Abstract
A pervasive finding in animal models of substance abuse is that associations form quickly between contexts and drugs of abuse, such as cocaine. Studies of conditioned place preference (CPP) demonstrate that animals approach cues previously paired with cocaine. This is a commonly used preparation, but the configuration of the CPP apparatus differs across laboratories. Two common apparatus configurations for CPP are one compartment (in which the animal has access to the entire apparatus and spatial cues are irrelevant) or two compartments (in which access is restricted to one half of the apparatus and spatial cues are relevant). We compared the effects of acquisition and extinction of cocaine-induced CPP as a function of configuration. During CPP acquisition, C57BL/6J mice received cocaine paired with one tactile floor (conditioned stimulus, CS+) and saline paired with the other (CS−). CS+ and CS− trials occurred on alternate days in one of three configurations: one-compartment (exposure to the entire apparatus during CS+ or CS−), two-compartment consistent position (exposure to CS+ or CS− in adjacent, spatially distinct compartments), or two-compartment alternating position (exposure to CS+ or CS− in adjacent compartments that alternated spatial locations across days). A stronger preference for the CS+ floor occurred in two- versus one-compartment groups, with the strongest preference observed when cocaine was paired with alternating chamber positions. In contrast, greater loss of preference occurred after extinction in a one-compartment procedure, regardless of one- or two-compartment acquisition history. These findings suggest that a two-compartment configuration facilitated acquisition but attenuated extinction of a cocaine-induced CPP. The use of different CPP configurations may distinguish the underlying substrates and relevant cues for acquisition and extinction processes in cocaine abuse.
Keywords: conditioned place preference, learning and memory, acquisition, extinction, cocaine, spatial learning
Conditioned place preference (CPP) is a tool for investigating how neutral environmental cues (conditioned stimuli, CSs) become associated with drugs of abuse (unconditioned stimuli, USs). The process of cocaine-induced CPP involves an animal associating cocaine with specific cues (e.g., tactile, spatial) within a CPP apparatus. When animals are subsequently given a choice between a place that was previously paired with cocaine (CS+) and a place paired with saline (CS−), they often choose to spend more time with the CS that was paired with cocaine. The animal’s performance (conditioned response) at this time of memory retrieval reflects the degree of CPP learning. Repeated exposure to the CS+ in the absence of cocaine (CS−no US) will result in extinction, during which the preference for the CS+ will be weakened. Extinction is thought to leave acquisition memories intact while new inhibitory learning occurs and suppresses expression of CPP.
CPP is widely used to assess the conditioned reinforcing properties of cues associated with drugs of abuse (Bardo & Bevins, 2000, Napier et al, 2013). Even a cursory reading of the CPP literature reveals that there are very different physical characteristics associated with the apparatus across laboratories. One of the more obvious differences is whether the apparatus is configured to have one or two compartments during conditioning. In a one-compartment configuration with no divider, one of two tactile floor types serves as the CS+ or CS−, which results in exposure to either cue across the entire apparatus (e.g., Bernardi et al, 2009; Cunningham et al, 2003; Raybuck et al, 2013; Vezina & Stewart, 1987a, 1987b). In a two-compartment configuration, the chamber is divided into at least two compartments and the animal is confined to one chamber position during CS+ trials and to another chamber position during CS− trials (e.g.; Fuchs et al, 2002; Malvaez et al, 2012; Shimosato & Watanabe, 2003). Thus, in both procedures, the tactile cues predict drug or saline reinforcement, but an additional spatial component is present in two-compartment procedures, with position of apparatus potentially predicting drug or saline delivery. This predictive spatial component can be eliminated in a third type of procedure, by alternating the spatial position of tactile cues over acquisition trials (Cunningham et al, 2006b).
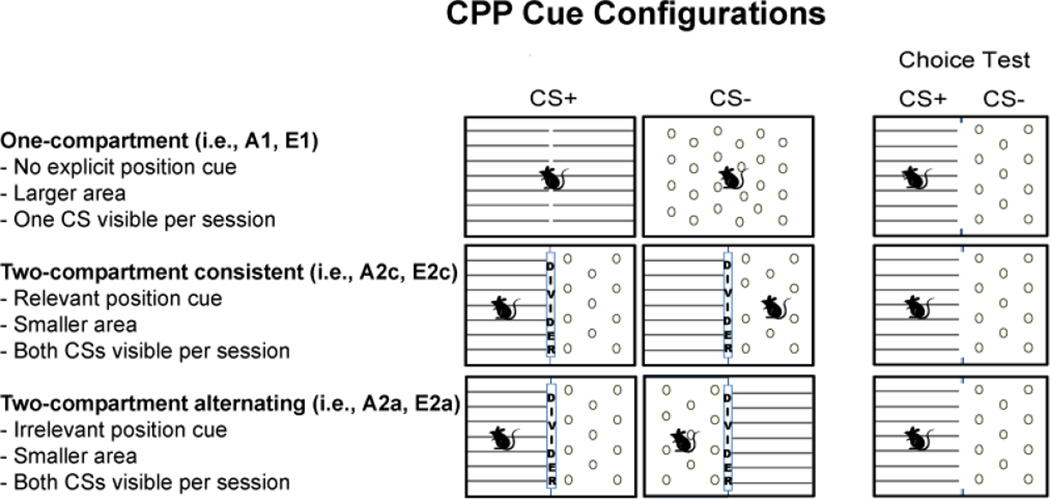
These three procedures differ in several ways (Figure 1). First, the two-compartment procedures expose the animal to only half of the chamber during each training trial, resulting in exposure to tactile cues in a more confined space compared to a one-compartment procedure. Second, the similarity between training and testing condition differs between procedures. A one-compartment procedure provides the same amount of apparatus space between training and testing (i.e., mice can explore the entire apparatus), but a different floor configuration between training and testing (i.e., only the CS+ or the CS− floor cue is present and accessible during training whereas both floor cues are present and accessible during testing). Third, a two-compartment procedure with drug delivered in a consistent location introduces a relevant spatial component to the task that is not present in either a one-or a two-compartment alternating position procedure.
Figure 1.
Conditioned Place Preference (CPP) Cue Configurations. Abbreviations for each configuration are based on the number of CPP compartments (one-,1 or two-compartments, 2) and the type of spatial cue (consistent, c or alternating, a) during CPP acquisition (A) and extinction (E). CPP preparations consisted of tactile cues (grid, G or hole, H floors) for all animals. Two-compartment chambers were identical to one-compartment chambers except that a clear divider bisected the chamber area, leaving the opposite conditioned stimulus (CS) flooring/position visible. During acquisition (A), mice were injected IP with 20 mg/kg cocaine (+) or saline (−) and placed on their assigned CS+ or CS− paired floor in a one- or two-compartment apparatus. Cocaine was paired with the grid floor (G+/H−) for half of the animals and with the hole floor (G−/H+) for the other half (not shown). During extinction (E), CPP was extinguished by placing mice on the previously paired CS+ floor without a cocaine injection (mice did not receive tactile exposure to the previous CS− floor during extinction). Preference for the CS+ paired floor was determined by a Choice Test before and after acquisition or extinction.
These configurations may differentially affect acquisition or extinction of CPP. During acquisition, more cues (e.g., tactile and spatial) may be associated with cocaine in the consistent two- versus one-compartment procedure, allowing better retrieval of the memory post-acquisition (Pearce and Bouton, 2001). In contrast, more cues could also lead to one cue overshadowing another cue, resulting in weaker expression post-acquisition depending on which cues are sampled at test (Rescorla and Wagner, 1972). If the dominant training cue is not also in the testing configuration then retrieval and performance will decline. An alternating two-compartment or one-compartment procedure may also increase CPP. In these procedures, the spatial cues are not predictive of drug state; therefore, animals may better isolate the tactile cues as the key predictive CS and increase performance.
Following acquisition, many studies have shown that extinction is specific to the context in which it occurs, with conditioned responding showing renewal when testing occurs in a different context (e.g., Bouton et al, 2004). It is possible that changes in CPP apparatus configuration from extinction to post-extinction testing may alter the expression of the extinguished preference. For instance, changes in cue configuration between extinction and testing may effectively change the context of testing, which will result in renewal of drug seeking, whereas similar configurations between extinction and testing would lead to greater generalization of the extinction context to the testing context and greater extinction expression. Therefore, the application of a one- or two-compartment procedure (with consistent or alternating cues) may change the similarity of cues between training (acquisition or extinction) and testing conditions and may ultimately influence CPP.
In the following experiments, we examined the effects of apparatus configuration on acquisition and extinction of cocaine-induced CPP. In Experiment 1, we show that a two-compartment procedure promotes acquisition, but impairs extinction, regardless of acquisition history. In Experiment 2, we show that an alternating two-compartment procedure promotes acquisition, relative to both one-compartment and consistent two-compartment procedures. In Experiment 3, we show that a one-compartment procedure promotes extinction, compared to either of the two compartment procedures. These findings have practical implications for how to generate and extinguish CPP in the laboratory, as well as theoretical implications for the processes that underlie acquisition, expression, and extinction of CPP. They also point to potentially different neurobiological mechanisms of CPP as a function of cue configuration during acquisition and extinction.
Experiment 1: CPP Acquisition and Extinction
This experiment examines the role of position cues in the acquisition and extinction of cocaine-induced CPP. Expression of drug preference may change depending on stimulus conditions and apparatus configuration during testing (White et al, 2005), though less is known about the effect of different conditions during acquisition and extinction. In addition, few direct comparisons have been made to determine the effects of configuration between the common one- and two-compartment CPP approaches (Cunningham et al, 2006b).
Methods
Subjects
Sixty male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed 4 per cage in a controlled environment (12 hr light dark cycle, lights on at 6 am). Mice (8–18 weeks of age) had ad libitum access to food and water and weighed 20–30 grams. Experimental events occurred between 7 am and 12 pm. All experiments were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Apparatus
CPP was generated using an unbiased apparatus, consisting of clear acrylic walls (30 cm × 15 cm × 15 cm). The apparatus included interchangeable halves (left and right) of two floor types (Grid, G and Hole, H; Cunningham et al, 2006a). Grid floors consisted of 2.3-mm stainless steel rods mounted 6.4-mm apart in an acrylic frame. Hole floors consisted of perforated stainless steel with 6.4-mm round holes on 9.5-mm staggered centers. The CPP test chambers were housed in melamine shells (McCarthy Manufacturing, Gresham, OR) with air vents around the side of the chamber, allowing low levels of light to enter each chamber. A camera was mounted inside the center of the shell. During two-compartment training, the CPP chamber was bisected by a clear acrylic barrier. This created a left and a right side and two positions for the mouse to be placed. During one-compartment training, the acrylic barrier was removed. The one-compartment chamber was 30 cm wide × 15 cm deep × 15 cm high; each side of the two-compartment chamber was 15 cm wide × 15 cm deep × 15 cm high. The opposite floor type was visible through the divider during two-compartment training.
Drug
Cocaine HCL (COC) was obtained from Sigma and dissolved in physiological saline (SAL, 0.9% NaCl) for intraperitoneal (IP) injection (10 ml/kg), and administered at a dose of 20 mg/kg on conditioned stimulus positive days (CS+). This dose was chosen based on our previous cocaine-induced CPP results in mice (Bernardi et al, 2010; Raybuck et al, 2013). Saline (SAL) was injected (IP) into animals on conditioned stimulus negative days (CS−), matching any volume and handling specific effects between COC CS+ and SAL CS− acquisition trials.
Cocaine-Induced CPP Protocol
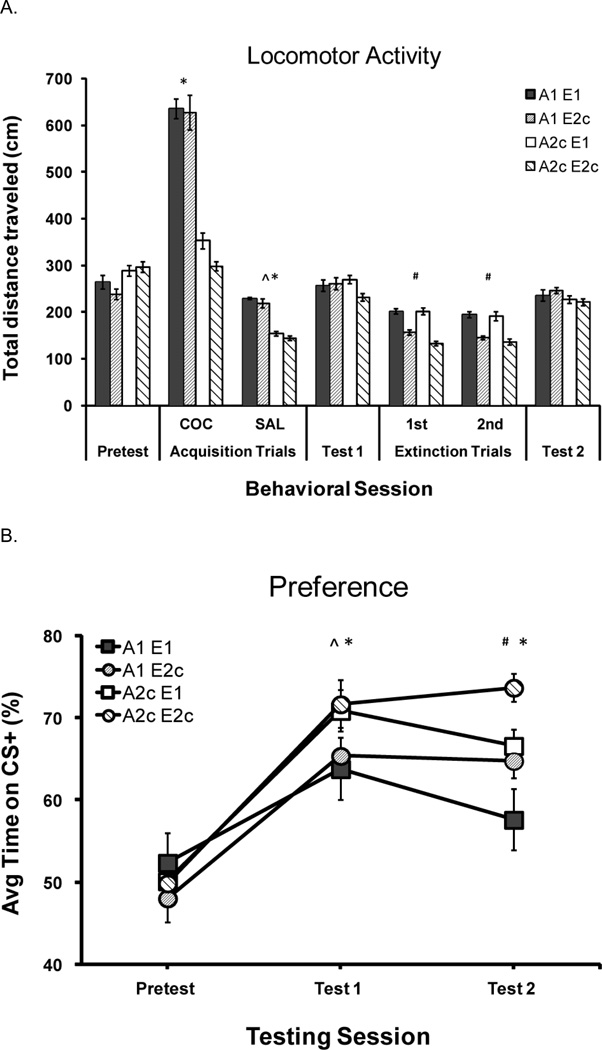
CPP involved the following phases: Habituation, Pretest, Acquisition (A), Post-Acquisition test (Test 1), Extinction (E), and Post-Extinction test (Test 2). Mice were assigned to one of four treatment groups that were categorized by the type of apparatus configuration used (one-compartment or two-compartments with consistent position cues) during acquisition (A) and extinction (E). We compared locomotion (Figure 2A) and cocaine-induced CPP (Figure 2B) in these four groups. Treatment groups based on apparatus configuration (1 or 2c) and learning phase (A or E) were designated as follows: A1 E1 (one-compartment acquisition and one-compartment extinction, N = 14); A1 E2c (one-compartment acquisition and two-compartment extinction with consistent position cues, N = 14); A2c E1 (two-compartment acquisition with consistent position cues and one-compartment extinction, N = 16), and A2c E2c (two-compartment acquisition and extinction with consistent position cues, N = 16).
Figure 2.
Experiment 1. Treatment group abbreviations are based on apparatus configuration (1 or 2c) during acquisition (A) and extinction (E): A1 E1 (N=14); A1 E2c (N=14), A2c E1 (N=16) and A2c E2c (N=16). Error bars indicate the standard error of the mean (±SEM). Total distance traveled in cm (mean, SEM) during behavioral sessions (Pretest, Acquisition, Test 1, Extinction, and Test 2). Acquisition data have been pooled over the two cocaine CS+ and two saline CS− trial types. Extinction data are from trials 1 (1st) and 2 (2nd). Note. ^Saline CS− < Cocaine CS+; *Two-compartment acquisition < One-compartment acquisition; #Two-compartment extinction < One-compartment extinction; p < 0.05, Fig 2A. Cocaine-induced CPP represented as the percent of time spent on the cocaine paired (CS+) floor at the Pretest (Pretest), Post-Acquisition test (Test 1) and Post-Extinction test (Test 2). Note. ^Pretest < Test 1; *A1 < A2c; #E1 < E2c; ps < 0.05, Fig 2B Please see Experiment 1 Results section (Locomotor Activity and Test Preference) for a description of statistical findings.
Habituation (three trials prior to Pretest)
Mice were habituated to the experimental room and to handling prior to cocaine-induced CPP pretesting. On each day, mice were transported in their home cage with three other mice from the colony to the experimental room. They were weighed, allowed to rest for one hour before and after handling (similar to subsequent experimental days), and returned to the colony room.
Testing [Pretest, Post-Acquisition test (Test 1); Post-Extinction test (Test 2)]
Testing (Pretest, Test 1, and Test 2) consisted of a 5-min session in which the mouse had access to both the CS+ and CS− floors. Floors (Grid and Hole) were configured the same for each individual mouse during all testing sessions. These were also consistent with the orientation from acquisition and/or extinction days if applicable (i.e., any two-compartment manipulation: A1 E2c, A2c E1, and A2c E2c groups), but without the divider in place. Conditioned stimulus assignments were counterbalanced within and between configuration groups by floor type (Grid or Hole) and position (left or right). Pretesting pre-exposed the animals to the apparatus and allowed a baseline (naïve) measure of preference in each animal for comparison to their Post-Acquisition Test 1 and Post-Extinction Test 2 preference. Testing was completed 24 hr after the last habituation, acquisition, and extinction trial.
Acquisition (A, four trials prior to Test 1)
Mice were moved to the experimental room, weighed, and allowed to habituate to the room for 1 hr prior to acquisition trials. Mice were then injected with COC or SAL, immediately placed back in their home cage for less than 30 s (while other mice within the same cage were injected) and then placed in their assigned acquisition chamber. Half of the mice were assigned to one of two floor subgroups (injected with COC and placed on a Grid floor = G+) and half were assigned to the other floor subgroup (injected with COC and placed on a Hole floor = H+). Thus, on alternate days over four acquisition trials (two COC, two SAL), mice in the G+ subgroup received COC immediately prior to two separate 15-min acquisition trials on the Grid floor and SAL immediately prior to two separate 15-min trials on the Hole floor. Alternatively, mice in the H+ subgroup received COC immediately prior to 15-min acquisition trials on the Hole floor and SAL immediately prior to 15-min trials on the Grid floor. In all three experiments, mice were counterbalanced as best as possible so that any residual effect of drug or CPP condition was balanced between groups and within each home cage. For example, there were two A1 treated mice and two A2c treated mice per home cage and on each conditioning day half of the mice treated with cocaine were in the A1 group and half were in the A2c group.
Mice were assigned to a one- or two-compartment acquisition (A) group and pre-test preference was counterbalanced between these groups prior to acquisition trials. The group of mice that acquired a CPP in a two-compartment configuration with consistent position cues is further identified as the A2c group. In this configuration, the left and right floor types were different during all phases of CPP (1 Grid side and 1 Hole side). A tall clear divider restricted mice to one floor type and one position of the apparatus per trial. Each position of the chamber was consistently paired with COC or SAL but not both; therefore, each position, as well as each floor, consistently predicted COC or SAL during two-compartment acquisition with consistent position cues. The group of mice that acquired a CPP in a one-compartment configuration is further identified as the A1 group. In this configuration, the left and right floor types were identical during acquisition trials and mice had access to both sides of the apparatus, with no divider separating each chamber position. The assignment of CS+ floor (Grid or Hole), CS+ position (left or right, if applicable), and the order of drug injection (Days 1–4: COC, SAL, COC, SAL or SAL, COC, SAL, COC) were counterbalanced within each acquisition group.
Extinction (E, 2 trials prior to Test 2)
During extinction (E), all mice were exposed to the previously paired (CS+) floor (Grid or Hole) for 30-min with no injection. This occurred over two consecutive days and was followed by a Post-Extinction test (Test 2). Each acquisition apparatus configuration group (A1 and A2c) was divided into two extinction configuration groups (E1 and E2c). For half of the mice, CPP was extinguished in a two-compartment configuration (E2c), resulting in exposure to the previously COC-associated floor on the CS+ chamber position. For the other half, CPP was extinguished in a one-compartment configuration (E1), resulting in exposure to the previously COC-associated floor, unrestricted by position. This resulted in four groups of mice, distinguished by the apparatus configuration applied during acquisition and extinction trials (A1 E1, A1 E2c, A2c E1 and A2c E2c). It should be noted that half of the mice were extinguished in exactly the same apparatus configuration as during CS+ acquisition trials (A1 E1 and A2c E2c), and half were extinguished on the same floor but not the same apparatus configuration as during CS+ acquisition trials (A1 E2c and A2c E1).
Data Analysis
The locomotor activity and position of each animal in the CPP box (left/right position) were recorded by a camera mounted on the CPP shell ceiling and analyzed by Ethovision software (Noldus, Leesburg, VA). Place preference was defined as the amount of time that each animal spent on the CS+ associated floor during each test session. This was represented first by comparing the average seconds per minute (sec/m) that each floor subgroup spent on the Grid floor (mice conditioned to Grid floor with COC, G+ subgroup, compared to mice conditioned to Hole floor with COC, G− subgroup). In all three experiments, a statistically significant difference in the time spent on the Grid floor by each floor subgroup (G+ versus G−, ps < 0.05, data not shown) verified a place preference in each treatment group following acquisition (Cunningham et al, 2003). There were no differences in results if G or H was used as the CS+ floor; therefore, the percent of total time that mice spent on their assigned CS+ paired floor during testing was used to present CPP data.
Behavioral data were analyzed with Microsoft Office Excel and SPSS software. Dependent variables were place preference (average time on CS+, %) and locomotion (total distance traveled in cm). Independent variables were configuration group (one- or two-compartment) during acquisition and/or extinction, test session (Pretest, Test 1, or Test 2), injection drug type (COC CS+ or SAL CS−), and extinction trial (1st or 2nd). Analysis of variance (ANOVA) and planned LSD follow-up tests analyzed datasets with a significance level set at 0.05. For repeated measures ANOVAs, a Greenhouse-Geisser correction adjusted the degrees of freedom and p value for violations to sphericity (Mauchly sphericity test, p < 0.05).
Results and Conclusions
There was no effect of configuration on distance traveled during any test session (ps > 0.05, Figure 2A, 3A, and 4A) or Pretest preference (ps > 0.05, Figures 2B, 3B, and 4B). These results were consistent in all three experiments.
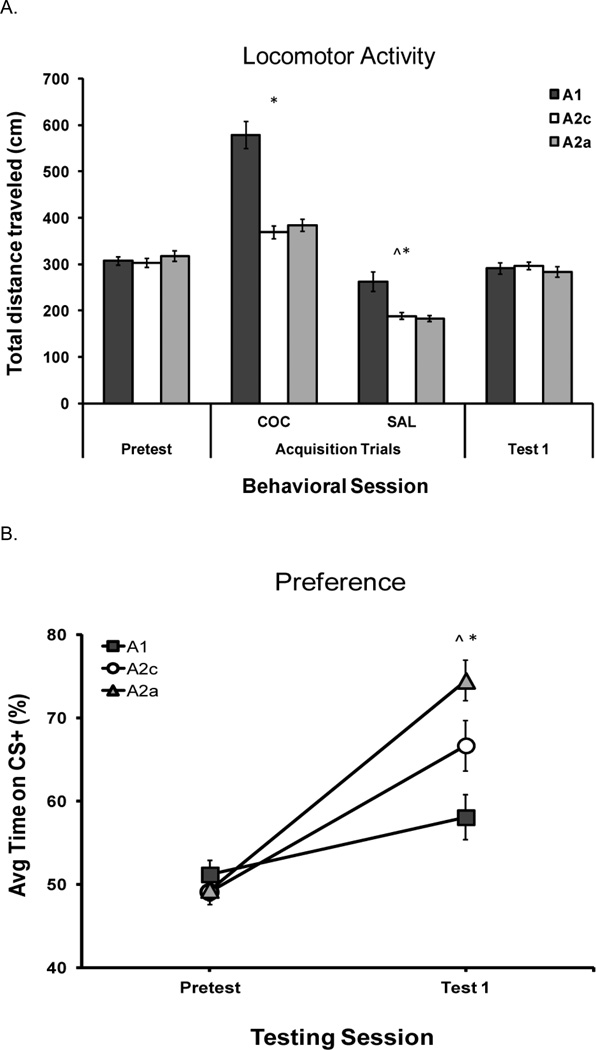
Figure 3.
Experiment 2. Treatment group abbreviations are based on apparatus configuration (1, 2c or 2a) during acquisition (A): A1 (N=16); A2c (N=16), A2a (N=16). Error bars indicate the standard error of the mean (±SEM). Total distance traveled in cm (mean, SEM) during behavioral sessions (Pretest, Acquisition, Test 1). Acquisition data have been pooled over the two cocaine CS+ and two saline CS− trial types. Note. ^Saline CS− < Cocaine CS+; *Two-compartment acquisition < One-compartment acquisition; p < 0.05, Fig 3A. Cocaine-induced CPP represented as the percent of time spent on the cocaine paired (CS+) floor at Pretest (Pretest) and Post-Acquisition test (Test 1). Note. ^Pretest < Test 1; *A1 < A2c < A2a; ps < 0.05, Fig 3B. Please see Experiment 2 Results section (Locomotor Activity and Test Preference) for a description of statistical findings.
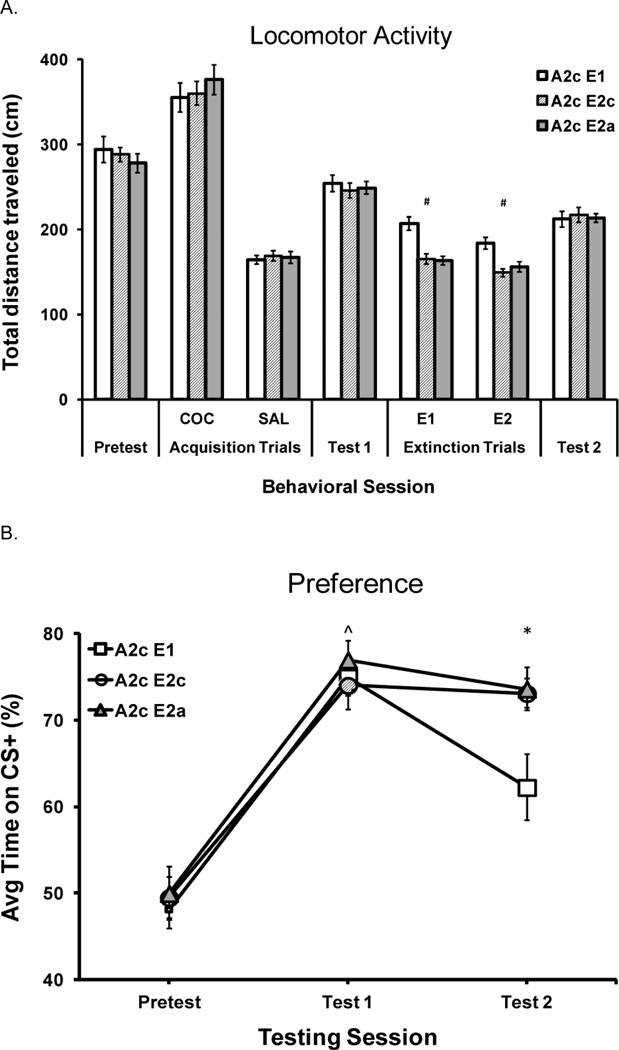
Figure 4.
Experiment 3. Treatment group abbreviations are based on apparatus configuration (1, 2c or 2a) during acquisition (A) and extinction (E): A2c E1 (N=16); A2c E2c (N=16), A2c E2a (N=16). Error bars indicate the standard error of the mean (±SEM). Total distance traveled in cm (mean, SEM) during behavioral sessions (Pretest, Acquisition, Test 1, Extinction, and Test 2). Acquisition data have been pooled over the two cocaine CS+ and two saline CS− trial types. Extinction data are from trials 1 (1st) and 2 (2nd). Note. ^Saline CS− < Cocaine CS+; *Two-compartment acquisition < One-compartment acquisition; #Two-compartment extinction < One-compartment extinction; p < 0.05, Fig 4A. Cocaine-induced CPP represented as the percent of time spent on the cocaine paired (CS+) floor at Pretest (Pretest), Post-Acquisition test (Test 1) and Post-Extinction test (Test 2). Note. ^Pretest < Test 1; *A2c E1 < A2c E2c and A2c E2a; ps < 0.05, Fig 4B Please see Experiment 3 Results section (Locomotor Activity and Test Preference) for a description of statistical findings.
Locomotor Activity
During acquisition, mice traveled a greater distance during CS+ trials (in the presence of cocaine) compared to CS− trials (in the presence of saline) and in the one-compartment configuration compared to the two-compartment configuration. These observations were confirmed by a 2 (acquisition configuration) × 2 (subsequent extinction configuration) × 2 (drug type-repeated) ANOVA, which revealed main effects of drug type and acquisition configuration, and an interaction of drug type × acquisition configuration [Fs (1, 56) > 96.9, ps < 0.001]. There was no effect of subsequent extinction configuration on distance traveled during acquisition. Follow-up tests revealed that the one-compartment group traveled a greater distance than the two-compartment group following either cocaine or saline injections, and that cocaine injections induced greater activity than saline injections in both configuration groups (ps < 0.001, Figure 2A).
Effects on activity by extinction configuration were similar to acquisition configuration effects (Figure 2A). Mice confined to a one-compartment extinction configuration traveled a greater distance than those confined to a two-compartment configuration. A 2 (acquisition configuration) × 2 (extinction configuration) × 2 (extinction trial-repeated) ANOVA revealed a significant main effect of extinction trial [F (1, 56) = 6.8, p = 0.01]. Separate 2 (acquisition configuration) × 2 (extinction configuration) ANOVAs on Extinction Days 1 and 2 revealed reliable main effects of extinction configuration [Fs > 55.7, ps < 0.001] on both days and an interaction between acquisition and extinction configuration on Day 1 [F (1, 56) = 4.2, p = 0.05]. Follow up tests revealed that distance decreased from extinction trial Day 1 to Day 2, the E1 groups traveled more than the E2c groups, and the E2c groups traveled more on extinction Day 1 after an A1 versus A2c conditioning procedure (ps ≤ 0.01, Figure 2A).
Test Preference
The effect of acquisition and extinction configuration on test preference is clear in the analysis of percent time spent on the CS+ floor (Figure 2B). The change in percent of preference across test session was different between configuration groups and supported with a repeated measures ANOVA [2 (acquisition configuration) × 2 (extinction configuration) × 3 (test session-repeated)]. This analysis indicated a reliable main effect of test session and interactions between test and acquisition configuration, and between test and extinction configuration (Fs > 8.4, ps < 0.005). Follow-up ANOVAs suggested a reliable difference between Pretest and Test 1 preference [F (1, 56) = 96.6, p < 0.001] but no interaction based on acquisition or extinction configuration. During Test 1 (Post-Acquisition), preference was higher after two-compartment acquisition (A2c) than after one-compartment acquisition (A1; Figure 2B; Test 1). This observation was supported by a two-way ANOVA (acquisition configuration × subsequent extinction configuration), which found a significant main effect of acquisition configuration [F (1, 56) = 5.6, p = 0.02] with no effect of subsequent extinction configuration (Test 1 in Figure 2B).
Between Test 1 (Post-Acquisition) and Test 2 (Post-Extinction), preference decreased in the E1 groups relative to the E2c groups. This was demonstrated by a 2 (acquisition configuration) × 2 (extinction configuration) × 2 (test session-repeated) ANOVA indicating a significant interaction of test × extinction configuration [F (1, 56) = 5.0, p = 0.03]. A two-way ANOVA for percent preference at Test 2 (Post-Extinction) revealed a significant main effect of acquisition [F (1, 56) = 9.2, p = 0.004] and a reliable main effect of extinction configuration [F (1, 56) = 14.4, p < 0.001] with no interaction. Therefore, the effect of extinction configuration was not altered by prior acquisition configuration treatment (Test 2 in Figure 2B).
Experiment 1 demonstrated that cocaine-induced CPP was higher after acquisition with a consistent two-compartment procedure compared to one-compartment procedure. In contrast, a one-compartment extinction procedure decreased preference compared to a consistent two-compartment extinction procedure. Therefore, the expression of cocaine-induced CPP was greater after acquisition in a two-compartment configuration with cocaine consistently paired to an apparatus position and floor type, whereas expression of this preference was decreased to a greater degree after extinction in a one-compartment apparatus (floor CS only), regardless of prior acquisition configuration.
Experiment 2: CPP Acquisition
In Experiment 1, we demonstrated stronger preference expression following a two-compartment relative to a one-compartment acquisition procedure. These acquisition results may be accounted for by at least two possible mechanisms. First, retrieval of cocaine-location pairings may be stronger when additional cues combine into one association, such as the tactile and CS+ position cues. In the two-compartment procedure, this would result in associations between either the spatial or the tactile cues and cocaine guiding a stronger test preference. Second, confinement in a smaller space during acquisition may increase expression of CPP at test, independent of the spatial cues that are available. There is some evidence that preference can be modulated by changes in available locomotion space (Swerdlow & Koob, 1984) or CS+ floor size (Vezina & Stewart, 1987b) between acquisition and testing. In Experiment 2, we attempted to make spatial cues irrelevant during acquisition by confining mice to one compartment, but alternating the location of the compartment within the apparatus during acquisition.
Methods
Unless noted otherwise, Experiment 2 was identical to Experiment 1.
Subjects
Forty-eight mice (8 weeks old) were used for this experiment.
Cocaine-Induced CPP Protocol
CPP involved the following phases: Habituation, Pretest, Acquisition (A), and Post-Acquisition test (Test 1).
Acquisition (A, four trials prior to Test 1)
Following the Pretest, mice were assigned to one of three acquisition groups based on apparatus configuration (A1 N = 16; A2c N = 16 and A2a N = 16). In each group, one tactile CS was always paired with cocaine (CS+) and the other was always paired with saline (CS−). One acquisition group was trained in a one-compartment apparatus (A1) and another in a two-compartment apparatus with a consistent CS+ position (A2c), as described in Experiment 1. The third group was conditioned in a two-compartment apparatus with the CS+ associated position alternated (A2a) every other day. This was designed to eliminate the consistent spatial contingency during cocaine pairings. This kept the total chamber area equal to that of the A2c group (in which each floor and position type was consistently paired to a COC and SAL injection in a two-compartment chamber) while also keeping only the floor type matched with each injection (similar to the A1 group). During each 15-min acquisition trial, A2c and A2a configurations contained a tall clear divider that confined animals to one of two chamber positions (left or right) and to one of two floor types (Grid or Hole). During Days 1 and 2 of acquisition, mice in the A2a group received CS+ and CS− trials in the same position of the chamber (e.g., right side). During Days 3 and 4, mice in the A2a group received CS+ and CS− trials in the opposite position of the chamber (e.g., left side). This treatment should have maintained the specific tactile CS−cocaine associations, while severing the predictive relation between positional cues and cocaine. The order of stimulus exposure (e.g., SAL or COC drug, left or right position, grid or hole texture) and CS+ testing configuration (e.g., consistent with Trial 1, 2, 3, or 4) was counterbalanced between groups to minimize any order effects on preference.
Data Analysis
Locomotor activity and test preference was measured during each test (Pretest and Test 1) and compared by acquisition configuration group (A1, A2c and A2a).
Results and Conclusions
Locomotor Activity
Figure 3A shows that activity was enhanced by cocaine injections and during a one-compartment acquisition procedure, similar to Experiment 1. A 3 (acquisition configuration) × 2 (drug type-repeated) ANOVA revealed reliable main effects of drug type [F (1, 45) = 464.9, p < 0.001] and acquisition configuration [F (2, 45) = 43.8, p < 0.001], as well as a reliable interaction of acquisition configuration × drug type [F (2, 45) = 15.1, p < 0.001]. Follow-up tests confirmed that the one-compartment procedure induced greater activity than either two-compartment procedure (ps < 0.001). In addition, mice traveled a greater distance after cocaine injections compared to after saline injections (ps < 0.001).
Test Preference
As demonstrated in Figure 3B, preference expression increased from Pretest to Test 1 in all groups, was greatest in the A2a group, and was least in the A1 group. This observation was supported by a 2 (test session-repeated) × 3 (acquisition configuration) ANOVA, revealing a significant main effect of test [F (1, 45) = 135.5, p < 0.001] and an interaction between test and acquisition configuration [F (2, 45) = 13.9, p < 0.001]. During Test 1, a main effect of acquisition configuration was supported by a one-way ANOVA [F (2, 45) = 10.4, p < 0.001)]. Follow up tests confirmed that A1 preference was significantly less than A2a (p < 0.001) and A2c groups (p = 0.02). In addition, A2c preference was significantly less than A2a preference (p = 0.04).
In conclusion, acquisition of a cocaine-induced CPP was enhanced in both groups that were confined during acquisition via a two-compartment configuration (A2a and A2c preference greater than A1). Preference increased further when the location of cocaine pairings alternated between the two compartments (A2a group), demonstrating that eliminating predictive spatial cues promoted preference. By alternating the mouse location during cocaine and saline delivery, spatial cues within the apparatus did not predict the location of cocaine delivery, which may have resulted in less overshadowing of the tactile cues by the spatial cues. In Experiment 3, we ask whether these same apparatus configuration effects occur in extinction of CPP.
Experiment 3: CPP Extinction
In Experiment 1 we found that a one-compartment procedure produced greater extinction compared to a consistent two-compartment procedure. In Experiment 2 we found that a two-compartment procedure with irrelevant spatial cues produced greater CPP expression compared to a one- or consistent two-compartment procedure. In Experiment 3, we examined these effects during extinction after training all mice in a consistent two-compartment procedure. We exposed mice to a large tactile area using a one-compartment procedure (Group E1) or to a small tactile area using a two-compartment procedure with consistent (Group E2c) or alternating (Group E2a) spatial locations.
Previous studies of extinction in many different procedures have demonstrated that the learning that occurs during extinction is specific to the context of extinction; changes in context between extinction and testing often reveal renewal of conditioned responding (Bouton & Bolles, 1979; Crombag et al, 2008). The design of Experiment 3 allowed us to assess whether renewal of cocaine seeking would occur when certain features of the context were held constant or changed between extinction and testing. We varied the size of the apparatus (whole apparatus in the one-compartment groups; half apparatus in the two-compartment groups), the size of the floors (whole floors in one compartment; half floors in two-compartment), and the informativeness of the positional cues within the apparatus (informative in the E2c group; uninformative in the E1 and E2a groups). All groups were tested in the whole apparatus with both floors present, as in Experiments 1 and 2.
If apparatus size is a salient feature of the context, then extinction in the one-compartment procedure should result in the lowest preference during testing in the same-sized apparatus. If CS position is a salient feature of the context, then extinction in the consistent two-compartment procedure should result in lower preference during testing than the alternating procedure with CS position the same.
Methods
Unless noted otherwise, Experiment 3 was conducted similar to Experiments 1 and 2.
Cocaine-Induced CPP Protocol
CPP involved the following phases: Habituation, Pretest, Acquisition (A), Post-Acquisition test (Test 1), Extinction (E), and Post-Extinction test (Test 2).
Acquisition (A, four trials prior to Test 1)
All animals (N = 48) acquired a CPP in a two-compartment apparatus with consistent CS+ position cues (A2c), as described in Experiments 1 and 2. Experiment 1 demonstrated no interaction between acquisition and extinction configuration groups on preference; therefore, only one acquisition procedure was applied prior to extinction group assignment in order to match groups for future comparisons.
Extinction (E, two trials prior to Test 2)
Following an A2c acquisition procedure, mice were assigned to one of three extinction groups categorized by apparatus configuration (E1 N = 16; E2c N = 16 and E2a N = 16). Groups E1 and E2c were handled identically to extinction procedures in Experiment 1. On both extinction days, mice were only permitted to stand on their previously conditioned floor type (either in a one- or a two-compartment configuration). No injections were given. In the E2a group, animal placement each day alternated between CS+ and CS− positions in a counterbalanced order while the opposing floor and position remained visible. This procedure was designed to resemble E1 extinction with exposure to CS+ floor cues on both positions of the apparatus and to resemble E2c extinction with a smaller extinction area. Therefore, E2a mice were exposed to the CS+ floor in both positions of the apparatus (similar to the E1 group), but were confined to one position of the apparatus per trial (similar to the E2c group). The order of CS+ testing configuration (e.g., consistent with Trial 1 or 2) was counterbalanced in the E2a group to minimize any order effects on preference.
Data Analysis
Locomotor activity and test preference was measured during each test (Pretest, Test 1 and Test 2) and compared by extinction configuration group (E1, E2c and E2a).
Results and Conclusions
Locomotor Activity
As previously demonstrated during acquisition trials, activity increased after cocaine injections compared to saline injections (Figure 4A). Supported by a 3 (extinction configuration) × 2 (drug type-repeated) ANOVA, reliable differences in activity emerged due to drug type [F (1, 44) = 131.2, p < 0.001), with no interaction due to subsequent extinction configuration (p > 0.8, Figure 4A).
During extinction, traveling distance increased in a one-compartment procedure compared to a two-compartment procedure, similar to Experiment 1. A 3 (extinction configuration) by 2 (extinction trial-repeated) ANOVA revealed a significant main effect of extinction trial [F (1, 45) = 40.6, p < 0.001], and a reliable interaction between extinction trial and configuration type [F (2, 45) = 3.1, p = 0.05; Figure 4A]. Separate ANOVAs on extinction trials 1 and 2 revealed reliable main effects of extinction configuration [Fs > 9.0, ps ≤ 0.001]. Follow up tests revealed that on both Days 1 and 2, more distance was traveled in the E1 group compared to the E2c and E2a groups (ps ≤ 0.002, Figure 4A).
Test Preference
Following acquisition, preference for the CS+ paired floor was expressed in all groups at Test 1 (Figure 4B). This was tested by a 3 (test-repeated) × 3 (extinction configuration) ANOVA indicating a significant main effect of test [F (2, 70) = 117.3, p < 0.001)] with no interaction of subsequent extinction configuration [F (2, 70) = 2.4, p = 0.07]. A follow up ANOVA confirmed that percent preference significantly differed from Pretest to Test 1 [F (1, 45) = 220.7, p < 0.001)] with no interaction of extinction configuration. As demonstrated in Figure 4B, preference for the CS+ paired floor was similar in all three groups during Test 1, prior to extinction treatment.
From Test 1 to Test 2 a reliable main effect of test [F (1, 45) = 17.8, p < 0.001] and a reliable interaction between extinction configuration and test session [F (2, 45) = 7.1, p = 0.002] was revealed. At Test 2 (Post-Extinction), a significant effect of extinction between groups was confirmed [F (2, 45) = 5.3, p = 0.009] by a one-way ANOVA (Figure 4B). Follow-up tests determined that there was no difference in preference between E2c and E2a groups (p = 0.897) and that preference decreased in the E1 group compared to both E2c and E2a groups (p = 0.009 and p = 0.006, respectively). These findings are consistent with greater extinction induced by a one-compartment procedure, compared to either of the two-compartment procedures.
These extinction results replicated and extended those from Experiment 1, with the E1 configuration inducing the greatest decrease in preference compared to either two-compartment procedure. In Experiment 3, a preference for the cocaine-paired floor was expressed in all subsequent extinction groups after A2c acquisition. Following acquisition, we tested whether consistent spatial cues (E2c group) or an increase to CS+ confinement without consistent spatial cues (E2a group) would influence extinction. Preference was extinguished differentially based on extinction apparatus configuration. One-compartment extinction facilitated the largest decreases in CPP and the two- compartment groups (E2c and E2a) demonstrated very little change in preference following extinction.
The large difference between the E2a and E1 groups suggest that experiencing the CS+ in different positions is not enough to promote extinction. One possible theoretical explanation for these findings is that the size of the apparatus becomes encoding as a salient feature of the extinction context. When this size was changed between extinction and testing, as was the case for the E2 groups, the extinguished preference showed renewal. Of course, there are other explanations, including total amount of exposure to the CS+ during extinction, which may account for differences in extinction. Whatever the mechanism, these results are clear in showing loss of preference following a one-compartment extinction procedure.
Discussion
In a series of three CPP experiments, we demonstrate that acquisition and extinction of cocaine-induced CPP are affected by the configuration of drug-associated cues. Expression of cocaine-seeking behavior was increased after acquisition in a two-compartment configuration compared to conditioning in a one-compartment configuration. When the location of the tactile CS+ and CS− cues were alternated in a two-compartment configuration, CPP was further increased. Finally, extinction treatment in a one-compartment configuration led to the greatest decrease in preference, compared to either two-compartment configuration. These findings suggest that cue configuration may have opposite effects during acquisition and extinction of cocaine-induced CPP.
Two-compartment acquisition enhances preference
Previous studies have confirmed that changes to CS configuration, either by modality (visual or tactile cues) or size, can alter the expression of preference for ethanol- or morphine-associated cues (Cunningham et al 2006b; Vezina &Stewart, 1987a 1987b; White et al, 2005). In the current study, preference for cocaine-associated cues was enhanced following a two-compartment acquisition procedure compared to a one-compartment procedure. Many theories expect that expression of associative learning will be affected by the similarity between the conditions of learning and the conditions of testing. To evaluate the contributions of CS configuration to the expression of preference after acquisition, we manipulated three variables: 1) the location of the CS in the chamber, 2) the size of floor, and 3) whether CS+ and CS− cues were present on all trials. In our two-compartment acquisition procedure with consistent spatial cues (Group A2c), the CS+ and CS− floors were visible and in the same location during acquisition and testing. The only difference between acquisition and testing was the presence of a clear divider during acquisition, which prevented the mice from making contact with the tactile cues on the opposite side of the apparatus. This resulted in a difference in size of the confined area during acquisition – in A2 groups, mice were confined to half of the apparatus and could see both CS+ and CS− cues, whereas in A1 groups, mice were allowed to explore the tactile cues over the entire apparatus but could only see one cue per trial.
One study that investigated the effect of confinement on CPP found that restrained rats learn amphetamine-induced conditioned locomotion (sensitization) but do not acquire CPP (Swerdlow & Koob, 1984). A difference between that study and ours is that Swerdlow and Koob (1984) confined animals to a much smaller area during conditioning than we used in our two-compartment apparatus. Extensive confinement in the Swerdlow and Koob study may have significantly decreased reward-related associations paired with the amphetamine environment and increased exploratory behavior in the testing environment, ultimately decreasing CPP. Our finding that confinement to half of the apparatus during acquisition resulted in greater CPP compared to the A1 group suggests that high preferences can be expressed with a moderate decrease in chamber size between acquisition and testing. In fact, the decrease in chamber area paired with unconditioned stimuli, and the related decrease in distance traveled by mice during training, may have promoted the acquisition of tactile cue associations. Although it is plausible that floor cues are better sensed and given more attention when activity on them is increased, our results suggest that activity alone does not predict the preference because the two-compartment configuration induced less locomotor activity, but higher levels of CPP.
Correspondingly, animals in a larger area (such as a one-compartment procedure) during acquisition may distribute a greater proportion of their cocaine association to the larger environment in general (due to more visible and tangible contextual cues). This may dilute the overall floor-cocaine association and ultimately lead to a decrease in preference expression during testing due to competing associations elicited by extraneous cues (e.g., irrelevant position and locomotor cues, distant visual and auditory cues). Thus, a smaller physical stimulus size in this experiment may have increased conditioned responding to the tactile CS+ by minimizing the development of associations with extraneous cues. This is analogous to results from many conditioning experiments that have found a promotion of conditioning by smaller temporal durations (e.g., Barela, 1999; Cunningham & Prather, 1992) or smaller stimulus size (Kosaki et al, 2013; Tommasi & Polli, 2004). A decrease in the continuous dimension of a CS, such as a shorter audible CS or a smaller place CS may increase the ability to identify it from other irrelevant background cues. Therefore, a smaller stimulus duration or size may be easier to identify or isolate as a predictive cue. Thus, in two-compartment groups, a decrease in size of the apparatus and proximal cues may generally promote preference, which may then be further promoted when spatial cues are made irrelevant in the alternating two-compartment group, leaving only proximal drug-predictive tactile cues.
We further observed that it is not confinement to half of the apparatus alone during acquisition that promotes CPP expression at test. The group that received confined exposure to alternating spatial locations (Group A2a) showed enhanced expression during testing compared to the group that received confined CS+ and CS− exposure to consistent spatial locations (Group A2c). By alternating cocaine exposure in the A2a group, the spatial location was eliminated as a predictive cue. Removal of this predictive spatial component may have allowed associative learning to the tactile cue to be enhanced further in the A2a animals over that of A2c animals and lead to increased CPP expression. Therefore, an increase in familiarity with the CPP configuration (CS+ and CS− cues visible during all trials) and a decrease in overall movement on the CS+ floor may have enhanced the association between cocaine and tactile cues in both A2c and A2a groups. When spatial cues were made irrelevant in the A2a group, this further increased conditioning to the tactile floor, resulting in increased CPP over that of the A2c group.
This result may seem contradictory to previous experiments that found impairments in CPP when cue configuration was alternated (Cunningham et al, 2006b). During conditioning and testing, Cunningham et al. (2006b) alternated the location of tactile cues in the dark or visual cues in the light within a two-compartment apparatus. In contrast, our study alternated the location of both tactile and visual cues in the light. Therefore, both tactile and visual cues remained relevant predictors of cocaine in our study, but the spatial cue relevance was eliminated. As demonstrated by Cunningham et al., visual cues in the absence of consistent spatial cues fail to produce CPP in rodents. In contrast, tactile cues alone or tactile cues in combination with visual or spatial cues effectively produce CPP. After spatial cues were alternated in the current study, the effective tactile and visual cue combination remained a predictive CS for mice. Contradictory findings between these alternating cue studies may be accounted for by the different cues that remained after spatial cues were made irrelevant.
Locomotion differences cannot fully account for post-acquisition preference
During acquisition, a one-compartment configuration consistently induced less preference and greater cocaine-induced locomotion compared to a two-compartment configuration. A combination of cocaine and an increase in activity has been shown to enhance catecholamine, glucose, and lactose plasma levels, both during and after activity (Han et al, 1996). This could alter reinforcement and withdrawal between one and two-compartment groups that significantly differed in locomotor activity. Although it was beyond the scope of this paper to determine if activity differences altered drug pharmacokinetics and drug-cue associations, our data demonstrate that activity alone cannot account for differences in preference expression. For example, if a decrease in activity induced pharmacokinetic changes that extended to preference results, we would have expected both consistent and alternating two-compartment acquisition groups, with similar activity levels, to express similar preference levels. This did not occur. During extinction, consistent and alternating groups expressed a similar amount of activity once again, but had similar levels of preference. Thus, the relation between activity and preference expression is not consistent.
One-compartment extinction inhibits preference
In contrast to acquisition (gain of preference), a one-compartment procedure enhanced extinction (loss of preference). There are different ways to think about this result. One could think of these findings as reflecting impairments in learning during acquisition and enhancements in learning during extinction. It also is possible that a performance process that generally leads to low levels of expression mediates preference after one-compartment acquisition or extinction. How this process works is unclear, but these effects could be mediated by context, with differential sensitivity to changes in context between acquisition, extinction, and testing. If apparatus size is the salient feature of the context, keeping size constant between extinction and testing may allow the size of the apparatus itself to serve as a retrieval cue for the learning that occurred during extinction, resulting in low levels of preference (i.e., weak renewal). Another way to think about the extinction results is that the one-compartment extinction procedure allowed the animal to associate extinction of the tactile cues with multiple spatial contexts, compared to when extinction is confined to a specific spatial location. This may have reduced the ambiguity of cues associated with extinction, creating additional retrieval cues for extinction (Bouton & Bolles, 1979; Bouton, 1988, 2004), similar to what might occur when extinction occurs in multiple contexts (e.g., Gunther et al, 1998; Holmes & Westbrook, 2013).
This interpretation is complicated, however, by the finding that confined exposure to alternating positions during extinction (E2a) did not promote extinction, relative to a group that received confined exposure to a consistent position (E2c). This result suggests that exposures to CS+ cues in multiple spatial locations do not alone promote extinction. Instead, exposure to a single large CS+ floor during extinction promoted the loss of preference. The change in CS floor size and CS position that the E1 group experienced between extinction and testing may have allowed the animals to better detect the extinction contingencies, resulting in a persistent extinction effect not observed with the two-compartment procedures.
These findings extend what is known about these commonly used CPP approaches and the underlying differences between acquisition and extinction. Our experiments leave several important issues unresolved. For example, in our two-compartment procedure, animals were confined to either the CS+ or CS−, but they were still able to see the opposite floor through the divider. It remains to be determined how observing the CS+ during CS− conditioning days (and vice versa) alters the strength of CPP, although previous work with visual cues suggests that the identity of the opposite floor does not influence preference expression (Cunningham et al, 2006b). Our results suggest that the same cue configuration may have different effects on the learning that occurs during acquisition and extinction of CPP. This lays the groundwork for future neurobiological studies that may investigate potentially different mechanisms that underlie acquisition and extinction of CPP with designs that may or may not include a spatial component.
Acknowledgments
This research was supported by NIDA grants DA025922 and DA018165 (to KML) and T32DA007262 (to LNH).
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Current Opinion in Neurobiology. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barela PB. Theoretical mechanisms underlying the trial-spacing effect in Pavlovian fear conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:177–193. doi: 10.1037//0097-7403.25.2.177. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuropharmacology and Neurotoxicology. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM. A role for α1-adreneergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behavioral Neuroscience. 2010;124:204–210. doi: 10.1037/a0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala β2- and α1-adrenergic antagonists. Learning and Memory. 2009;16:777–789. doi: 10.1101/lm.1648509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: Implications for exposure therapy. Behaviour Research Therapy. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Crombag H, Bossert J, Koya E, Shaham Y. Context-induced relapse to drug-seeking: a review. Philosophical Transactions of the Royal Society. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006a;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behavioral Neuroscience. 2006b;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Prather LK. Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Anim Learn Behav. 1992;20:187–194. [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Research. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Gunther LM, Denniston JC, Miller RR. Conducting exposure treatment in multiple contexts can prevent relapse. Behaviour Research and Therapy. 1998;79:75–91. doi: 10.1016/s0005-7967(97)10019-5. [DOI] [PubMed] [Google Scholar]
- Han DH, Kelly P, Fellingham W, Conlee RK. Cocaine and exercise: temporal changes in plasma levels of catecholamines, lactate, glucose, and cocaine. American Physiological Society. 1996;270:E438–E444. doi: 10.1152/ajpendo.1996.270.3.E438. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Westbrook RF. Extinction of reinstated or ABC renewed fear responses renders them resistant to subsequent ABA renewal. J Exp Psychol Anim Behav Process. 2013;39:208–220. doi: 10.1037/a0031986. [DOI] [PubMed] [Google Scholar]
- Kosaki Y, Austen J, McGregor A. Overshadowing of geometry learning by discrete landmarks in the water maze: effects of relative salience and relative validity of competing cues. Journal of Experimental Psychology: Animal Behavior Processes. 2013;39:126–139. doi: 10.1037/a0031199. [DOI] [PubMed] [Google Scholar]
- Malvaez M, McQuown S, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proceedings of the National Academy of Sciences, USA. 2012;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, De Wit H. Using conditioned place preference to identify relapse prevention medications. Neuroscience and Biobehavioral Reviews. 2013;37:2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annual review of Psychology. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, McCleery EJ, Cunningham CL, Wood MA, Lattal KM. The histone deacetylase inhibitor sodium butyrate modulates acquisition and extinction of cocaine-induced conditioned place preference. Pharmacology Biochemistry and Behavior. 2013;106:109–116. doi: 10.1016/j.pbb.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. In: Black AH, Prosky WF, editors. Classical Conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. Journal of Neuroscience Methods. 2003;128:103–110. doi: 10.1016/s0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow, Koob Restrained rats learn amphetamine-conditioned locomotion, but not place preference. Psychopharmocology. 1984;84:163–166. doi: 10.1007/BF00427440. [DOI] [PubMed] [Google Scholar]
- Tomassi L, Polli C. Representation of two geometric features of the environment in the domestic chick (Gallus gallus) Animal Cognition. 2004;7:53–59. doi: 10.1007/s10071-003-0182-y. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology. 1987a;91:375–380. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharmacology. 1987b;93:257–260. doi: 10.1007/BF00179944. [DOI] [PubMed] [Google Scholar]
- White NM, Chai SC, Hamdani S. Learning the morphine conditioned cue preference: Cue configuration determines effects of lesions. Pharmacology, Biochemistry & Behavior. 2005;81:786–796. doi: 10.1016/j.pbb.2005.06.002. [DOI] [PubMed] [Google Scholar]