Abstract
The evolutionally conserved transforming growth factor β (TGFβ) affects multiple cell types in the immune system by either stimulating or inhibiting their differentiation and function. Studies using transgenic mice with ablation of TGFβ or its receptor have revealed the biological significance of TGFβ signaling in the control of T cells. However, it is now clear that TGFβ is more than an immunosuppressive cytokine. Disruption of TGFβ signaling pathway also leads to impaired generation of certain T cell populations. Therefore, in the normal physiological state, TGFβ actively maintains T cell homeostasis and regulates T cell function. However, in the tumor microenvironment, TGFβ creates an immunosuppressive milieu that inhibits antitumor immunity. Here, we review recent advances in our understanding of the roles of TGFβ in the regulation of T cells and tumor immunity.
Introduction
TGFβ proteins are a family of pleiotropic cytokines that regulate diverse biological processes, including development of organs and tissues, carcinogenesis and immune responses. TGFβ is synthesized in a latent form with a homodimer of TGFβ that is noncovalently linked with the latency-associated protein (LAP). The activation of latent form TGFβ is promoted by a TGFβ activator via LAP degradation or conformational changes. Active TGFβ binds to TGFβ type 2 receptor (TGFβRII) and induces the assembly of the tetrameric TGFβ receptor complex composed of TGFβRII and TGFβ type 1 receptor (TGFβRI), which activates the kinase activity of TGFβRI. Activated TGFβRI phosphorylates transcription factors, mothers against decapentaplegic homolog (SMAD)2 and SMAD3. Phosphorylated SMAD2 and/or SMAD3 form complexes with the common SMAD (SMAD4) that are translocated into the nucleus where they associate with DNA-binding cofactors to regulate the transcription of target genes [1]. In addition, TGFβ can also activate SMAD-independent pathway, including those mediated by mitogen-activated kinase (MAPK), Rho family proteins, Par6 and PP2A phosphatase to induce different cell type-specific SMAD-independent responses [2].
In mammals, three members of TGFβ family have been identified: TGFβ1, TGFβ2, and TGFβ3, with TGFβ1 being the major regulator in the immune system. TGFβ is involved in the regulation of development, survival and function of many types of immune cells. However, the role of TGFβ in T cell regulation has attracted the most interest due to the discovery of uncontrolled T cell activation and expansion in TGFβ1-deficeint mice [3, 4]. Given that TGFβ is produced in abundance by many types of tumor cells, it is without surprise that TGFβ facilitates evasion of immune surveillance by regulating T cells and other immune cell types in the tumor microenvironment [5]. In this review, we discuss the current understanding of TGFβ regulation of T cell biology and tumor immunity.
The role of TGFβ in T cell biology
TGFβ was initially defined as a negative regulator of T cells by early studies since addition of TGFβ to T cell culture inhibited T cell proliferation [6]. Consequently, mice that lack TGFβ1 and mice with T cell-specific deletion of either TGFβRI or TGFβRII die early of age from systemic autoimmune disorder caused by hyperactivation and enhanced proliferation of T cells [3, 4, 7–9]. These findings thus suggest TGFβ signaling to T cells is critically associated with the maintenance of T cell tolerance. Intriguingly, recent studies have provided evidence to demonstrate that TGFβ also promotes the differentiation, homeostasis and responses of certain T cell populations (Figure 1). This section focuses on a major role of TGFβ in regulation of T cell differentiation and tolerance. We also address the potential of TGFβ-based therapeutics for the treatment of autoimmune disease.
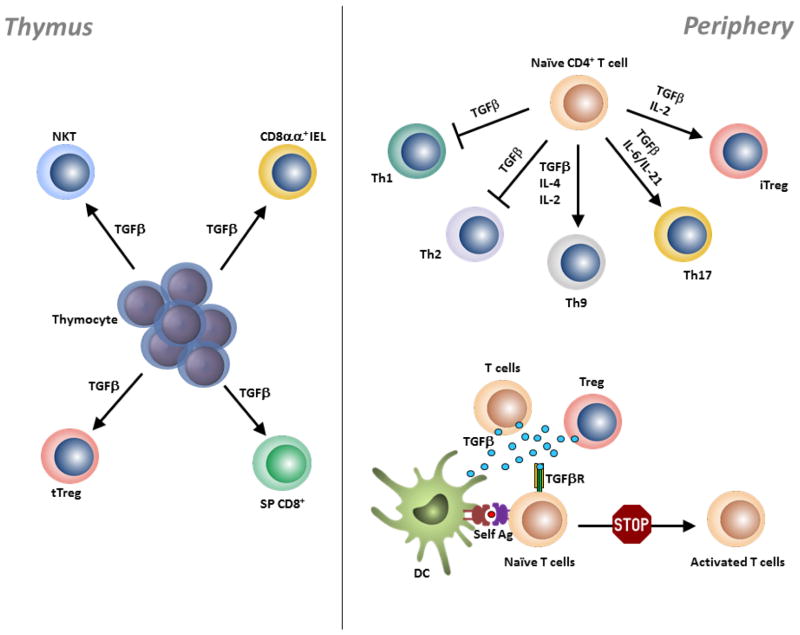
Figure 1. TGFβ regulation of T cells in the thymus and periphery.
During T cell development in the thymus, TGFβ supports the differentiation of thymocytes into tTreg cells, CD8 T cells, NKT cells and TCRαβ+CD8αα+ IEL precursors. In the periphery, TGFβ inhibits Th1 and Th2 cell differentiation by repressing T-bet and GATA-3 expression, respectively. In other scenarios, TGFβ acts synergistically with other cytokines to promote the differentiation of Th9, Th17 and iTreg cells. DCs, T cells and Treg cells serve as a source of TGFβ, which is critically required for the maintenance of peripheral T cell tolerance by inhibiting activation and proliferation of self-reactive T cells.
T cell differentiation
TGFβ has been shown to implicate on the development of T cell precursors into mature T cells in the thymus, as well as differentiation of effector T cells in the periphery. In this section, we focus on a major role of TGFβ in the differentiation of conventional T cells (CD4+ and CD8+), regulatory T (Treg) cells, and non-conventional T cells (NKT, and CD8αα+ intestinal intraepithelial lymphocytes [IELs]).
CD4+ T cells
CD4+ helper T (Th) cells play a major role in establishing and augmenting immune responses against pathogens. This is achieved through their production of cytokines that provide help to other cells in the innate and adaptive immune systems. After activation by engagement of TCR to peptide-MHC complex and co-stimulatory signals, naïve CD4+ T cells undergo proliferation and differentiation into various effector Th subsets, which depends on the nature of antigens and cytokine environment. As TGFβ inhibits the differentiation and function of Th1 and Th2 cells (discussed later), we focus on the stimulatory role of TGFβ in the differentiation of Th17 cells, Treg cells and the recently identified Th9 cells.
Th17 cell differentiation
TGFβ has been shown to be required for the differentiation of Th17 cells from naïve CD4+ T cells, as Th17 cells were profoundly diminished or absent in TGFβ-deficient mice [10]. Moreover, T cells that are deficient in TGFβ receptors, and therefore cannot respond to TGFβ, are impaired in Th17 cell differentiation resulting in mice that are protected from EAE [11]. It was found that TGFβ and IL-6 together induce the differentiation of Th17 cells from naïve CD4+ T cell precursors [10, 12, 13]. In addition to IL-6, IL-21 together with TGFβ provided an alternative pathway for Th17 cell development in the absence of IL-6 [14].
However, some studies argue the necessity for TGFβ in driving Th17 differentiation under certain circumstances. For example, it was reported that TGFβ indirectly promotes Th17 cell differentiation by inhibiting STAT4 and GATA3 expression, which are required for Th1 and Th2 cell differentiation, respectively. Accordingly, IL-6 alone was sufficient to induce Th17 response in STAT6−/−.Tbet−/− mice [15]. In another study, IL-6 or IL-23 in combination IL-1β was shown to induce Th17 cell differentiation from naïve T cells [16]. These results suggest that TGFβ is dispensable for generating Th17 cells under contain circumstances. Although TGFβ/IL-6 and IL-23/IL-6/IL-1β both induce T cells capable of producing IL-17, the pathogenicity of Th17 cells that arise from these two cytokine environments are strikingly different. Th17 cells generated by stimulation with IL-6/IL-1β/IL-23 efficiently caused severe EAE upon transfer, whereas TGFβ/IL-6-induced Th17 cells had no effect [16]. This was likely due to the high level of IL-10 produced by TGFβ and IL-6-induced Th17 cells [17]. Nevertheless, it is generally believed that TGFβ is critical for the differentiation of Th17 cells at least in rodents. The important questions ahead are the underlying molecular mechanisms downstream of TGFβ signaling that mediate IL-17 gene transcription in T cells.
As TGFβ is a differentiation factor for both Treg (discussed below) and Th17 cells, TGFβ synergizes with other cytokines to regulate Treg and Th17 cell development. It was shown that exposure of naïve CD4+ T cells to TGFβ can result in expression of both Foxp3 and RORγt. However, Foxp3 drives the differentiation of Treg cells by inhibition of RORγt function. In contrast, IL-6, IL-21 and IL-23 release RORγt from Foxp3-mediated inhibition, thus inducing Th17 cell differentiation [18]. It has also been shown that TGFβ regulates the differentiation of Treg and Th17 cells in a concentration-dependent manner. Low concentrations of TGFβ together with IL-6 and IL-23 promoted the expression of IL-23R, thus inducing Th17 cell differentiation. However, at high concentrations, TGFβ suppresses IL-23R and favors Treg cell differentiation [18]. Therefore, the developmental pathways of Th17 and Treg cells are mutually exclusive even though they share a common differentiation factor, TGFβ.
Th9 cell differentiation
Apart from the well-characterized Th1, Th2 and Th17 cells, Th9 cells which preferentially produce IL-9 were recently discovered and added to the family of helper T cells [19, 20]. Th9 cells are involved in host immunity against gastrointestinal parasites [21]. In addition, Th9 cells are involved in the antitumor immune response by stimulating tumor-specific CD8+ cytotoxic T lymphocytes (CTLs) differentiation and function [22, 23]. However, Th9 cells are also capable of causing autoimmunity and allergic inflammation [19, 24].
Long before the discovery of Th9 cells, it was shown that TGFβ combined with IL-4 induced IL-9 production in naïve CD4+ T cells and IL-2 was also essential [25]. Unlike other helper T cell subset, transcription factor(s) specific to Th9 cells have not yet been identified. Given that, Th2 cells are capable of producing IL-9 upon exposure to TGFβ [20], it remains to be determined if IL-9-producing CD4+ T cells represent a separate lineage of helper T cells.
Regulatory T cells
Treg cells, which constitute 5% to 10% of CD4+ T cells, are generated in the thymus (tTreg) and can also be induced from naïve CD4+ T cells in the periphery (iTreg). Treg cells have been considered as the major mediator of peripheral tolerance ever since Sakaguchi and colleagues discovered that adoptive transfer of CD4+CD25+ T cells suppressed the development of autoimmunity [26]. Apart from suppressing the autoimmune response, Treg cells also have a pivotal role in limiting anti-tumor immunity (discussed in tumor immunity section of this review) and excessive immune responses to non-self antigens such as commensal bacteria in the gut. This is indicated by eradication of tumors or the occurrence of inflammatory bowel disease, followed by removal of Treg cells.
tTreg cell differentiation
The production of functional Treg cells by the thymus was first demonstrated by Sakaguchi and colleagues. They showed that transfer of CD4+CD25+-depleted mature thymocytes produced various autoimmune diseases in athymic nude mice and that CD4+ splenocytes which contained CD4+CD25+ T cells completely inhibited the development of autoimmunity [27]. Moreover, manipulation of the thymus such as neonatal thymectomy or adult thymectomy combined with cyclophosphamide induced a variety of autoimmune diseases in genetically susceptible mouse strains [28, 29].
TGFβ signaling was previously thought to be dispensable for the development of tTreg cells. This is because 8–17-day-old TGFβ-deficient or TGFβRII-deficient mice have similar numbers of tTreg cells in the thymus compared to wildtype mice and tTreg cells from TGFβ-deficient mice maintain a normal Foxp3 expression [9, 30]. In contrast to these conclusions, we have shown that T cell-specific deletion of TGFβRI inhibited the development of thymic Treg cells in young mice between 3 to 5 days of age [7], which was confirmed by another study using TGFβRII conditional knockout mice [31]. In reconciliation with earlier studies, we showed that thymic Treg cells rapidly expand in the absence of TGFβ signaling as a result of increased production of, and responsiveness to IL-2 in the thymus, since thymic Treg cells were completely absent in mice deficient in both TGFβRI and IL-2. Furthermore, we recently validated a critical function of TGFβ in the induction of Foxp3 gene transcription in the thymic Treg cell precursors by utilizing multiple physiological experimental approaches in mice in vivo. We have revealed that thymic apoptosis drives tTreg development and demonstrated a previously unrecognized apoptosis-TGFβ-Foxp3 axis in the tTreg generation in the thymus. [32]. One study has suggested that TGFβ signaling is crucial for the survival of tTreg cells instead, rather than for their lineage commitment. However, increased tTreg cell death was not observed in neonate or adult thymi of Tgfbr2f/f.Foxp3-Cre mice, in which TGFβ signaling was abrogated after Foxp3 gene expression was switched on, and their tTreg cell levels were comparable to their wildtype counterparts [32]. Collectively, these data provide a compelling evidence for the vital role of TGFβ signaling in the generation of tTreg cells.
iTreg cell differentiation
Similar to other lineages of T cells, naïve CD4+ T cells can also differentiate into Treg cells in the periphery as shown in several experimental settings. For example, continuous delivery of peptide using osmotic pump or targeting of antigen to DCs by means of the DEC-205 endocytosis receptor have both been shown to successfully transform naïve T cells into Treg cells in the peripheral lymphoid organs [33, 34]. These periphery-induced Treg cells or iTreg cells display the tTreg cell phenotype (expressing tTreg cell-associated cell surface molecules and transcriptional signature) and possess suppressive activity.
TGFβ plays a pivotal role in the generation and expansion of Treg cells in the periphery. We discovered that TGFβ induced Foxp3 gene transcription in the context of TCR stimulation from peripheral naïve CD4+ T cells [35]. Consistently, transient expression of TGFβ specifically in the pancreatic islets increased the number of Treg cells in the islets and protected against diabetes [36]. In vivo induction of Treg cell generation by low dose antigen delivery is also dependent on TGFb [33, 34]. In our most recent studies, we have successfully generated antigen-specific Treg cells in mice with established autoimmunity, which could potently suppress the autoimmune diseases [37]. Mechanistically, binding of SMAD3 and NFAT to the Foxp3 enhancer and/or Basic HLH protein E2A binding at the Foxp3 promoter are required to switch on Foxp3 expression [35, 38, 39]. The induction of Foxp3 was further proven to be mediated by TGFβ-SMAD singling pathway as T cells that lack CNS1 (contains a SMAD-NFAT response element) or SMAD3 binding sites showed reduced iTreg generation [40, 41].
In addition, retinoic acid produced by CD103+ DCs in the gut-associated lymphoid tissue facilitates the generation of iTreg cells in the presence of TGFβ [42–44]. Retinoic acid is suggested to enhance TGFβ-induced Foxp3 expression in naïve CD4+ T cells by suppressing CD44hi effector/memory T cells, which secrete IL-4, IL-21 and IFNγ to restrain iTreg cell generation [45], although it remains in debate [46]. Thus, it is possible that the gut preferentially promotes the generation of iTreg cells through the release of TGFβ and retinoic acid so that iTreg cells can dampen any excessive immune response in the microbe-rich environment.
Although TGFβ signaling is crucial for the development of both tTreg and iTreg cells, it may not be absolutely required for Treg cell function. This was demonstrated in a recent report that TGFβRII-deficient Treg cells prevented the development of diabetes when cotransferred with diabetogenic BDC T cells into lymphopenic Scid/NOD mice [47]. However, we have shown that Treg cells that lack TGFβ signaling (isolated from Tgfbr1f/f.Foxp3-Cre mice) suppress the development of EAE and asthma but fail to confer their suppressive function in the gut (Konkel, unpublished data). Our results suggest that the requirement of TGFβ signaling in Treg cells function is contingent on the site of action. The exact function of TGFβ signaling in Treg suppressive activity still remains to be elucidated.
CD8+ T cell differentiation
The role of TGFβ signaling in CD8+ T cell differentiation in the thymus is still unclear. We showed a decade ago that mice with TGFβ1 null mutation show reduced frequency of CD8+ T cells in the thymus and periphery [48]. Similarly, mice with T cell-specific deletion of TGFβRII (Tgfbr2f/f.CD4-Cre) showed a reduced number of single-positive CD8+ thymocytes [8]. This is consistent with our finding that TGFβ signaling induced and maintained CD8α expression in T cells [49]. Nevertheless, another study reported that Tgfbr2f/f.CD4-Cre mice developed normal frequencies of single-positive CD4 and CD8 thymocytes [9]. The disparity between the two studies may result from the timing of examination and development of systemic inflammation in Tgfbr2f/f.CD4-Cre mice, which could mask the effect of TGFβ on the development of CD8+ T cells. Consistent with this notion, a recent study showed that in the absence of autoimmunity, female Tgfbr2f/f.CD4-Cre.HY mice had significantly less CD8+ T cells compared to HY mice with intact TGFβ signaling [50]. CD8+ T cells from Tgfbr2f/f.CD4-Cre.HY mice expressed much lower level of IL-7Rα compared to their wildtype counterparts [50]. As IL-7 signaling is critically required for the differentiation of thymic CD8+ T cells [51], TGFβ signaling is likely to induce CD8+ T cell differentiation through the regulation of IL-7Rα expression in thymocytes. Taken together, these results confirm the requirement of TGFβ signaling in CD8+ T cell differentiation. Interestingly, we showed that TGFβ signaling was capable of switching on CD8α expression in mature CD4+ T cells by inhibition of Th-POK expression. This is consistent with another study demonstrating that Th-POK actively maintains CD4+ T cell phenotype by repressing genes of the CD8 lineage [52].
Non-conventional T cell differentiation
Apart from CD4+ and CD8+ T cells, TGFβ signaling has also been shown to be essential in the development of non-conventional T cells including NKT cells and TCRαβ+CD8αα+ IELs. Natural killer T (NKT) cells develop from CD4+CD8+ thymocytes. In contrast to conventional T cells, NKT cells do not recognize antigens presented by MHC molecules. NKT cells express a semi-invariant TCR that recognize lipid antigens presented by the MHC class I-related CD1d molecule. A significant decrease of NKT cell precursors was observed in the thymus of mice with T cell-specific deletion of TGFβRII (Tgfbr2f/f.CD4-Cre) [8, 9]. Although the mechanisms that regulate NKT cell differentiation are not well understood, it has been demonstrated that TGFβ signaling is involved in multiple stages of invariant NKT (iNKT) cell (a subset of NKT cells) development through both SMAD-dependent and independent pathways [53]. Furthermore, TGFβ signaling is specifically required for the survival and function of IL-17-producing, RORγt + iNKT cells [54].
TGFβ signaling also controls the development of TCRαβ+CD8αα+ IELs. Mice with TGFβ1 deficiency or T cell-specific deletion of TGFβRI showed a reduced population of TCRαβ+CD8αα+ IELs due to an impaired development of the TCRαβ+CD8αα+ IEL thymic precursors. The reduction of TCRαβ+CD8αα+ IEL thymic precursors resulted from enhanced expression of the proapoptotic molecule Bim. In contrast, mice with T cell-specific overexpression of TGFβ1 had an increased population of TCRαβ+CD8αα+ IELs and TGFβ was found to induce CD8α expression in TCRαβ+CD8αα+ IEL thymic precursors by repressing Th-POK expression [49].
The role of TGFβ in maintenance of peripheral T cell tolerance
TGFβ signaling to T cells plays an important role in the maintenance of T cell tolerance. This is evidenced by the development of early onset lethal autoimmune diseases caused by hyperactivation of T cells, and overproduction of proinflammatory cytokines in TGFβ1-deficient mice and mice with T cell-specific deletion of TGFβR [3, 7–9]. The pathology of these mouse models closely resembles that of mice that lack Treg cells [55]. It has been shown that TGFβ maintains T cell tolerance through intrinsic and extrinsic mechanisms.
Intrinsic mechanisms
The importance of TGFβ in controlling T cell tolerance has been demonstrated using TCR transgenic mice that harbor autoreactive T cells. As expected, T cell-specific deletion of TGFβR in NOD mice that carry BDC2.5 TCR transgene led to accelerated autoimmune diabetes with elevated Th1 cells [47]. However, the deletion of TGFβR in both Treg cells and effector CD4+ T cells (Ox40-Cre), but not Treg cells alone (Foxp3-Cre), induced diabetes in BDC2.5-NOD mice. Furthermore, similar to observation in Tgfbr2f/f.CD4-Cre mice, transferred Treg cells were not able to inhibit diabetes caused by TGFβRII-deficient BDC2.5 T cells. Collectively, these results suggest that direct regulation of T cells by TGFβ is critical for the maintenance of T cell tolerance.
Although TGFβ has been shown to be critically required for T cell tolerance, the molecular mechanisms by which TGFβ regulates T cells are not well understood. Several reports have proposed the following mechanisms that may contribute to TGFβ-mediated regulation of T cells; TGFβ inhibited the proliferation of target T cells by blocking the production of IL-2 in a SMAD3-dependent mechanism [56]. TGFβ also inhibited the expression of T-bet and GATA-3 which are required for the differentiation of Th1 and Th2 cells, respectively [57, 58]. This is consistent with findings that CD4+ T cells deficient in TGFβ signaling differentiated into Th1 and Th2 cells [8, 9, 59]. Apart from inhibiting T cell proliferation and differentiation, TGFβ has also been shown to prevent T cell activation by blocking TCR signaling events, including the activation of the Tec kinase Itk, calcium influx and translocation of NFAT [60]. TGFβ also directs the differentiation of naïve T cells into Treg cells which further enforce peripheral T cell tolerance [35]. A recent report has demonstrated that TGFβ prevented an inflammatory response in the gut by downregulating ThPOK and upregulating Runx3. The transition between CD4 and CD8-lineage transcription factors switched on the expression of CD8α in the colitogenic CD4+ T cells and modulated intestinal inflammatory response [49, 61].
Extrinsic mechanisms
CD4+Foxp3+ Treg cells play an important role in the maintenance of peripheral T cell tolerance as evidenced by the development of catastrophic autoimmune disease in mice and humans with Foxp3 mutations [62, 63]. Treg cells have been shown to produce inhibitory cytokines such as TGFβ and IL-10, which have potent immunosuppressive effects. It has been demonstrated that Treg cells mediate suppression in vitro by delivering membrane-bound TGFβ to responder T cells via a cell contact-dependent manner [64, 65]. Moreover, Treg cells that express membrane TGFβ delay the progress of diabetes by inhibiting the migration of CD8+ T cells into pancreatic islets [66]. It has also been suggested that Treg cells require membrane-bound TGFβ to convert naïve T cells to Treg cells via the mechanism of infectious tolerance [67]. These findings are consistent with studies in vivo. T cells deficient in TGFβRII are resistant to Treg cell suppression and cause colitis or diabetes, suggesting that TGFβ responsiveness in self-reactive T cells is crucial for Treg cell suppression [47, 66, 68]. Importantly, Treg cells from TGFβ-deficient mice retain the ability to protect against colitis but the protection is abrogated by anti-TGFβ antibody [68]. This result therefore raises the possibility that TGFβ is required for Treg cell-mediated suppression in vivo but that TGFβ may be derived from non-Treg cells. One study demonstrated that TGFβ−/− Treg cells were as suppressive as wildtype Treg cells when these cells were cultured with wildtype antigen presenting cells (APCs) [30]. However, a significantly reduced suppression by TGF-β−/− Treg cells was observed when the cells were stimulated with TGF-β−/− APCs, indicating that TGFβ produced by APCs may aid Treg suppressive activity. However, other studies have revealed that TGFβ produced by Treg cells is required for their suppressive function in vivo, as Treg cells from mice with T cell-specific deletion of TGFβ1 failed to inhibit colitis [69]. Furthermore, anti-TGFβ antibody blocked the therapeutic effect of transferred Treg cells on colitis or airway allergic responses [70, 71]. The discrepancies between these in vivo studies remain unresolved. More work is needed to define the role of secreted and membrane-bound TGFβ, as well as the source of secreted TGFβ in Treg suppression.
TGFβ-based therapeutics for the treatment of autoimmune disease
Given the role of TGFβ in regulating T cell tolerance, TGFβ represents a potential therapeutic target in the treatment of autoimmunity. Early studies have demonstrated that in vivo administration of TGFβ decreased the incidence and severity of EAE, even when TGFβ was administered after disease onset [72, 73]. However, systemic treatment of TGFβ may not be practical in clinical practice due to the pleiotropic roles of TGFβ in many cellular pathways, including differentiation, proliferation, function and homeostasis. Importantly, administration of TGFβ in patients may lead to worsening of existing autoimmune inflammation due to increased differentiation of pathogenic Th17 cells in the presence of proinflammatory cytokines such as IL-6.
An alternative method to apply TGFβ in the treatment of autoimmune disease would be through adoptive transfer of Treg cells, as TGFβ produced by Treg cells is critical for controlling T cell tolerance with the added advantage of antigen specificity while avoiding overall immunosuppression. Indeed, adoptive transfer of Treg cells has resulted in successful prevention of graft-versus-host disease in human clinical trials [74, 75]. However, the effectiveness of Treg cells to treat established autoimmune diseases is less satisfactory. Mice with ongoing disease were not completely cured after treatment with Treg cells due to the persistence of pathogenic activated/memory T cells that are more resistant to suppression by Treg cells [76–78]. This is likely to be caused by intrinsic properties of the effector T cells, such as production of high levels of pro-inflammatory cytokines. Indeed, previous studies have shown that while Treg cells are effective in suppressing naive autoreactive T cells, they fail to control pathogenic effector T cells that secrete IL-17, IFNγ, IL-6 and TNFα [79–81]. Some autoimmune diseases like type 1 diabetes can be predicted or diagnosed at an early pre-clinical stage by the presence of islet autoantibodies in individuals with high-risk genetic markers. However, many others such as autoimmune gastritis, autoimmune hepatitis, and multiple sclerosis are difficult to diagnose since they may be asymptomatic at the early stages or share symptoms in common with diseases without an autoimmune basis. As a result, an early diagnosis for autoimmune diseases is not always possible and treatment that can reprogram dysregualted immune response in advanced disease will be critically required. Furthermore, the generation and expansion of Treg cells (especially autoantigen-specific Treg cells) in adequate numbers for adoptive transfer may be difficult in a human setting.
To this end, we have recently developed a new therapeutic approach that induces the generation of autoantigen-specific Treg cells in vivo [37]. We initially induced apoptosis of immune cells in mice with established autoimmune diseases by systemic sub-lethal irradiation, or depleted B and CD8+ T cells with specific antibodies. This removes a substantial proportion of pathogenic cells before reestablishment of immune tolerance. Also, apoptotic cells induced professional phagocytes to produce TGFβ that contributed to an immunosuppressive milieu, as shown previously [82]. Auto-antigenic peptides were then administered into the treated mice to promote the generation of autoantigen-specific Treg cells. We have demonstrated that this therapeutic approach successfully ameliorates disease in EAE and NOD models.
TGFβ in tumor immunity
TGFβ is a powerful cytokine whose overall actions greatly depend on the physiological setting. While the requirement for TGFβ in maintaining self-tolerance is undisputable, it is also a critical component in the aetiology of cancer. As a testament to its pleiotropic effects on immune and non-immune cells alike, TGFβ can function both as a tumor suppressor and a tumor promoter. TGFβ initially functions as a tumor suppressor in the early stages of carcinogenesis by inhibiting cancer cell proliferation. However, the tumor later becomes refractory to the growth-inhibitory effects of TGFβ due to the accumulation of mutations that inactivate TGFβ receptors or downstream signaling and it ultimately fosters an environment conducive for tumor progression [83, 84].
The high level of TGFβ in the tumor microenvironment (TME) also plays another major role: evasion of immune surveillance [85]. Local immunosuppression in the TME appears to underlie the failure of a vast array of cancer therapies, and is highly specific in nature, since tumor-bearing animals respond normally to challenge with non-tumor antigens [86]. TGFβ in the TME can suppress or alter activation, maturation and differentiation of both innate and adaptive immune cells, including NK cells, DCs, macrophages, neutrophils and CD4+ and CD8+ T cells [87, 88]. Additionally, TGFβ-induced Treg cells in the TME further contribute to the tolerizing environment. This section will outline the effects of TGFβ on various populations of immune cells in the TME, as well as outline efforts to block TGFβ signaling in combination with immunotherapy.
Effect of TME TGFβ on innate immunity
Natural killer cells
Natural killer (NK) cells represent a critical component of the innate immune system, and function by inducing cytolysis in infected cells through granzyme or perforin release, and boosting the maturation and activation of DCs, macrophages and T cells through IFN-γ and TNFα secretion [89]. TGFβ has been shown to inhibit expression of NK cell activating receptors and this downregulation is associated with reduced cytotoxic granule release, IFN-γ secretion and tumor killing, and an overall poor clinical prognosis [90–92]. This can be attributed to a direct effect of TGFβ or might result indirectly from interaction between NK cells and Treg cells which produce this cytokine [93]. The finding that TGFβ can suppress IFN-γ through SMAD3-dependent signaling provides evidence for the former mechanism [92].
Tumor antigen-specific NK cells have demonstrated effective anti-tumor activity in vitro and are able to infiltrate solid tumors in vivo, however, their activity is considerably weakened in the tumor microenvironment [94, 95]. It has been further demonstrated that TGFβ blunts the activation of NK cells in human ovarian cancer by antagonizing IL-15 induced proliferation and gene expression (normally associated with NK cell activation) [96]. Expectedly, TGFβ-blockade restored NK cell activation and effector function in this context. Intriguingly, TGFβ seems to only affect activation, and not survival of NK cells, in contrast to its pro-apoptotic effects on T cells [97]. Thus the TGFβ-rich TME maintains an inactive but viable population of NK cells, whose activity can be restored by TGFβ-blockade. Indeed, antagonism of TGFβ signaling has been shown to result in restoration of normal levels of NKG2D and concomitant anti-tumor functionality in NK cells [98]. Increased secretion of IFN-γ in these NK cells may also aid in anti-tumor immunity, as antagonism of TGFβ signaling has been shown to promote the accumulation of NK cells that are able to secrete high levels of IFN-γ [99]. These findings suggest that NK cells may serve as an untapped subset of cells that can be used to restore anti-tumor immunity.
DCs
Dendritic cells (DCs) are unique in their ability to induce primary immune responses in the establishment of immunological memory and are considered the most effective antigen-presenting cells [100]. A number of reports have demonstrated that tumor-infiltrating DCs are defective in their ability to activate anti-tumor T cell responses in a variety of human cancers [101–103]. This may be due in part to high TGFβ levels in the tumor microenvironment. Tumor-derived TGFβ has been shown to immobilize DCs and prevent migration to tumor-draining lymph nodes in mouse and human skin cancers [104, 105] and may also directly induce DC apoptosis in tumor-draining lymph nodes [106]. Importantly, DCs can present antigen in an immunogenic or tolerogenic manner, depending on the local micro milieu and cytokine environment, and thus play a key role in determining the overall response to tumors [107, 108]. In TGFβ-rich environments like the TME, DCs take up tumor cells, become tolerogenic TGFβ-secreting cells and promote the induction of tumor-specific Treg cells in both mice and humans [109–112] that in turn act as potent inhibitors of anti-tumor T cell responses [113, 114].
DCs serve as an attractive target in cancer immunotherapy due to their potency as APCs. Indeed, since Treg cells are one of the major obstacles to successful antitumor immunity, the ability of DCs to promote Treg development in the TME presents the possibility of indirectly downregulating these immunosuppressive cells by targeting DCs. So far, a number of protocols for the generation of clinical-grade DCs for use in cancer vaccines have been generated [115, 116]. However, only modest clinical efficacy has been observed, likely due to high TGFβ levels in the TME, which can significantly affect the responsiveness of T cells to DC priming [117]. Thus simultaneous neutralization of TGFβ during administration of DC-based vaccines may further enhance the ability of DC-primed T cells in tumor eradication. However, since TGFβ can inhibit tumor proliferation in certain contexts, systemic inhibition of TGFβ may lead to accelerated tumor growth. The challenge now is to design strategies for localized neutralization of TGFβ in the TME, so as to reverse immunosuppression without blocking the inhibitory effect of TGFβ on tumorigenesis.
Macrophages and myeloid-derived suppressor cells
Macrophages exhibit a remarkable degree of plasticity and adopt either pro- or anti-inflammatory phenotypes in response to environmental stimuli [118]. Classically activated macrophages (M1 macrophages) mediate host defense to invading pathogens and elicit anti-tumor immunity. Alternatively activated macrophages (M2 macrophages) have anti-inflammatory functions and regulate wound healing. Macrophages constitute a major component of immune cell infiltrate in the TME, and can constitute up to 50% of tumor mass. In the vast majority of human cancers, high frequencies of tumor-associated macrophages (TAMs) correlate with poor prognosis [119, 120]. These TAMs are generally believed to have an M2-like phenotype [118, 121], although it is not unanimously accepted. In addition, the absence of M1-orienting signals such as IFN-γ as well as pro-M2 stimuli such as IL-10 and TGFβ in the tumor TME may skew differentiation of macrophages toward the M2 phenotype [122]. TAMs are best characterized by their ability to suppress anti-tumor immunity and are associated with increased expression of arginase 1 and indoleamine 2,3-dioxygenase (IDO) that inhibit T cell proliferation and survival, and themselves contribute to high IL-10 and TGFβ levels [123]. Global gene profiling of TAMs highlighted upregulation of a number of genes. In particular, migration-stimulating factor (MSF) is induced in TAMS by CSF-1, IL-4 and TGFβ [124]. TAM-derived MSF strongly stimulated tumor cell migration, contributing to the increased motility of neoplastic cells. Thus the TGFβ-rich TME may favor M2-polarization of macrophages, which in turn reinforces the metastatic properties of the tumor. Another mechanism by which TGFβ can circumvent anti-tumor responses is by negatively regulating Toll-like receptor (TLR) signaling in macrophages. Recognition of microbial signatures such as LPS, DNA and lipopeptides by TLRs activates MyD88 and TRIF signaling pathways in macrophages, which result in secretion of several cytokines involved in the anti-tumor responses, such as TNFα, IL-12 and IFN-γ [125]. TGFβ can induce expression of the interleukin receptor associated kinase (IRAK-M), a key negative regulator of TLR signaling [126]. While IRAK-M is critical for preventing excessive inflammatory responses [127], its presence is detrimental to in the context of cancer due to its potential to induce evasion of host immune surveillance [128]. Indeed, IRAK-M gene expression was found to correlate with poor survival in lung cancer sufferers [126]. Thus, taken together, TGFβ in the TME may act first to polarize macrophages towards an immunosuppressive subtype, and in continuous manner to reinforce this phenotype through crosstalk with TLRs.
It is easy to dismiss the fact that macrophages are essentially “eaters”, and their anticancer potential has until more recently been underestimated. Tumor cells subvert engulfment by transmission of “don’t eat me” signals to macrophages [129]. Indeed, the voracious phagocytic properties of macrophages have been harnessed in the treatment of pancreatic cancer in humans [130] and in the engulfment of various mouse tumors [131]. However, phagocytosis of apoptotic tumor cells by TAMs may also outcompete tumor antigen uptake by DCs, indirectly limiting anti-cancer T cell responses [132]; TGFβ has been shown to enhance these phagocytic properties of TAMs [133]. Fortunately, the differentiation of macrophages is possibly reversible depending on the microenvironment [134–136], suggesting that TAMs have the potential to be deconverted into an anti-tumor M1-like phenotype with a change in cytokine environment. Thus one can envision a macrophage-based anti-cancer therapy in which neutralization of TGFβ can 1) prevent tumor immune evasion due to reduced competition for tumor antigen, and 2) reprogram TAMs into an anti-tumoral M1-phenotype to activate adaptive immune responses to the tumor. Simultaneous administration of therapies to enhance “eat me” or downregulate “don’t eat me” signals in cancer cells would then unleash the phagocytic properties of these cells, which, given their large numbers in the TME, are ideally placed to “eat cancer”.
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of activated immature myeloid cells that are characterized by a mixture of granulocytic and monocytic cells but lack the expression of cell-surface markers associated with fully differentiated monocytes, macrophages or DCs [137]. MDSCs have been found to undergo expansion and accumulate in the tumor host [138, 139]. As its name suggests, this population of cells is highly immunosuppressive [138, 140, 141], which may contribute greatly to the tumor progression. MDSC represent an important component of the immune-suppressive network responsible for defective anti-cancer T cell responses and also contribute to tumor progression via regulation of angiogenesis and tumor cell motility. These cells are found in increased numbers in the TME, and the peripheral blood, liver, and tumor-draining lymph nodes of the cancer-bearing host, with their frequency correlating with increased stage and metastatic disease [142, 143]. It is thought that MDSCs are recruited and undergo expansion in response to tumor-secreted cytokines like IL-6, GM-CSF and IL-1β, PGE2, VEGF, IDO, IL-10, SCF and importantly TGFβ [144, 145]. At the TME, MDSCs mediate immunosuppression through involve arginase 1 (ARG-1)-mediated depletion of L-arginine, inducible nitric oxide synthase (iNOS) and NADPH oxidase (NOX2)-mediated production of reactive nitrogen and oxygen species, VEGF overexpression, cysteine depletion and secretion of TGFβ [146–150]. Thus MDSCs can both respond to and secrete TGFβ. In addition to directly limiting T cell responses, emerging evidence supports a role for MDSCs in the local expansion and functional maturation of CD4+CD25+Foxp3+ Treg cells in the TME, through TGFβ-dependent and independent pathways [109, 112, 151], suggesting that MDSCs can further reinforce the immunosuppressive environment at the tumor site by maintaining Treg cells.
MDSC depletion in the TME has shown promise in mouse models of breast and lung cancer by greatly enhancing CD8+ T cell responses and decreasing Treg cell infiltration [152, 153]. Thus, a major mechanism through which TGFβ-blockade might work is through reduced recruitment and maintenance of this mixture of immunosuppressive cells at the TME.
Neutrophils
Neutrophils are the predominant leukocyte subset in human blood and have a well-established role in first line defense against microbial pathogens. However, due to their short life span and fully differentiated phenotype, neutrophils were thought to have negligible impact on cancer immunology. Neutrophils have more recently emerged as new tumor-infiltrating myeloid cells; named tumor-associated neutrophils (TANs). High TAN infiltration has been associated with poor clinical outcome in several human cancers, such as renal cell and hepatocellular carcinomas, colorectal cancer, and head and neck cancer [154]. Indeed neutrophils have been found to regulate key mechanisms of tumor progression such as angiogenesis, invasion and metastasis. For example, neutrophils can have strong pro-angiogenic activities via release of matrix metalloprotease (MMP9) and vascular endothelial growth factor (VEGF) [155]. Neutrophils can also promote tumor motility through the release of proinflammatory cytokines that enhance the invasive and migratory potential of tumor cells [156–159]. However, TANs can also be associated with better prognosis in other cancers, such as gastric carcinomas [160]. TANs can exert an anti-tumoral effect through a direct cytotoxic activity against tumor cells and can release a range of mediators (cytokine, chemokines and growth factors) to recruit and activate cells of the adaptive immune system. It is thought that the dichotomous roles of TANs in tumor suppression and promotion can be resolved by careful characterization of TAN subtypes. Analogous to macrophages, TANs can take on a tumor-inhibiting N1 phenotype or a tumor-promoting N2 phenotype [161]. The polarization process appears to be dependent on the microenvironment with TGFβ playing a key role in this respect [162]. While earlier studies initially showed that TGFβ acts directly as a potent chemotactic factor for neutrophils [163], and could also influence neutrophil migration indirectly by regulating the expression of adhesion molecules in the endothelium [164], more recent studies have revealed that TGFβ can also influence the polarization of TANs. In particular, Albelda and colleagues showed that TGFβ drives resident TANs to become tumor-promoting N2 neutrophils associated with an immunostimulatory profile (TNFαhigh, CCL3high, ICAMhigh, arginaselow); in contrast, TGFβ-blockade promoted acquisition of the anti-tumor N1 phenotype [161]. Depletion of this N2 subpopulation in tumor-bearing mice was sufficient to inhibit tumor growth, highlighting the impressive immunosuppressive potential of N2 TANs [161, 165, 166]. Acquisition of the anti-tumor N1 phenotype has also been shown to promote cell death and inhibits tumor growth [161, 167, 168]. In addition, it was shown that removal of N2 TANs or N1 TANs increased or decreased the activation status of intratumoral CD8+ T cells respectively, in further support of the different immunosuppressive or stimulatory functions of TAN subtypes [161].
The use of monoclonal antibodies in cancer therapy has increased dramatically in the past decade [169], of which a central mechanism is the recognition of IgG Fc domains by Fcγ receptors on NK cell, monocytes and neutrophils, to elicit tumor cell killing [170]. Neutrophils offer advantages for use in tumor therapy due to their abundance, which takes away the need for ex vivo expansion. Simultaneous localized administration of TGFβ antagonists may polarize resident TANs into an anti-tumor N1 phenotype, with concomitant increase in CD8+ T cell activation at tumor sites, to reinforce tumor eradication.
Effects of TME TGFβ on adaptive immunity
T cells
CD8+ CTLs are a critical component of anti-tumor immunity due to their ability to carry out cytolytic killing of tumor cells in a tumor-antigen specific manner [171]. Several studies have shown a direct correlation between the ratio of cytotoxic CD8+ T cells to Treg cells and cancer survival [172–175]. TGFβ in the TME has been shown to reduce antitumor CD8+ T cell response by inhibiting the expression of cytotoxic genes, including perforin, granzyme A, granzyme B, Fas ligand and IFNγ. Neutralization of TGFβ restored CD8+ T cell cytotoxicity and led to tumor clearance [176]. In consistence with this study, CD8+ T cells with impaired TGFβ signaling elicited a strong antitumor immune response and inhibited tumor development. The protective effect was associated with enhanced tumor infiltration and increased proliferation and activity of tumor-infiltrated CTLs [59, 177, 178]. Interestingly, TGFβ can also influence the anti-tumor effect of CTLs by upregulating IL-17 production, although the effect of IL-17 on tumor growth versus immune surveillance remains controversial [179, 180].
CD4+ T cells have been largely overlooked in their involvement in cancer progression, due to the fact that most tumor cells express MHC class I but not class II. Thus, while CD8+ T cells can induce direct tumor cell killing by recognition of tumor peptides presented in an appropriate fashion on MHC class I complexes on tumor cells, the action of CD4+ T cells is unnoticeable in this respect. However, CD4+ T cells are central to adaptive immunity, and a growing number of studies have emphasized these cells in the induction, maintenance and regulation of antitumor immune responses. Tumor-reactive CD4+ T cells have shown efficacy in tumor eradication or slowed tumor progression in a number of mouse cancers [181–185], even in cases where tumors were resistant to CD8+ T cell-mediated rejection [186]. Moreover, studies in TCR-transgenic mice have supported a role for anti-tumor CD4+ T cells in the activation of memory CTLs in vivo [187]. However, differential effects of CD4+ T cells have been documented in other tumor models [188]; and are thought to be attributed to heterogeneity within the CD4+ T cell population.
Th1 cells are thought to play an important role in anti-tumor-immunity, through the release of IFNγ, TNFα and cytolytic granules. In addition, they are thought to aid in the expansion of tumor-antigen specific CTL populations, through CD40/CD40L interaction and release of IL-2 [189]. Indeed, analysis of immune infiltrates in cancer patients has revealed that anti-tumor immunity is typically polarized towards Th1 responses [190], with increased numbers correlating with improved prognosis [191]. In contrast, Th2 cells are thought to impair tumor-specific responses by secreting cytokines that induce T cell-anergy and inhibit T-cell mediated cytotoxicity. Accordingly, increased numbers of Th2 mostly correlate with tumor progression [191]. In addition, tumor-derived TGFβ was shown to inhibit Th1 responses by skewing polarization of infiltrating T cell towards the Th2 cell phenotype, resulting in a less efficient anti-tumor response [192]. However, more recent studies have suggested that both Th1 and Th2 cells can support CTL responses against cancer cells, although Th1 cells seemed more effective by promoting activation of antigen presenting cells [193, 194].
Although the role of Th17 cells in a number of autoimmune diseases is well established, the activity of this T cell subset in cancer is controversial [12]. Higher numbers of Th17 cells have been detected in various human cancers, such as ovarian, pancreatic, renal cell and gastric cancers [195, 196]. In some cases, a direct correlation between Th17 frequencies in the TME and cancer stage has been documented [197]. Other studies suggest that Th17 cells may instead have anti-cancer effects, as cancer survivors and patients with early-stage cancer have high Th17 levels [198].
Treg cells are generally thought to antagonize protective immunity in cancer [199, 200]. Treg cells suppress a wide range of anti-tumor responses, including CD4+ T cells, CD8+ T cells, NK cells and natural killer T (NKT) cells. Several studies have documented an accumulation of Treg cells at peripheral sites and TME of tumor patients, which correlates with increased tumor burden and poor anti-tumor effector response [201]. Importantly, this is often associated with low CD8+ T cell frequencies at these sites, suggesting that tumor-infiltrating Treg cells dampen anti-tumor responses through suppression of CTLs [201].
The composition of Foxp3+ Treg cells in the TME is poorly understood, though a number of studies suggest that tTreg cells are recruited to the tumor site where they undergo expansion. It was shown that specific recruitment of pre-existing human Treg cells was mediated by high levels of the chemokine CCL22, produced by tumor cells and macrophages in the TME [202]. Importantly, tumor-associated Treg cells were shown to undergo substantial proliferation, in response to TGFβ produced by MDSCs at the tumor site [109]. In further support of this model, the TCR repertoires of tumor-infiltrating Treg and conventional T cells were found to be non-overlapping, indicating that tumor-infiltrating Treg cells were likely tTreg cells, as a significant overlap in TCR repertoires would have been observed from de novo differentiation of naïve T cells into iTreg cells [203]. In contrast, other studies suggest that tumor-infiltrating Treg cells are mostly iTreg cells resulting from the conversion of naïve T cells in response to high levels of TGFβ at the TME [204–206]. However, rather than being mutually exclusive, it is likely that both iTreg and nTreg cells contribute to the total Treg pool in the TME [207].
Not all Treg cells express Foxp3; Foxp3− Treg cells such as IL-10-secreting Tr1 cells can likewise exert immune-suppressive effects [208, 209]. In particular, Tr1 cells are found to be enriched at the TME in a number of cancers and demonstrate a prominent antitumor response in vitro [210–212]. Increased Tr1 frequencies, with concomitant decreases in FoxP3+ Treg cells correlated with a better survival rate in a study using ex vivo stimulated PBMCs for treatment of ovarian cancer [213]. Consistent with this, Tr1 cells have shown efficacy in tumor eradication in a murine glioma model by augmenting CTL and NK cell responses [214]. Thus, it has been postulated that the ratio of Foxp3+ Treg cells versus IL-10+ Tr1 cells may affect antitumor responses, although further studies are required to study the role of Tr1 cells in antitumor immunity.
Important considerations in TGFβ-based therapies for cancer
The tumor site represents a unique microenvironment with a variety of cell types, including neoplastic cells, stem-like cells, fibroblasts, endothelial cells and immune cells, all engaged in some level of cross-talk with each other. Although tumor cells are known to secrete TGFβ, immune cells such as effector T cell, Treg cells, APCs and MDSCs may well represent a large source of this cytokine. Identifying the most relevant source of TGFβ remains an important quest, albeit a difficult one given that this may vary with stage and site of the cancer. Moreover, given its pleiotropic effects on a large range of cells, immune and non-immune alike, it is unclear as to which of the TGFβ-mediated effects dominates in the TME. Nonetheless, the effect of TGFβ on immune cell infiltrate at the tumor site seems to be an overall suppressive and anti-proliferative one. Thus, TGFβ-blockade may act to unleash the antitumor effects of key components of the innate and adaptive immune system. For example, simultaneous inhibition of TGFβ-signaling with adoptive T cell therapy has been shown to significantly improve T cell survival and anti-tumor T cell cytotoxicity [215]. Further study is then needed to identify the ideal timing of TGFβ blockade in the host, as well as optimize delivery of inhibitors in a localized fashion at the TME to avoid deleterious systemic effects. Importantly, TGFβ is instrumental in the differentiation programs of numerous immune cell types, for example, instructing the M1/M2-polarization of macrophages and conversion of naïve T cells into Treg cells, further reinforcing an immune cell environment conducive to tumor progression. Clearly, more studies are needed to clearly delineate the functions of different immune cell subsets in cancer, particularly, the ambiguous role of Th17 cells at the TME. Once this is elucidated, however, localized TGFβ-inhibition would then provide the potential to skew the polarization of different immune cells towards a more immunogenic, anti-tumoral phenotype at the TME, effectively allowing us to harness our own inherent defenses to combat this devastating disease.
Concluding remarks
It has been two decades since Doetschman and colleagues made the seminal observation of catastrophic inflammatory disease in TGFβ1-deficient mice [3]. In the meantime, significant progress has been made in our understanding of immune regulation by TGFβ. It is now clear that TGFβ plays an indispensable role in the immune system, and can act in either a stimulatory or inhibitory manner, depending on the nature and differentiation status of immune cells, as well as the microenvironment and cytokine milieu. However, many fundamental questions remain unaddressed. It is not completely understood as to how TGFβ signaling coordinates Treg, Th17 and Th9 cell differentiation. Furthermore, the mechanisms which dictate whether the TGFβ signal results in T cell tolerance or the differentiation of effector T cells are not yet determined. Although much effort has been directed towards elucidating the role of TGFβ in the context of T cell, TGFβ undeniably plays a major regulatory role for other immune cell types and should not be overlooked in this regard. Understanding the mechanisms by which TGFβ regulates T cells and other components of the immune system will valuable provide insight into the aetiology of autoimmune disease and cancer, knowledge of which is essential for the design of more efficient therapies.
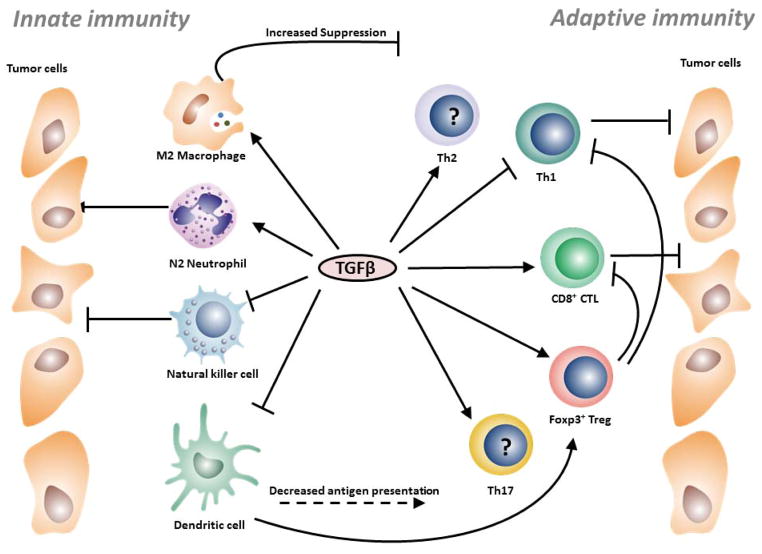
Figure 2. The effect of TGFβ on innate and adaptive immune cells.
TGFβ has an overall inhibitory effect on innate immune cells. TGFβ may skew M2-polarization of macrophages, which inhibits T cell proliferation and survival. TGFβ can also convert N1 neutrophils into the less cytotoxic N2 phenotype. The expression of activating receptors in NK cells is inhibited in response to this cytokine, resulting in reduced tumor cell killing. TGFβ in the TME decreases antigen presentation of DCs and provides a tolerogenic environment in which DCs promote tumor-specific Treg cells. Cells of the adaptive immune system display differential responses to TGFβ. Th1 cells are thought to have stronger anti-tumor responses and TGFβ may skew polarization infiltrating T cells at the TME towards the less-efficient Th2 phenotype. Importantly, TGFβ downregulates cytolytic killing of tumor cells by CD8+ CTLs by suppressing their cytotoxic program or blocking TCR signaling. TGFβ promotes differentiation of Th17 and Treg cells, although the role of the former subset in cancer remains controversial. In contrast, Treg cells suppress a wide array of antitumor activities, notably, by inhibiting Th1 and CD8+ CTL responses.
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the NIH, NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 2.Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell and tissue research. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 3.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nature reviews Immunology. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. The Journal of experimental medicine. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nature immunology. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 8.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–54. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 11.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature immunology. 2006;7:1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, et al. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. The Journal of experimental medicine. 2009;206:2407–16. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 21.Licona-Limon P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limon I, Ishigame H, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39:744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, et al. Th9 cells promote antitumor immune responses in vivo. The Journal of clinical investigation. 2012;122:4160–71. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nature medicine. 2012;18:1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38:360–72. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. Journal of immunology. 1994;153:3989–96. [PubMed] [Google Scholar]
- 26.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology. 1995;155:1151–64. [PubMed] [Google Scholar]
- 27.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. Journal of immunology. 1999;162:5317–26. [PubMed] [Google Scholar]
- 28.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 29.Barrett SP, Toh BH, Alderuccio F, van Driel IR, Gleeson PA. Organ-specific autoimmunity induced by adult thymectomy and cyclophosphamide-induced lymphopenia. European journal of immunology. 1995;25:238–44. doi: 10.1002/eji.1830250139. [DOI] [PubMed] [Google Scholar]
- 30.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. The Journal of experimental medicine. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konkel JE, Jin W, Abbatiello B, Grainger JR, Chen W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E465–73. doi: 10.1073/pnas.1320319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. The Journal of experimental medicine. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Science translational medicine. 2014;6:241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 38.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature immunology. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama T, Konkel JE, Zamarron BF, Chen W. The molecular mechanisms of Foxp3 gene regulation. Seminars in immunology. 2011;23:418–23. doi: 10.1016/j.smim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. The Journal of experimental medicine. 2012;209:1529–35. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. The Journal of experimental medicine. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–2. doi: 10.1016/j.immuni.2009.03.008. author reply 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishigame H, Zenewicz LA, Sanjabi S, Licona-Limon P, Nakayama M, Leonard WJ, et al. Excessive Th1 responses due to the absence of TGF-beta signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6961–6. doi: 10.1073/pnas.1304498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, et al. Requirement for transforming growth factor beta1 in controlling T cell apoptosis. The Journal of experimental medicine. 2001;194:439–53. doi: 10.1084/jem.194.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nature immunology. 2011;12:312–9. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouyang W, Oh SA, Ma Q, Bivona MR, Zhu J, Li MO. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity. 2013;39:335–46. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nature immunology. 2010;11:257–64. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nature immunology. 2013;14:281–9. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doisne JM, Bartholin L, Yan KP, Garcia CN, Duarte N, Le Luduec JB, et al. iNKT cell development is orchestrated by different branches of TGF-beta signaling. The Journal of experimental medicine. 2009;206:1365–78. doi: 10.1084/jem.20090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havenar-Daughton C, Li S, Benlagha K, Marie JC. Development and function of murine RORgammat+ iNKT cells are under TGF-beta signaling control. Blood. 2012;119:3486–94. doi: 10.1182/blood-2012-01-401604. [DOI] [PubMed] [Google Scholar]
- 55.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 56.McKarns SC, Schwartz RH. Distinct effects of TGF-beta 1 on CD4+ and CD8+ T cell survival, division, and IL-2 production: a role for T cell intrinsic Smad3. Journal of immunology. 2005;174:2071–83. doi: 10.4049/jimmunol.174.4.2071. [DOI] [PubMed] [Google Scholar]
- 57.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. The Journal of experimental medicine. 2002;195:1499–505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. Journal of immunology. 2000;165:4773–7. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 59.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 60.Chen CH, Seguin-Devaux C, Burke NA, Oriss TB, Watkins SC, Clipstone N, et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. The Journal of experimental medicine. 2003;197:1689–99. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nature immunology. 2013;14:271–80. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 63.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. The Journal of experimental medicine. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine & growth factor reviews. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 66.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10878–83. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. The Journal of experimental medicine. 2008;205:1975–81. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. The Journal of experimental medicine. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. Journal of immunology. 2007;178:1433–42. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 71.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. The Journal of experimental medicine. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. Journal of immunology. 1991;146:3012–7. [PubMed] [Google Scholar]
- 73.Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. Journal of immunology. 1991;147:1792–6. [PubMed] [Google Scholar]
- 74.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 75.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. The Journal of experimental medicine. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. The Journal of experimental medicine. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu E, Bourges D, Gleeson PA, Ang DK, van Driel IR. Pathogenic T cells persist after reversal of autoimmune disease by immunosuppression with regulatory T cells. European journal of immunology. 2013;43:1286–96. doi: 10.1002/eji.201242771. [DOI] [PubMed] [Google Scholar]
- 79.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. Journal of immunology. 2009;182:1247–52. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature medicine. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. Journal of immunology. 2003;171:4040–7. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 82.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 83.Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer research. 2008;68:9107–11. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Investigational new drugs. 2003;21:21–32. doi: 10.1023/a:1022951824806. [DOI] [PubMed] [Google Scholar]
- 86.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Molecular medicine. 2000;6:465–79. [PMC free article] [PubMed] [Google Scholar]
- 87.Li MO, Flavell RA. TGF-beta, T-cell tolerance and immunotherapy of autoimmune diseases and cancer. Expert review of clinical immunology. 2006;2:257–65. doi: 10.1586/1744666X.2.2.257. [DOI] [PubMed] [Google Scholar]
- 88.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5262–70. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 89.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 90.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. Journal of immunology. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 91.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-oncology. 2010;12:7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. Journal of immunology. 2008;181:3784–92. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wahl SM, Wen J, Moutsopoulos NM. The kiss of death: interrupted by NK-cell close encounters of another kind. Trends in immunology. 2006;27:161–4. doi: 10.1016/j.it.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, et al. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 95.Le Maux Chansac B, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, et al. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. Journal of immunology. 2005;175:5790–8. doi: 10.4049/jimmunol.175.9.5790. [DOI] [PubMed] [Google Scholar]
- 96.Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, et al. Human tumour immune evasion via TGF-beta blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PloS one. 2011;6:e22842. doi: 10.1371/journal.pone.0022842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–44. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer research. 2009;69:7775–83. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 99.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature immunology. 2005;6:600–7. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 100.Banchereau J, Pulendran B, Steinman R, Palucka K. Will the making of plasmacytoid dendritic cells in vitro help unravel their mysteries? The Journal of experimental medicine. 2000;192:F39–44. doi: 10.1084/jem.192.12.f39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, et al. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer immunology, immunotherapy: CII. 2008;57:1665–73. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. Journal of immunology. 2007;178:2763–9. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 103.Gigante M, Blasi A, Loverre A, Mancini V, Battaglia M, Selvaggi FP, et al. Dysfunctional DC subsets in RCC patients: ex vivo correction to yield an effective anti-cancer vaccine. Molecular immunology. 2009;46:893–901. doi: 10.1016/j.molimm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weber F, Byrne SN, Le S, Brown DA, Breit SN, Scolyer RA, et al. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer immunology, immunotherapy: CII. 2005;54:898–906. doi: 10.1007/s00262-004-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halliday GM, Le S. Transforming growth factor-beta produced by progressor tumors inhibits, while IL-10 produced by regressor tumors enhances, Langerhans cell migration from skin. International immunology. 2001;13:1147–54. doi: 10.1093/intimm/13.9.1147. [DOI] [PubMed] [Google Scholar]
- 106.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, et al. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. Journal of immunology. 2006;176:5637–43. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 107.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 108.Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Frontiers in immunology. 2013;4:82. doi: 10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. The Journal of experimental medicine. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. Journal of immunology. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 111.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. Journal of immunology. 2008;181:6923–33. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. Journal of immunology. 2009;182:2795–807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Huang H, Yuan J, Sun D, Hou WS, Gordon J, et al. CD4−8− dendritic cells prime CD4+ T regulatory 1 cells to suppress antitumor immunity. Journal of immunology. 2005;175:2931–7. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 114.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. The Journal of experimental medicine. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gigante M, Mandic M, Wesa AK, Cavalcanti E, Dambrosio M, Mancini V, et al. Interferon-alpha (IFN-alpha)-conditioned DC preferentially stimulate type-1 and limit Treg-type in vitro T-cell responses from RCC patients. Journal of immunotherapy. 2008;31:254–62. doi: 10.1097/CJI.0b013e318167b023. [DOI] [PubMed] [Google Scholar]
- 116.Ranieri E, Gigante M, Storkus WJ, Gesualdo L. Translational mini-review series on vaccines: Dendritic cell-based vaccines in renal cancer. Clinical and experimental immunology. 2007;147:395–400. doi: 10.1111/j.1365-2249.2006.03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. Journal of immunology. 2003;170:3806–11. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 118.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 120.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et biophysica acta. 2009;1796:11–8. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 121.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 122.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of immunology. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 123.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Seminars in cancer biology. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. Journal of immunology. 2010;185:642–52. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 125.Seya T, Akazawa T, Uehori J, Matsumoto M, Azuma I, Toyoshima K. Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer research. 2003;23:4369–76. [PubMed] [Google Scholar]
- 126.Standiford TJ, Kuick R, Bhan U, Chen J, Newstead M, Keshamouni VG. TGF-beta-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene. 2011;30:2475–84. doi: 10.1038/onc.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 128.del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. Journal of immunology. 2005;174:3032–40. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]
- 129.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weiskopf K, Ring AM, Schnorr PJ, Volkmer JP, Volkmer AK, Weissman IL, et al. Improving macrophage responses to therapeutic antibodies by molecular engineering of SIRPalpha variants. Oncoimmunology. 2013;2:e25773. doi: 10.4161/onci.25773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reiter I, Krammer B, Schwamberger G. Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. Journal of immunology. 1999;163:1730–2. [PubMed] [Google Scholar]
- 133.Byrne SN, Knox MC, Halliday GM. TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunology and cell biology. 2008;86:92–7. doi: 10.1038/sj.icb.7100116. [DOI] [PubMed] [Google Scholar]
- 134.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and experimental immunology. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. Journal of leukocyte biology. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of immunology. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Current opinion in immunology. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 140.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy: CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer research. 2009;69:5514–21. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer metastasis reviews. 2006;25:323–31. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. International journal of cancer Journal international du cancer. 2009;124:2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews Immunology. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 147.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of immunology. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. International immunopharmacology. 2009;9:937–48. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 149.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer research. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. The Journal of experimental medicine. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer research. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE., 3rd Myeloid-derived suppressor cells in breast cancer. Breast cancer research and treatment. 2013;140:13–21. doi: 10.1007/s10549-013-2618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sawant A, Schafer CC, Jin TH, Zmijewski J, Tse HM, Roth J, et al. Enhancement of antitumor immunity in lung cancer by targeting myeloid-derived suppressor cell pathways. Cancer research. 2013;73:6609–20. doi: 10.1158/0008-5472.CAN-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Seminars in cancer biology. 2013;23:200–7. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 155.Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Seminars in cancer biology. 2013;23:159–70. doi: 10.1016/j.semcancer.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 156.Dumitru CA, Gholaman H, Trellakis S, Bruderek K, Dominas N, Gu X, et al. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. International journal of cancer Journal international du cancer. 2011;129:859–69. doi: 10.1002/ijc.25991. [DOI] [PubMed] [Google Scholar]
- 157.Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Experimental cell research. 2010;316:138–48. doi: 10.1016/j.yexcr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 158.Imai Y, Kubota Y, Yamamoto S, Tsuji K, Shimatani M, Shibatani N, et al. Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: an in vitro study. Journal of gastroenterology and hepatology. 2005;20:287–93. doi: 10.1111/j.1440-1746.2004.03575.x. [DOI] [PubMed] [Google Scholar]
- 159.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. Journal of cellular physiology. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 160.Caruso RA, Muda AO, Bersiga A, Rigoli L, Inferrera C. Morphological evidence of neutrophil-tumor cell phagocytosis (cannibalism) in human gastric adenocarcinomas. Ultrastructural pathology. 2002;26:315–21. doi: 10.1080/01913120290104593. [DOI] [PubMed] [Google Scholar]
- 161.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical reviews in oncology/hematology. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Reibman J, Meixler S, Lee TC, Gold LI, Cronstein BN, Haines KA, et al. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6805–9. doi: 10.1073/pnas.88.15.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. Journal of immunology. 1996;157:360–8. [PubMed] [Google Scholar]
- 165.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. The Journal of experimental medicine. 1995;181:435–40. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–45. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 168.Colombo MP, Lombardi L, Stoppacciaro A, Melani C, Parenza M, Bottazzi B, et al. Granulocyte colony-stimulating factor (G-CSF) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between G-CSF-producing and G-CSF-nonproducing tumor cells. Journal of immunology. 1992;149:113–9. [PubMed] [Google Scholar]
- 169.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer immunity. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 170.van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Seminars in cancer biology. 2013;23:190–9. doi: 10.1016/j.semcancer.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 171.Kershaw MH, Trapani JA, Smyth MJ. Cytotoxic lymphocytes: redirecting the cell-mediated immune response for the therapy of cancer. Therapeutic immunology. 1995;2:173–81. [PubMed] [Google Scholar]
- 172.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer research. 2001;61:3932–6. [PubMed] [Google Scholar]
- 173.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer research. 2001;61:5132–6. [PubMed] [Google Scholar]
- 174.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 175.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 176.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 177.Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA, et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35:123–34. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nature medicine. 2001;7:1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 179.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–9. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. The Journal of experimental medicine. 2010;207:637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, Hanahan D. Immune enhancement of skin carcinogenesis by CD4+ T cells. The Journal of experimental medicine. 2003;197:1017–28. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 184.Girardi M, Oppenheim D, Glusac EJ, Filler R, Balmain A, Tigelaar RE, et al. Characterizing the protective component of the alphabeta T cell response to transplantable squamous cell carcinoma. The Journal of investigative dermatology. 2004;122:699–706. doi: 10.1111/j.0022-202X.2004.22342.x. [DOI] [PubMed] [Google Scholar]
- 185.Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, et al. CD4+ T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer. Cancer research. 2005;65:2018–25. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 186.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–54. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer research. 2002;62:6438–41. [PubMed] [Google Scholar]
- 188.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lai YP, Lin CC, Liao WJ, Tang CY, Chen SC. CD4+ T cell-derived IL-2 signals during early priming advances primary CD8+ T cell responses. PloS one. 2009;4:e7766. doi: 10.1371/journal.pone.0007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lowes MA, Bishop GA, Crotty K, Barnetson RS, Halliday GM. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. The Journal of investigative dermatology. 1997;108:914–9. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 191.Fridman WH. The immune microenvironment as a guide for cancer therapies. Oncoimmunology. 2012;1:261–2. doi: 10.4161/onci.19651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. Journal of immunology. 1996;156:73–8. [PubMed] [Google Scholar]
- 193.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer immunology, immunotherapy: CII. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Current opinion in immunology. 2009;21:200–8. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. Journal of immunology. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 196.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15505–10. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochemical and biophysical research communications. 2008;374:533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 198.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. European journal of immunology. 2009;39:216–24. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 200.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. International journal of biological sciences. 2011;7:651–8. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. International journal of cancer Journal international du cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 202.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 203.Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer research. 2011;71:736–46. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. Journal of immunology. 2007;178:320–9. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 205.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer research. 2006;66:4488–95. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 206.Gomella LG, Sargent ER, Linehan WM, Kasid A. Transforming growth factor-beta inhibits the growth of renal cell carcinoma in vitro. The Journal of urology. 1989;141:1240–4. doi: 10.1016/s0022-5347(17)41230-4. [DOI] [PubMed] [Google Scholar]
- 207.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. Journal of immunology. 2007;178:2155–62. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 208.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 209.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunological reviews. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 210.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert opinion on biological therapy. 2012;12:1383–97. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3706–15. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. Journal of immunology. 2004;173:4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 213.Dobrzanski MJ, Rewers-Felkins KA, Samad KA, Quinlin IS, Phillips CA, Robinson W, et al. Immunotherapy with IL-10- and IFN-gamma-producing CD4 effector cells modulate “Natural” and “Inducible” CD4 TReg cell subpopulation levels: observations in four cases of patients with ovarian cancer. Cancer immunology, immunotherapy: CII. 2012;61:839–54. doi: 10.1007/s00262-011-1128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Segal BM, Glass DD, Shevach EM. Cutting Edge: IL-10-producing CD4+ T cells mediate tumor rejection. Journal of immunology. 2002;168:1–4. doi: 10.4049/jimmunol.168.1.1. [DOI] [PubMed] [Google Scholar]
- 215.Connolly EC, Saunier EF, Quigley D, Luu MT, De Sapio A, Hann B, et al. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer research. 2011;71:2339–49. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]