Abstract
Rationale
Creatine is thought to be involved in the spatial and temporal buffering of ATP in energetic organs such as heart and skeletal muscle. Creatine depletion affects force generation during maximal stimulation, while reduced levels of myocardial creatine are a hallmark of the failing heart, leading to the widely held view that creatine is important at high workloads and under conditions of pathological stress.
Objective
We therefore hypothesised that the consequences of creatine-deficiency in mice would be impaired running capacity, and exacerbation of heart failure following myocardial infarction.
Methods and Results
Surprisingly, mice with whole-body creatine deficiency due to knockout of the biosynthetic enzyme (guanidinoacetate N-methyltransferase – GAMT) voluntarily ran just as fast and as far as controls (>10km/night) and performed the same level of work when tested to exhaustion on a treadmill. Furthermore, survival following myocardial infarction was not altered, nor was subsequent LV remodelling and development of chronic heart failure exacerbated, as measured by 3D-echocardiography and invasive hemodynamics. These findings could not be accounted for by compensatory adaptations, with no differences detected between WT and GAMT−/− proteomes. Alternative phosphotransfer mechanisms were explored; adenylate kinase activity was unaltered, and although GAMT−/− hearts accumulated the creatine pre-cursor guanidinoacetate, this had negligible energy-transfer activity, while mitochondria retained near normal function.
Conclusions
Creatine-deficient mice show unaltered maximal exercise capacity and response to chronic myocardial infarction, and no obvious metabolic adaptations. Our results question the paradigm that creatine is essential for high workload and chronic stress responses in heart and skeletal muscle.
Keywords: Cardiac contractility and energetics, cardiovascular physiology, heart failure, metabolism, transgenic mice
INTRODUCTION
Creatine is ubiquitous among vertebrates where it is commonly considered essential for maximal performance in cells with dynamic energy demands, such as neurons and all types of muscle cell. In omnivores, 50% of the daily creatine requirement is met from dietary sources, but the remainder (~1g in humans) has to be synthesised,1 representing a significant metabolic investment.
Creatine has no metabolic role other than the reversible conversion to phosphocreatine (PCr) under the influence of the creatine kinase (CK) enzyme 2: Cr + ATP ↔ PCr +ADP + H+
This reaction is the only phosphagen system in vertebrates and has several putative functions, (i) it provides control over localised concentrations of adenosine triphosphate and adenosine diphosphate (ATP/ADP ratio), which is important to maximise the chemical energy from ATP hydrolysis (ΔGATP), i.e. the energy available to perform work; (ii) PCr is more readily diffusible than ATP and can perform an energy transfer role linking ATP-producing mitochondria to sites of ATP utilisation; and (iii) as an energy buffer, with PCr present in relatively high concentrations and available for rapid regeneration of ATP when energy demand outstrips supply.3–6
Interest in the role of creatine in the heart is driven by the consistent observation that both total creatine and CK activity are down-regulated in heart failure regardless of aetiology,3, 7, 8 and that the ratio of PCr/ATP represents an excellent prognostic marker in dilated cardiomyopathy.9 The widely held view is that creatine is only of marginal importance for normal baseline cardiac function, but is essential for attaining very high workloads and becomes critical under conditions of pathological stress.3, 6 For example, chronic treatment of rats with β-guanidinopropionic acid (β-GPA) reduces total creatine by 50–70% but has minimal effect on resting cardiac function in vivo.10, 11 However, maximal stimulated responses are blunted and the severe stress of an experimental myocardial infarction causes death in 93–100% of treated rats within 24 hours.11, 12
However, β-GPA has many limitations, such as potential off-target effects, slow and incomplete creatine depletion, and the ability to partially compete in the creatine kinase reaction.13 Genetically manipulated mouse models circumvent these limitations and provide a new approach to assess the functional significance of the PCr/CK system. Guanidinoacetate methyl-transferase (GAMT) is the second of two essential enzymes in the biosynthesis of creatine. Therefore, GAMT knockout mice fed a creatine-free diet have a chronic, and absolute, deficiency of creatine and PCr in all organs.14 We have previously shown that GAMT−/− mice have normal LV ejection fraction up to one year of age,15 and mildly reduced systolic pressure development.16 Furthermore, contractile reserve is impaired, as is recovery from acute myocardial ischemia, in line with the PCr/CK system being particularly important under conditions of acute stress.17, 18
However, the chronic functional significance of these defects (measured acutely under general anaesthesia or in isolated hearts) has not been tested in intact conscious mice where workload is higher. We therefore hypothesised that creatine-deficient GAMT−/− mice would have reduced exercise capacity and develop more severe heart failure post-myocardial infarction. To our surprise, this was not the case, and we therefore looked for major compensatory adaptations. However, comparison of GAMT−/− and WT proteomes, mitochondrial function, and auxillary phosphotransfer pathways all failed to identify adaptations that could explain these results, questioning the importance of creatine for physiological and pathological stress responses in the intact laboratory mouse.
METHODS
Full details of all methods are included in the online Supplemental Material.
Animal husbandry
GAMT knockout mice on a pure C57BL/6J genetic background (backcrossed for >10 generations) were genotyped by PCR as previously described.14 Breeding was by heterozygous mating to provide littermates as wild-type controls (WT). Post-weaning, GAMT−/− and WT mice were housed separately to prevent creatine absorption via coprophagia. Mice were fed standard chow (which is naturally creatine-free), water ad libitum and kept in specific pathogen-free conditions, with 12h light–dark cycle at 20–22°C. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Voluntary wheel running
Individually caged female mice were provided with a running wheel in the home cage and exercise parameters continuously data-logged over a 3 week period as previously described.19 Maximum running speed and distance were compared between n=8 WT, n=6 GAMT−/− mice, and n=6 creatine-supplemented GAMT−/− mice (0.75% creatine mixed with standard chow started 7 days before the running protocol). At the end of the protocol, mice were killed by cervical dislocation with LV and hind-limb skeletal muscle snap frozen in liquid nitrogen for determination of creatine content by HPLC as described previously.20
Forced treadmill running
Running capacity was tested in WT and creatine-free GAMT−/− mice (n=8 per group, with 4 males and 4 females of each) using an Exer-6M treadmill with Simplex II control unit and Shock detection unit (Columbus Instruments, Ohio, USA). Mice were familiarised to the apparatus on two consecutive days for 10 minutes at 8.5m/min and 5° incline. All mice learned to run on the treadmill to avoid electric shocks from the grid (1.4mA; 200ms duration). Mice were then tested to exhaustion, defined as the point when mice would no longer run for more than 2s at a time preferring to sit on the shock grid even when touched by a gloved hand. Mice were started running at 8.5m/min for the first 9 minutes, which increased to 10m/min for 3 min, then increased by 2.5m/min increments every 3 min thereafter (5° incline throughout). GAMT−/− mice were changed to creatine-supplemented chow (as above) and all mice retested 7 days later. Vertical work was calculated as described in.21
Chronic infarct study
Surgery to induce permanent myocardial infarction (MI) was performed as previously described.22 A total of 104 mice underwent surgery: WT n=41 (25M/16F) and GAMT−/− n=38 (18M/20F) to induce MI; WT n=13 (5M/8F) and GAMT−/− n=12 (5M/7F) sham-operated. Mice were aged 21–45 weeks, with average age comparable between all groups. 3D-echocardiography was performed at 1 and 6 weeks post-surgery under 1.5% isoflurane anesthesia as previously described.23 Hemodynamic measurements were obtained at 6 weeks as previously described,16 with contractile reserve tested by dobutamine infusion via the jugular vein. Isoflurane anaesthesia was used for all protocols at 1–2% in medical oxygen, with adequate depth of anesthesia assessed by lack of pedal reflex. Buprenorphine 1mg/kg subcutaneous injection was given for post-surgical analgesia.
β-GPA treatment and acute MI survival
WT and GAMT−/− (n=6 of each) were given 200μl intraperitoneal injection of 0.5mol/L solution of β-guanidinopropionic acid (Sequoia Research Products Ltd, Reading, UK) 24 hours prior to myocardial infarction (as above). Injections were repeated immediately before surgery and at 24 hour intervals thereafter. Surviving mice were euthanized 48 hours post-surgery, LV perfused with vital dye to estimate infarct size, and RV used to determine myocardial levels of β-GPA by HPLC.
31P-NMR magnetization transfer
Flux through the CK reaction was measured in ex vivo perfused hearts from male GAMT−/− and WT mice (n=4/group) using a 31P-NMR magnetization (saturation) transfer protocol 24 on a Bruker Avance 500 spectrometer equipped with a 11.7 T magnet and a 20 mm 1H-/31P-crosscage resonator.
Proteomics
Powdered LV tissue from WT and age-matched male 16 week old GAMT−/− siblings was used. Two-dimensional difference in-gel electrophoresis (DIGE) was performed on 4 paired samples, as previously described.25 Non-Label Quantitative Mass Spectroscopy was performed on LV proteins from 3 GAMT−/− and 3 WT male mice as previously described.26
Enzyme activities and immunoblots
LV homogenates from GAMT−/− and WT hearts were used to determine adenylate kinase (AK), glycolytic enzymes, and pyruvate dehydrogenase activities spectrophotometrically as described in the Online Supplement. The relative abundance of respiratory chain enzymes in LV homogenates was determined using the MitoProfile® Rodent Total OXPHOS Complexes Detection Kit (Cambridge BioScience, UK). Antibodies were detected with the ECL Advance chemiluminescence kit (GE Health Care, UK), and a FluorChem 8800 imager.
Mitochondrial function experiments
Saponin–permeabilized cardiac fibers isolated from the left ventriclar endocardium were used to measure respiration of the total mitochondrial population in situ using a Clark-type oxygen electrode (Strathkelvin Instruments, UK). Superoxide production was measured by incubation of LV homogenates with dihydroethidium (DHE) and subsequent fluorescent detection of 2-hydroxyethidium (2-OHE) by HPLC.
Data analysis
All measurements were performed independently and analysed blind. Data are expressed as mean ± sem. Statistical analysis for the infarct study was by one-way ANOVA with Bonferroni’s correction for multiple comparisons. Kaplan-Meier survival curves were compared by log-rank test. Data comparing two groups was performed by unpaired Student’s t test. Differences were considered significant when P<0.05. Statistical analysis of proteomics data is detailed in the on-line supplement.
RESULTS
Voluntary exercise capacity
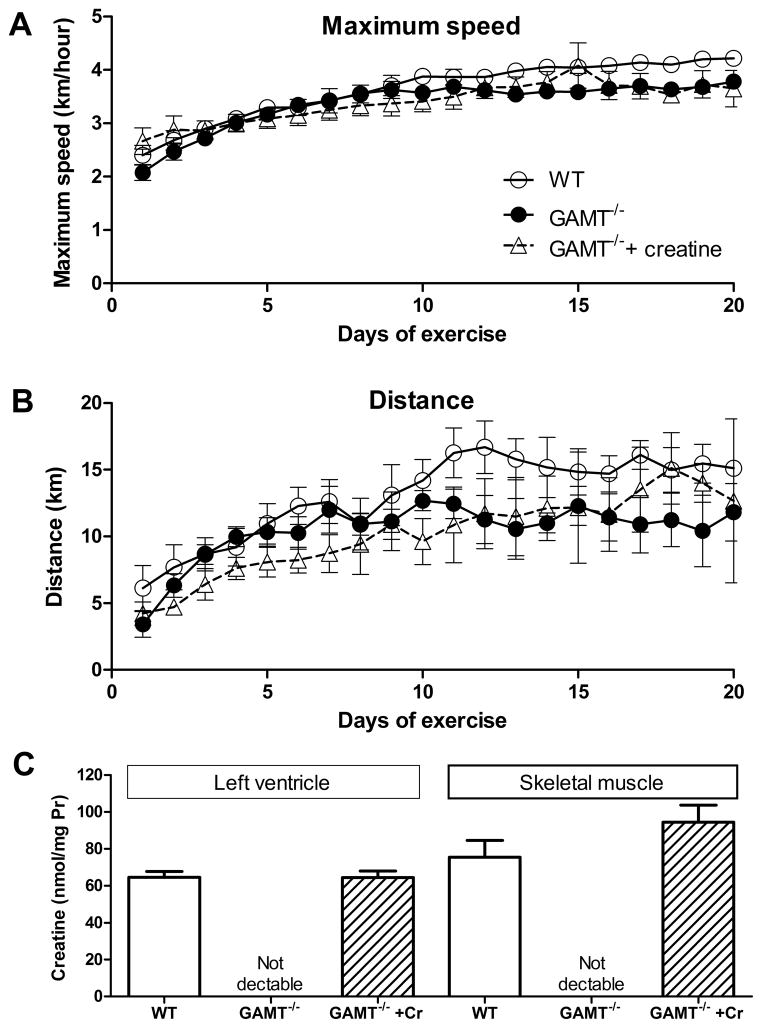
Voluntary exercise capacity in GAMT−/− and WT mice was tested by providing running wheels in the home cage. There was no difference in maximum speed (Figure 1A), or in cruising speed and average speed (data not shown). There was a non-significant trend for GAMT−/− to run less distance per night, however this difference remained even in GAMT−/− with creatine supplementation (Figure 1B), suggesting it is unrelated to creatine deficiency. The absence of creatine in GAMT−/− LV and skeletal muscle was confirmed by HPLC and creatine supplementation was shown to be effective at increasing tissue creatine levels to normal WT values (Figure 1C). Therefore, creatine deficiency did not limit the ability of GAMT−/− mice to run as fast and as far as controls, with mice actively choosing to run >10km per night.
Figure 1. Assessment of exercise capacity by voluntary wheel running.
Each point represents mean ± s.e.m. from 8 wild-type (WT), 6 GAMT−/−, and 6 creatine-supplemented GAMT−/− female mice (0.75% creatine in chow started 7 days before the running protocol). Maximum speed was not significantly different between groups (A). There was a non-significant trend for GAMT−/− mice to run less distance per night, however this was not directly related to creatine depletion since creatine supplementation did not alter distance run (B). Tissue creatine levels were undetectable in left ventricle and skeletal muscle of GAMT−/− mice using HPLC, whereas creatine supplementation returned tissue creatine to normal WT levels (C).
Forced exercise capacity
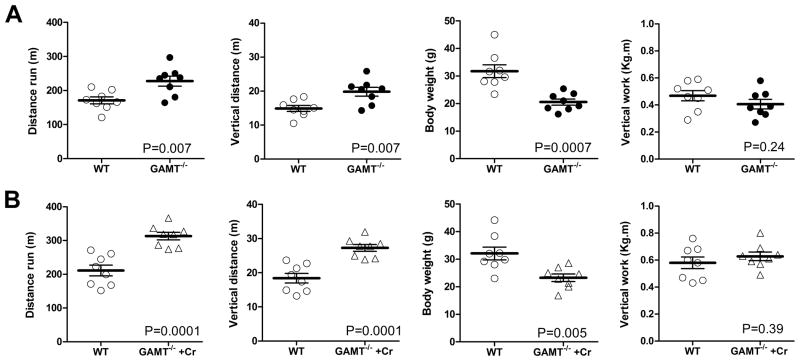
To corroborate these findings, mice were tested to exhaustion on a treadmill by using forced continuous running with an escalating velocity protocol. Creatine-free GAMT−/− mice ran further than WT controls, gaining more vertical distance (Figure 2A) and attaining maximum speeds of 22.5m/min on a 5° incline. Vertical work was calculated in order to account for the reduced body weight in GAMT−/− mice, showing that both WT and creatine-free mice performed the same amount of work. The treadmill test was repeated after 7 days of creatine-supplementation in the GAMT−/− mice and similar results were obtained (Figure 2B). Both groups improved running capacity suggesting a learning/training effect, however there was no additional benefit from replenishing tissue creatine levels in GAMT−/− mice. This indicates that creatine deficiency did not impair the ability of GAMT−/− mice to run.
Figure 2. Assessment of maximal exercise capacity by forced treadmill running.
Eight wild-type (WT) and GAMT−/− mice were forced to run to exhaustion using a standardised protocol of escalating speeds and 5° incline. Creatine-free GAMT−/− mice ran further than control mice covering more vertical distance (panel A). However, there was no difference between genotypes when vertical work was calculated, which takes into account the lower body weight of GAMT−/− mice. GAMT−/− mice were then changed to chow containing 0.75% creatine and the running test repeated after 7 days (panel B). Both groups improved running capacity suggesting a learning/training effect, however there was no additional benefit from replenishing tissue creatine levels in GAMT−/− mice.
Chronic heart failure secondary to myocardial infarction
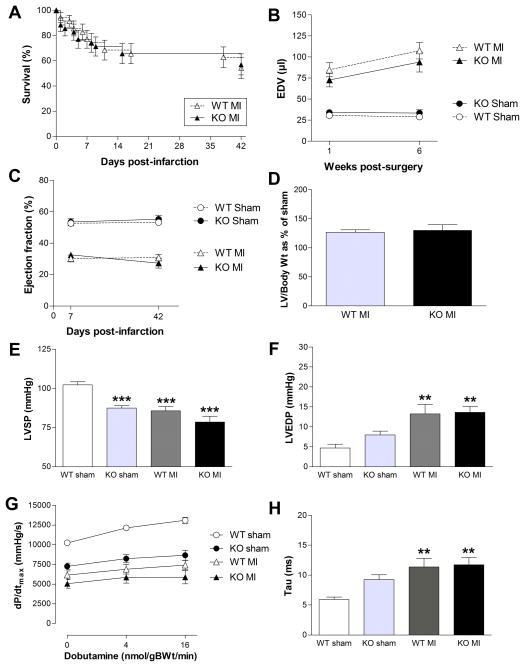
Six weeks after permanent MI, survival was not different between genotypes (54% WT vs. 57% KO; Figure 3A). For subsequent analysis, infarct size was retrospectively matched between groups, an important requirement to observe the effect on chronic heart failure (CHF) independent of any effect on ischemic damage. Mean infarct size was 40% of LV circumference in both groups (WT range 26–57%; KO range 26–55%). 3-D echocardiography revealed significant LV dilatation and impaired ejection fraction compared to sham (P<0.0001 for both), but with no effect of genotype (Figure 3B–C). LV hypertrophic response measured at post-mortem was also indistinguishable (Figure 3D). Likewise, LV hemodynamics showed post-MI changes commensurate with development of CHF, but to an equal extent in WT and GAMT−/− mice (Figure 3E–H). This was despite impaired baseline function in KO sham mice, with significantly lower LV systolic pressure (P<0.0001) and impaired contractile reserve compared to WT sham (Figure 3G; P<0.0001). That creatine-free mice survive MI at all is a highly surprising result since rats fed β-GPA to deplete creatine stores have 93–100% mortality within 24 hours11, 12. To investigate the reason for this discrepancy, WT and KO mice were administered with β-GPA 24 hours prior to infarct, repeated daily until 2-days post-infarct. Mean plasma β-GPA was 1.3±0.1mM and myocardial tissue levels 195±43 nmol/mg protein (n=3). For comparison, normal myocardial creatine levels are ~70 nmol/mg protein. All six WT and 5/6 KO mice survived this protocol (infarct size 39±3% and 36±3% respectively), suggesting there are species differences in response to depleted creatine or β-GPA rather than off-target effects.
Figure 3. Response to chronic myocardial infarction (MI) in WT and GAMT KO mice.
(A) Kaplan-Meier survival curves were not significantly different for post-MI survival. (B–C) 3D-echocardiography at 1 and 6 weeks post-MI showing significant dilatation (enlarged end-diastolic volume, EDV) and impaired ejection fraction in both infarct groups compared to sham, but with no differences between genotypes. (D) Post-mortem LV weights (% of sham control) indicate no difference in hypertrophic response. (E–F) LV systolic pressure (LVSP) and end-diastolic pressure (LVEDP) respectively. (G) Inotropic response to β-adrenergic stimulation was significantly lower in all groups compared to WT sham (2-way ANOVA; P<0.001), but was not different between infarct WT and KO groups. (H) Isovolumetric constant of relaxation (tau) was significantly prolonged in both MI groups. *** denotes P<0.001, and ** denotes P<0.01 compared to WT sham group.
Search for compensatory mechanisms in GAMT−/− mice
We looked for adaptive or compensatory mechanisms that might mitigate the negative effects of chronic creatine-deficiency.
Wall stress
One explanation could be differences in LV wall stress, which is an important driving force for myocardial remodelling. GAMT−/− mice have slightly smaller hearts compared to littermate controls, and lower LV systolic pressure, however, end-systolic meridional wall stress was not significantly lower in GAMT−/− mice (45±15 g/cm2) compared to WT (51±11 g/cm2; P=0.34; n=10).
Role for alternative phosphagens?
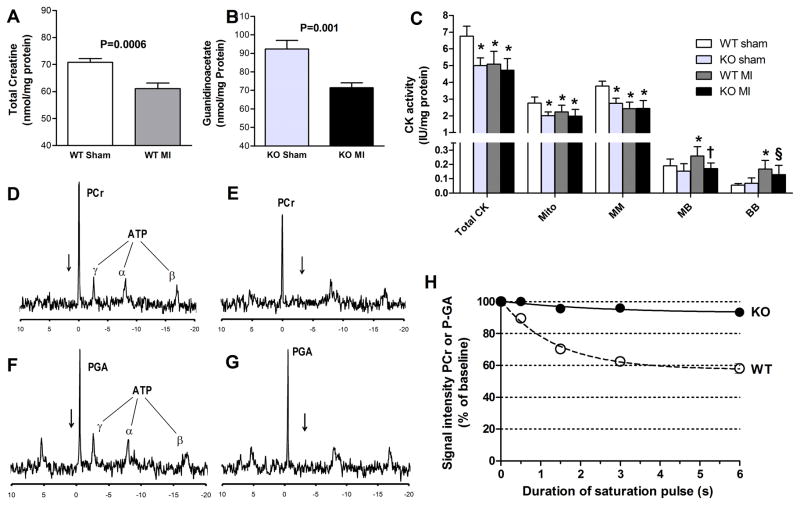
Characteristic of chronic heart failure, LV total creatine levels were 14% lower in infarcted versus sham WT mice (Figure 4A). GAMT−/− mice were creatine-free, but accumulated the creatine precursor guanidinoacetate, which was similarly reduced post-MI (23% lower than Sham; Figure 4B). Creatine kinase activity was 26% lower in sham operated KO versus sham WT hearts. Following myocardial infarction, mito- and MM- isoenzyme activities were lower in WT, but did not fall further in KO hearts, whereas activity of MB-isoenzyme was higher in WT and BB-isoenzyme higher in both groups (Figure 4C). To determine to what extent guanidinoacetate can act as an alternative phosphagen by participating in the creatine kinase reaction we measured CK flux in perfused hearts by 31P-MRS magnetization transfer. An example spectrum from WT heart shows a large PCr peak and three phosphoryl groups from ATP (Figure 4D). Selective saturation at the frequency of the [γ-P]ATP peak results in reduced amplitude of the PCr peak, indicating significant phosphoryl transfer between ATP and PCr in WT hearts (Figure 4E). In GAMT−/− mice, there is a large phospho-guanidinoacetate (PGA) peak (Figure 4F), but equivalent flux of [γ-P] between ATP and guanidinoacetate was not detectable (Figure 4G). Figure 4H shows averaged data (n=4/group) as a function of saturation pulse duration (effect of genotype P<0.0001).
Figure 4. Utilization of phosphogens in the heart.
(A) Myocardial creatine concentration is reduced in post-MI heart failure in WT hearts. (B) Analogous reduction in guanidinoacetate (GA) in GAMT KO mice. (C) activity of total CK and constituent isoenzymes; * denotes P<0.01 compared to WT sham; † denotes P<0.05 for WT MI versus KO MI; and § denotes P<0.05 for KO sham versus KO MI. (D–G) Representative 31P-MRS spectra in Langendorff perfused hearts. Panels D and F are from WT and KO hearts and indicate the large phosphocreatine (PCr) and phosphoguanidinoacetate (PGA) peaks respectively, with three smaller peaks representing the phosphoryl groups of ATP. The arrow shows frequency of selective saturation in magnetization transfer protocol. When this is applied at the specific frequency of the [γ-P]ATP peak, a reduction in PCr peak is observed, indicating phosphoryl flux between ATP and PCr in WT (E). In KO hearts there is no change in PGA peak (G), indicating no measurable flux of [γ-P]-between ATP and GA. (H) Quantification of change in PCr/PGA signal intensity as a function of saturation pulse duration (n=4/group).
Comparison of LV proteomes
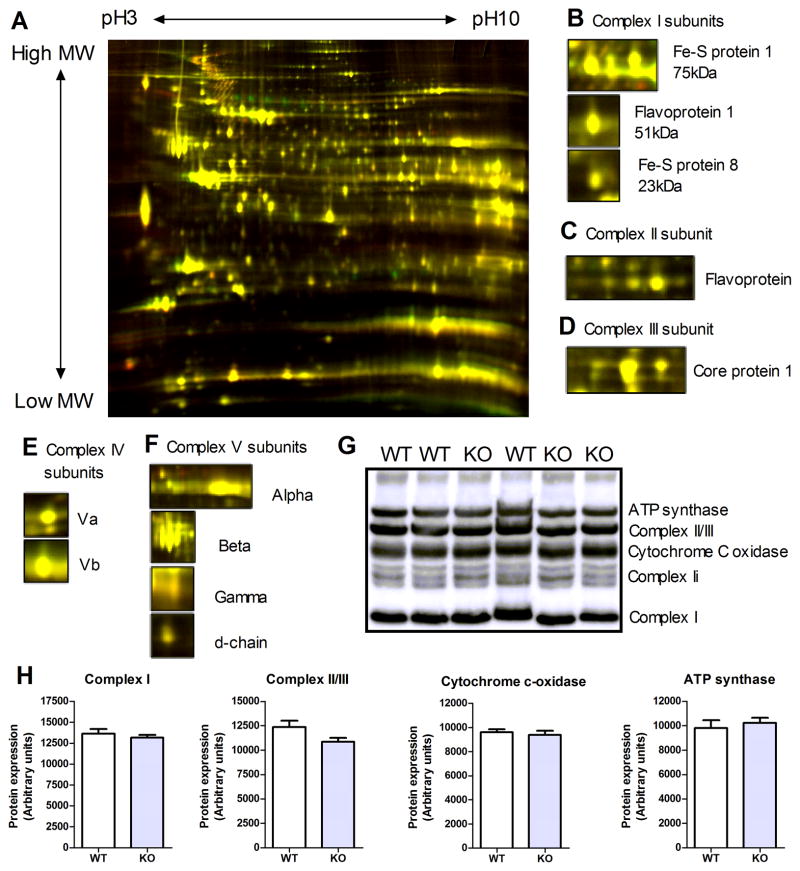
2-D difference in-gel electrophoresis (DIGE) was used as a hypothesis-free approach to identify compensatory mechanisms. However, the image shown in Figure 5A illustrates that there was very little variation in the proteomes of 16-week old WT and GAMT−/− hearts. This was confirmed by quantitative mass spectrometry using iTRAQ protein labeling, which revealed no statistically significant differences in protein expression for the 546 proteins identified (see Online Table II). Particular attention was paid to respiratory chain enzymes (Figure 5B–F), but Western blotting confirmed no significant differences in protein expression of complex I, complex II/III, cytochrome-c oxidase, or ATP synthase (Figure 5G–H).
Figure 5. Proteomics analysis.
(A) Example of 2-D difference in-gel electrophoresis from age-matched WT and GAMT KO hearts. Expansion of several protein subunits from Complex I (B), Complex II (C), Complex III (D), Complex IV (E), and Complex V (F). WT proteins were labelled with Cy3 (red) and GAMT KO proteins were labelled with Cy5 (green) for n=4 paired samples. Proteins were separated in the horizontal direction by isoelectric focusing point (~pH 3 to pH 10) and in the vertical direction by molecular weight (~150 kDa to 10 kDa). Protein subunits were identified by mass spectrometry. Western blots for respiratory chain enzymes (G), with corresponding densitometric analysis (H) confirming no difference in protein expression (n=7 WT and n=8 KO).
Alternative phosphotransfer and other metabolic pathways
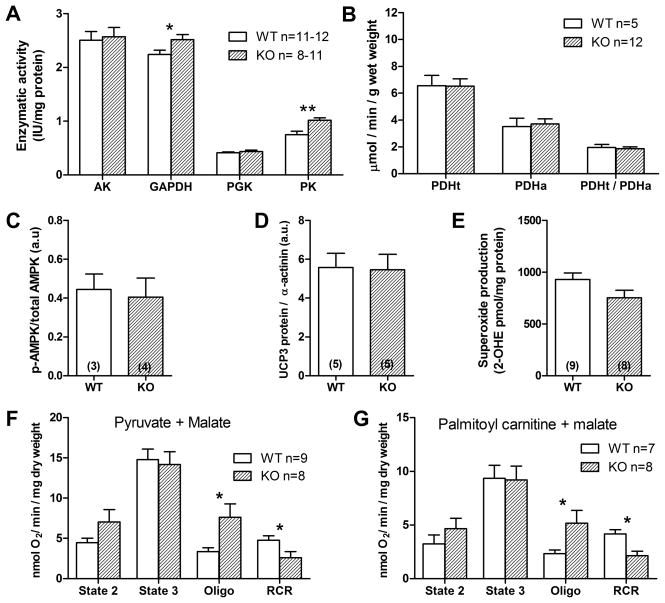
Adenylate kinase (AK) can function as an auxillary high-energy phosphotransfer system, but activity was not significantly different between WT and GAMT−/− hearts (Figure 6A). Activity of key glycolytic enzymes were measured since glycolysis could compensate by localizing to ATPases or performing auxillary phosphotransfer. 3-phosphoglycerate kinase (PGK) was not altered, while activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was elevated by 12% (P=0.03) and pyruvate kinase (PK) activity by 36% (P=0.001) compared to WT (Figure 6A). Activity of pyruvate dehydrogenase (PDH) was unchanged, suggesting that glycolytic contribution to the Krebs cycle was unaltered. Since the CK system helps to maintain normal ratios of adenosine nucleotides, we also ruled out changes in AMPK activation (Figure 6C).
Figure 6. Cardiac energetic profile in LV homogenates from 6 month old WT and GAMT KO mice.
(A) enzyme activites for total adenylate kinase (AK) and the major glycolytic enzymes: glyceraldehyde- 3-phosphate dehydrogenase (GAPDH), 3-phosphoglycerate kinase (PGK) and pyruvate kinase (PK). (B) Activity of total and active fractions of pyruvate dehydrogenase (PDHt and PDHa respectively). (C) Phosphorylated fraction of AMP-activated protein kinase (AMPK) protein. (D) Protein expression of uncoupling protein-3 (UCP3) and superoxide production (E) is not significantly different. (F–G) Oxygen consumption from permealised LV fibres reflecting mitochondrial respiration in response to different substrates. State 2 is basal unstimulated respiration, state 3 is maximal ADP-stimulated respiration, Oligo is uncoupled respiration (state 4o) in presence of oligomycin to inhibit ATP synthase. RCR is the respiratory control ratio (i.e. state 3/4). All data are mean±sem, sample numbers in parenthesis. * denotes P<0.05, ** P<0.01 for WT versus KO.
Mitochondrial function
Oxygen consumption was measured in permeabilised cardiac fibres as a measure of mitochondrial respiration and therefore ATP production. The response to pyruvate + malate and palmitoyl carnitine + malate as substrates was typical (Figure 6F–G; results for other substrates are shown in Online Table I), with a trend for increased baseline respiration (state 2) that could be accounted for by increased proton leak (oligo), and no change in maximal stimulated respiration (State 3). Since the difference between oligo and state 3 represents coupled oxygen consumption, this suggests that mitochondrial ATP production is, if anything, impaired and certainly not a compensatory mechanism for the loss of creatine. Despite increased proton leak, we observed no differences in protein expression of uncoupling protein 3 in LV homogenates (Figure 6D), or in superoxide production (Figure 6E).
DISCUSSION
Here we show that, contrary to expectations, creatine is not essential for maximal exercise capacity, or for acute survival from myocardial infarction, nor in the setting of chronic heart failure. Previous findings have indicated impaired cardiac contractile reserve (confirmed in this study) and skeletal muscle force generation in response to maximal stimulation in anaesthetised mice,11, 16, 27 but this is apparently non-limiting when the long term consequences are tested in conscious animals or in the chronic setting.
A key question is whether the running regimes truly achieved maximal exercise capacity. It has previously been shown that C57BL/6 mice perform best on voluntary wheel running, with higher maximal speed and exercise intensity compared to treadmill,28 and this is exactly the pattern we observed. Furthermore, the maximum speed attained during voluntary exercise in our study was 3.5km/hour (= 58m/min), which is in good agreement with the reported functional maximum for mice of ~50m/min.29 These findings were confirmed in a forced exercise protocol where mice were tested to exhaustion and where it was possible to correct for differences in body weight by calculating vertical work. In both exercise regimes, creatine-free mice performed just as well as wild-type and this was not altered by creatine feeding. However, we cannot rule out that higher exercise capacities might be possible under non-laboratory conditions in the wild.
Several studies have previously used the creatine analogue β-guanidinopropionic acid (β-GPA) to deplete creatine levels in rodents. β-GPA competes with creatine for cellular uptake, but is a poor substrate for CK. Chronic β-GPA feeding results in creatine depletion of between 40–87% depending on dose and length of treatment,11, 12, 30, 31 and results in exceptionally high mortality after MI, e.g. 93%11 to 100%12 mortality within 24 hours. Since the GAMT−/− mouse model represents 100% creatine depletion, it is therefore highly surprising that mortality was not increased. To provide further insight we injected mice with β-GPA immediately before and after myocardial infarction. This resulted in high levels of myocardial β-GPA but did not adversely affect 48 hour survival, indicating that the discrepancy in mortality is not due to off-target effects of β-GPA, but rather species differences between mice and rats. Since β-GPA treatment in rats increased the incidence of post-MI ventricular arrhythmia,11 our findings may simply reflect that mice are much more resistant to this type of arrhythmia,22 in part, due to the small myocardial surface area that is less able to sustain re-entry events.32
We observed an increase in state 4 respiration in GAMT−/− mice suggesting protein leak and mitochondrial uncoupling, that may be a direct effect of creatine depletion.33 It has previously been demonstrated that mild mitochondrial uncoupling protects against ischemic injury,34 so could this compensate for creatine-loss in the MI model? This is unlikely since protection was dependent on increased production of reactive oxygen species, while we observed no difference in superoxide generation between WT and KO hearts. In addition, our infarct model uses permanent coronary artery ligation, which means there is very little potential for rescuing ischemic tissue and we retrospectively match experimental groups for infarct size in order to control for potential differences in ischemic injury.
A key question is to what extent the creatine precursor guanidinoacetate can compensate by acting as a phosphagen. GAMT−/− mice accumulate guanidinoacetate (GA),14 forming phosphorylated (P-)GA by interaction with cytosolic CK,13 and this can then act as an energy reservoir during acute ischemia.16 We therefore tested CK reaction velocity in isolated perfused hearts using 31P-NMR saturation transfer, but were unable to detect ATP synthesis from PGA. This suggests that use of PGA is neither rapid nor efficient in the intact heart, consistent with in vitro studies where flux through the CK reaction was 100x lower compared to creatine.13 While it is not possible to completely rule out a small compensatory effect of PGA, it is highly unlikely to explain the discrepancy in mortality with the β-GPA model, since β-GPA can also be phosphorylated by CK with similarly slow reaction kinetics.13
We did not detect any significant differences in protein expression between GAMT−/− and WT hearts, strongly arguing against the existence of major compensatory mechanisms. Furthermore we ruled out changes in activity of key metabolic proteins such as adenylate kinase and pyruvate dehydrogenase and in AMPK activation. One minor change that could be interpreted as compensatory was an increased activity for the glycolytic enzymes GAPDH and PK in GAMT−/− hearts. However, the functional significance of these small changes in some, but not all, glycolytic enzymes is questionable. This could be related to stimulation of glycolysis by elevated inorganic phosphate,35 but PGK activity remained unchanged. A more likely explanation is the absence of PCr, which would usually exert direct inhibitory effects, specifically on these two enzymes.36
Another approach in evaluating the role of creatine is to knockout the isoforms of creatine kinase, i.e. normal total creatine levels but non-functioning CK system. These mice have impaired exercise capacity in voluntary running experiments37, 38 and eventually develop baseline dysfunction and cardiac hypertrophy 39 in some, but not all studies 38, dependent on genetic background.40 This represents a more severe phenotype than creatine-deficiency models, which suggests that loss of substrate and loss of enzyme are not biochemically equivalent. It seems likely that some of the effects of removing creatine kinase may be related to the properties of the protein and unrelated to the CK reaction. Creatine kinase represents a significant fraction of the total muscle protein and may be involved in multi-protein complexes,41 furthermore, mitochondrial-CK may have a structural role within the mitochondrial inter-membrane space.4 In contrast, GAMT is not expressed in muscle cells since creatine biosynthesis occurs in kidney and liver,6 therefore there is no associated loss of protein.
Impaired inotropic reserve is one of the few differences we observed between WT and GAMT−/− mice and recapitulates our previous findings.16 There are multiple potential underlying mechanisms, such as changes in calcium handling, calcium-sensitivity of contractile proteins, or in β-adrenergic signalling. Energetic reserve is another important determinant of inotropic reserve,18 suggesting that energy substrate metabolism and the free energy available from ATP hydrolysis (ΔGATP) may also be of interest for further study.
There are some limitations to our study. GAMT −/− mice are lighter than WT, with changes in body composition and long bone length14 which may alter biomechanical properties and therefore running capacity. We attempted to control for this by including a group of creatine-supplemented GAMT −/− mice. It seems likely that the non-significant trend for distance run observed between these mice and WT is accounted for by differences in body size and biomechanics. Nor can we rule out that creatine confers a survival benefit under maximum stress conditions in the wild, which laboratory stress-tests fail to emulate. However, when considering the heart alone, it is difficult to inflict greater stress than myocardial infarction affecting 40% of the ventricle. Nor can we rule out all possible adaptive changes that might compensate in GAMT−/− mice, although we have tried hard to achieve this in a non-biased way using proteomics. Our search for adaptations used non-infarcted mice and it remains a possibility that we missed adaptations that are relevant only to the post-infarct setting. Specifically, our findings should not be extrapolated to the brain, where neurological defects are consistently observed in patients with in-born errors of creatine biosynthesis.42
Similarly, our results do not mean that augmenting the creatine / CK system could not still be beneficial. Ischemia / reperfusion injury is a clinically relevant scenario where creatine deficiency does apparently matter. Reperfused hearts from both GAMT−/− and CK−/− mice exhibit impaired functional recovery,16, 17 and increasing PCr has recently been shown to protect against ischemia/reperfusion injury.43 It is also notable that over-expression of M-CK was protective in a murine model of heart failure due to pressure-overload.44 However, our data does suggest that loss of total creatine during chronic heart failure does not directly contribute to disease progression.
Furthermore, it questions the central tenant that creatine is essential for high workload and stress responses in heart and skeletal muscle, which prompts the question of why, in an evolutionary sense, it is there at all? It seems likely that close control of ATP/ADP ratios and the buffering of inorganic phosphate confer a certain level of metabolic fine tuning, however, this is clearly not essential. One argument, supported by our data, is that the creatine / CK system originally evolved in cells with a large diffusion distance between mitochondria and energy utilisation sites.2, 45 As an energy-intensive organ, the heart has inherited this system by default, but with mitochondrial cell density higher than in any other cell type, it has apparently become physiologically redundant. The role of creatine and the CK reaction may be associated with levels of workload not attainable in laboratory studies, or may play hitherto unidentified long-term roles in energetic cells, which presumably makes the metabolic cost of creatine biosynthesis worthwhile.
CONCLUSIONS
Contrary to expectations, we have shown that creatine is not essential to obtain maximum exercise capacity when tested in conscious animals over a prolonged period. Furthermore, we have demonstrated for the first time that chronic creatine deficiency is compatible with survival post-myocardial infarction, and need not be detrimental to development of chronic heart failure, suggesting that reduced total creatine levels in the failing heart are not pathophysiological. The lack of an obvious adaptive response supports the conclusion that creatine is not, after all, essential to support energy demand under laboratory exercise and pathophysiological stress in heart or skeletal muscle.
Supplementary Material
Novelty and Significance.
What Is Known?
Creatine plays a key role in buffering chemical energy (ATP) in organs with high energy demands such as heart and skeletal muscle.
Partially depleting creatine in rodents using non-functional analogues blunts force generation in response to maximal stimulation and results in excessive early mortality following myocardial infarction.
Reduced levels of myocardial creatine are characteristic of the failing heart.
What New Information Does This Article Contribute?
Mice with total whole-body creatine deficiency due to knockout of an essential biosynthetic enzyme (guanidinoacetate N-methyltransferase – GAMT) are capable of running just as far and as fast as control mice.
Complete creatine deficiency did not affect survival following myocardial infarction and subsequent development of chronic heart failure was not exacerbated.
These findings suggest that creatine is not essential for high workload and stress responses in muscle cells when tested in conscious, intact, laboratory animals.
This study was designed to extend observations, obtained from experiments using partial creatine depletion in anaesthetised animals, into conscious creatine-free mice where maximal obtainable workload is highest. Contrary to expectations, creatine-deficient GAMT knockout mice exhibited normal exercise capacity and maximal running velocity in both voluntary and forced running protocols. We demonstrate that chronic creatine deficiency is compatible with survival after-myocardial infarction, and need not be detrimental to development of chronic heart failure, suggesting that reduced total creatine levels in the failing heart may have little pathophysiological impact. A total absence of creatine was not associated with any significant proteomic change in enzymes associated with energy conversion and contraction, while mitochondria retained near normal function. The lack of an adaptive response supports the conclusion that creatine may not be essential to support energy demand under laboratory exercise and pathophysiological stress in heart or skeletal muscle.
Acknowledgments
GAMT−/− mice were originally generated and gifted by Prof Dirk Isbrandt, Centre for Molecular Neurobiology, Hamburg, Germany. The authors thank Dr Svetlana Reilly for help and advice with the measurement of superoxide generation.
SOURCES OF FUNDING
This work was supported by British Heart Foundation grant RG/10/002/28187 and Wellcome Trust Core Award, Grant 090532/Z/09/Z.
Non-standard Abbreviations
- β-GPA
β-guanidinopropionic acid
- CHF
Chronic heart failure
- Cr
Creatine
- GAMT
Guanidinoacetate N-methyltransferase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- MI
Myocardial infarction
- PCr
Phosphocreatine
- PDH
Pyruvate dehydrogenase
- PGA
Phospho-guanidinoacetate
- PGK
3-phosphoglycerate kinase
- PK
Pyruvate kinase
- WT
Wild-type
Footnotes
DISCLOSURES
None
References
- 1.Brosnan J, da Silva R, Brosnan M. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- 2.Ellington WR. Evolution and physiological roles of phosphagen systems. Annu Rev Physiol. 2001;63:289–325. doi: 10.1146/annurev.physiol.63.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 4.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- 6.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 7.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol (Lond) 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lygate CA, Fischer A, Sebag-Montefiore L, Wallis J, Ten Hove M, Neubauer S. The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol. 2007;42:1129–1136. doi: 10.1016/j.yjmcc.2007.03.899. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S, Hu K, Horn M, Remkes H, Hoffmann KD, Schmidt C, Schmidt TJ, Schnackerz K, Ertl G. Functional and energetic consequences of chronic myocardial creatine depletion by beta-guanidinopropionate in perfused hearts and in intact rats. J Mol Cell Cardiol. 1999;31:1845–1855. doi: 10.1006/jmcc.1999.1016. [DOI] [PubMed] [Google Scholar]
- 11.Lorentzon M, Ramunddal T, Bollano E, Soussi B, Waagstein F, Omerovic E. In vivo effects of myocardial creatine depletion on left ventricular function, morphology, and energy metabolism--consequences in acute myocardial infarction. J Card Fail. 2007;13:230–237. doi: 10.1016/j.cardfail.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Horn M, Remkes H, Stromer H, Dienesch C, Neubauer S. Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation. 2001;104:1844–1849. doi: 10.1161/hc3901.095933. [DOI] [PubMed] [Google Scholar]
- 13.Boehm EA, Radda GK, Tomlin H, Clark JF. The utilisation of creatine and its analogues by cytosolic and mitochondrial creatine kinase. Biochim Biophys Acta. 1996;1274:119–128. doi: 10.1016/0005-2728(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt A, Marescau B, Boehm EA, Renema WK, Peco R, Das A, Steinfeld R, Chan S, Wallis J, Davidoff M, Ullrich K, Waldschutz R, Heerschap A, De Deyn PP, Neubauer S, Isbrandt D. Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet. 2004;13:905–921. doi: 10.1093/hmg/ddh112. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JE, Stork LA, Bell JT, Hove MT, Isbrandt D, Clarke K, Watkins H, Lygate CA, Neubauer S. Cardiac structure and function during ageing in energetically compromised Guanidinoacetate N-methyltransferase (GAMT)-knockout mice - a one year longitudinal MRI study. J Cardiovasc Magn Reson. 2008;10:9. doi: 10.1186/1532-429X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Sebag-Montefiore L, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005;111:2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- 17.Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, Neubauer S. Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol. 2004;287:H1039–1045. doi: 10.1152/ajpheart.01016.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol. 1996;270:H1207–1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 19.De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, Friedrich J, Gaudron P, Schnackerz K, Ingwall JS. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest. 1995;95:1092–1100. doi: 10.1172/JCI117756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1006–R1013. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- 22.Lygate C. Surgical models of hypertrophy and heart failure: Myocardial infarction and transverse aortic constriction. Drug Disc Today Dis Models. 2006;3:283–290. [Google Scholar]
- 23.Dawson D, Lygate CA, Saunders J, Schneider JE, Ye X, Hulbert K, Noble JA, Neubauer S. Quantitative 3-dimensional echocardiography for accurate and rapid cardiac phenotype characterization in mice. Circulation. 2004;110:1632–1637. doi: 10.1161/01.CIR.0000142049.14227.AD. [DOI] [PubMed] [Google Scholar]
- 24.Bittl JA, Ingwall JS. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985;260:3512–3517. [PubMed] [Google Scholar]
- 25.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DT, Harris RA, French S, Aponte A, Balaban RS. Proteomic changes associated with diabetes in the BB-DP rat. Am J Physiol Endocrinol Metabol. 2009;296:E422–432. doi: 10.1152/ajpendo.90352.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan HE, Buse-Pot TE, Peco R, Isbrandt D, Heerschap A, de Haan A. Lower force and impaired performance during high-intensity electrical stimulation in skeletal muscle of GAMT-deficient knockout mice. Am J Physiol Cell Physiol. 2005;289:C113–C119. doi: 10.1152/ajpcell.00040.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 29.Desai KH, Bernstein D. Exercise and oxygen consumption in the mouse. In: Hoit BD, Walsh RA, editors. Cardiovascular Physiology in the Genetically Engineered Mouse. Boston: Kluwer; 2002. pp. 277–302. [Google Scholar]
- 30.Lindbom M, Ramunddal T, Camejo G, Waagstein F, Omerovic E. In vivo effects of myocardial creatine depletion on left ventricular function morphology and lipid metabolism: study in a mouse model. J Card Fail. 2008;14:161–166. doi: 10.1016/j.cardfail.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Boehm E, Chan S, Monfared M, Wallimann T, Clarke K, Neubauer S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am J Physiol Endocrinol Metabol. 2003;284:E399–406. doi: 10.1152/ajpendo.00259.2002. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ Res. 1999;85:174–181. doi: 10.1161/01.res.85.2.174. [DOI] [PubMed] [Google Scholar]
- 33.Brand MD, Kesseler A. Control analysis of energy metabolism in mitochondria. Biochem Soc Trans. 1995;23:371–376. doi: 10.1042/bst0230371. [DOI] [PubMed] [Google Scholar]
- 34.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Renema WKJ, Kan HE, Wieringa B, Heerschap A. In vivo magnetic resonance spectroscopy of transgenic mouse models with altered high-energy phosphoryl transfer metabolism. NMR Biomed. 2007;20:448–467. doi: 10.1002/nbm.1117. [DOI] [PubMed] [Google Scholar]
- 36.Kupriyanov VV, Seppet EK, Emelin IV, Saks VA. Phosphocretine production coupled to the glycolytic reactions in the cytosol of cardiac cells. Biochim Biophys Acta. 1980;592:197–210. doi: 10.1016/0005-2728(80)90181-4. [DOI] [PubMed] [Google Scholar]
- 37.Momken I, Lechene P, Koulmann N, Fortin D, Mateo P, Doan BT, Hoerter J, Bigard X, Veksler V, Ventura-Clapier R. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol. 2005;565:951–964. doi: 10.1113/jphysiol.2005.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lygate CA, Hunyor I, Medway D, de Bono JP, Dawson D, Wallis J, Sebag-Montefiore L, Neubauer S. Cardiac phenotype of mitochondrial creatine kinase knockout mice is modified on a pure C57BL/6 genetic background. J Mol Cell Cardiol. 2009;46:93–99. doi: 10.1016/j.yjmcc.2008.09.710. [DOI] [PubMed] [Google Scholar]
- 39.Nahrendorf M, Streif JU, Hiller KH, Hu K, Nordbeck P, Ritter O, Sosnovik DE, Bauer L, Neubauer S, Jakob PM, Ertl G, Spindler M, Bauer WR. Multimodal functional cardiac MR imaging in creatine kinase deficient mice reveals subtle abnormalities in myocardial perfusion and mechanics. Am J Physiol Heart Circ Physiol. 2006;290:H2516–H2521. doi: 10.1152/ajpheart.01038.2005. [DOI] [PubMed] [Google Scholar]
- 40.Lygate CA, Medway DJ, Ostrowski PJ, Aksentijevic D, Sebag-Montefiore L, Hunyor I, Zervou S, Schneider JE, Neubauer S. Chronic creatine kinase deficiency eventually leads to congestive heart failure, but severity is dependent on genetic background, gender and age. Basic Res Cardiol. 2012;107:276. doi: 10.1007/s00395-012-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maughan DW, Henkin JA, Vigoreaux JO. Concentrations of Glycolytic Enzymes and Other Cytosolic Proteins in the Diffusible Fraction of a Vertebrate Muscle Proteome. Mol Cell Proteomics. 2005;4:1541–1549. doi: 10.1074/mcp.M500053-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Nasrallah F, Feki M, Kaabachi N. Creatine and Creatine Deficiency Syndromes: Biochemical and Clinical Aspects. Pediatr Neurol. 2010;42:163–171. doi: 10.1016/j.pediatrneurol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Lygate CA, Bohl S, Ten Hove M, Faller KM, Ostrowski PJ, Zervou S, Medway DJ, Aksentijevic D, Sebag-Montefiore L, Wallis J, Clarke K, Watkins H, Schneider JE, Neubauer S. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res. 2012;96:466–475. doi: 10.1093/cvr/cvs272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol Cell Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.