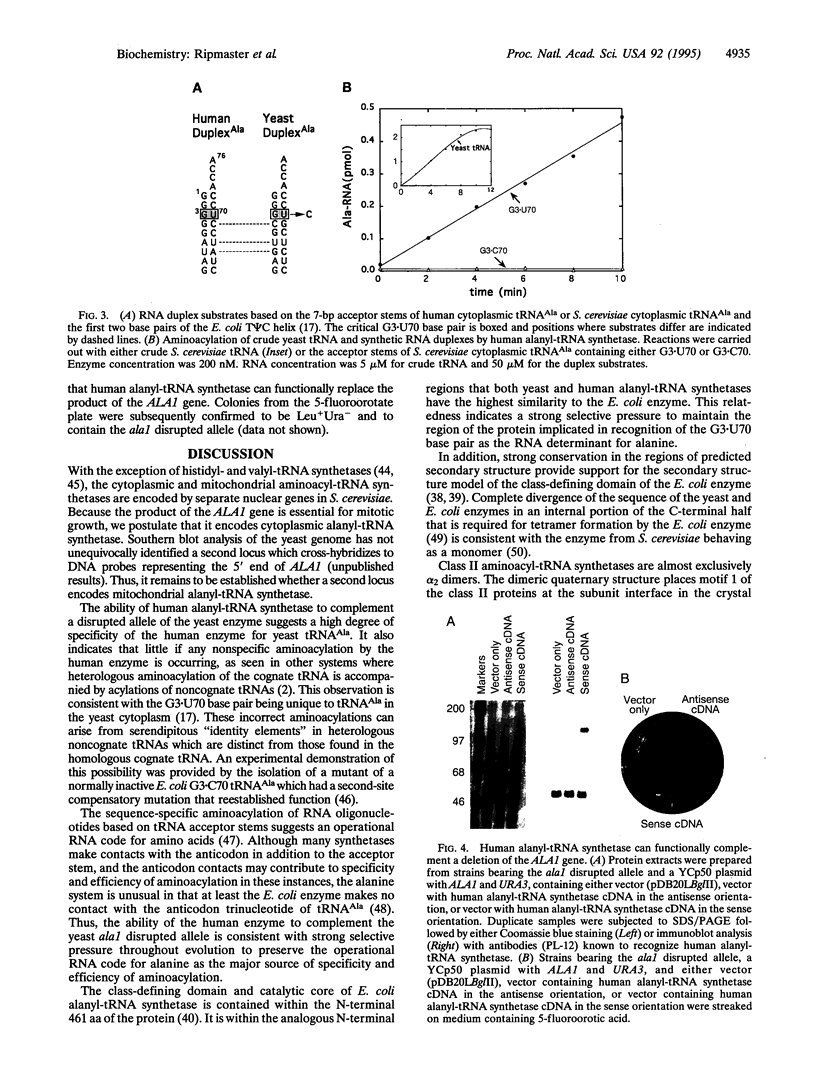
Abstract
Because of variations in tRNA sequences in evolution, tRNA synthetases either do not acylate their cognate tRNAs from other organisms or execute misacylations which can be deleterious in vivo. We report here the cloning and primary sequence of a 958-aa Saccharomyces cerevisiae alanyl-tRNA synthetase. The enzyme is a close homologue of the human and Escherichia coli enzymes, particularly in the region of the primary structure needed for aminoacylation of RNA duplex substrates based on alanine tRNA acceptor stems with a G3.U70 base pair. An ala1 disrupted allele demonstrated that the gene is essential and that, therefore, ALA1 encodes an enzyme required for cytoplasmic protein synthesis. Growth of cells harboring the ala1 disrupted allele was restored by a cDNA clone encoding human alanyl-tRNA synthetase, which is a serum antigen for many polymyositis-afflicted individuals. The human enzyme in extracts from rescued yeast was detected with autoimmune antibodies from a polymyositis patient. We conclude that, in spite of substantial differences between human and yeast tRNA sequences in evolution, strong conservation of the G3.U70 system of recognition is sufficient to yield accurate aminoacylation in vivo across wide species distances.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedouelle H., Guez V., Vidal-Cros A., Hermann M. Overproduction of tyrosyl-tRNA synthetase is toxic to Escherichia coli: a genetic analysis. J Bacteriol. 1990 Jul;172(7):3940–3945. doi: 10.1128/jb.172.7.3940-3945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Piña B., Silverman N., Marcus G. A., Agapite J., Regier J. L., Triezenberg S. J., Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992 Jul 24;70(2):251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Biou V., Yaremchuk A., Tukalo M., Cusack S. The 2.9 A crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNA(Ser). Science. 1994 Mar 11;263(5152):1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Buechter D. D., Schimmel P. Dissection of a class II tRNA synthetase: determinants for minihelix recognition are tightly associated with domain for amino acid activation. Biochemistry. 1993 May 18;32(19):5267–5272. doi: 10.1021/bi00070a039. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Autoreactive epitope defined as the anticodon region of alanine transfer RNA. Science. 1987 Nov 20;238(4830):1116–1119. doi: 10.1126/science.2446387. [DOI] [PubMed] [Google Scholar]
- Chang P. K., Dignam J. D. Primary structure of alanyl-tRNA synthetase and the regulation of its mRNA levels in Bombyx mori. J Biol Chem. 1990 Dec 5;265(34):20898–20906. [PubMed] [Google Scholar]
- Chatton B., Walter P., Ebel J. P., Lacroute F., Fasiolo F. The yeast VAS1 gene encodes both mitochondrial and cytoplasmic valyl-tRNA synthetases. J Biol Chem. 1988 Jan 5;263(1):52–57. [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Cusack S., Härtlein M., Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489–3498. doi: 10.1093/nar/19.13.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H., Schimmel P. An E. coli aminoacyl-tRNA synthetase can substitute for yeast mitochondrial enzyme function in vivo. Cell. 1987 Nov 20;51(4):643–649. doi: 10.1016/0092-8674(87)90133-4. [DOI] [PubMed] [Google Scholar]
- Edwards H., Trézéguet V., Schimmel P. An Escherichia coli tyrosine transfer RNA is a leucine-specific transfer RNA in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1153–1156. doi: 10.1073/pnas.88.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Ge Q., Trieu E. P., Targoff I. N. Primary structure and functional expression of human Glycyl-tRNA synthetase, an autoantigen in myositis. J Biol Chem. 1994 Nov 18;269(46):28790–28797. [PubMed] [Google Scholar]
- Hill K., Schimmel P. Evidence that the 3' end of a tRNA binds to a site in the adenylate synthesis domain of an aminoacyl-tRNA synthetase. Biochemistry. 1989 Mar 21;28(6):2577–2586. doi: 10.1021/bi00432a035. [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature. 1988 May 12;333(6169):140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. Evidence that a major determinant for the identity of a transfer RNA is conserved in evolution. Biochemistry. 1989 Aug 22;28(17):6800–6804. doi: 10.1021/bi00443a003. [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. Functional compensation of a recognition-defective transfer RNA by a distal base pair substitution. Biochemistry. 1992 Oct 27;31(42):10310–10314. doi: 10.1021/bi00157a019. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Madern D., Leberman R. Cloning and characterization of the gene for Escherichia coli seryl-tRNA synthetase. Nucleic Acids Res. 1987 Feb 11;15(3):1005–1017. doi: 10.1093/nar/15.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi H., Hoben P., Yamao F., Ozeki H., Söll D. Transfer RNA mischarging mediated by a mutant Escherichia coli glutaminyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5076–5080. doi: 10.1073/pnas.81.16.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Modular arrangement of functional domains along the sequence of an aminoacyl tRNA synthetase. Nature. 1983 Dec 1;306(5942):441–447. doi: 10.1038/306441a0. [DOI] [PubMed] [Google Scholar]
- Keng T., Webster T. A., Sauer R. T., Schimmel P. Gene for Escherichia coli glycyl-tRNA synthetase has tandem subunit coding regions in the same reading frame. J Biol Chem. 1982 Nov 10;257(21):12503–12508. [PubMed] [Google Scholar]
- McClain W. H., Foss K. Changing the identity of a tRNA by introducing a G-U wobble pair near the 3' acceptor end. Science. 1988 May 6;240(4853):793–796. doi: 10.1126/science.2452483. [DOI] [PubMed] [Google Scholar]
- Musier-Forsyth K., Scaringe S., Usman N., Schimmel P. Enzymatic aminoacylation of single-stranded RNA with an RNA cofactor. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):209–213. doi: 10.1073/pnas.88.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsoulis G., Hilger F., Fink G. R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986 Jul 18;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- Park S. J., Schimmel P. Evidence for interaction of an aminoacyl transfer RNA synthetase with a region important for the identity of its cognate transfer RNA. J Biol Chem. 1988 Nov 15;263(32):16527–16530. [PubMed] [Google Scholar]
- Plotz P. H., Dalakas M., Leff R. L., Love L. A., Miller F. W., Cronin M. E. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med. 1989 Jul 15;111(2):143–157. doi: 10.7326/0003-4819-111-2-143. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Royal N. J., Neuman de Vegvar H., Herlihy W. C., Biemann K., Schimmel P. Primary structure of a large aminoacyl-tRNA synthetase. Science. 1981 Sep 25;213(4515):1497–1501. doi: 10.1126/science.7025207. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Sauer R. T., Schimmel P. R. Purification and properties of alanine tRNA synthetase from Escherichia coli A tetramer of identical subunits. J Biol Chem. 1981 Jan 10;256(1):198–204. [PubMed] [Google Scholar]
- Racher K. I., Kalmar G. B., Borgford T. J. Expression and characterization of a recombinant yeast isoleucyl-tRNA synthetase. J Biol Chem. 1991 Sep 15;266(26):17158–17164. [PubMed] [Google Scholar]
- Ribas de Pouplana L., Buechter D. D., Davis M. W., Schimmel P. Idiographic representation of conserved domain of a class II tRNA synthetase of unknown structure. Protein Sci. 1993 Dec;2(12):2259–2262. doi: 10.1002/pro.5560021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schimmel P., Giegé R., Moras D., Yokoyama S. An operational RNA code for amino acids and possible relationship to genetic code. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8763–8768. doi: 10.1073/pnas.90.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. P., Musier-Forsyth K., Schimmel P. Region of a conserved sequence motif in a class II tRNA synthetase needed for transfer of an activated amino acid to an RNA substrate. Biochemistry. 1994 May 3;33(17):5312–5318. doi: 10.1021/bi00183a039. [DOI] [PubMed] [Google Scholar]
- Shiba K., Schimmel P., Motegi H., Noda T. Human glycyl-tRNA synthetase. Wide divergence of primary structure from bacterial counterpart and species-specific aminoacylation. J Biol Chem. 1994 Nov 25;269(47):30049–30055. [PubMed] [Google Scholar]
- Shiba K., Suzuki N., Shigesada K., Namba Y., Schimmel P., Noda T. Human cytoplasmic isoleucyl-tRNA synthetase: selective divergence of the anticodon-binding domain and acquisition of a new structural unit. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7435–7439. doi: 10.1073/pnas.91.16.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Berman L., O'Brien C., Reichlin M. Anti-KJ: a new antibody associated with the syndrome of polymyositis and interstitial lung disease. J Clin Invest. 1989 Jul;84(1):162–172. doi: 10.1172/JCI114136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trézéguet V., Edwards H., Schimmel P. A single base pair dominates over the novel identity of an Escherichia coli tyrosine tRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1991 May;11(5):2744–2751. doi: 10.1128/mcb.11.5.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygand-Durasevic I., Johnson-Burke D., Söll D. Cloning and characterization of the gene coding for cytoplasmic seryl-tRNA synthetase from Saccharomyces cerevisiae. Nucleic Acids Res. 1987 Mar 11;15(5):1887–1904. doi: 10.1093/nar/15.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygand-Durasević I., Ban N., Jahn D., Söll D. Yeast seryl-tRNA synthetase expressed in Escherichia coli recognizes bacterial serine-specific tRNAs in vivo. Eur J Biochem. 1993 Jun 15;214(3):869–877. doi: 10.1111/j.1432-1033.1993.tb17990.x. [DOI] [PubMed] [Google Scholar]
- Wiesenberger G., Waldherr M., Schweyen R. J. The nuclear gene MRS2 is essential for the excision of group II introns from yeast mitochondrial transcripts in vivo. J Biol Chem. 1992 Apr 5;267(10):6963–6969. [PubMed] [Google Scholar]