Abstract
People must constantly select among potential thoughts and actions in the face of competition from (a) multiple task-relevant options (underdetermined competition) and (b) strongly dominant options that are not appropriate in the current context (prepotent competition). These types of competition are ubiquitous during language production. In this work, we investigate the neural mechanisms that allow individuals to effectively manage these cognitive control demands and to quickly choose words with few errors. Using fMRI, we directly contrast underdetermined and prepotent competition within the same task (verb generation) for the first time, allowing localization of the neural substrates supporting the resolution of these two types of competition. Using a neural network model, we investigate the possible mechanisms by which these brain regions support selection. Together, our findings demonstrate that all competition is not alike: resolving prepotent competition and resolving underdetermined competition rely on partly dissociable neural substrates and mechanisms. Specifically, activation of left ventrolateral prefrontal cortex is specific to resolving underdetermined competition between multiple appropriate responses, most likely via competitive lateral inhibition. In contrast, activation of left dorsolateral prefrontal cortex is sensitive to both underdetermined competition and prepotent competition from response options that are inappropriate in the current context. This region likely provides top-down support for task-relevant responses, which enables them to out-compete prepotent responses in the selection process that occurs in left ventrolateral prefrontal cortex.
People often face competition in selecting among potential thoughts and actions. For example, when we speak, we must constantly select words in the face of multiple possible alternatives. In some cases, multiple words are compatible with the intended message. For example, you might be pleased with the couch you recently purchased or happy with the sofa you just bought. These phrases are equally good ways of expressing the same idea, but share few words in common. Selecting among appropriate options is underdetermined, with no clear single correct response. Underdetermined competition occurs when multiple task-relevant options are automatically activated, and competition among the options must be resolved to select a single word during language production (e.g., Kan & Thompson-Schill, 2004; Nelson, Reuter-Lorenz, Persson, Sylvester, & Jonides, 2009; Snyder et al., 2010), select a product to buy (e.g., Iyengar & Lepper, 2000; Tversky & Shafir, 1992), or make a decision when there is no clear best option (e.g., Diederich, 2003; Redelmeier & Shafir, 1995). Underdetermined competition increases with the number of options and the similarity between those options.
In other cases, selecting an appropriate response requires over-riding a strongly dominant, but inappropriate response. For example, this prepotent competition occurs when one must avoid using a word that is not appropriate in the current context (e.g., a New Yorker calling the Metro in D.C. “the subway”, or more consequentially, one researcher continuing to refer to his wife as his “girlfriend” after their marriage (Anderson & Levy, 2007)). Prepotent competition must also be resolved to override a habit (e.g., stopping at the store instead of driving home as usual), or to make a decision when there is conflict from a tempting option not consistent with one’s goals (e.g., choosing fruit instead of chocolate cake for dessert). The more strongly an inappropriate response is activated relative to appropriate responses, the higher the prepotent competition is.
Both underdetermined and prepotent competition slow responding, even in healthy adults. For example, people are slower to say a verb that goes with a noun when there is high underdetermined competition between multiple verb associates (e.g., ball, associated with kick, hit, throw, etc. vs. scissors, associated only with cut; e.g., Nelson et al., 2009; Snyder & Munakata, 2008; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997). People are also slower to complete sentences with multiple possible endings (e.g., Nathaniel-James & Frith, 2002; Snyder & Munakata, 2008), and to name pictures with multiple names (e.g., Kan & Thompson-Schill, 2004). Likewise, people are slower to respond when there is high prepotent competition, for example when naming pictures paired with semantically related competitor words in the picture-word interference task (e.g., dog picture with the word cat; e.g., Lupker, 1979; Maanen, Rijn, & Borst, 2009; Schriefers, Meyer & Levelt, 1990), and naming the ink color of incongruent color words in the Stroop task (e.g., Kane & Engle, 2003; Stroop, 1935).
However, for the most part we can manage these cognitive control demands as demonstrated by our ability to quickly choose words and make few errors while speaking. What mechanisms allow us to do so? We hypothesize that competitive inhibitory dynamics in left ventrolateral prefrontal cortex (VLPFC) play a key role in resolving underdetermined competition. Left VLPFC is more active when such competition is high, whether people are generating verbs (e.g., Crescentini, Shallice, & Macaluso, 2010; Nelson et al., 2009; Snyder, Banich, & Munakata, 2011; Thompson-Schill et al., 1997), naming pictures (e.g., Kan & Thompson-Schill, 2004), or generating items from categories (e.g., Hirshorn & Thompson-Schill, 2006). Psycholinguistic theories and models have long posited that lexical selection during speech occurs via competitive processes (e.g., Levelt, Roelofs, & Meyer, 1999). A unified, biologically plausible computational model of the verb generation task by our group has demonstrated how competitive, inhibitory (GABAergic) dynamics among neurons in VLPFC can support underdetermined selection by amplifying activity of the most active representation and suppressing competing representations (Snyder et al., 2010).
Specifically, as excitatory neurons become active, they activate inhibitory interneurons, which in turn diffusely inhibit excitatory neurons (e.g., Douglas & Martin, 2004). Thus, excitatory neurons that are less active are suppressed below firing threshold, while the most active excitatory neurons remain above threshold, such that the strongest representation comes to dominate neural firing (e.g., Douglas & Martin, 2004; for implications for cognitive processes see Munakata et al., 2011). Our computational model simulates this mechanism. A simulated population of excitatory neurons represents one response option, which starts out only slightly more active than simulated neural populations representing other response options. Through lateral inhibition, this slightly more active representation is gradually able to suppress the activity of competing representations. This model generated predictions about underdetermined competition that have been tested and confirmed through levels of brain activity as assessed by fMRI, neuropharmacological manipulation, and links to psychopathology (Snyder et al., 2010; 2013).
However, the need to resolve prepotent competition highlights an important limitation to how well the competitive lateral inhibition mechanism can accomplish selection. Namely, it will always result in selection of the response that is most active in the VLPFC, as described above. When there is competition among multiple valid task responses this mechanism is sufficient, since the most active will be appropriate. However, when there is competition from task-inappropriate responses that must not be selected, this mechanism would allow these task-inappropriate responses to win the competition if they are more strongly activated than task-relevant responses. Nonetheless, healthy adults generally make few errors on tasks involving prepotent selection (e.g., Stroop). How are we able to over-ride such prepotent responses to make a task-appropriate response? Many theories have proposed mechanisms that bias competition towards task-relevant information or responses (e.g., Banich, 2009; Cohen, Dunbar, & McClelland, 1990; Desimone & Duncan, 1995; Thompson-Schill & Botvinick, 2006), but there has been disagreement as to the neural mechanisms supporting this biasing process.
One possibility is that VLPFC plays a role in both underdetermined and prepotent selection. For example, in one framework the pattern of activation across response options is a function of both of the stimulus and task, such that the stimulus initially induces a pattern of activation resembling that in a free association task, and this pattern is then modulated by a control signal that increases activation of task-relevant responses and decreases the activation of task-irrelevant responses (Thompson-Schill, 2005; Thompson-Schill & Botvinick, 2006). This framework proposes that left VLPFC is the source of this control signal, but the authors note that it could potentially come from other prefrontal areas.
Indeed, others have posited that this control signal arises not from VLPFC, but from dorsolateral prefrontal cortex (DLPFC). Specifically, several frameworks propose that portions of DLPFC maintain abstract representations of the task goal, which provide top-down support for task-relevant representations, biasing the system towards the correct response (e.g., Banich, 2009; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Kane & Engle, 2002; Miller & Cohen, 2001; Munakata et al., 2011). This framework is supported by evidence that tasks requiring overriding a prepotent response (e.g. Stroop) activate left DLPFC (e.g., see Nee, Wager, & Jonides, 2007 for meta-analysis). For example, the cascade-of-control model (e.g., Banich, 2009) predicts that left DLPFC is key for resolving prepotent competition, while left VLPFC will be sensitive to prepotent competition only if task-set maintenance and top-down biasing from DLPFC is inadequate to prevent activation of task-irrelevant representations.
Thus, we hypothesize that lateral inhibition in VLPFC alone may be sufficient for resolving underdetermined competition, while resolving prepotent competition requires active maintenance of task goals in DLPFC to bias competition towards task-relevant responses. Specifically, boosting the activation of task-relevant responses could enable them to out-compete prepotent responses via competitive lateral inhibition in VLPFC. We test this possibility by directly contrasting underdetermined and prepotent competition within the same task (verb generation) for the first time, which allows us to localize the neural substrates supporting the resolution of these two types of competition. We find that activation of left VLPFC is sensitive only to underdetermined competition, while activation of left DLPFC is sensitive to both underdetermined and prepotent competition. We then explore computational mechanisms by which these regions interact to resolve underdetermined and prepotent competition, using a model of the verb generation task. Neural network modeling provides a valuable tool for investigating the mechanisms underlying the effects of competition, by allowing us to directly manipulate excitatory activity and competitive inhibition in simulated prefrontal regions, to determine the resulting neural and behavioral effects.
Method
Participants
Participants were 19 healthy, right-handed, young adults (11 women). Four additional participants were excluded from analysis due to excessive movement during fMRI (> 2 mm translation/2° rotation). All participants were native English speakers, had no history of neurological conditions or head injury, and were not taking psychoactive medications. Participants gave informed consent and were treated in accordance with procedures approved by the University of Colorado Boulder Institutional Review Board.
Design and Stimuli
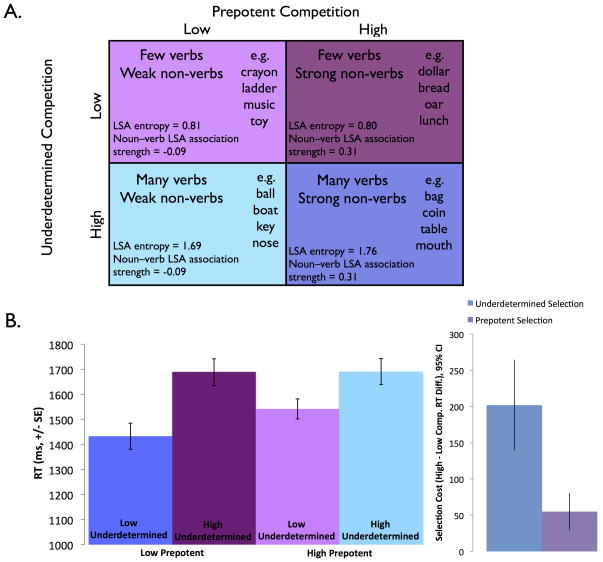
Stimuli were 100 nouns in a 2 × 2 design (Figure 1) crossing high vs. low underdetermined competition and high vs. low prepotent competition, with 25 trials/condition. Underdetermined competition were defined as in previous experiments using latent semantic analysis (LSA) entropy, computed over LSA association values, which reflects competition between all alternative responses (Snyder & Munakata, 2008; Snyder et al., 2010, 2011). Because nouns with high prepotent competition were not available from previous studies, they were selected from a large set of nouns normed for this study by a separate sample of participants (n = 49). In the high prepotent competition condition, task-irrelevant non-verb responses (generated by two or more participants in the free-association norming sample) were more strongly associated with the noun stimuli than task-relevant verb responses (based on higher LSA cosine), whereas in the low prepotent competition condition the reverse was true. All conditions were matched on retrieval demands (calculated as in Snyder et al., 2010, 2011).
Figure 1.
(A) Verb generation task design with example items. Underdetermined competition (high versus low competition among possible verb responses) is crossed with prepotent competition (high versus low competition from non-verb associates). Nouns in the high underdetermined competition conditions have multiple possible verb responses, while nouns in the low underdetermined competition conditions have few possible verb responses (quantified as the LSA entropy, see Methods). Nouns in the high prepotent competition conditions have strong non-verb associates, while nouns in the low prepotent competition conditions have stronger verb than non-verb associates (quantified as the LSA cosine, see Methods). All conditions are matched on retrieval demand. (B) Participants take longer to respond when there is competition among verb responses (underdetermined selection, significant by subjects and items) or competition from prepotent non-verb responses (prepotent selection, significant by subjects and items), and these factors interact (significant by subjects only).
Procedure
Participants were instructed to say the first verb that came to mind for each noun (e.g., cat)–either something it does (e.g., meow), or something you do with it (e.g., feed). They were given an example and eight practice trials prior to entering the scanner, and reminded of the instructions prior to starting the task. Each noun was presented on a screen for 3500 ms with a 500 ms ITI. This stimulus timing was selected based on piloting procedures to minimize omission errors and allow participants to take a breath before the next trial. Participants responded with a fiber-optic noise-canceling microphone (Optoacoustics Ltd., Or-Yuhuda, Israel). A blocked paradigm was used to encourage participants to maintain higher cognitive control during high-competition conditions and lower control during low-competition conditions. Participants completed 5 blocks of 5 trials each per condition, plus 11 baseline fixation blocks, lasting 20 seconds each, in one functional run. Blocks were presented in two counterbalanced orders across participants. Within-condition, item order was randomized across participants.
Image Acquisition and Processing
Data were acquired with a 3T Siemens Magnetom TrioTim whole-body MRI scanner, with T2*-weighted echo, echo-planer imaging (EPI; TR= 2000 ms, TE= 29 ms, flip angle= 75°). Functional data were collected in one run of 316 EPI volumes, each consisting of 28 ascending 4 mm thick slices (gap=1 mm, filed-of-view (FOV)=220 mm, in-plane matrix= 64 × 64, in-plane resolution= 3.4 × 3.4 mm2), angled parallel to the inferior surface of the orbital frontal cortex. High-resolution 3D multiecho MPRAGE full head anatomical images were acquired along the transverse plane (TR=2530 ms, TE1=1.64 ms, TE2=3.50 ms, TE3= 5.36 ms, TE4=7.22 ms, TE5= 9.08 ms, flip angle=7°, inversion time=1200 ms; 220 mm FOV, 256 × 256 matrix, 1 mm × 1 mm in-plane resolution, 192 slices, 1 mm slice thickness). A standard head coil was used and head motion minimized with moldable pillows.
Image pre-processing and analysis were conducted with FSL (FMRIB’s Software Library). The first five volumes of the run were discarded to allow the MR signal to reach steady state. Images were motion corrected using MCFLIRT, non-brain voxels removed using BET, spatially smoothed with a 3D Gaussian kernel (FWHM = 8 mm), intensity normalized for all volumes by the same factor, and high-pass filtered to remove high-frequency noise (σ=120 sec). Statistical analyses were conducted using FEAT (FMRIB’s Easy Analysis Tool). GLM analyses of the time series data were conducted, then subjected to group-level random effects analysis. For group analyses, statistical maps were normalized into the common MNI-152 stereotaxic space, using FLIRT (FMRIB’s Linear Image Registration Tool).
Results
Behavioral Results
Reaction times (RTs) were analyzed with a 2 × 2 repeated-measures ANOVA, and plotted in Figure 1B. Replicating previous findings (e.g., Snyder et al., 2010; 2011; Snyder & Munakata, 2008), participants were slowed by underdetermined competition among possible verb responses, F(1,17) = 80.37, p < .001, high underdetermined competition RT M = 1690 ms, SE = 52 ms, low underdetermined competition M = 1488, SE = 34 ms. Participants were also slowed by prepotent competition from non-verb associates, F(1,17) = 8.37, p = .01, high prepotent competition M = 1617 ms, SE = 43 ms, low prepotent competition M = 1562 ms, SE = 40 ms. In addition, prepotent and underdetermined competition interacted, F(1,17) = 5.91, p = .026: the effect of prepotent competition was greater when underdetermined competition was low (RT difference (diff.) M = 109 ms, SE = 31 ms) than when it was high (RT diff. M = 0 ms, SE = 23 ms), and the effect of underdetermined competition was greater when prepotent competition was low (RT diff. M = 257 ms, SE= 44 ms) than when it was high (RT diff. M= 148 ms, SE= 34 ms). Results were similar when RTs were analyzed by item rather than by subject: significant effects of underdetermined competition, F(1, 24) = 82.31, p < .001, and prepotent competition, F(1, 24) = 4.71, p = .04, although the interaction did not reach significance, F(1,24) = 1.82, p = .191.
fMRI Results
First, we report whole-brain analyses, with follow-up region of interest (ROI) analyses to further probe the activation patterns in the clusters identified in the whole-brain analysis. Specifically, we test the opposite contrast in each peak (i.e., the prepotent competition contrast in an ROI around the underdetermined competition peak, and the underdetermined competition contrast in an ROI around the prepotent competition peak). Second, to allow direct comparison to previous research on underdetermined and prepotent selection, we report results from a priori ROI analyses for left VLPFC areas previously implicated in underdetermined competition and a left DLPFC area previously implicated in prepotent competition. For all significant ROI analyses we report false discovery rate (FDR, Benjamini, Brieger, & Yekutieli, 2006) adjusted p values in addition to unadjusted p values.
Whole-Brain Analysis
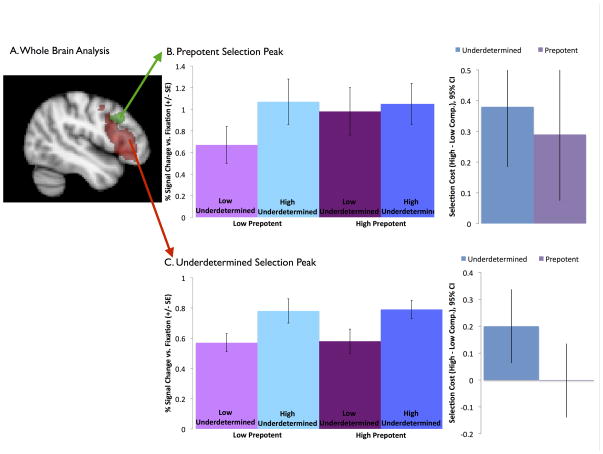
Whole-brain analyses were conducted for: (1) underdetermined competition (high vs. low underdetermined competition, collapsing across levels of prepotent competition) and (2) prepotent competition (high vs. low prepotent competition, collapsing across levels of underdetermined competition). As predicted, underdetermined competition activated a large area of left VLPFC (left inferior frontal gyrus, centered on BA 47), while prepotent competition activated an area of left DLPFC (left middle frontal gyrus, centered on BA 8/9; Table 1, Figure 2). In addition, underdetermined competition activated the cingulate/supplementary motor area, middle temporal gyrus, and cerebellum (Table 1), which have also been implicated in previous studies (e.g., Crescentini et al., 2010; Snyder et al., 2010).
Table 1.
Peak MNI Voxel Coordinates, Anatomical Locations, and Approximate Brodmann’s Areas from Whole-Brain Random Effects Analysis
| Contrast | Region | BA | Max Z | No. of Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Underdetermined Selection | Inferior Frontal Gyrus (L) | 47 | 4.51 | 3493 | −42 | 32 | −8 |
| Superior Frontal Gyrus (L) | 8 | 3.29 | 439 | −6 | 16 | 54 | |
| Middle Temporal Gyrus (L) | 21 | 3.64 | 219 | −58 | −54 | 2 | |
| Cerebellum Posterior Lobe (R) | NA | 4.08 | 3676 | 34 | −60 | −32 | |
| Cerebellum Posterior Lobe (L) | NA | 3.18 | 190 | −32 | −58 | −34 | |
| Caudate (R) | NA | 3.29 | 368 | 20 | 14 | 24 | |
| Precuneus (R) | 7 | −3.94 | 1951 | 6 | −64 | 34 | |
| Superior Temporal Gyrus (R) | 39 | −3.24 | 456 | 48 | −58 | 22 | |
|
| |||||||
| Prepotent Selection | Middle Frontal Gyrus (L) | 8/9 | 3.62 | 205 | −48 | 18 | 36 |
Note. All clusters z>2.58, minimum cluster size=154 voxels, p<.01, two-tailed. BA= Brodmann’s area, L= left, R=right.
Figure 2.
Whole Brain Analysis. (A) Prepotent competition significantly activates a cluster in left DLPFC (green), while underdetermined competition significantly activates a cluster in left VLPFC (red). Follow-up ROI analyses for each activation peak reveal (B) equally high activation for all high-competition conditions in DLPFC, with significant effects of both prepotent and underdetermined competition while (C) VLPFC is sensitive only to underdetermined competition.
To further explore the profile of activation in the key left VLPFC and DLPFC clusters, spherical ROIs (radius = 10 mm) were created around their peak coordinates, and the opposite contrast was tested in each (Figure 2, Table 2). While there was no effect of prepotent competition in the VLPFC ROI around the underdetermined competition peak in the whole-brain analysis, there was, as expected greater activation for the high underdetermined competition than low underdetermined competition conditions, across both levels of prepotent competition (Figure 2). In contrast, in the DLPFC ROI around the prepotent competition peak in the whole-brain analysis, there was also a significant effect of underdetermined competition. As expected there was greater activation for the high prepotent competition than low prepotent competition conditions; however, activation for the low prepotent competition condition with a high level of underdetermined competition was just as high as for the high prepotent competition conditions, as compared to the low prepotent/low underdetermined competition condition (Figure 2).
Table 2.
Results from ROI Analyses
| ROI(s) | Center MNI Coordinates | Variable | Statistical Test | p | FDR adjusted p |
|---|---|---|---|---|---|
| Whole-brain underdetermined competition peak (left VLPFC) | −42, 32, −8 | Prepotent competition | t(18) = 0.27 | .79 | ns |
| Whole-brain prepotent competition peak (left DLPFC) | −48, 18, 36 | Underdetermined competition | t(18) = 3.35 | .004* | .014* |
| A priori left VLPFC (aVLPFC & mid-VLPFC, 2 × 2 × 2 ANOVA) | −48, 30, −6 (aVLPFC) | Underdetermined competition | F(1,16) = 24.68 | < .001* | < .005 * |
| −50, 25, 14 (mid-VLPFC) | Prepotent competition | F(1,16) = 0.04 | .85 | ns | |
| Underdetermined competition X Prepotent competition | F(1,16) = 0.07 | .79 | ns | ||
| Region | F(1,16) = 1.41 | .25 | ns | ||
| Region X Underdetermined competition | F(1,16) = 4.09 | .06# | .10 | ||
| Region X Prepotent competition | F(1,16) = 0.03 | .86 | ns | ||
| Region X Underdetermined competition X Prepotent competition | F(1,16) = 2.63 | .12 | ns | ||
| A priori left aVLPFC | −48, 30, −6 | Underdetermined competition | F(1,16) = 21.06 | < .001* | < .005 * |
| Prepotent competition | F(1,16) = 0.01 | .93 | ns | ||
| Underdetermined competition X Prepotent competition | F(1,16) = 0.05 | .83 | ns | ||
| A priori left mid-VLPFC | −50, 25, 14 | Underdetermined competition | F(1,18) =24.90 | < .001* | < .005 * |
| Prepotent competition | F(1,18) = 0.26 | .61 | ns | ||
| Underdetermined competition X Prepotent competition | F(1,18) = 1.04 | .32 | ns | ||
| A priori left DLPFC | −42, 16, 28 | Underdetermined competition | F(1,15) = 9.64 | .007* | .019* |
| Prepotent competition | F(1,15) = 6.38 | .023* | .045* | ||
| Underdetermined competition X Prepotent competition | F(1,15) = 7.22 | .017* | .038* |
Note:
p < .05,
p < .10
Left VLPFC ROI Analyses
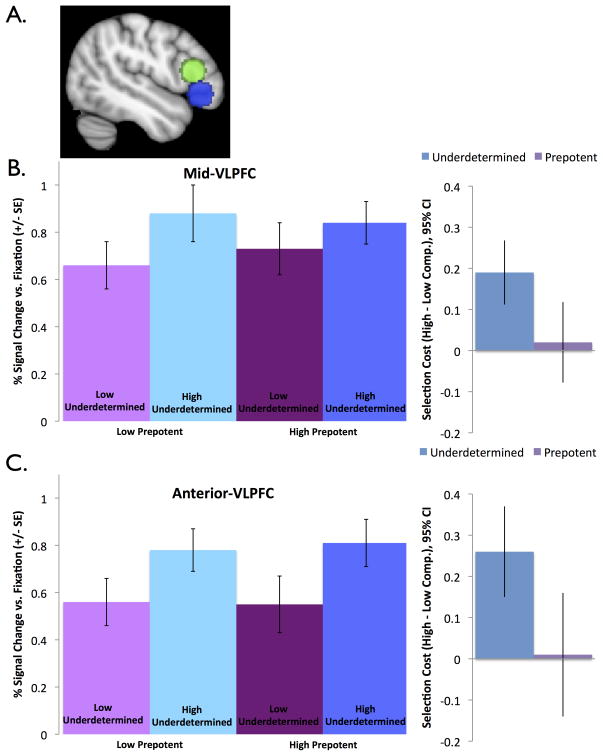
A priori ROI analyses were conducted for the left VLPFC ROIs implicated in underdetermined selection in Snyder et al. (2011). These ROIs were defined around the mean coordinates identified in Badre and Wagner (2007) for left anterior and mid VLPFC (radius =10 mm; Table 2, Figure 3).1 Activation for each condition versus fixation were analyzed with a 2 × 2 × 2 (underdetermined competition x prepotent competition x region) repeated measures ANOVA and follow-up ANOVAs in each region. Outliers with Cook’s d > 3 SD above the mean were excluded, leading to the exclusion of no more than two participants per analysis. Significance of results remained the same with these outliers included.
Figure 3.
VLPFC ROI activation. (A) ROIs were defined in anterior VLPFC (blue) and mid-VLPFC (green). Both (B) mid-VLPFC and (C) anterior VLPFC are sensitive to underdetermined competition but not prepotent competition.
Results are reported in Table 2 and shown in Figure 3. There was significantly greater activation in the high than low underdetermined competition conditions. There was no significant effect of prepotent competition, and no underdetermined x prepotent competition interaction. There was a marginal underdetermined competition x region interaction, with a larger effect of underdetermined competition in anterior-VLPFC than mid-VLPFC. There was no main effect of region or any other interactions with region. Within each ROI, mid and anterior VLPFC showed the same pattern of results: significant effects of underdetermined competition, with no effect of prepotent competition or interaction.
Left DLPFC ROI Analysis
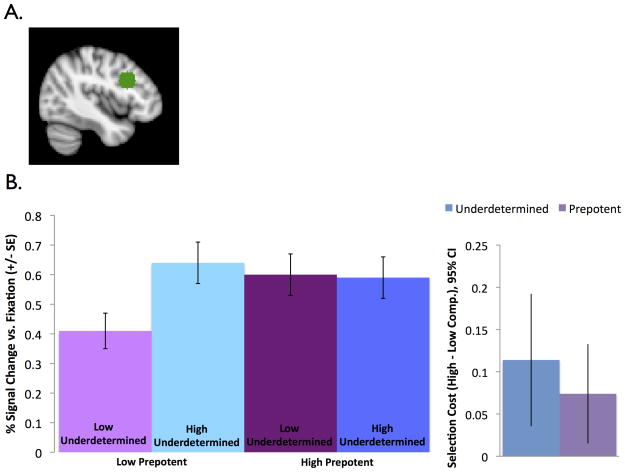
A spherical ROI was defined around the mean coordinates identified in a meta-analysis of Stroop fMRI studies (Nee et al., 2007), which is in posterior portions of DLPFC near the inferior frontal junction (radius= 10 mm, Table 2, Figure 4). The Stroop contrast was chosen because it is the most widely used task involving prepotent competition. Activation for each condition versus fixation was analyzed with a 2 × 2 (underdetermined competition x prepotent competition) repeated measures ANOVA. Three outliers with Cook’s d > 3 SD above the mean were excluded. With these outliers included the pattern of results was the same but the effect of prepotent competition did not reach significance.
Figure 4.
DLPFC ROI activation. (A) The ROI was defined in left DLPFC based on a Stroop meta-analysis (Nee et al., 2007), as the Stoop task involves prepotent competition. (B) This area of DLPFC is sensitive to both prepotent and underdetermined competition, with similar activation levels for the three high-competition conditions.
Results are reported in Table 2 and shown in Figure 4. There was a significant main effect of prepotent competition with greater activation in the high prepotent than low prepotent competition conditions. There was also a significant main effect of underdetermined competition, with greater activation in the high underdetermined than low underdetermined competition conditions. Underdetermined and prepotent competition interacted: the effect of prepotent competition was larger when underdetermined competition was low, and the effect of underdetermined competition was larger when prepotent competition was low.
Discussion of Neuroimaging Results
In sum, the whole-brain analysis identified a left DLPFC area (BA 8/9) sensitive to prepotent competition, while the left VLPFC was sensitive to underdetermined competition, along with a broader network also found in prior studies of underdetermined competition (e.g., Crescentini et al., 2010; Snyder et al., 2010). Follow-up ROI analyses in these areas indicated that the left DLPFC area was sensitive to both underdetermined and prepotent competition, suggesting that it becomes active whenever competition is high. In contrast, left VLPFC was only activated during underdetermined selection. These findings were replicated in a priori DLPFC and VLPFC ROIs previously implicated in prepotent and underdetermined selection respectively. This pattern suggests that VLPFC cannot be the source of the control signal that biases competition towards task-relevant responses (although it may be sensitive to prepotent competition when top-down cognitive control is inadequate). Rather, these results suggest that an area of left DLPFC is the key source of the control process that supports prepotent selection (e.g. Banich, 2009). Also replicating of Snyder et al. (2011), both anterior and mid-VLPFC were activated during underdetermined selection, counter to accounts that posit that they subserve controlled retrieval and selection respectively (Badre & Wagner, 2007).
Several questions remain. First, prepotent competition did not activate VLPFC, suggesting that non-verb competitors do not contribute to activation in VLPFC, yet prepotent competition slowed RTs, suggesting that non-verb responses do compete with verb responses and thus slow selection of an appropriate response. What might account for this apparent discrepancy? Second, the area of left DLPFC sensitive to prepotent competition was also sensitive to underdetermined competition. Previous theories have posited that this area is involved in maintaining task goals to bias competition towards task-relevant responses. Thus, it is not clear what role left DLPFC may play in underdetermined selection (where competition is among task-relevant responses), and how it may differ from the role of VLPFC in underdetermined selection. We use neural network simulations to explore possible answers to these questions and generate predictions for future empirical research.
Computational Mechanisms for Prepotent Selection
We extended a computational model of verb generation (Snyder et al., 2010) to explore mechanisms supporting selection. Specifically, the model tests the theory that input from DLPFC to task-relevant responses in VLPFC can enable them to out-compete prepotent responses (e.g., Banich, 2009; Miller & Cohen, 2001; Munakata et al., 2011). The model further allows us to explore how this DLPFC mechanism might affect underdetermined selection (given that DLPFC was also sensitive to underdetermined competition in the current study), and how it might interact with the competitive lateral inhibition mechanism in VLPFC, which was the key mechanism supporting underdetermined selection in the earlier model. The model implements a verb generation task in a biologically-plausible neural network using the Leabra framework (O’Reilly & Munakata, 2000) as implemented in Emergent (grey.colorado.edu/emergent). Details of the original model are given in Snyder et al. (2010). We first describe the model architecture, including an overview of the mechanisms implemented by each simulated brain region (layer). We next describe how the model was tested by manipulating those mechanisms, and how the results provide insight into potential neural mechanisms for resolving competition.
Verb Generation Model Architecture
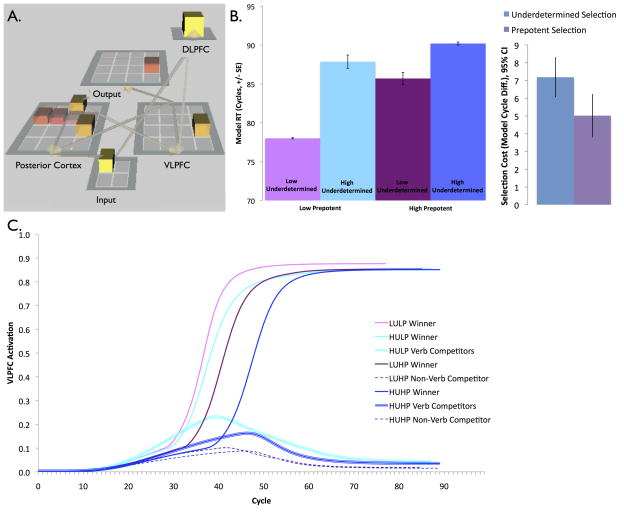
The model contains layers that simulate the following: (1) presentation of noun stimuli, (2) activation of associated verb and non-verb responses in the posterior cortex, (3) selection of responses in VLPFC, (4) maintenance of task goals in DLPFC and top-down biasing towards goal-relevant responses in VLPFC, and (5) output of the selected response (Fig 5A). The strength of connections between nouns and associated responses and between alternative responses were set according to the known association strengths observed in humans using LSA (Landauer, Foltz, & Laham, 1998). These connections support spreading activation between related semantic representations like that observed in posterior cortex (c.f., Levelt et al., 1999). Simulated neurons in the posterior cortex layer then activate representations in the VLPFC layer, which implements competitive lateral inhibition, selecting one response for output. We adapted the model to simulate prepotent competition by (a) adding units representing non-verb competitors to the posterior cortex and VLPFC layers, and (b) adding a DLPFC layer (Figure 5A). These changes to the model are detailed below. Unless otherwise noted, all other aspects of the model are identical to those in the previous version described in Snyder et al (2010).
Figure 5.
Neural network model. (A) Network architecture, with added non-verb competitor units in the posterior cortex and VLPFC layers to simulate prepotent competition, and added DLPFC layer that provides top-down support for relevant verb responses in the VLPFC to test this mechanism for prepotent selection. (B) Model simulates human RTs, showing effects of underdetermined and prepotent competition and their interaction. (C) Activation of the VLPFC units in each simulation condition. Both underdetermined and prepotent competition delay and reduce activation of winning verb responses (thin solid lines), due to competition from alternative responses (thick and dashed lines). Activation of non-verb competitors in the high prepotent competition conditions is reduced by top-down biasing from the DLPFC, which boosts activation of verb responses, helping them to out-compete non-verbs. LULP= low underdetermined competition/low prepotent competition, HULP= high underdetermined competition/low prepotent competition, LUHP= low underdetermined competition/high prepotent competition, HUHP= high underdetermined competition/high prepotent competition.
Input layer
The input layer has four units, representing each condition of the fMRI study: low and high underdetermined competition crossed with low and high prepotent competition (Figure 1). Weights between input units and their response units in the posterior cortex layer are LSA cosines (see fMRI Methods regarding LSA), averaged across all items in that condition (scaled to 75%). The input units for the high underdetermined competition conditions each project to six verb units in posterior cortex, while those for the low underdetermined competition conditions each project to only one verb unit. The input units for the high prepotent competition conditions also project to non-verb competitor units in posterior cortex, specifically their strongest non-verb. In addition, input units project to the DLPFC layer. For simplicity, the DLPFC is activated directly from the input, with weights scaled according to the activation pattern of the DLPFC ROI in the fMRI study (1/3 higher for the high than low competition condition) to simulate the greater activation of DLPFC in the high competition conditions. In the brain, DLPFC may instead be activated by other brain areas depending on task demands (e.g., Banich, 2009), but this is beyond the scope of the current model.
Posterior cortex layer
The posterior cortex layer has one unit for each verb response, plus two non-verb competitor units (one for each high prepotent competition condition). Verb units in the high underdetermined competition conditions have bidirectional lateral connections to one another. The non-verb units in the high prepotent competition conditions have bidirectional lateral connections to the verb units. All lateral connection strengths are set according to the LSA association values, with equal weights in each direction. Thus, this layer simulates spreading semantic activation in posterior cortex. Each posterior cortex layer unit projects to one unit in the VLPFC layer and one unit in the output layer.
VLPFC layer
The VLPFC layer has one unit for each verb response, plus two non-verb competitors, as in the posterior cortex. Units compete through kWTA inhibition, with the kWTA pt parameter set to the standard inhibition level used in the previous model (.66) to fit behavioral data, then manipulated to the low (.62) and high (.68) inhibition levels to test effects of competitive inhibition. VLPFC units are recurrently connected to themselves, and project to their respective posterior cortex and output units. Added to the model are inputs to VLPFC verb units from the DLPFC layer, as described in the next section. The recurrent connection strength was reduced to .60 to prevent over-activation given the additional inputs from the new DLPFC layer.
DLPFC layer
The main addition to the model is a DLPFC layer, which provides top-down support for task-relevant responses. For simplicity, the DLPFC layer has a single unit, simulating populations of neurons representing the task goal (i.e., say verbs). The DLPFC layer is activated by the input layer (see Input layer), and then activates verb, but not non-verb, response units in the VLPFC layer. To prevent verbs not associated with the current noun input from becoming activated, the code was modified such that only verb units that were already becoming active (due to input from the posterior cortex layer) received added excitatory input from the DLPFC, simulating the role of voltage-gated NMDA receptors (see Discussion of Modeling Results). Specifically, at each time-step of the settling process, the DLPFC unit activation level was multiplied by a weight term (to simulate different levels of DLPFC input, see next section) and added to the existing activation level of all VLPFC verb units with activation levels > 0.
Model–Testing Procedure
To explore potential mechanisms involved in resolving underdetermined and prepotent competition, model parameters were first adjusted to simulate the RT effects in the verb generation task: effects of underdetermined and prepotent competition and an interaction between them.2 Vm trial noise was added (Gaussian distribution with M = 0, var = 0.00005) and 30 simulations run at each of five levels of DLPFC input (DLPFC pt. = .003–.007). To test the effects of neural inhibition, the kWTA pt parameter in the VLPFC layer (.66) was reduced to 0.62 and increased to .68, and 30 simulations were again run at each level of DLPFC input.
Model Results
Like human participants, the model generates longer response times when underdetermined competition is high than when it is low (average selection cost = 7.2 cycles, Figure 5B). Also like humans, the model has longer response times when prepotent competition is high than when it is low (average selection cost = 5.0 cycles). The model also replicates the interaction in the human RT data: prepotent selection costs are higher when underdetermined competition is low (7.7 cycles) than when it is high (2.3 cycles), and underdetermined selection costs are higher when prepotent competition is low (9.9 cycles) than when it is high (4.5 cycles).3
The effects of competition can be understood in terms of the activation dynamics of units in the VLPFC layer (Figure 5C). First, both types of competition affect the slope and asymptote of VLPFC unit activations, with units becoming active more gradually and reaching a lower asymptote under high competition. This pattern occurs because all active representations in VLPFC inhibit one another, and thus suppress one another’s activation levels via competitive inhibitory dynamics, which simulate the effects of GABAergic interneurons. The magnitude of these effects is consistent with the order of RTs in the human data (compare solid lines). Second, both verb and non-verb competitors become active in the VLPFC, but non-verbs have a lower asymptote and are active for a shorter period of time than verbs (compare thick to dashed lines), even though they are more active in the posterior cortex. This reflects the influence of top-down biasing from the DLPFC, which boosts activation of verbs, but not non-verbs. Finally, verb competitors have a lower slope and asymptote in the presence of non-verb competitors than in their absence (compare light blue and dark blue thick lines) and non-verb competitors likewise have a lower slope and asymptote in the presence of verb competitors (compare dark blue and dark purple dashed lines), consistent with the interaction in the human RT data. This reflects the fact that verb and non-verb competitors also compete with (and thus suppress) one another.
In the model, DLPFC top-down biasing of VLPFC is critical for resolving prepotent competition by boosting the activation of task-relevant representations. However, this activation has the side effect of also increasing underdetermined competition among task-relevant options. When VLPFC competitive lateral inhibition is at a normal level (Figure 6A) and DLPFC influence is inadequate (below .003), non-verb responses win the competition–that is, the model reliably makes non-verb errors. As the amount of DLPFC input to VLPFC verb units increases, model RTs in the high prepotent competition conditions decrease, as verbs are able to more easily out-compete non-verbs. For example, for the stimulus word bread, DLPFC input helps boost the activation of associated verbs like eat, making them more active than non-verb competitors like butter, thus allowing a verb to win the competition and be selected as the response. However, as DLPFC input increases, model RTs in the high underdetermined/low prepotent competition condition increase, as DLPFC input increases activation of all verb responses, thus increasing competition among them. For example, for the stimulus word ball, which has many associated verbs (e.g., kick, throw, hit), strong top-down input from DLPFC makes all of those associated verbs more active, increasing competition among them.
Figure 6.
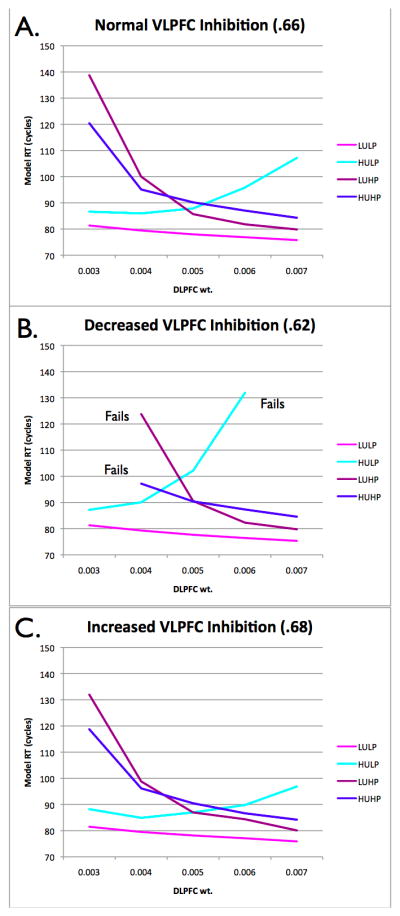
Effects of DLPFC top-down biasing and VLPFC competitive lateral inhibition. (A) As DLPFC input increases, increasing activation of possible verb responses, response times in the high prepotent competition conditions decrease (verbs more easily out-compete non-verbs), but underdetermined competition increases (there is more competition among verbs). This pattern is (B) stronger when VLPFC competitive inhibition is reduced and (C) weaker when VLPFC inhibition is increased.
These patterns interact with the level of competitive lateral inhibition in the VLPFC layer. When inhibition is low (Figure 6B), DLPFC biasing becomes more critical for resolving prepotent competition, while the negative effects of DLPFC biasing on underdetermined competition are increased. Specifically, when DLPFC input is weak, the activation level of verb responses is boosted only slightly higher than those of non-verb responses. When competitive lateral inhibition in VLPFC is weak, it may be inadequate to resolve this strong competition from non-verbs. Indeed, at the lowest level of DLPFC input, the model cannot resolve competition in the high prepotent competition conditions when VLPFC inhibition is low, and fails to settle on a response. Conversely, higher levels of DLPFC input, which increase the activation of all task-relevant verb responses, are particularly problematic for underdetermined selection when VLPFC lateral inhibition is weak–at the highest level of DLPFC input, the model cannot resolve competition among verbs, and fails to settle on a response. In contrast, when VLPFC competitive inhibition is relatively high (Figure 6C), this has the opposite effects of low competitive inhibition: the negative effects of low DLPFC input on prepotent selection are reduced because strong competitive inhibition in VLPFC helps to suppress prepotent competitors, and the negative effects of high DLPFC input on underdetermined selection are reduced because strong competitive inhibition helps to resolve the increased competition among task-relevant responses.
Discussion of Modeling Results
The model simulates the RT results of the current experiment and suggests that competitive lateral inhibition in VLPFC is adequate for resolving underdetermined competition. Specifically, inhibition allows the most active representation to suppress activation of alternative responses and thus be selected for production. However, top-down biasing of VLPFC from DLPFC is essential for resolving prepotent competition: it allows initially weaker task-relevant responses to become more active and thus out-compete the formerly stronger task-irrelevant responses. When DLPFC input to VLPFC is too weak, the model makes errors, generating the prepotent response rather than a task-relevant response, as do patients with left PFC damage (e.g., Noonan, Jefferies, Corbett, & Ralph, 2009).
Our verb generation model makes a number of predictions, some supported by previous research, and some which remain to be tested by future research. First, the model predicts that competitive lateral inhibition in VLPFC is critical for resolving underdetermined competition. The key role of left VLPFC in underdetermined selection is supported by the current and many previous fMRI studies (e.g., Kan & Thompson-Schill, 2004; Nelson, et al., 2009; Snyder et al., 2010), as well as lesion studies. Left VLPFC lesions cause dynamic aphasia with severe reductions in spontaneous speech, and studies find impairment only when there is competition among response options, with normal performance when competition is low (e.g., Robinson et al., 2010; Thompson-Schill et al., 1998). The prediction that inhibition is the key mechanism allowing VLPFC to resolve underdetermined competition has been less extensively tested, but is supported by findings that pharmacological increases in GABA improve inhibition (Snyder et al., 2010), while anxiety, associated with reduced inhibition, impairs selection (Snyder et al., 2010; 2013). Recent evidence also shows that higher levels of GABA (relative to glutamate) in lateral prefrontal cortex predict better selection (de la Vega et al., under review). This prediction could be further tested with future research directly manipulating GABA function (e.g., with TMS).
Second, the model predicts that VLPFC will only be sensitive to prepotent competition in neuroimaging studies when biasing from DLPFC is inadequate to allow prepotent competitors to be quickly suppressed, either because DLPFC function is impaired, or prepotent competition is very strong. Consistent with this model, left VLPFC was not sensitive to prepotent competition in the current study, where competing responses are internal representations in semantic memory, but was sensitive to prepotent competition in the some studies where competition may be stronger because it is present in the external stimulus (e.g., Roberts & Hall, 2008; Snyder, Feigenson, & Thompson-Schill, 2007). This prediction could be further tested by future research using parametric manipulations of prepotent competition and studies of VLPFC response to prepotent competition when DLPFC function is impaired (e.g., by a lesion or TMS).
Third, the model predicts that while top-down biasing from DLPFC is essential for resolving prepotent competition, it may contribute to underdetermined competition by making all task-relevant responses more active. Thus, while our fMRI study found that the left DLPFC area sensitive to prepotent competition was also sensitive to underdetermined competition, the model suggests that DLPFC biasing would ideally be engaged only when prepotent competition is high, but not when underdetermined competition is high. There are two broad possibilities for this discrepancy. First, brain mechanisms may not be ideal. Individuals may not successfully detect sources of competition, and instead simply detect that competition in general, or even cognitive control demands more broadly, have increased. In tasks context in which increased demands often involve prepotent competition, more strongly maintaining task goals whenever the going gets tough is a reasonable strategy. Second, there may be additional neural mechanisms not included in the model. The model provides input to all task-relevant responses active in VLPFC, thus increasing competition among them. However, the brain may have mechanisms to target DLPFC input to only the most active VLPFC neurons, for example, with a stronger version of the voltage gating mechanism. This prediction could be tested by manipulating DLPFC function (e.g., by TMS or tDCS): if DLPFC input is detrimental during underdetermined competition, then increased DLPFC activation should impair underdetermined selection (but improve prepotent selection), whereas if the model is incorrect and DLPFC in fact targets only the most active task-relevant representations in VLPFC, increased DLPFC activation should improve both underdetermined and prepotent selection. In either case, the model is informative: either there are negative side effects of DLPFC biasing, or the biasing mechanism must be more complex.
The potential downside of top-down excitation is illustrated by an informative early failure of the model. The DLPFC layer, as first implemented, simply provided excitation to all verb units in the VLPFC layer. This leads to diffuse excitation of all verbs, including those not associated with the current noun stimulus, massively increasing competition. While this may seem obvious in retrospect, it has not been noted by any of the previous conceptual models, which have assumed that a simple DLPFC mechanism providing top-down support for task-relevant representations should always improve performance. The model demonstrates that this need not the case, and at minimum it is necessary to restrict DLPFC input to representations that are already active in VLPFC due to input from posterior cortex. In the brain, this may be achieved by voltage-gated NMDA receptors, which allow active neurons to become more so, while relatively quiet cells remain inactive (e.g., Raffone, Murre, & Wolters, 2003). Thus, the model predicts that NMDA synapses may play a crucial role in biased competition, a possibility that could be tested in future studies using pharmacological manipulation of NMDA function.
Another important area for future investigation is the mechanisms that allow DLPFC task goal representations to be linked to task-relevant responses in VLPFC without positing hardwired, symbolic representations. Indeed, the broader question of how PFC is able to flexibly represent and bind an almost limitless combination of representations has long perplexed the field. Recently, a computational model has been developed that can flexibly bind PFC representations (e.g., a word with its sentence role) via indirection, in which one part of PFC maintains the location of information maintained in another part of PFC, and can regulate it via the basal ganglia (Kriete, Noelle, Cohen, & O’Reilly, 2013). Such a mechanism could plausibly account for the ability of DLPFC task goal representations to regulate VLPFC representations, but this remains speculative given that this model has not yet been extensively empirically tested.
General Discussion
All competition is not alike. Namely, unbiased competitive lateral inhibition in VLPFC may be sufficient for underdetermined selection (e.g., resolving competition among possible verb responses in the verb generation task), but will allow inappropriate prepotent competitors to win (e.g., non-verbs in the verb generation task). Thus, the current fMRI experiment and neural network simulations were designed to explore the neural substrates and mechanisms that allow us to resolve these two types of competition, by contrasting underdetermined and prepotent competition within the same task for the first time. We propose that left DLPFC and VLPFC implement different computational mechanisms, which interact to affect prepotent and underdetermined selection. Taken together, the current model and neuroimaging evidence are consistent with the view that left DLPFC plays a key role in increasing activation of task-relevant representations in left VLPFC, and that this top-down support must be limited to already active representations. In contrast, the results are not consistent with the proposal that VLPFC is itself the source of the control signal that biases competition towards task-relevant responses. Rather, we suggest that all associated responses (both task relevant and irrelevant) compete in VLPFC, with input from DLPFC biasing competition in favor of the task-relevant responses.
The current fMRI study found that left VLPFC was more active when underdetermined competition was high, replicating previous findings (e.g., Crescentini et al., 2010; Snyder et al., 2011; Thompson-Schill et al., 1997). However, left VLPFC was not more active when prepotent competition was high. These results are consistent with the cascade-of-control model (Banich, 2009) and our computational model, which predict that left VLPFC will be sensitive to prepotent competition only when top-down biasing from DLPFC is inadequate to quickly reduce the activation of prepotent competitors, either because competition is too high or cognitive control is impaired. When DLPFC input is adequate, prepotent competitors may become only briefly and/or weakly active in VLPFC, and so may not drive the BOLD signal.
In contrast to the VLPFC, in the current study an area of left DLPFC (middle frontal gyrus, BA 8/9, in the vicinity of the inferior frontal junction) was more active under conditions of prepotent competition, consistent with previous evidence from the Stroop task (e.g., for meta-analysis see Nee et al., 2007), and other prepotent selection tasks (e.g., Snyder et al., 2007). This finding is also consistent with the cascade-of-control model, which posits that portions of DLPFC provide top-down support for task-relevant representations when there is high prepotent competition (e.g., Banich, 2009; Herd, Banich, & O’Reilly, 2006), and is also consistent with research that suggests that task instructions (in this case, to produce verbs) are gated into, and maintained by active firing in, working memory networks that include DLPFC (e.g., Dumontheil, Thompson, & Duncan, 2011). We thus expanded our previous neural network model to operationalize a version of this conceptual model. The expanded model includes a DLPFC layer that increases activation of task-relevant responses, but not task-irrelevant competitors, in the VLPFC layer. This mechanism is also similar to that proposed by Thompson-Schill and colleagues (e.g., Thompson-Schill & Botvinick, 2006), although the current data do not support their speculation that VLPFC itself is the source of the biasing mechanism. When individuals detect competition, they may attempt to increase control by more strongly maintaining task goals in left DLPFC, which in turn boosts activation of initially weaker task-relevant responses in left VLPFC, thus allowing them to out-compete the formerly stronger task-irrelevant responses.
Somewhat surprisingly, the DLPFC area activated by prepotent competition was also sensitive to underdetermined competition. Activation of left DLPFC by underdetermined competition has been found in some studies using blocked paradigms (e.g., Nathaniel-James & Frith, 2002; Persson et al., 2004), but has generally not been found in event related paradigms (e.g., Crescentini et al, 2010; Snyder et al., 2011). This pattern of findings is consistent with the theory that the presence of competition on the first trial of blocks may serve as cue to increase DLPFC activation on subsequent trials, regardless of the type of competition.4 This theory could be further tested in fMRI studies using a conflict adaptation paradigm. The neural network model suggests that this strategy of increasing control when conflict is detected is essential for resolving prepotent competition, but as with many cognitive and neural processes, there is an inherent trade-off: when underdetermined competition arises, such top-down biasing may actually impair performance, because DLPFC boosts the activation of all task-relevant responses, increasing competition among them. Thus, moderate levels of DLPFC input to VLPFC may be ideal, consistent with the inverted U-shaped curves found for the relation between cognition and many neural processes (e.g., Cools & D’Esposito, 2011).
In sum, the current study and neural network simulations suggest that all competition is not alike: resolving prepotent competition from options that are inappropriate in the current context and underdetermined competition between multiple appropriate responses relies on partly dissociable neural substrates and mechanisms. Better understanding how these processes and brain areas interact during language production may ultimately have implications for better understanding and treating impairments associated with prefrontal damage and psychopathology. For example, strategies or interventions that improve prepotent selection (e.g., increasing task goal maintenance) may be detrimental to underdetermined selection. For an individual with aphasia who has left VLPFC damage, strongly maintaining the goal of naming a fruit may counterproductively increase competition among the names of all fruits in the fruit bowl, making it difficult to select the name of the fruit he or she wants. Finally, these findings may have broader implications for understanding the organization of prefrontal cortex and fundamental trade-offs between excitatory and inhibitory neural mechanisms supporting cognitive control.
Acknowledgments
This research and preparation of the manuscript was supported by grants from the National Institutes of Health (P50-MH079485, F31-MH087073, F32-MH098481, T32-MH15442). We thank Harry R. Smolker and Kathy L. Pearson for assistance with data collection and analysis and members of the P50 center on Executive Function and Dysfunction, Cognitive Development Center, and Banich Lab for valuable discussions.
Footnotes
Nearly identical results were also found for anatomical ROIs consisting of left inferior frontal gyrus pars triangularis and pars orbitalis (mid and anterior VLPFC).
A simple manual search was conducted over (1) VLPFC recurrent connection strength (0.5–1) and (2) the DLPFC wt parameter (.001–.01) to achieve a qualitative fit to RTs, while keeping all other parameters identical to the previous model (Snyder et al., 2010). The basic pattern (effects of underdetermined and prepotent competition) was never violated within this set of parameters.
The goal was to achieve a qualitative fit that provides insight into neural mechanisms, rather than a precise quantitative fit. The interaction in the model is a reasonable match for the human RT data: it slightly underestimates the human RT difference between prepotent selection under high vs. low underdetermined competition, and slightly overestimates it for underdetermined selection under high vs. low prepotent competition. We do not focus on these quantitative differences, given the individual differences across human participants in the size of the interaction, and that other combinations of model parameters could change the quantitative fit without fundamentally changing the model.
Consistent with this theory, participants had significantly slower RTs on the first trial of high competition blocks than on subsequent trials (t(17) = 2.29, p = .035), while there was no difference between first and subsequent trials for low competition blocks (t(17) = 0.64, p = .53).
References
- Anderson MC, Levy BJ. Theoretical issues in inhibition: Insights from research on human memory. In: Gorfein DS, MacLeod CM, editors. Inhibition in Cognition. Washington, DC: American Psychological Association; 2007. pp. 81–102. [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Benjamini Y, Krieger A, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescentini C, Shallice T, Macaluso E. Item retrieval and competition in noun and verb generation: An fMRI study. Journal of Cognitive Neuroscience. 2010;22:1140–1157. doi: 10.1162/jocn.2009.21255. [DOI] [PubMed] [Google Scholar]
- de la Vega A, Brown MS, Snyder HR, Singel D, Munakata Y, Banich MT. Individual differences in the balance of GABA to glutamate in prefrontal cortex predict the ability to select among competing options. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diederich A. Decision making under conflict: Decision time as a measure of conflict strength. Psychonomic Bulletin & Review. 2003;10:167–176. doi: 10.3758/BF03196481. [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. Neural circuits of the neocortex. Annual Review of Neuroscience. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Thompson R, Duncan J. Assembly and use of new task rules in fronto-parietal cortex. Journal of Cognitive Neuroscience. 2011;23:168–182. doi: 10.1162/jocn.2010.21439. [DOI] [PubMed] [Google Scholar]
- Herd SA, Banich MT, O’Reilly RC. Neural mechanisms of cognitive control: An integrative model of Stroop task performance and fMRI data. Journal of Cognitive Neuroscience. 2006;18:22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Lepper M. When choice is demotivating: Can one desire too much of a good thing? Journal of Personality and Social Psychology. 2000;79:995–1005. doi: 10.1037//0022-3514.79.6.995. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive Affective & Behavioral Neuroscience. 2004;4:43–57. doi: 10.3758/CABN.4.1.43. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/BF03196323. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kriete T, Noelle DC, Cohen JD, O’Reilly RC. Indirection and symbol-like processing in the prefrontal cortex and basal ganglia. Proceedings of the National Academy of Sciences. 2013;110:16390–16395. doi: 10.1073/pnas.1303547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer T, Foltz P, Laham D. An introduction to latent semantic analysis. Discourse Processes. 1998;25:259–284. doi: 10.1080/01638539809545028. [DOI] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lupker SJ. The semantic nature of response competition in picture-word interference. Memory & Cognition. 1979;7:485–496. [Google Scholar]
- Maanen L, Rijn H, Borst JP. Stroop and picture-word interference are two sides of the same coin. Psychonomic Bulletin & Review. 2009;16:987–999. doi: 10.3758/PBR.16.6.987. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: Evidence from the effects of contextual constraint in a sentence completion task. NeuroImage. 2002;16:1094–1102. doi: 10.1006/nimg.2002.1167. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/CABN.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Persson J, Sylvester CYC, Jonides J. Mapping interference resolution across task domains: a shared control process in left inferior frontal gyrus. Brain Research. 2009;1256:92–100. doi: 10.1016/j.brainres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Ralph MAL. Elucidating the nature of deregulated semantic cognition in semantic aphasia: Evidence for the roles of prefrontal and temporo-parietal cortices. Journal of Cognitive Neuroscience. 2009;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Munakata Y. Computational explorations in cognitive neuroscience: Understanding the mind by simulating the brain. Cambridge, MA: The MIT Press; 2000. [Google Scholar]
- Raffone A, Murre JMJ, Wolters G. NMDA synapses can bias competition between object representations and mediate attentional selection. Behavioral and Brain Sciences. 2003;26:100–101. doi: 10.1017/S0140525X03400022. [DOI] [Google Scholar]
- Redelmeier D, Shafir E. Medical decision making in situations that offer multiple alternatives. Journal of the American Medical Association. 1995;273:302–305. doi: 10.1001/jama.1995.03520280048038. [DOI] [PubMed] [Google Scholar]
- Roberts K, Hall D. Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory Stroop tasks. Journal of Cognitive Neuroscience. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Bozzali M, Cipolotti L. Conceptual proposition selection and the LIFG: Neuropsychological evidence from a focal frontal group. Neuropsychologia. 2010;48:1652–1663. doi: 10.1016/j.neuropsychologia.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Schriefers H, Meyer AS, Levelt WJM. Exploring the time course of lexical access in language production: Picture-word interference studies. Journal of Memory and Language. 1990;29:86–102. doi: 10.1016/0749-596X(90)90011-N. [DOI] [Google Scholar]
- Snyder HR, Munakata Y. So many options, so little time: The roles of association and competition in underdetermined responding. Psychonomic Bulletin & Review. 2008;15:1083–1088. doi: 10.3758/PBR.15.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Banich MT, Munakata Y. Choosing our words: Retrieval and selection processes recruit shared neural substrates in left ventrolateral prefrontal cortex. Journal of Cognitive Neuroscience. 2011;23:3470–3482. doi: 10.1162/jocn_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hutchison N, Nyhus E, Curran T, Banich MT, O’Reilly RC, Munakata Y. Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16483–16488. doi: 10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, Munakata Y. Cognition & Emotion. 2013. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Thompson-Schill SL. Dissecting the language organ: A new look at the role of Broca’s area in language processing. In: Cutler A, editor. Twenty-first century psycholinguistics: Four cornerstones. Mahwah, NJ: Lawrence Erlbaum; 2005. pp. 173–189. [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: A response to Martin and Cheng (2006) Psychonomic Bulletin & Review. 2006;13:402–408. doi: 10.3758/BF03193860. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Shafir E. Choice under conflict: The dynamics of deferred decision. Psychological Science. 1992;3:356–361. doi: 10.1111/j.1467-9280.1992.tb00047.x. [DOI] [Google Scholar]