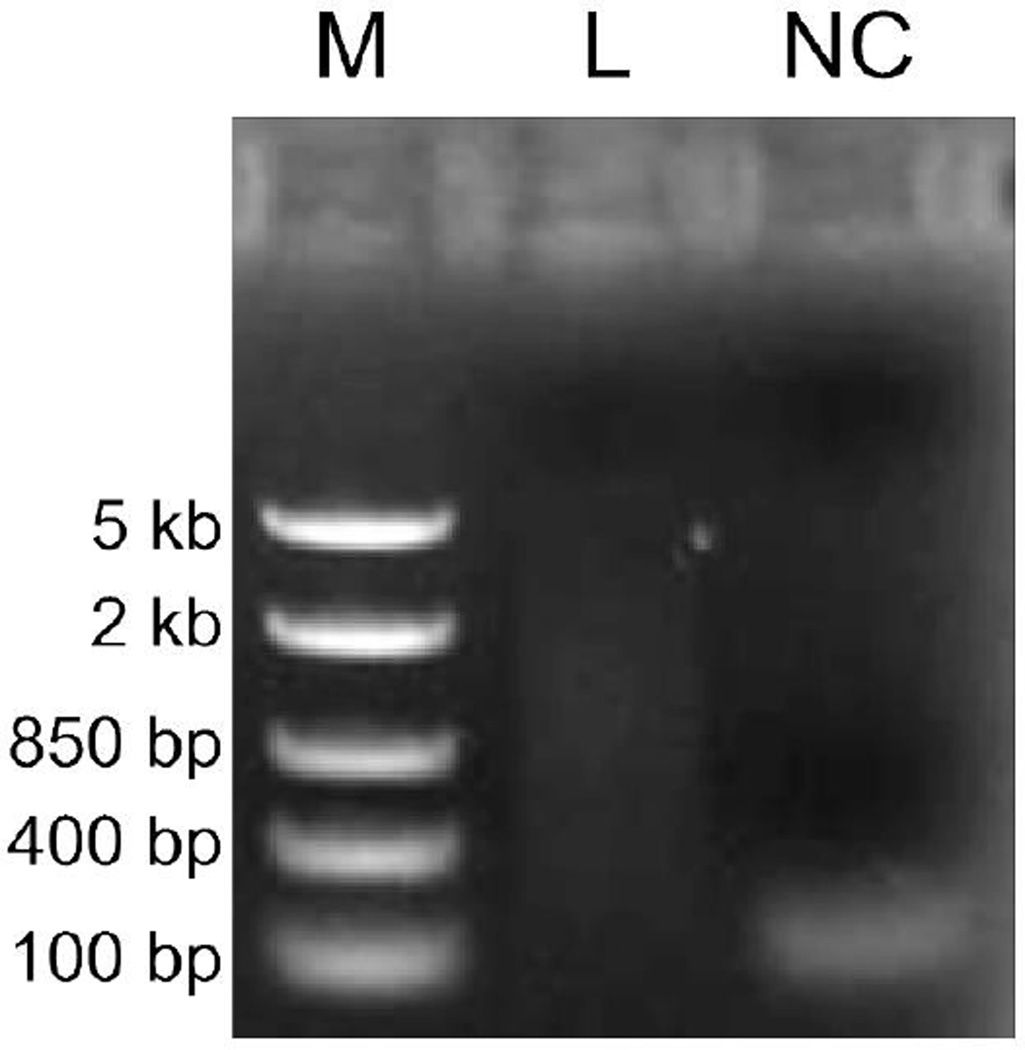
Primer-dimer or artifacts should not be present in the purified cDNAs. Their elimination can be confirmed by comparing the purified cDNAs with the non-purified negative control. -
11Measurement of the concentration of purified cDNAs by NanoDrop (15 min).
- Measure the concentration of the purified cDNAs according to the manufacturer's instruction. Measure in triplicates (1 µl × 3) and calculate the average.
- Dilute all the samples to 10 ng / µl by nuclease-free water.
The concentration of the purified semi- suppressive PCR libraries should be higher than 20 ng / µl to avoid artificial redundancy (see Discussion). -
12PCR to add sequencing adapters (library PCR, 1 h 30 min).
- Perform PCR in large scale: 3 × 100 µl per sample and 100 µl for a control reaction without template.
- Prepare 92 µl of PCR mixture per reaction by mixing 10 µl 10× Ex Taq Buffer, 8 µl dNTP Mixture (2.5 mM each), 2 µl 10 µM Forward Library PCR Primer, 2 µl 10 µM Reverse Library PCR Primer, 0.5 µl TaKaRa Ex Taq HS (5 U / µl) and 69.5 µl nuclease-free water.The concentration of PCR primers is lower than usual because of the small number of cycles, which results in a large leftover.
- Add 92 µl PCR reaction mixture to each tube.
- Add 8 µl (80 ng) purified DNA per sample and 8 µl nuclease-free water to the control reaction without template.
- Start PCR with the following PCR program: 95 °C for 1 min, (95 °C 15 s, 55 °C 10 s, 68 °C 2 min) × 1 cycle, (95 °C 15 s, 65 °C 10 s, 68 °C 2 min) × 6 cycles, hold at 4 °C.
At this step, adapter sequence are added to the nanoCAGE libraries for binding to the Genome Analyzer's flow cell and amplification by bridge PCR. -
13Purification of the library PCRs by Agencourt AMPure XP (30 min).Do not purify the control reaction without template.Before performing the AMPure XP purification, pool the multiple PCR reactions from the same starting cDNA. Follow the manufacturer's instructions, using pipette mixing, washing with 900 µl 70% ethanol and eluting with 30 µl 0. 1% v/v Tween20 in nuclease-free water.Before eluting, remove as much washing solution as possible, and do not let the beads dry, as it may reduce the recovery.
-
14
Measurement of the concentration of the purified libraries by NanoDrop (15 min).
Measure the concentration according to the manufacturer's instructions. Measure in triplicate (1 µl × 3) and calculate the average.The concentration of the purified library PCR products is usually higher than 20 ng / µl and in a typical range from ~20 to 30 ng / µl. -
15Analysis of the nanoCAGE libraries with Agilent Bioanalyzer (1 h 30 min).It is important to check the removal of PCR primer-dimers/artifacts as well as to know the profile of the amplified libraries (size distribution, molar concentration) to calculate the amount of molecules to apply in the sequencing reaction.
- Dilute 1 µl of each purified library to 5 ng / µl with 0.1 % v/v Tween20 and use 1 µl of the diluted library samples for the analysis.
- Follow manufacturer's instructions for the analysis of the libraries by High Sensitivity DNA Kit (Agilent Biotechnologies, Cat #5067-4626).Analyze the libraries in triplicate.
- This protocol assumes that only the cDNAs in the 200–700 bp range are efficiently sequenced in the Illumina GA II sequencers. Therefore, calculate the nanomolar concentration of the size-range of 200–700 bp (average ~500 bp) from the Agilent Bioanalyzer data (Figure 5). Calculate the average of this concentration. Multiply this concentration with the dilution factor (Step 15 i) to obtain the original library concentration.There should be no peak visible shorter than 150 bp on the Bioanalyzer data.
-
16
Sequencing of the nanoCAGE libraries.
The prepared libraries are ready for sequencing on the Illumina GA IIx platform for single or paired-end reads. Sequence the libraries at a 15 pM final concentration.One lane on a GA IIx sequencer generally yields more than 15 millions reads, which although not tested will be larger with HiSeq2000. -
17
Store the prepared nanoCAGE library in a −20 °C refrigerator until sequenced. Clean up working bench.
Figure 4.
Confirmation of semi-suppressive PCR amplification and purification. The purified nanoCAGE library (L) is visible as a smear with small molecular weight artifacts. The non-purified negative control (NC) is used to visualize the artifacts before removal. The electrophoresis was carried out on 1 % agarose gel at 100 V for 15min. M: DNA marker (FastRuler DNA Ladders Middle Range, Fermentas).
Figure 5.
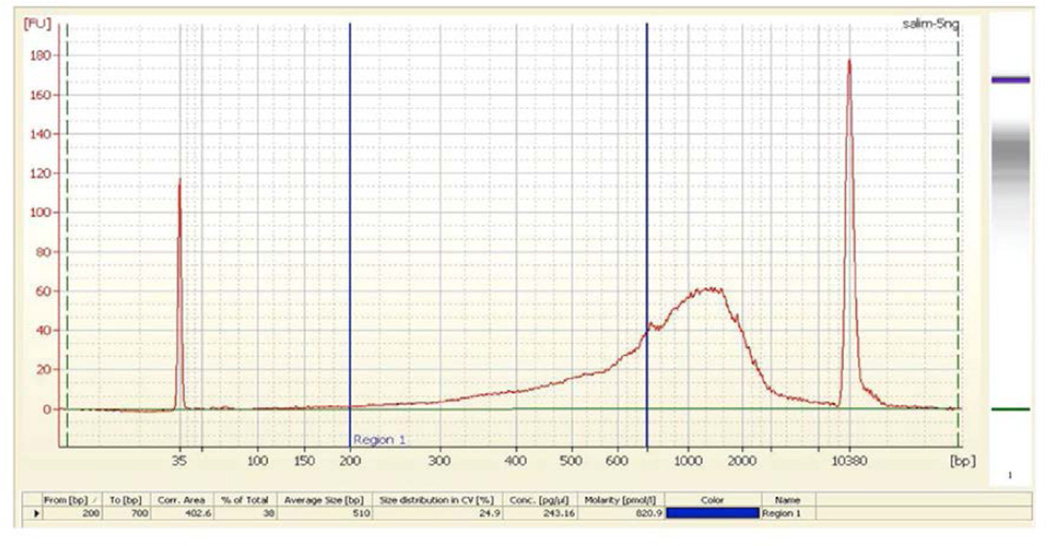
Determination of the molarity of a nanoCAGE library with a Bioanalyzer for sequencing. The picture shows the profile of a typical nanoCAGE library analyzed with a Bioanalyzer (5 ng / µl). This protocol assumes that only the cDNAs in the 200–700 bp range are efficiently sequenced in the Genome Analyzer II as the longer templates are likely to be out-competed by the shorter ones for binding to the flow cell. In addition, they may be difficult to amplify with the bridge PCR. To determine the molarity of the 200–700 bp range, do the following steps: Open the library DNA analysis file (.xad). Double click on the electrophoregram data of the sample to be analyzed. Click on the ‘Region table’ icon under the page. Click right button of mouse after scrolling it into the table, then choose ‘Modify region’. Input 200 and 700 on the box ‘From[bp]’ and ‘To[bp]’ respectively. Click ‘OK’ button to find the molarity. Multiply the obtained molarity with dilution factor to get the original library concentration.
TROUBLESHOOTING
Problem: CT value is low compared to usual (16–20).
[Step 5.i]
Solution: Perform a small scale PCR (20 µl) with the extra purified cDNA solution (step 7) as described in step 6.ii. Take aliquots after 20, 22 and 24 cycles. Confirm amplification and detect optimal cycle number by running the PCR aliquots on a 1 % agarose gel. The number of cycles of the large scale reaction is defined by the moment where signal become visible on gel for sample and the negative control still has no detectable products.
Problem: CT value is higher (> 20) compared to usual.
[Step 5.i]
Solution: The most probable cause is partial degradation of RNA, reverse transcriptase inactivation, impurities in the RNA, presence of inhibitory substances in the RNA, or high concentration (~50 ng / µl) of the smaller fraction (< 200 bp). If possible, re-check the RNA quality with Bioanalyzer. Check reverse transcriptase with a control template. Check for any possible source of contamination during RT preparation. Perform RT reaction with shortest possible time.
Problem: Primer-dimers or short artifacts could not be removed from the library
[Step 9 and 13]
Solution: It may be experienced with the nanoCAGE libraries stared with < 50 ng of total RNA. However, re-purification with AMPure kit may solve this problem.
Problem: The concentration of the purified semi-suppressive PCR library is less than 20 ng / µl.
[Step 11.i]
Solution: Although the library can be sequenced, it may be highly redundant (see Discussion). Therefore, we recommend re-making the library if there is backup sample. Alternatively, the library can be used for promoter discovery (see Discussion).
DISCUSSION
This protocol allows nanoCAGE library preparation from only 50 ng of total RNA, with a level of sensitivity one thousand times higher than CAGE (Kodzius et al, 2006) within only two working days. nanoCAGE overcomes the difficulty of obtaining large quantity of cells for studying such as microdissected sample and future diagnosing diseases such as cancer.
The protocol for nanoCAGE library preparation described here is comprehensively revised (Table 1) and simplified from the original one (Plessy et al, 2010), as it does not use anymore the enzymatic cleavage step that was time consuming and limiting the informative length of the tags to ~25 bp. To revise the original protocol, we prepared libraries with different reverse transcriptases and found that they require different conditions for efficient template switching (Table 1 and Figure 2). We hypothesize that the efficiency of template switching—which depends on the reverse-transcription of the cap (Plessy et al, 2010)—plays a significant role in these differences. We evaluate our libraries according to the fraction of aligned reads corresponding to the rRNA (< 10 %) and redundancy (< 3; defined by the total number of aligned reads divided by the number of unique aligned reads). We observed that libraries prepared with PrimeScript contain approximately twice more reads aligning to promoter regions as libraries prepared using SuperScript III (Figure 2). Therefore, we have selected PrimeScript for reverse transcription for this protocol.
A high redundancy usually indicates a molecular bottleneck, where only a small fraction of the original mRNA molecules contribute reads in the libraries. We observed that libraries where the concentration of the purified cDNAs after semi-suppressive PCR is lower than 20 ng/µl often have a redundancy higher than 5 (Table 1). This could be explained by the preferential amplification a subset of templates, or by initial rarity of PCR templates, for instance because of a low efficiency in the reverse-transcription or template-switching. However, libraries with a high redundancy can still be used for promoter discovery keeping in mind that rare transcripts may not appear and the shape of the transcription start site may become artificially sharpened even with broad promoters (Carninci et al, 2006).
This nanoCAGE protocol is designed for Illumina platform. However, it may be adapted for other sequencing platforms by designing primers suitable for them. NanoCAGE libraries have been successfully prepared from non-polyadenylated RNA, from polysomal RNA, from RNA extracted from histological sections that were stained by immunohistochemistry, and from microdissected samples with laser capture. In general, nanoCAGE will also work on degraded RNA, to the progressive expense of its accuracy of detecting promoters. With de-capped and fragmented samples, nanoCAGE still detects gene expression, but similarly to RNA-seq protocols (Plessy et al., 2010). It may require a higher number of PCR cycles (> 30) to prepare nanoCAGE libraries from total RNA sample which contains a large fraction of small RNAs (< 150 bp), when their molarity becomes in the same order of magnitude as the oligonucleotides used in the reverse-transcription. NanoCAGE libraries may be prepared with less than 50 ng of total RNA, with the risk of a molecular bottleneck and need a high number of PCR cycles (see above).
The transcripts detected by nanoCAGE are strand-specific and once prepared, the library can be sequenced for both 5′-end single read and paired end. Single read sequencing can pinpoint the gene's product—the mRNA molecule—from the transcription start site (TSS) where transcription begins, whereas paired end sequencing can provide insights into the architecture of transcripts and thus into their possible functions by linking TSSs to downstream sequences (Plessy et al., 2010). The ability to fully scan the 3′ end of long transcripts is now limited in range by the length of the paired-end reads. However, further development of sequencing technology will overcome this limitation.
Depending on the source of total RNA, the fraction of sequencing reads that align to ribosomal RNA man bye higher than usual (>8 %). Ribosomal RNA is not capped and hence high quantity of reads matching ribosomal RNA may suggest that the template-switching reaction was not highly specific, and that the detection of the promoters will not be accurate at a base pair level. Some RNA samples contain a larger fraction of rRNAs (polysomal RNA, non-polyadenylated RNA, etc), like Hela cells and the nanoCAGE libraries prepared from these samples will always contain more reads matching the rDNA. We also have the experience that some cell lines contain more ribosomal RNA than others (HeLa > THP-1). Conversely, for many organisms, a full-length sequence of the rDNA repeated unit is not available, and the percentage of reads matching rRNA will not be an accurate measurement of the efficiency of template-switching. The hallmark of CAGE (Carninci, 2010; Kodzius et al, 2006; Valen et al, 2009) is the addition of extra guanosines at the 5′ end of the tags, but for nanoCAGE these guanosines become part of the 5′ linker, and most of them are removed as linker sequence. Nevertheless, mismatches at the 5′ end of the tags are expected to be G in most of the cases. The contrary of this may indicate a problem in the library, for instance degradation of the starting material and therefore a much lower fraction of capped 5′ ends.
The nanoCAGE method has the ability to grasp the complexity of the promoter landscape from fewer cells, without the need to rely on predetermined gene models such as RefSeq. nanoCAGE libraries prepared by this protocol can be exploited for new applications in drug screening, biopsy analysis and whole-transcriptome association studies.
[R] sorbitol/trehalose (3.3 M / 0.66 M) stock solution.
Perform all procedures under RNase-free conditions. Prepare all reagents with nucleasefree H2O.
To prepare Sorbitol/Trehalose stock solution, perform the following steps:
- Preparation of saturated trehalose solution (about 74.4 % for trehalose anhydride):
- Prepare a glass bottle and label it “Saturated trehalose solution”.
- Balance 7.27 g trehalose in a micro balance.
- Transfer 7.27 g trehalose into the glass bottle.
- Add nuclease-free water to the trehalose and make the volume 10 ml.
- Autoclave the saturated trehalose solution for 30 min at 121 °C.
- Preparation of 4.9 M sorbitol stock solution.
- Prepare a glass bottle and label it “4.9 M sorbitol solution”.
- Balance 17.8 g sorbitol using a micro balance.
- Transfer 17.8 g sorbitol into the glass bottle.
- Add 15 ml nuclease-free distilled water to the sorbitol and dissolve.
- Adjust the total volume of the solution to 20 ml.
- Autoclave “4.9 M sorbitol solution” for 30 min at 121 °C.
- Preparation of sorbitol/trehalose stock solution.
- Prepare a 15 ml tube and label it “Setup 3.3 M sorbitol/0.66 M trehalose stock solution”.
- Add 10 ml 4.9 M sorbitol solution to the labeled tube.
- Add 5 ml Saturated trehalose solution to the tube containing the 4.9 M sorbitol solution.
- Add Chelex 100 resin under 1 cm using a disposable pipette tip.
- Mix solution well by using a vortex.
- Leave the solution for 3 h at room temperature (25 °C) without further agitation.
- Centrifuge solution using a Allegra X-12R Centrifuge at 2,000 rpm for 10 min.
- Transfer the supernatant to a new tube labeled “3.3 M sorbitol / 0.66 M trehalose stock solution”.
Solutions treated with Chelex 100 for deionization may turn yellow even after centrifugation. The color does not affect the solution for use in nanoCAGE library production.Store sorbitol/trehalose stock solution at −20 °C. The sorbitol/trehalose stock solution can be used for 6 months.
ACKNOWLEDGMENTS
This work was funded by a Grant-in-Aids for Scientific Research (A) 20241047 for P.C., a grant of the 7th Framework of the European Union commission to P.C. (Dopaminet), a U.S. National Human Genome Research Institute grant U54 HG004557 to P.C, and a Research Grant for RIKEN Omics Science Center from the Japanese Ministry of Education, Culture, Sports, Science and Technology to Y. Hayashizaki. The authors wish to acknowledge RIKEN GeNAS for the sequencing of the libraries J53-GA to J55-GA and J61-GA to J65-GA using the Genome Analyzer IIx (Illumina), as well as for subsequent data processing.
REFERENCES
- Carninci P, Kvam C, Kitamura A, Ohsumi T, Okazaki Y, Itoh M, Kamiya M, Shibata K, Sasaki N, Izawa M, et al. High-efficiency full-length cDNA cloning by biotinylated CAP trapper. Genomics. 1996;37:327–336. doi: 10.1006/geno.1996.0567. [DOI] [PubMed] [Google Scholar]
- Carninci P, Westover A, Nishiyama Y, Ohsumi T, Itoh M, Nagaoka S, Sasaki N, Okazaki Y, Muramatsu M, Schneider C, et al. High efficiency selection of full-length cDNA by improved biotinylated cap trapper. DNA Res. 1997;4:61–66. doi: 10.1093/dnares/4.1.61. [DOI] [PubMed] [Google Scholar]
- Carninci P, Sandelin A, Lenhard B, Katayama S, Shimokawa K, Ponjavic J, Semple CA, Taylor MS, Engström PG, Frith MC, et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet. 2006;38:626–635. doi: 10.1038/ng1789. [DOI] [PubMed] [Google Scholar]
- Carninci P. The science of decoding genes transcription. In: Carninci P, editor. Cap-Analysis Gene Expression (CAGE) Temasek Boulevard, Singapore: Pan Stanford Publishing; 2010. [Google Scholar]
- Chenchik A, Zhu YY, Diatchenko L, Li R, Hill J, Siebert PD. Generation and use of high quality cDNA from small amounts of total RNA by SMART PCR. In: Natick MA, editor. RT-PCR Methods for Gene Cloning and Analysis. Westborough, MA: Eaton Publishing; 1998. pp. 305–319. [Google Scholar]
- Hirzmann J, Luo D, Hahnen J, Hobom G. Determination of messenger RNA 5′-ends by reverse transcription of the cap structure. Nucleic Acids Res. 1993;21:3597–3598. doi: 10.1093/nar/21.15.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodzius R, Kojima M, Nishiyori H, Nakamura M, Fukuda S, Tagami M, Sasaki D, Imamura K, Kai C, Harbers M, et al. CAGE: cap analysis of gene expression. Nat Methods. 2006;3:211–222. doi: 10.1038/nmeth0306-211. [DOI] [PubMed] [Google Scholar]
- Plessy C, Bertin N, Takahashi H, Simone R, Salimullah M, Lassmann T, Vitezic M, Severin J, Olivarius S, Lazarevic D, et al. Linking promoters to functional transcripts in small samples with nanoCAGE and CAGEscan. Nat Methods. 2010;7:528–534. doi: 10.1038/nmeth.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Forrest AR, van Nimwegen E, Daub CO, Balwierz PJ, Irvine KM, Lassmann T, Ravasi T, Hasegawa Y, de Hoon MJ, et al. The transcriptional network that controls growth arrest and differentiation in a human myeloid leukemia cell line. Nat Genet. 2009;41:553–562. doi: 10.1038/ng.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valen E, Sandelin A, Winther O, Krogh A. Discovery of regulatory elements is improved by a discriminatory approach. PLoS Comput Biol. 2009;5:e1000562. doi: 10.1371/journal.pcbi.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitezic M, Lassmann T, Forrest AR, Suzuki M, Tomaru Y, Kawai J, Carninci P, Suzuki H, Hayashizaki Y, Daub CO. Building promoter aware transcriptional regulatory networks using siRNA perturbation and deepCAGE. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq729. [DOI] [PMC free article] [PubMed] [Google Scholar]