Abstract
Trans sodium crocetinate (TSC) is a synthetic carotenoid that improves the diffusion of oxygen in animal models of ischemia/hypoxia. This study evaluated multiple doses of TSC in patients with peripheral artery disease (PAD) and hypothesized that a preliminary dose–response relationship could be identified on peak walking time (PWT). Forty-eight patients with symptomatic PAD and an ankle–brachial index < 0.90 were included, while critical limb ischemia, recent revascularization, and exercise limited by symptoms other than claudication were exclusionary. Patients were randomized to placebo or eight dosing levels of TSC ranging from 0.25 mg/kg to 2.0 mg/kg given intravenously once daily for 5 days. Subjects were tested on a graded treadmill protocol to claudication-limited PWT with the change to Day 5 as primary. A cubic regression was fit to detect a pre-specified inverted U-shaped dose–response relationship (65% power). Patient-reported walking distance from the Walking Impairment Questionnaire was a secondary endpoint. Adverse events were not predominant on any drug dose relative to placebo. Changes in PWT demonstrated a cubic trend for dose (p = 0.07, r = 0.39, r2 = 0.15) with morphologic signals of benefit at doses above 1.00 mg/kg after both the first and fifth dosing days. Similar improvements occurred with the walking distance score at doses above 1.00 mg/ kg. In conclusion, TSC was safe and well tolerated at all doses. Notable signals of benefit were observed at higher doses for both PWT and patient-perceived walking distance. These results support a phase II study to define the optimal dose for longer-term therapy with TSC.
Keywords: claudication, dose–response relationship, drug, peripheral artery disease
Introduction
Peripheral artery disease (PAD) is a major manifestation of systemic atherosclerosis and as a consequence a strong predictor of cardiovascular morbidity and mortality.1 Patients with PAD commonly manifest the symptom of claudication that is cramping, aching, or fatigue in the calf muscles provoked by walking activity and relieved by rest.2 However, all patients with PAD (whether they manifest classic symptoms or not) have a profound limitation in physical performance measures as defined by peak exercise performance, the 6-minute walk, and integrated measures of physical activity over a week.3,4
The pathophysiology of claudication in PAD is primarily related to atherosclerotic arterial occlusion(s) in the lower extremity that limit the increase in blood flow induced by walking exercise.5 This limits the supply of oxygen required to meet the metabolic demands of exercising skeletal muscle, leading to the symptom of claudication during exercise. In addition, PAD is associated with abnormal skeletal muscle mitochondrial function and altered skeletal muscle oxidative metabolism.6 Restoration of blood flow and oxygen delivery by revascularization can significantly relieve claudication symptoms and improve exercise performance.7 However, there are no pharmacologic therapies that directly modify oxygen delivery for the treatment of claudication.
Trans sodium crocetinate (TSC) is a novel bipolar synthetic carotenoid that is designed to enhance the oxygenation of hypoxic tissues. The putative effect of TSC is to increase the diffusivity of oxygen (the ability of oxygen to move from a higher to a lower concentration).8 An increased diffusivity is hypothesized to be the primary mechanism by which TSC exerts its pharmacodynamic effects, as shown in models of ischemic injury where TSC increased tissue oxygen concentration.9,10
The current study tested the hypothesis that a range of doses of TSC would be safe and well tolerated in patients with PAD and that a dose–response could be identified on the functional endpoint of treadmill peak walking time.
Methods
Design and dosing
This was a randomized, double-blinded, placebo-controlled, dose escalation study in patients with claudication due to PAD. It was designed as a proof-of-concept and dose-range-finding study to evaluate the safety, pharmacokinetics, and efficacy of TSC. Four cohorts of patients, consisting of a total of 12 patients each, were enrolled, with patients in each cohort randomized to placebo (n = 2) or one of two drug doses (n = 5/dose). The first cohort tested doses of 0.25 and 0.50 mg/kg, the second doses of 0.75 and 1.00 mg/kg, the third 1.25 and 1.50 mg/kg, and the forth 1.75 and 2.00 mg/kg. Study drug and placebo were dosed intravenously as a bolus injection over 2 minutes, once daily for 5 consecutive days.
The maximum allowed pre-treatment phase was up to 39 days, including a 21-day wash-out period if a patient needed to discontinue an exclusionary medication after being consented into the trial. Prior to randomization at the Dose 1 Visit, the patient was asked to complete two visits: the Screening Visit and the Baseline Visit. For all patients enrolled, regardless of whether a wash-out period was needed, the Screening Visit was conducted within 24 days prior to the Dose 1 Visit. The Baseline Visit was conducted after laboratory tests from the Screening Visit had been assessed, and within 3–10 days prior to the Dose 1 Visit. The expected enrollment timeframe was 12 months, allowing for an accrual rate of approximately three to four patients per month. The actual enrollment took 18 months.
Inclusion and exclusion criteria
Patients included were at least 40 years old and had at least a 6-month history of walking limitation due to stable claudication. PAD was diagnosed by a resting ankle– brachial index (ABI) ≤ 0.90 in the symptomatic leg. Patients with a resting ABI between 0.90 and 1.00, or ABI > 1.30 could enter with alternate hemodynamic evidence of PAD including a decrease in ABI of 20% after exercise or a toe–brachial index (TBI) ≤ 0.70. Other concomitant diseases and therapy must have been stable for at least 3 months. Patients must not have been considering a change in smoking and/or exercise habits (formal exercise program) throughout the study and follow-up.
Patients taking stable antihypertensive therapy, cholesterol-lowering therapy (e.g. statins), chronic oral nitrates, and diabetic therapy (if applicable) were eligible for the study. Those taking cilostazol or pentoxifylline must have discontinued those medications 21 days prior to screening. An inclusion criterion was time on a graded treadmill with a peak walking time (PWT) of at least 1 minute, but no more than 12 minutes at the Baseline Visit.
Patients were excluded with a history of critical limb ischemia (CLI) manifested by rest pain, ulceration, gangrene, lower limb amputation or non-atherosclerotic causes of vascular disease. Also excluded were patients whose exercise performance was limited by venous claudication, chronic compartment syndrome, peripheral nerve pain (e.g. severe peripheral neuropathy), pseudo-claudication caused by spinal cord compression, or acute limb ischemia. Conditions other than claudication of significant severity that could have impacted the exercise test measurements were excluded, including angina, heart failure, chronic obstructive pulmonary disease, orthopedic disease, neurological disorders, rheumatologic disorders and prior lower limb amputation (except for minor toe amputations not expected to interfere with exercise testing). All prior revascularizations must have been performed at least 3 months prior to enrollment. Patients were excluded who were exposed to angiogenic therapies within 12 months of screening.
Safety monitoring
Safety and tolerability were evaluated by an independent Safety Monitoring Committee (SMC), which consisted of three blinded independent reviewers, all with prior expertise in clinical trials. The SMC reviewed safety data after each cohort of 12 subjects had completed dosing. The decision to progress to the next dosing cohort was based on this independent review of adverse events and laboratory data that were judged to be acceptable to allow for exposure to increasing doses. The SMC chose to remain blinded during their deliberations despite the option to become unblinded as to drug group assignment.
Endpoints
The primary efficacy endpoint was PWT, with claudication onset time (COT) as a secondary efficacy measure. Testing was performed on a standardized graded exercise treadmill test (Gardner protocol, conducted at a walking speed of 2.0 mph (3.2 km/h) at a defined grade ranging from 0 and increasing 2% every 2 minutes to a maximum of 18%). This test has been used in previous trials of pharmacological agents and is, thus, useful for making comparisons.
Patient-reported outcomes were assessed with the Walking Impairment Questionnaire (WIQ), which also served as secondary endpoints. The questionnaire measures pain severity, walking distance, walking speed, and stair climbing, and was used to evaluate community-based walking ability.11 Owing to the short time frame of this study, a modified (mWIQ) was used to ask about the patient’s experience in the previous 5 days, rather than 30 days. The WIQ has previously been shown to correlate with PWT in patients with PAD.12
Study populations
The intention-to-treat (ITT) population included all patients who were dosed and had post-dosing efficacy data. Data were presented by the dosing arm to which the patient was randomized. The second was the per-protocol (PP) population and included all patients randomized who received all five doses of TSC or placebo.
Sample size and statistical analysis
Since the primary objective of this study was to evaluate the safety and pharmacokinetics of TSC, the study was not powered to definitively establish efficacy. Despite this limitation, signals of benefit were evaluated across the entire dose range of TSC on PWT based on the coefficient of multiple correlations. For these efficacy analyses, PWT values at each time point were log-transformed to generate a normal distribution of the data. Further, percent changes in the geometric means were determined from a back-calculation of the log-transformed values. While the data were not normally distributed, percent changes in the arithmetic means were also calculated as a means to illustrate the degree of linear increase in PWT.
An interesting facet of TSC-dosing, as shown in preclinical studies of stroke,10 is that the most beneficial dose is an intermediate one. It is thought that this phenomenon arises from the fact that TSC increases the hydrogen-bonding of the water molecules in the plasma.8,9 Dose-dependent amounts of hydrogen-bonding formation are a well-known characteristic of compounds known as kosmotropes, compounds that, like TSC, increase the hydrogen-bonding among water molecules.
Based on preclinical dose-dependence results, quadratic and cubic relationships were pre-specified as possible models to evaluate an anticipated response to TSC that would be maximal at a mid-range dose with less response at low and high doses. Therefore, a symmetric quadratic dose–response curve for the mean change from baseline of log PWT, with a peak at 1.00 mg/kg, was assumed. The standard deviation of the change from baseline of log PWT was set to 0.46. One thousand samples of the change from baseline of log PWT were simulated according to the study design. The average value of r2 from these simulated samples was 0.1338. Thus, if the change from baseline to Day 5 in PWT was twofold higher at the optimal dose compared to placebo, then there was approximately 65% power to detect this difference with 48 patients.
Results
A total of 110 patients were screened for the study: 37 for Cohort 1; 19 for Cohort 2; 31 for Cohort 3; and 23 for Cohort 4 (Figure 1). Sixty-two patients were screening failures, with the two most common reasons including not meeting ABI or TBI criteria (10.9%) and a non-qualifying peak walking time of less than 1 minute or more than 12 minutes at the Baseline Visit (6.4%).
Figure 1.
Subject disposition.
Forty-eight patients were randomized into the study and included in the ITT population: 12 into each cohort. Within each cohort, 10 patients received TSC (five patients per intra-cohort TSC dosing arm) and two patients received placebo. Two patients withdrew from the study (both from the 1.00 mg/kg arm): one for missing several visits following an adverse event of nausea and diarrhea and the other withdrew owing to work commitments. Four patients were excluded from the PP population, including the two who withdrew and an additional two patients for receiving < 85% of the study medication. Accounting for these excluded patients, there were 44 patients in the PP population: eight in the placebo group; four in the 0.25 mg/kg dosing arm; three in the 1.00 mg/kg dosing arm; four in the 1.75 mg/kg dosing arm; and five in the remaining TSC dosing arms.
The overall mean age of the population was 65 years and was similar across the groups except for patients randomized to the 1.00 mg/kg dosing arm who had a mean age of 55.0 years (Table 1). The majority of the population was male and non-Hispanic white. The majority of subjects were current or former smokers. The mean ABI values ranged from 0.50 to 0.80 and were typical of a population of patients with claudication.
Table 1.
Subject demographics
| Placebo n = 8 |
0.25 mg/kg n = 5 |
0.50 mg/kg n = 5 |
0.75 mg/kg n = 5 |
1.00 mg/kg n = 5 |
1.25 mg/kg n = 5 |
1.50 mg/kg n = 5 |
1.75 mg/kg n = 5 |
2.00 mg/kg n = 5 |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 8 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Age, years | Mean ± SD | 69.0 ± 7.3 | 67.0 ± 8.8 | 65.2 ± 13.2 | 63.4 ± 8.1 | 55.0 ± 5.2 | 65.4 ± 8.3 | 65.8 ± 10.8 | 64.0 ± 6.4 | 67.4 ± 7.5 |
| Age | Min–max | 61–82 | 54–78 | 46–77 | 58–76 | 49–61 | 59–75 | 51–79 | 57–72 | 56–75 |
| Ethnicity / race | Hispanic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| White | 7 (87.5) | 4 (80.0) | 4 (80.0) | 4 (80.0) | 3 (60.0) | 4 (80.0) | 3 (60.0) | 5 (100.0) | 3 (60.0) | |
| African | 1 (12.5) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 2 (40.0) | 1 (20.0) | 2 (40.0) | 0 (0.0) | 2 (40.0) | |
| American | ||||||||||
| Sex | Male | 6 (75.0) | 5 (100.0) | 3 (60.0) | 5 (100.0) | 3 (60.0) | 3 (60.0) | 4 (80.0) | 3 (60.0) | 3 (60.0) |
| Female | 2 (25.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) | 2 (40.0) | 2 (40.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) | |
| Smoking status | None | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) |
| Former | 3 (37.5) | 2 (40.0) | 4 (80.0) | 0 (0.0) | 1 (20.0) | 3 (60.0) | 3 (60.0) | 2 (40.0) | 2 (40.0) | |
| Current | 5 (62.5) | 2 (40.0) | 1 (20.0) | 4 (80.0) | 3 (60.0) | 2 (40.0) | 2 (40.0) | 3 (60.0) | 2 (40.0) | |
| ABI | Mean ± SD | 0.65 ±0.14 | 0.80 ± 0.24 | 0.68 ± 0.32 | 0.57 ±0.17 | 0.67 ± 0.09 | 0.65 ±0.15 | 0.53 ±0.13 | 0.79 ± 0.08 | 0.55 ±0.17 |
ABI, ankle–brachial index.
As seen in Table 2, after 5 days of treatment, there was a 9.6% geometric mean increase in PWT on placebo. The TSC dosing arms demonstrated a differential dose–response on PWT with a decrease in PWT from baseline at low doses of TSC (maximum decrease of 21.5% for the 0.25 mg/kg dosing arm) and substantial increases from baseline in PWT at the higher doses (peak increase of 29.3% for the 1.50 mg/kg dosing arm). Similar results were observed for percent change in geometric mean PWT as early as the Dose 1 Visit, with a decrease in PWT from the baseline in the lowest dose group (−12.6%, 0.25 mg/kg dosing arm) and an increase in PWT for higher dose groups (17.3%, 1.25 mg/kg dosing arm). The log ratio change in PWT was used for statistical analysis to normalize the data. These changes are shown in Figure 2 and demonstrated a cubic trend for dose (p = 0.07, r = 0.39, r2 = 0.15), with morphologic signals of benefit at doses above 1.00 mg/kg after both the first and fifth dosing days.
Table 2.
Percent change in geometric mean of PWT from baseline by dosing arm
| Time point | Placebo | 0.25 mg/kg |
0.50 mg/kg |
0.75 mg/kg |
1.00 mg/kg |
1.25 mg/kg |
1.50 mg/kg |
1.75 mg/kg |
2.00 mg/kg |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Dose 1 | n | 8 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| mean | 5.2% | −12.6% | 1.9% | −6.1% | 10.2% | 17.3% | 11.3% | 8.5% | 13.0% | |
| Dose 5 | n | 8 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 5 |
| mean | 9.6% | −21.5% | −16.0% | 4.5% | −14.7% | 8.9% | 29.3% | 7.2% | 6.5% | |
| 5-Day post | n | 8 | 5 | 4 | 4 | 3 | 5 | 5 | 5 | 5 |
| mean | 2.8% | −16.3% | −8.8% | 24.4% | −19.8% | −11.9% | 10.5% | 23.2% | −0.7% |
Figure 2.
Log ratio of change in peak walking time from baseline by treatment group at the Dose 5 Visit.
The arithmetic percent change in PWT was also calculated to provide a more clinically relevant representation of the data. This demonstrated a 38% increase in PWT at the 1.5 mg/kg dose (representing a difference of 21% above the placebo response of 17%) at Dose 5.
Relative increases in claudication onset time from baseline were observed after 5 days of dosing (Dose 5 Visit), with the greatest increase observed at the lowest dose of TSC (0.25 mg/kg dosing arm) and modest signals of improvement for the dosing arms between 1.25 mg/kg and 1.75 mg/kg.
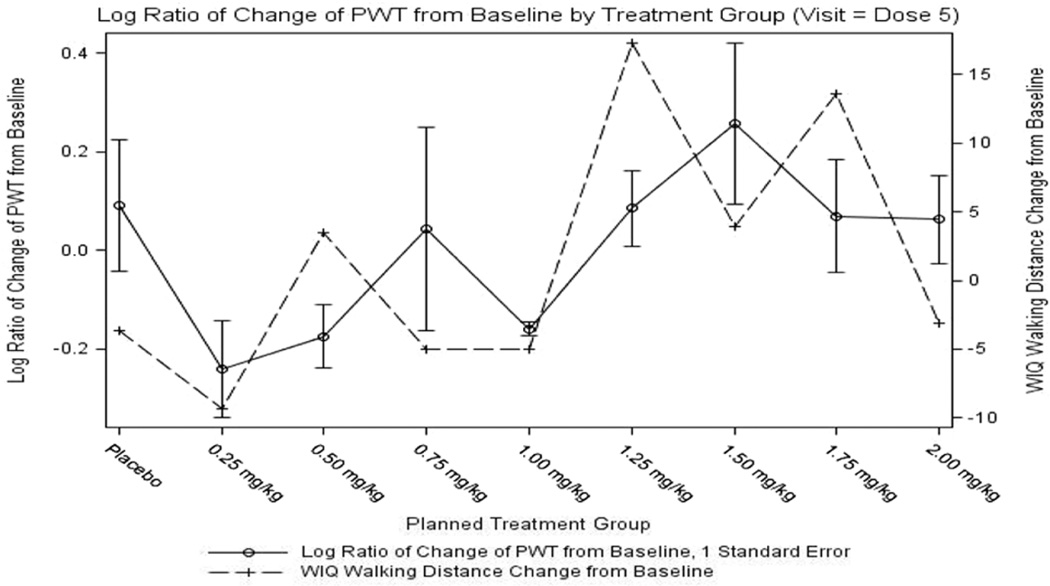
The change from baseline for the walking distance scale of the WIQ at the Dose 5 Visit for the ITT population was −3.6 for the placebo group but in treated subjects a trend was observed for an improvement in walking distance at doses greater than 1.00 mg/kg, with a peak of 17.3 in the 1.25 mg/kg dosing arm. Results for walking speed score appeared to follow a similar pattern with regard to signals of improvement at doses between 1.25 and 1.75 mg/kg. Overall, the WIQ data were notable for an overlap of dose– response with the objective measure of PWT, suggesting patient-reported perceived benefit of treatment in the active dosing arms with doses higher than 1.00 mg/kg (Figure 3).
Figure 3.
Log ratio of change in peak walking time and change in Walking Impairment Questionnaire (WIQ) walking distance score.
The most common adverse events (AEs) were in the gastrointestinal disorder, musculoskeletal and connective tissue, and investigations (laboratory) body systems. The occurrence of these AEs was not predominant in any TSC dosing arm when compared to the placebo group (Table 3). Serious adverse events were observed in five patients. Two patients on placebo had an SAE including angina and a malignant lung neoplasm. One patient on 0.25 mg/kg developed a small bowel obstruction, one patient on 0.50 mg developed a deep vein thrombosis, and one patient on 0.75 mg/kg developed unstable angina. There were two deaths that occurred over 30 days post dosing and these were one placebo patient who had lung cancer and the patient with a deep vein thrombosis who died of a pulmonary embolus.
Table 3.
Adverse and serious adverse events
| Placebo (n = 8) |
0.25 mg/kg (n = 5) |
0.50 mg/kg (n = 5) |
0.75 mg/kg (n = 5) |
1.00 mg/kg (n = 5) |
1.25 mg/kg (n = 5) |
1.50 mg/kg (n = 5) |
1.75 mg/kg (n = 5) |
2.00 mg/kg (n = 5) |
|
|---|---|---|---|---|---|---|---|---|---|
| Patients with AEs |
7 | 3 | 3 | 4 | 2 | 3 | 2 | 4 | 3 |
| Patients with SAEs |
2 | 1 | 1 | 1 |
Discussion
This was an early-stage study of TSC in patients with PAD, with the primary aim to evaluate safety and tolerability. The exposure to TSC demonstrated that the drug was well-tolerated when given as a parenteral administration over 5 days across a wide range of doses. A dose–response for adverse or serious adverse events was not observed in any particular organ system. Thus, these data support the further development of extended dosing schemes.
The number of placebo patients showing serious adverse events was 2/8 (25%) versus 3/40 (7.5%) of the TSC-treated patients. Patients showing adverse events were 7/8 (87.5%) for the placebo group and 24/40 (60%) for the TSC-treated patients. Thus, TSC appears to be a safe treatment. It should be noted that all of the patients were still on all of their previously prescribed medications during the entire duration of this trial.
TSC demonstrated signals of clinical benefit in a pre-specified dose–response fashion. The primary efficacy endpoint was peak walking time on the treadmill, a validated endpoint for both clinical and regulatory considerations.11 The observed increase in PWT at higher doses of TSC is of a magnitude consistent with approved pharmacotherapy for claudication.13 In addition, a patient-reported disease-specific outcome measure was administered to assess changes in each patient’s assessment of their walking ability. The results from the WIQ also demonstrated a similar dose–response relationship to PWT. Taken together these results provide suggestions of efficacy that would also warrant further study.
TSC increases the diffusion coefficient of oxygen through water and other aqueous solutions, such as blood plasma.8 Preclinical pharmacology studies conducted in multiple animal models with TSC have demonstrated improvement in a number of important physiological parameters and outcome variables. When TSC was evaluated in a three-vessel occlusion stroke model in rats, the results demonstrated a reduction in the infarct volume and an increase in brain oxygen levels in the ischemic penumbra tissue.10 Hemorrhagic shock animal models have shown that TSC increased whole body oxygen consumption, increased mean arterial blood pressure (BP), and greatly increased survival rates when compared to control animals.14 These effects are all hypothesized to be due to enhanced tissue oxygenation. Since TSC is a carotenoid, and carotenoids are known radical scavengers, it might be suggested that the mechanism of action of TSC could also involve such a phenomenon. The radical scavenging properties of TSC have been studied previously, and it was found that although TSC does scavenge free radicals, it only does so when the plasma TSC concentration is relatively high.14 In fact, TSC does not show significant radical scavenging properties unless the plasma concentration is about 100 times greater than the highest TSC plasma concentration occurring in this PAD trial.
Given the promising findings of TSC on exercise performance in PAD, a potential mechanism could be improved oxygen availability. If this compound is developed further, additional studies would need to evaluate the clinical benefits in a larger group of patients treated for a longer duration at a target dose of around the 1.5 mg/kg level. In addition, mechanistic insights relevant to the putative mechanism of action of TSC could be gained by evaluating changes in oxygen consumption and tissue hemoglobin saturation kinetics at the onset of exercise.15,16 Since this trial was designed to prove whether or not TSC could safely benefit patients having intermittent claudication, even when given on a short-term basis, the emphasis was placed on the improvement in the patients and not on discerning more about other physiological changes caused by TSC. This will be the subject of future trials, which will also be of a longer duration and with a different method of administering TSC.
Acknowledgements
This trial was registered on the ClinicalTrials.gov website as NCT00725881. The authors thank the patients and study sites for their commitment to this study. We also thank Kim Whitten at Diffusion Pharmaceuticals LLC as the Study Director.
Funding
Tim Bauer is a senior scientist from CPC clinical research, a non-profit academic research organization that received funding from Diffusion Pharmaceuticals to conduct this trial. John Gainer is the inventor of trans sodium crocetinate and is the Chief Scientific Consultant for Diffusion Pharmaceuticals. Luis H Eraso’s and a portion of Emile Mohler’s salary were supported by grant K12 HL-083772 from the National Institutes of Health. Dr Thanaporn was supported in part by grant K12 HL087746, and by the NIH Clinical and Translational Science Award to Stanford University.
References
- 1.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–181. doi: 10.1046/j.1525-1497.1999.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt WR, Nawaz D, Brass EP. Carnitine metabolism during exercise in patients with peripheral vascular disease. J Appl Physiol. 1987;62:2383–2387. doi: 10.1152/jappl.1987.62.6.2383. [DOI] [PubMed] [Google Scholar]
- 5.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion. Vasc Med. 2004;9:293–301. doi: 10.1191/1358863x04vm572ra. [DOI] [PubMed] [Google Scholar]
- 6.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Hargarten ME, Rutherford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 8.Stennett AK, Dempsey GL, Gainer JL. trans-Sodium crocetinate and diffusion enhancement. J Phys Chem B. 2006;110:18,078–18,080. doi: 10.1021/jp064308+. [DOI] [PubMed] [Google Scholar]
- 9.Gainer JL. Trans-sodium crocetinate for treating hypoxia/ ischemia. Expert Opin Investig Drugs. 2008;17:917–924. doi: 10.1517/13543784.17.6.917. [DOI] [PubMed] [Google Scholar]
- 10.Manabe H, Okonkwo DO, Gainer JL, Clarke RH, Lee KS. Protection against focal ischemic injury to the brain by trans-sodium crocetinate. Laboratory investigation. J Neurosurg. 2010;113:802–809. doi: 10.3171/2009.10.JNS09562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 12.Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 13.Pande RL, Hiatt WR, Zhang P, Hittel N, Creager MA, McDermott M. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med. 2010;15:181–188. doi: 10.1177/1358863X10361545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stennett AK, Murray RJ, Roy JW, Gainer JL. Trans-sodium crocetinate and hemorrhagic shock. Shock. 2007;28:339–344. doi: 10.1097/shk.0b013e3180487b2d. [DOI] [PubMed] [Google Scholar]
- 15.Bauer TA, Regensteiner JG, Brass EP, Hiatt WR. Oxygen uptake kinetics during exercise are slowed in patients with peripheral arterial disease. J Appl Physiol. 1999;87:809–816. doi: 10.1152/jappl.1999.87.2.809. [DOI] [PubMed] [Google Scholar]
- 16.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO(2) kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol. 2007;100:143–151. doi: 10.1007/s00421-007-0412-0. [DOI] [PubMed] [Google Scholar]