Abstract
A tibia fracture cast immobilized for 4 weeks can induce exaggerated substance P (SP) and CGRP signaling and neuropeptide-dependent nociceptive and inflammatory changes in the hindlimbs of rats similar to those seen in complex regional pain syndrome (CRPS). Four weeks of hindlimb cast immobilization can also induce nociceptive and vascular changes resembling CRPS. To test our hypothesis that immobilization alone could cause exaggerated neuropeptide signaling and inflammatory changes we tested 5 cohorts of rats; 1) controls, 2) tibia fracture and hindlimb casted, 3) hindlimb casted, no fracture, 4) tibia fracture with intrameduallary pinning, no cast, and 5) tibia fracture with intrameduallary pinning and hindlimb casting. After 4 weeks the casts were removed and hindlimb allodynia, unweighting, warmth, edema, sciatic nerve neuropeptide content, cutaneous and spinal cord inflammatory mediator levels, and spinal c-Fos activation were measured. After fracture with casting there was allodynia, unweighting, warmth, edema, increased sciatic nerve SP and CGRP, increased skin NK1 receptors and keratinocyte proliferation, increased in inflammatory mediator expression in the hindpaw skin (TNF-α, IL-1β, IL-6, NGF) and cord (IL-1β, NGF), and increased spinal c-Fos activation. These same changes were observed after cast immobilization alone, except spinal IL-1β levels were not increased. Treating cast only rats with an NK1 receptor antagonist inhibited development of nociceptive and inflammatory changes. Four weeks after fracture with pinning all nociceptive and vascular changes had resolved and there were no increases in neuropeptide signaling or inflammatory mediator expression.
Keywords: fracture, immobilization, cytokines, pain, complex regional pain syndrome
Introduction
Complex regional pain syndrome (CRPS) is characterized by allodynia, pain with movement, warmth, and edema in the injured extremity. The mechanisms mediating CRPS are unknown, but several observations suggest that limb immobilization plays a role. The traumatized limb is frequently immobilized in casts, splints, or fixators prior to the development of CRPS 1, 38 and CRPS patients tend to immobilize the limb to prevent movement-induced pain 3, 30. Furthermore, aggressively mobilizing the CRPS limb with physical therapy can alleviate symptoms 29, 30. Intriguingly, cast immobilization of the wrist for 4 weeks in normal experimental subjects causes skin warmth, hyperalgesia, and movement-evoked pain, symptoms partially mimicking CRPS 42. Collectively, these data suggest that prolonged immobilization can contribute to the development of CRPS.
Population-based studies indicate that distal limb fracture is the most common cause of CRPS11, 36 and we have developed a rat tibia fracture CRPS model. After tibia fracture with 4 weeks cast immobilization the rats developed chronic hindlimb von Frey allodynia, warmth, increased protein extravasation, edema, and periarticular osteopenia, changes paralleling those observed in CRPS patients.14 Surprisingly, control rats that had no fracture but were casted for 4 weeks also developed hindlimb allodynia, warmth, increased protein extravasation, edema, and periarticular osteopenia, similar to the effects of tibia fracture with casting, but these changes resolved much more quickly in the cast only rats.14 Treatment with a substance P (SP) NK1 receptor antagonist partially reversed the allodynia, warmth, spontaneous protein extravasation, and edema in both cast only and tibia/casted rats.14 These results suggest that SP signaling contributes to the CRPS-like changes observed with both cast immobilization and tibia fracture with casting.
The nociceptive and vascular changes characteristic of early CRPS suggest an inflammatory process and there is extensive evidence that neurogenic inflammatory responses mediated by sensory afferent release of SP and calcitonin gene-related peptide (CGRP) are exaggerated in the CRPS limb5, 6, 24, 31, 37, 44 and in the rat fracture/cast CRPS model.14, 46 Furthermore, inflammatory cytokine levels are up-regulated in CRPS skin blister fluid19, 20 and skin,23 and levels of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), interleukin 6 (IL-6), and nerve growth factor (NGF) are elevated in the hindpaw skin of fracture/casted rats.34, 35, 47 Treating fracture/cast rats with a TNF inhibitor (etanercept), an IL-1 receptor antagonist (anakinra), an IL-6 receptor antagonist (TB-2-081), or an anti-NGF antibody (tanezumab) reduced allodynia and unweighting.25, 27, 34, 35 In addition, treating fracture/casted rats with an SP NK1 receptor antagonist prevented the up-regulation of inflammatory mediators in the skin and reversed pain behavior.45 These data suggest that facilitated SP signaling after fracture with casting caused increased inflammatory mediator expression in the fracture limb, resulting in nociceptive sensitization.
The aims of current study were to test the hypotheses that prolonged immobilization can facilitate neuropeptide signaling, causing inflammatory and nociceptive changes similar to those observed after tibia fracture with casting, and to determine whether early mobilization after fracture with intramedullary pinning can prevent or reverse those changes.
Methods
These experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA) and followed the animal subjects guidelines of the International Association for the Study of Pain. One hundred forty five adult (9-month-old) male Sprague-Dawley rats (Simonsen Laboratories, Gilroy, CA) were used in these experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were given food and water ad libitum. During the experimental period the animals were fed Lab Diet 5012 (PMI Nutrition Institute), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g vitamin D3, and were kept under standard conditions with a 12-h light-dark cycle.
Study design
All cohorts of rats utilized in this study (other than the untreated controls) were enrolled in the same experimental protocol. First, all rats underwent right tibia fracture or hindlimb cast immobilization, then 4 weeks later all casts were removed. The following day all rats underwent hindpaw behavior testing (bilateral von Frey withdrawal thresholds, weight bearing, temperature, and thickness measurements), and then the rats were sacrificed and the hindpaw skin, sciatic nerve, and lumbar spinal cord were collected for EIA, western immunoblot, and immunohistochemical assays. The tibia fracture rats were divided into 3 different treatment cohorts at the time of fracture, 1) fracture stabilized by 4 weeks cast immobilization, 2) fracture stabilized by intramedullary pinning, and 3) fracture stabilized by intramedullary pinning and 4 weeks cast immobilization. One group of cast only immobilized rats was injected with a substance P NK1 receptor antagonist (LY303870, 20mg/kg, i.p.) 4 hours before behavior testing.
Drugs
The effects of an SP NK1 receptor antagonist (LY303870) were evaluated in hindlimb cast immobilized rats. After 4 weeks cast immobilization the casts were removed and the next day LY303870 (20mg/kg) was injected intraperitoneally. Approximately 4 hours later hindpaw von Frey thresholds, weight bearing, temperature, and thickness were determined and at 8 hours after LY303870 injection the rats were euthanized and the hindpaw skin collected for TNF-α, IL-1β, IL-6 and NGF EIA assays. LY303870 has nanomolar affinity for the rat NK1 receptor, has no affinity for 65 other receptors and ion channels, has no sedative, cardiovascular or core body temperature effects in rats at systemic dosed up to 30mg/kg, and is physiologically active for up to 24 h after a single systemic dose of 10 mg/kg 13, 18, 21.
Surgery
Tibia fracture was performed under 2–4% isoflurane to maintain surgical anesthesia as we have previously described.14, 15 The right hind limb was wrapped in a stockinet (2.5cm wide) and the distal tibia was fractured using pliers with an adjustable stop (Visegrip, Petersen Manufacturing) that had been modified with a three-point jaw. The hind limb was wrapped in casting tape (Delta-Lite, Johnson & Johnson) so the hip, knee, and ankle were flexed. The cast extended from the metatarsals of the hind paw up to a spica formed around the abdomen, thus insuring that the cast did not slip off the hind limb. The cast over the paw was applied only to the plantar surface; a window was left open over the dorsum of the paw and ankle to prevent constriction when post-fracture edema developed. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh. The rats were given subcutaneous saline and buprenorphine immediately after procedure (0.03 mg/kg) and on the first day after fracture for postoperative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. All rats used in this study had mechanical union at the fracture site after 4 weeks of cast immobilization.
The cast only rats underwent right hindlimb cast immobilization for 4 weeks as described above, without tibia fracture or pinning, and after 4 weeks the was cast removed.
The fracture with pinning rats underwent right tibia fracture followed by intramedullary nailing to provide an alternative method of fracture stabilization that allows for early hindlimb movement and weight bearing. The intramedullary nailing technique was based on the procedure described by Sigurdsen et al.40 To expose the right tibia, a 15 mm long incision was in the muscle and skin overlaying the anterior aspect of the right proximal tibia. A small hole was drilled through the cortex at approximately 5 mm below tibia plateau and 3 mm medial to the tibial tuberosity. A 20-gage stainless steel needle (38 mm in length with a 1 mm outer diameter) was first inserted into the bone marrow cavity until the tip reached the distal tibia, then the needle was retracted halfway out of the tibia. Using a fine tooth circular metal saw blade mounted on an electric drill a small notch was cut into the tibia cortex just distal to the boney union of the tibia and fibula. The distal tibia was then manually fractured and the needle was reinserted down the medullary canal across the fracture site until the tip of the needle reached the distal tibia. The proximal needle was cut flush to the tibial periosteum and the muscle incision was sutured shut and the skin incision closed with wound clips.
Another group of fracture and pinning rats had the right hindlimb casted after the skin incision was closed with wound clips. The cast was removed after 4 weeks.
Hindpaw nociception, temperature and edema
All hindpaw behavior testing was performed in the following sequential order; 1) von Frey allodynia, 2) unweighting, 3) temperature, and 4) paw thickness.
To measure hindpaw plantar allodynia in the fracture rats an up-down von Frey testing paradigm8 was used as we have previously described.14, 15. Hind paw mechanical nociceptive thresholds were analyzed as the difference between the treatment side and the contralateral untreated side.
An incapacitance device (IITC Inc. Life Science) was used to measure hind paw unweighting. The rats were manually held in a vertical position over the apparatus with the hind paws resting on separate metal scale plates, and the entire weight of the rat was supported on the hind paws. The duration of each measurement was 6s, and 10 consecutive measurements were taken at 60s intervals. Eight readings (excluding the highest and lowest) were averaged to calculate the bilateral hind paw weight bearing values.15, 35.
The temperature of the hindpaw was measured using a fine wire thermocouple (Omega) applied, as previously described.14, 15 Temperature testing was performed over the hindpaw dorsal skin between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). The measurements for each hindpaw were averaged for the mean paw temperature.
Hindpaw edema was determined by measuring the hindpaw dorsal-ventral thickness over the midpoint of the third metatarsal with a LIMAB laser measurement sensor (Goteborg, Sweden) while the rat was briefly anesthetized with isoflurane. 14, 15 Temperature and hindpaw thickness data were analyzed as the difference between the fracture side and the contralateral intact side.
Hindpaw von Frey thresholds, temperature, and thickness data were analyzed as the difference between the treatment side and the contralateral untreated side. Right hindpaw weight bearing data were analyzed as a ratio between the right hindpaw weighting and the mean of right and left hindpaws’ values ((2R/(R + L)) · 100%).
Western blotting assays for neuropeptide receptors in hindpaw skin
These experiments examined the effects of fracture and immobilization on levels of SP (neurokinin1, NK1) and CGRP (calcitonin receptor-like receptor, CRLR and receptor activity modifying protein 1,RAMP1) receptor proteins in the hindpaw skin. At 4 weeks after fracture and casting, cast only, or fracture and pinning, the ipsilateral hindpaw dorsum skin was collected under isoflurane anesthesia and was homogenized in modified RIPA buffer (50mM Tris-HCl, 150 mM NaCl, 1 mMEDTA, 1% Igepal CA-630, 0.1% SDS, 50 mM NaF, and 1 mM NaVO3) containing protease inhibitors (aprotinin (2 g/ml), leupeptin (5 g/ml), pepstatin (0.7 g/ml), and PMSF (2mM); Sigma). The homogenate was centrifuged at 13,000g for 30 min at 4°C. Total protein concentration of the homogenate was measured using a Coomassie Blue Protein Assay and BSA protein standard (Pierce). The supernatant was subjected to western blot analysis as we have previously described46 to elucidate changes in NK1, CRLR and RAMP1 protein expression in hindpaw skin. In short, equal amounts of protein (30 μg) were subjected to SDS-PAGE (12% Tris-HCl acrylamide gel, Bio-Rad) and electrotransferred onto a polyvinylidene difluorided membrane (Millipore). The blots were blocked with 5% non-fat dry milk in tris-buffered saline, incubated with various indicated primary antibody against specific proteins overnight at 4 °C and further incubated with HRP-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 hr at room temperature. Primary antibodies against NK1, CRLR, and β-actin, as well as HRP-conjugated secondary antibodies, were from Santa Cruz Biotechnology, whereas goat anti-RAMP1 was from Novus Biologicals. After washing in TBST three times, the blot was then incubated in ECL plus chemoluminescence reagents (Amersham) and scanned by PhosphoImager (Typhoon, GE Healthcare) and the band intensity was analyzed using ImageQuant 5.2 software (Molecular Dynamics) and normalized with the corresponding internal loading control band, β-actin. The specific protein/actin band intensity ratio represents the change of the specific protein after treatments.
ELISA assays for SP and CGRP in the sciatic nerve
These experiments examined the effects of fracture and immobilization on SP and CGRP protein expression in the sciatic nerve. At 4 weeks after fracture and casting, cast only, or fracture and pinning the right sciatic nerve was collected under isoflurane anesthesia, immediately frozen, and weighed. Nerve samples were minced in 1 ml of 3:1 ethanol/0.7 M HCl and homogenized for 20 s. The homogenates were shaken for 2 h at 4 °C and centrifuged at 3000g for 20 min at 4 °C. The supernatant was frozen and lyophilized, and the lyophilized product was stored at −80 °C. All nerve and dialysis samples were assayed in duplicate using EIA kits for SP (Assay Designs) and for CGRP (Caymen Chemicals) as we have previously described.46 Both assays were carried out following the manufacturer's protocols.
In vivo BrdU labeling and BrdU immunohistochemistry in hindpaw skin
Bromodeoxyuridine (BrdU) labeling was utilized to evaluate keratinocyte proliferation as we have previously described.45 Starting at 3 weeks after tibia fracture and/or casting or pinning, animals were injected intraperitoneally once daily with 50 mg/kg BrdU (Sigma-Aldrich) for 8 days. One day after the last injection the animals were sacrificed and the hind paw skin was harvested, fixed, and processed for immunostaining. Skin sections were pretreated in 2 N HCl for 30 min at 37 °C, followed by neutralization in 0.1 M borate buffer (pH 8.5) for 10 min and blocking with 10% normal donkey serum for 1 hour at room temperature, after which immunohistochemistry was performed using a rat anti-BrdU monoclonal antibody (1:300, Accurate Chemical) and donkey anti-rat fluorescein isothiocyanate secondary antibody (1:400, Jackson ImmunoResearch Laboratories). After three rinses with PBS, the sections were immunostained with the monoclonal anti-rat keratin. BrdU immunostaining was observed using a Leica DM 2000 fluorescent microscope and imaged using Spot Camera (version 4.0.8, Diagnostic Instruments). The number of BrdU-positive cells was counted, specifically those in keratin positive cells in the epidermis, with a minimum of six sections per animal. Cell densities were calculated by dividing cell numbers by the area.
ELISA assays for TNF-α, IL-1β, IL-6 and NGF in hindpaw skin and spinal cord
These experiments examined the effects of fracture and immobilization on cytokine and NGF levels in the hindpaw skin and spinal cord (L4-L5 lumbar enlargement). The hindpaw dorsal skin and spinal cord was collected after behavioral testing at 4 weeks post-fracture, casting or pinning, and frozen immediately on dry ice. Skin tissue and spinal cord were cut into fine pieces in ice-cold phosphate buffered saline (PBS), pH 7.4, containing protease inhibitors (aprotinin (2μ g/ml), leupeptin (5 μg/ml), pepstatin (0.7 μg/ml), and PMSF (2mM); Sigma) followed by homogenization using a rotor/stator homogenizer. Homogenates were centrifuged for 5 min at 14 000 g, 4°C. Supernatants were transferred to precooled Eppendorf tubes. Triton X-100 (Boehringer) was added at a final concentration 0.01 %. The samples were centrifuged again for 30 min at 8 000 g at 4°C. The supernatants were aliquoted and stored at −80°C. TNF-α, IL-1β, and IL-6 protein levels were determined using EIA kits (R&D Systems). The NGF concentrations were determined by using the NGF Emax® ImmunoAssay System kit (Promega) according to the manufacturer's instructions. The OD of the reaction product was read on a microplate reader at 450 nm, and values were normalized per mg of protein assayed. The concentrations of TNF-α, IL-1β, IL-6, and NGF proteins were calculated from the standard curve at each assay. Positive and negative controls were included in each assay. Each protein concentration was expressed as pg/mg total protein. Total protein contents in all tissue extracts were measured by the Coomassie Blue Protein Assay Kit and BSA protein standard (Pierce).
c-Fos spinal cord immunohistochemistry
This experiment examined the effects of fracture and immobilization on c-Fos spinal cord immunohistochemistry. At 4 weeks after fracture and casting, cast only, or fracture and pinning the rats were euthanized with CO2 and perfused intracardially with 200 ml 0.1 M PBS followed by 200 ml neutral 10% buffered formaldehyde as previously. Because the sciatic nerve projects heavily to the L3-L5 segments of the spinal cord, we analyzed the numbers of c-Fos immunoreactive (c-Fos-IR) neurons at that level. Spinal cord segments (L3-L5) were removed, post-fixed in the perfusion fixative overnight and cryoprotected in 30% sucrose at 4 °C for 24 h. Serial frozen spinal cord sections, 40-mm thick, were cut on a coronal plane using a sliding microtome, collected in PBS, and processed as free floating sections. c-Fos immunostaining was performed as previously described.35 To evaluate and compare the distribution of c-Fos immunoreactive neurons in the lumbar spinal cord, a Bioquant image analysis system attached to Nikon Eclipse 80i microscope was used. Images were captured using 10x magnification, converted to digital and c-Fos-IR neurons were identified by dense black staining of the nucleus. The c-Fos-IR neurons were plotted and counted with Bioquant Automated Imaging module in the superficial lamina (laminae I-II), the nucleus proprius (laminae III-IV), and the deep laminae (laminae V-VI) of the spinal gray matter of the L3-L5 segments, according to the cytoarchitectonic organization of the spinal cord dorsal horn.28, 33 For each section, the c-Fos-IR neurons were counted in each lamina, the counts were pooled, and the average number was calculated giving a count that was the mean of all stained neurons in three sections. The investigator responsible for plotting and counting of the c-Fos-IR neurons was blinded to treatment groups.
Statistical analysis
Statistical analysis was accomplished using a one-way analysis of variance (ANOVA), followed by post-hoc Newman-Keuls multiple comparison testing to compare between the control cohort and all other cohorts unless indicated as unpaired two-tailed t-test. Sample sizes were based on a power analysis of our previously published data generated from using each of the proposed assays in fracture animals. Based on this analysis we calculated that the proposed experiments would require 8 to 14 mice per cohort to provide 80% power to detect 25% differences between groups. The large numbers of animals described for each cohort in Figure 1 are the result of pooling the 4 weeks behavioral testing results of all the rats required for the various biochemical assays used in this study (Figs. 2,4,5) and the separate groups of rats required for the immunohistochemistry assays (Figs. 3,6). All data are presented as the mean ± SE of the mean, and differences are considered significant at a p value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA).
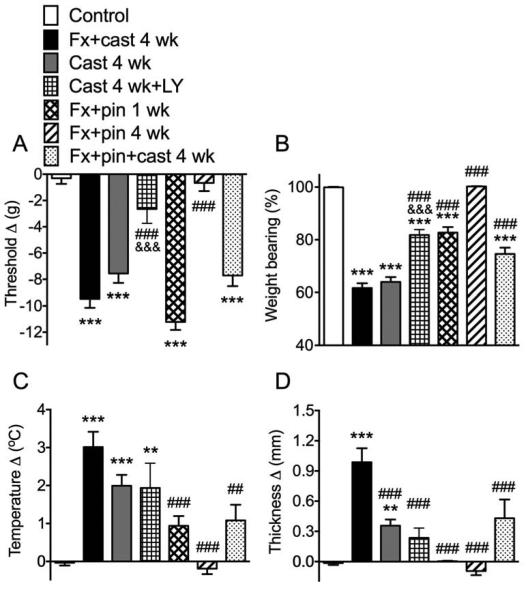
Fig. 1.
Hindpaw nociceptive and vascular changes in 7 cohorts of rats; 1) controls (Controls, n = 25), 2) a tibia fracture with 4 weeks cast immobilization (Fx + cast 4 wk, n = 28), 3) hindlimb cast immobilization for 4 weeks (Cast 4 wk, n = 39), 4) cast immobilization for 4 weeks treated with the substance P (SP) neurokinin 1 (NK1) receptor antagonist LY303870 once prior to testing (Cast 4 wk + LY, n = 14), 5) tibia fracture treated with intramedullary pinning for 1 week (Fx + pin 1 wk, n = 18), 6) tibia fracture treated with intramedullary pinning for 4 weeks (Fx + pin 4 wk, n = 28), 7) tibia fracture treated with intramedullary pinning and casting for 4 weeks (Fx + pin + cast 4 wk, n = 19). Fx+cast and Cast only treatments both resulted in significant hindpaw allodynia (A), unweighting (B), warmth (C) and edema (D) after 4 weeks. Cast rats treated with a single dose of SP NK1 antagonist had reduced hindpaw allodynia, unweighting and edema, but no change in warmth. Fx + pin treatment caused allodynia and unweighting at 1 week, but not at 4 weeks, and had no effect on hindpaw temperature or thickness. Fx + pin + cast caused hindpaw allodynia and unweighting, but no significant warmth or edema. Measurements for withdrawal thresholds (A), temperature (C), and paw thickness (D) represent the difference between the treatment side and the contralateral paw, thus a positive value represents an increase in temperature or thickness on the fracture side; a negative value represents a decrease in mechanical nociceptive thresholds on the affected side. Measurements for (B) represent weight-bearing on the fracture hindlimb as a ratio to 50% of bilateral hindlimb loading, thus a percentage lower than 100% represents hindpaw unweighting. *P<0.05, **P<0.01, ***P<0.001 vs. control values; ## P<0.01, ### P<0.001 vs. FX + cast; &&& P<0.001 Cast 4 wk + LY vs. Cast 4 wk.
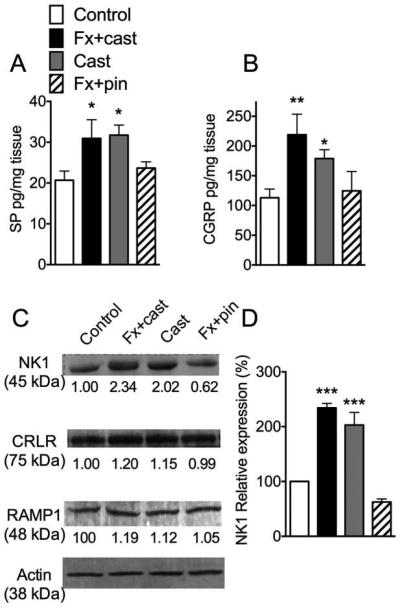
Figure 2.
Substance P (SP) (A) and calcitonin gene-related protein (CGRP) (B) expression in the sciatic nerve were measured by EIA. Both SP and CGRP protein levels were increased in the ipsilateral sciatic nerve after 4 weeks cast immobilization of a tibia fracture (Fx + cast) and after 4 weeks cast immobilization alone (Cast), but not 4 weeks after fracture with intramedullary pinning with early mobilization (Fx + pin) (n =8-14 per cohort). The SP neurokinin 1 (NK1) receptor and the CGRP co-receptors calcitonin receptor-like receptor (CRLR) and receptor activity modifying protein 1 (RAMP1) were measured in hindpaw skin by western blotting (C). Only NK1 receptor expression was increased after Fx + cast or Cast treatment, while the Fx + pin treatment had no effect on receptor expression in the skin (n=3 in each cohort). These data indicate that neuropeptide signaling in the skin is amplified after Fx + cast and Cast treatments (Fx + pin) (D). *P<0.05, **P<0.01, ***P<0.001 vs. control.
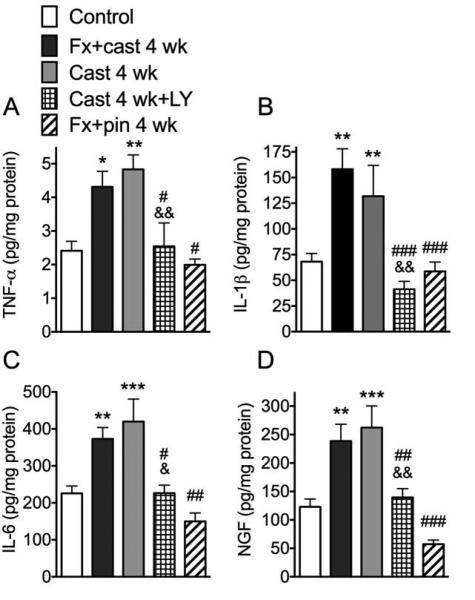
Figure 4.
Hindpaw skin levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) and nerve growth factor (NGF) were measured by EIA at 4 weeks after treatment (n = 8-16 per cohort). TNF-α (A), IL-1β (B), IL-6 (C) and NGF (D) protein levels were increased at 4 weeks after Fx + cast and at 4 weeks after Cast only treatment. Treating the 4-week Cast only rats with a single dose of the NK1 receptor antagonist LY303870 (Cast 4 wk + LY) reversed the expected cast-induced increase in skin cytokine and NGF levels. At 4 weeks after fracture treated with intramedullary pinning (Fx + pin 4 wk) there were no increases in cytokine or NGF expression in the skin. *P<0.05, **P<0.01, ***P<0.001 vs. control values; # P<0.05, ## P<0.01, ### P<0.001 vs. FX + cast; & P<0.05, && P<0.01 vs. Cast 4 wk.
Figure 5.
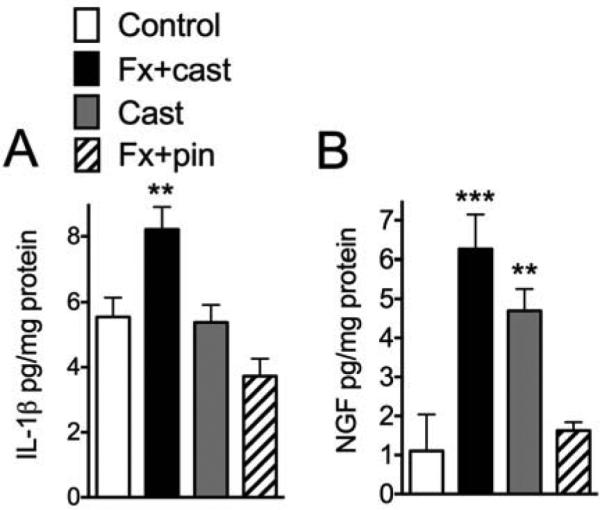
Lumbar spinal cord levels of IL-1β and NGF were measured by EIA assays at 4 weeks after treatment (n=8-15 per cohort). IL-1β (A) levels were elevated after Fx + cast, but not after Cast immobilization alone, whereas NGF (B) levels were elevated after Fx + cast and Cast only treatments. Fx + pin had no effect on spinal IL-1β or NGF levels. **P<0.01, ***P<0.001 vs. control.
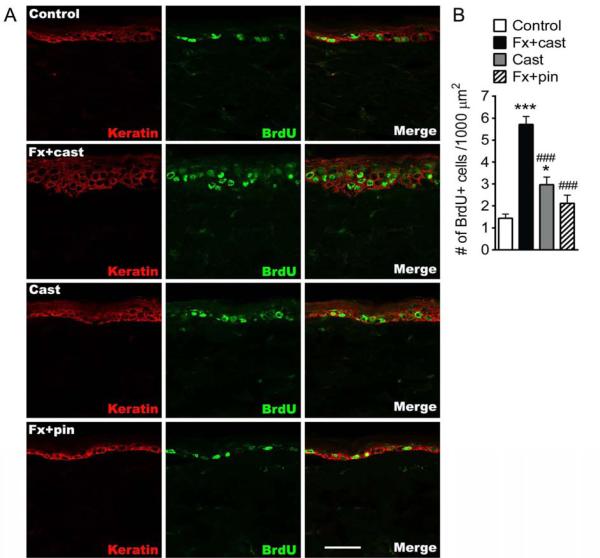
Figure 3.
Immunostaining for the cellular proliferation marker bromo-2-deoxyuridine (BrdU, green) and for the keratinocyte marker keratin (red) was used to quantify the number of proliferating keratinocytes in the hindpaw skin at 4 weeks after treatment (A). Top row of panels are representative images from a normal control rat, the second row of panels are from a Fx + cast rat, the third row of panels are from a Cast rat, and the fourth row of panels are from a Fx + pin rat (scale bar = 50 μm). BrdU-positive cell numbers increased by 299% at 4 weeks after Fx + cast and by 107% at 4 weeks after cast. Fx + pin had no effect on keratinocyte proliferation (n=6 in each cohort) (B). *P<0.05, ***P<0.001 vs. control; ###P<0.001 vs. FX + cast.
Figure 6.
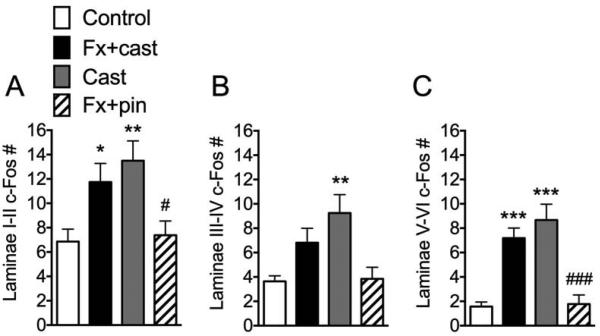
L4, L5 lumbar spinal cord segments were sectioned and stained for c-Fos immunoreactivity, allowing quantification of activated c-Fos positive cells in the ipsilateral dorsal horn laminae I + II (A), III + IV (B) and V + VI (C). Both Fx + cast and Cast only treatments increased c-Fos expression in laminae I + II and V + VI. Cast only also increased c-Fos expression in laminae III + IV. Fx + pin did not change c-Fos expression in any of the dorsal horn laminar regions ipsilateral to fracture. (n=12-16 in each cohort) * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control; # P<0.05, ### P<0.001 vs. FX + cast.
Results
Immobilization contributed to post-fracture nociceptive and vascular abnormalities
These experiments tested the hypothesis that prolonged immobilization of the hindlimb contributes to the nociceptive and vascular abnormalities observed in the rat fracture CRPS model and that intramedullary pinning of the tibia fracture without casting allows earlier hindlimb weight bearing and prevents the development of CRPS-like symptoms. When the hindlimb was immobilized in a cast for 4 weeks in the tibia fracture cohort (Fx + cast 4 wk), on the day after cast removal there was hindpaw von Frey allodynia (Fig. 1A), unweighting (Fig. 1B), warmth (Fig. 1C), and edema (Fig. 1D). Similarly, in rats that were not fractured, but underwent hindlimb cast immobilization for 4 weeks (Cast 4 wk), on the day after cast removal there was hindpaw allodynia, unweighting, warmth, and edema (Fig. 1). When cast only rats were treated with a SP NK1 receptor antagonist (LY303870, 20mg i.p., Cast 4 wk + LY) 4 hours prior to testing, it inhibited immobilization induced allodynia and unweighting, but had no significant effect on hindpaw warmth and edema (Fig. 1).
After tibia fracture with intramedullary pinning there was hindpaw allodynia and unweighting at 1 week but not at 4 weeks post-fracture (Fx + pin 1 wk and Fx + pin 4 wk, Fig. 1A,B). No significant hindpaw warmth (Fig. 1C) or edema (Fig. 1D) were observed at either 1 or 4 weeks after fracture with pinning. These results suggest that prolonged hindlimb cast immobilization contributes to the development of the nociceptive and vascular changes observed at 4 weeks after fracture with casting, although cast immobilization by itself appears to induce less robust hindpaw warmth and edema than that observed after tibia fracture with casting. Interestingly, tibia fracture stabilized with intramedullary pinning caused hindpaw von Frey allodynia and unweighting at 1 week post-fracture (but not warmth and edema), and these nociceptive changes resolved by 4 weeks post-fracture, suggesting that early weight bearing and mobilization in the fracture hindlimb can reverse the development of the nociceptive and vascular changes observed after fracture with cast immobilization.
When the rats underwent tibia fracture with both intramedullary pinning and cast immobilization for 4 weeks (Fx + pin + cast 4 wk), they developed allodynia and unweighting (Fig. 1A,B), but hind paw warmth and edema failed to reach significant levels (Fig. 1C,D) and the hind paw unweighting was significantly less than that observed with fracture and cast immobilization alone (Fig. 1D). The attenuated CRPS-like changes observed in the fracture/pinning/cast group compared to the fracture with cast alone treated rats suggests that the additional fracture stabilization proved by intramedullary fixation was beneficial.
Immobilization contributed to exaggerated neuropeptide signaling in the hindpaw skin
Previously we observed that at 4 weeks after tibia fracture with cast immobilization there was ipsilateral up-regulated expression of SP and CGRP in the sciatic nerve, increased NK1 receptor expression in the hindpaw keratinocytes and endothelial cells, and increased spontaneous and SP-evoked protein extravasation and edema in the hindpaw skin.46 The current study tested the hypothesis that neuropeptide signaling is also enhanced in the cast immobilized hindlimb. Both tibia fracture with casting and casting alone without fracture caused increased SP protein levels in the sciatic nerve, compared to nonfractured controls (Fig. 2A). Similarly, sciatic nerve CGRP levels were also increased at 4 weeks after tibia fracture with casting or after cast alone (Fig. 2B). Fracture rats treated with intramedullary pinning to allow early mobilization exhibited no increase in SP or CGRP levels in the sciatic nerve (Fig. 2A,B). Representative specific bands of NK1, CRLR, RAMP1 and actin extracted from hindpaw skin are shown in Figure 2C. The average digitized band intensities of NK1 normalized against actin and the relative intensity to the control values are also shown. Compared to controls, fracture with cast immobilization or just cast alone both caused increased NK1 expression in the hindpaw skin (Fig. 2D). Fracture with pinning had no effect on NK1 receptor levels, compared to controls. None of the treatments affected expression of the CGRP co-receptors CRLR or RAMP1 (Fig. 2C).
Immobilization stimulated keratinocyte proliferation in the hindpaw skin
In a prior study we demonstrated that distal tibia fracture with cast immobilization led to chronic keratinocyte proliferation and epidermal hyperplasia in the hindpaw skin,26 and we postulated that immobilization might contribute to post-fracture keratinocyte proliferation. To test this hypothesis BrdU was used to label DNA synthesis in proliferating keratinocytes. Figure 3 shows representative confocal images of BrdU (green) and keratin (a keratinocyte marker, red) in hindpaw skin sections from control, fracture with cast, cast immobilization only, and fracture with intramedullary pinning at 4 weeks post-fracture. BrdU-positive cell numbers in hindpaw skin were increased (299% and 107%, respectively) in the fracture with cast and the cast only rats, compared to controls. BrdU-positive cell numbers were not significantly increased at 4 weeks after tibia fracture with pinning, compared to controls.
Immobilization also contributed to inflammatory mediator expression in hindpaw skin
We previously observed that tibia fracture with cast immobilization stimulated TNF-α, IL-1β, IL-6, and NGF expression in the hindpaw skin and demonstrated that these mediators could induce hyperalgesia when injected intradermally in the hindpaw skin of rats 26, 27. Furthermore, we had observed that treating the fracture and casted rats with a SP NK1 receptor antagonist blocked these increases in inflammatory mediators, indicating neuropeptide signaling was contributing to inflammatory mediator expression in the paw skin.45 To examine the effects of immobilization on hindpaw inflammatory mediator levels, TNF-α, IL-1β, IL-6, and NGF protein levels in hindpaw skin were determined by EIA at 4 weeks after fracture and casting, cast only treatment, and fracture with intramedullary pinning (Fig. 4). Fracture with cast immobilization for 4 weeks caused increased TNF-α, IL-1β, IL-6, and NGF protein levels in hindpaw skin (Fig. 4). Similarly, when the hindlimb was just cast immobilized for 4 weeks, with no fracture, skin levels of TNF-α, IL-1β, IL-6 and NGF were increased (Fig. 4). When 4-week cast immobilized rats were treated with a SP NK1 receptor antagonist (LY303870 20 mg/kg i.p., injected once 8 hours before testing), it reversed immobilization induced increases in hindpaw skin TNF-α, IL-1β, IL-6, and NGF (Fig. 4). At 4 weeks after fracture with intramedullary pinning there were no changes in inflammatory mediator levels in the hindpaw skin, compared to controls, except for a slight reduction in NGF levels.
Immobilization contributed to increased NGF, but not IL-1β expression in the spinal cord
To elucidate the effect of fracture with cast immobilization and cast immobilization alone on spinal inflammatory mediator expression we used EIA to measure TNF-α, IL-1β, IL-6 and NGF in L4-L5 lumbar enlargement of spinal cord at 4 weeks post-treatment. TNF-α expression level in the spinal cord was below the EIA detection range under these experimental conditions. Figure 5 illustrates that at 4 weeks after fracture with cast immobilization there was an increase in IL-1β and NGF protein expression in the ipsilateral spinal cord, compared to controls. Cast immobilization alone for 4 weeks had no effect on spinal IL-1β expression (Fig. 5A) but did upregulate NGF protein levels in the ipsilateral cord (Fig. 5B). Neither fracture with cast nor cast only treatment had any effect on IL-6 levels in the cord (data not shown). Tibia fracture treated with 4 weeks of intramedullary pinning had no effect on IL-1β, IL-6, or NGF expression in the spinal cord (Fig. 5).
Immobilization evoked c-Fos immunoreactivity in the spinal cord
Previously we demonstrated that at 4 weeks after fracture with casting there were increased numbers of c-Fos IR neurons in the ipsilateral laminae I-II, III-IV, and V-VI of the dorsal horn34. Therefore, we postulated that immobilization alone could induce c-Fos activation in the spinal cord and in the present study we compared c-Fos immunoreactivity among all cohorts to examine the effects of cast immobilization and fracture with early mobilization on spinal c-Fos levels. At 4 weeks after fracture with cast immobilization or cast alone there was a significant increase in c-Fos positive neurons in Laminae I-II, III-IV, and V-VI (Fig. 6). Tibia fracture with 4 weeks intramedullary pinning had no effect on spinal c-Fos IR neuron numbers, compared to controls (Fig. 6).
Discussion
The vascular and nociceptive changes characteristic of the early stages of CRPS suggest an inflammatory process and there is convincing evidence that neurogenic inflammatory responses are exaggerated in the CRPS limb and in the rat fracture/cast model. Neurogenic inflammation is mediated by the depolarization of small sensory afferents in the skin, triggering the release of substance P (SP) and calcitonin gene-related peptide (CGRP), neurotransmitters that activate their receptors in the dermal vasculature to induce protein extravasation and vasodilatation. Electrically evoked extravasation and vasodilatation responses are enhanced in CRPS patients and when SP is microdialyzed in the skin there is exaggerated protein extravasation.24, 44 Furthermore, serum levels of SP and CGRP are elevated in CRPS patients.5, 6, 37
Previously we observed that after tibia fracture with 4 weeks cast immobilization in rats there is increased SP and CGRP expression in the sciatic nerve and in serum, up-regulated SP NK1 receptor expression in the endothelial cells and keratinocytes of the hindpaw skin, and SP evoked extravasation and edema responses are enhanced in the fracture hindlimb.46 Treating fracture/casted rats with an SP NK1 receptor antagonist reduced hindlimb allodynia, warmth, spontaneous protein extravasation, and edema, suggesting that exaggerated SP signaling contributes to the development of CRPS-like changes after fracture.14 When rats underwent hindlimb immobilization in a cast for 4 weeks they developed hindpaw allodynia, warmth, increased spontaneous protein extravasation, and edema, similar to the fracture casted rats, but these CRPS-like symptoms spontaneously resolved within 2 weeks.14 Treating casted rats with the NK1 receptor antagonist LY303870 (40 mg/kg i.p.) on the day after cast removal completely blocked the cast-induced increase in hindpaw spontaneous protein extravasation and partially reduced hindpaw warmth.14 The NK1 receptor antagonist also reduced cast-induced allodynia and edema in the hindpaw, but this did not reach significance.
In the current investigation we observed hindpaw von Frey allodynia, unweighting, warmth, and edema after 4 weeks of hindlimb cast immobilization (Fig. 1). Furthermore, neuropeptide signaling was up-regulated after 4 weeks of cast immobilization (Fig. 2). Both SP and CGRP levels in the sciatic nerve were increased in the casted limb and skin NK1 receptor expression was elevated. On the day after cast removal, a single treatment with the NK1 receptor antagonist LY303870 (20 mg/kg i.p.) partially reversed cast-induced nociceptive and vascular changes (Fig. 1). These findings indicate that exaggerated neuropeptide signaling contributes to the development of pain behavior and vascular inflammatory changes in the casted rats, and suggests that immobilization contributes to the facilitated neuropeptide signaling and nociceptive sensitization that we observe in the cast immobilized fracture model of CRPS.
When SP or CGRP is microdialyzed through the skin of normal volunteers48 or in CRPS skin24 there is no immediate painful response, evidence supporting the hypothesis that SP and CGRP act as intermediate mediators in the development of post-traumatic inflammatory pain. Intriguingly, inflammatory cytokines such as TNF-α and IL-6 are up-regulated in CRPS skin and skin blister fluid.2, 17, 19, 20, 23 Recently we observed that in patients with early CRPS there is keratinocyte proliferation in the affected skin and these activated keratinocytes express TNF-α and IL-6.4 Similarly, at 4 weeks after fracture/casting in rats the hindpaw skin keratinocytes proliferate and express increased levels of TNF-α, IL-1β, IL-6, and NGF in the hindpaw skin.26, 34, 35, 47 Previously we demonstrated that SP and CGRP can directly stimulate keratinocyte proliferation and inflammatory mediator expression in cell culture.39 Keratinocyte proliferation and up-regulated inflammatory mediator production in fracture/casted rats is inhibited by 8 days of treatment with an NK1 receptor antagonist or in transgenic fracture/casted mice deficient for SP or CGRP receptors, indicating a crucial role for neuropeptide signaling in post-fracture keratinocyte activation and inflammatory mediator expression.16, 45 In the current study keratinocyte proliferation, measured by BrdU incorporation in the epidermis, was dramatically increased at 4 weeks after fracture/casting and also increased, to a lessor extent, after 4 weeks of cast immobilization without fracture (Fig. 3). We also observed that after 4 weeks hindlimb cast immobilization in intact rats the levels of TNF-α, IL-1β, IL-6, and NGF are up-regulated in the hindpaw skin, similar to the effects of fracture with casting (Fig. 4). Furthermore, treatment with the NK1 receptor antagonist LY303870 reversed the cast-induced increase of TNF-α, IL-1β, IL-6, and NGF levels in the hindpaw skin (Fig. 4). Collectively, these data suggest that cast immobilization alone can induce exaggerated SP signaling that stimulates keratinocyte proliferation and inflammatory mediator expression in the fracture limb, resulting in nociceptive sensitization. Interestingly, it has been previously reported in mice that after 3, 7 and 14 days hindlimb immobilization there is up-regulate gene-expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the immobilized anterior tibialis muscle.7
Treating fracture/cast rats with the TNF inhibitor etanercept, the IL-1 receptor antagonist anakinra, or the anti-NGF antibody tanezumab reduced hindpaw allodynia and unweighting at 4 weeks post-fracture.27, 34, 35 Pro-inflammatory cytokines and NGF can immediately evoke spontaneous firing and sensitization in primary sensory afferents10, 22, 32 and we have observed that intraplantar injection of these inflammatory mediators into normal hindpaw skin rapidly induces nociceptive sensitization.26 Collectively, these data indicate that up-regulated inflammatory cytokines and NGF in the skin contribute to nociceptive sensitization in the fracture model of CRPS and potentially to painful sensitization in CRPS patients.
We also attempted to measure inflammatory mediators in the lumbar spinal cord at 4 weeks post-fracture/casting or 4 weeks cast immobilization (Fig. 5). We were unable to detect TNF-α protein levels and there were no changes in IL-6 protein levels in the spinal cord of the fracture/casted or cast only rats. Four weeks after fracture and casting both IL-1β and NGF levels were increased in the spinal cord. Interestingly, after 4 weeks of cast immobilization with no fracture only NGF levels were elevated. There is an extensive body of literature linking elevated spinal IL-1β levels to pain behaviors in various type of inflammatory and neuropathic models,43 and IL-1β can also directly induce pain following spinal administration.41 The post-fracture increase in spinal IL-1β levels could potentially contribute to the differences in duration of nociceptive sensitization in fracture/casted and cast only rats.14
Previously, we demonstrated that at 4 weeks after fracture with casting there were increased numbers of c-Fos immunoreactive neurons in the ipsilateral laminae I-II, III-IV, and V-VI of the dorsal horn, and this increase in c-Fos labeling inversely correlated with von Frey thresholds in the hindpaw, suggesting that c-Fos immediate-early gene activation in the spinal cord neurons is a marker for nociceptive sensitization after fracture and casting.34 In the present study we observed that 4 weeks after fracture with cast immobilization or after 4 weeks of cast immobilization alone there was a significant increase in dorsal horn c-Fos positive neurons (Fig. 6).
In another series of experiments the effects of early mobilization after fracture were investigated in tibia fracture rats that underwent intramedullary pinning to stabilize the fracture site, thus requiring no cast immobilization. The fracture with pinning rats did develop hindpaw allodynia and unweighting at 1 week post-fracture, but this resolved by 4 week post-fracture (Fig. 1). Unlike the tibia fracture rats that were cast immobilized for 4 weeks, the fracture/pin rats did not exhibit hindpaw warmth or edema at either 1 or 4 weeks after fracture (Fig. 1). These results correspond with previously published reports in femur fractured rats stabilized with intramedullary pinning that exhibited hindpaw unweighting that peaked at day 1 post-fracture and resolved by 15 to 18 days post-fracture.9, 12
After 4 weeks cast immobilization the fracture/cast rats exhibited increased sciatic nerve SP and CGRP expression, increased hindpaw skin NK1 levels (Fig. 2), hindpaw skin keratinocyte proliferation (Fig. 3), increased inflammatory mediator expression in the hindpaw skin (Fig. 4) and in the ipsilateral lumbar spinal cord (Fig. 5), and increased in Fos activation in the corresponding lumbar spinal cord (Fig. 6). In contrast, at 4 weeks after fracture with pinning there was no increase in sciatic nerve SP or CGRP expression or hindpaw skin NK1 levels (Fig. 2), there was no hindpaw skin keratinocyte proliferation (Fig. 3), there was no increase in inflammatory mediator expression in the hindpaw skin (Fig. 4) or in the ipsilateral lumbar spinal cord (Fig. 5), and there was no increase in c-Fos activation in the corresponding lumbar spinal cord (Fig. 6). These results indicate that early weight bearing in the fracture hindlimb inhibits the development and maintenance of ipsilateral facilitated neuropeptide signaling, keratinocyte proliferation and inflammatory mediator expression, IL-1β and NGF expression in the lumbar spinal cord, spinal c-Fos activation, hindlimb nociceptive sensitization, warmth, and edema. Unfortunately the clinical treatment of fractures with intramedullary rods does not always allow for early mobilization of the limb as it does in the rat, sometimes patients are restricted to non-weight bearing on the fracture limb for 3-6 weeks. The results of this study suggest that whenever possible fractures should be stabilized to allow early mobilization and weight bearing in the fracture limb, thus avoiding CRPS sequelae.
In conclusion, these data support the hypothesis that prolonged cast immobilization without fracture has effects similar to those observed after fracture with cast immobilization, including exaggerated neuropeptide signaling in the immobilized limb, keratinocyte proliferation and inflammatory mediator expression, increased NGF expression in the lumbar spinal cord, spinal c-Fos activation, hindlimb nociceptive sensitization, warmth, and edema. Unlike fracture with casting, the cast immobilized rats failed to develop increased levels of spinal IL-1β. Early weight bearing after tibia fracture with pinning prevented or reversed these nociceptive and inflammatory changes, suggesting that the early mobilization of an injured limb inhibits the development of CRPS.
Perspective.
Collectively, these data indicate that immobilization alone increased neuropeptide signaling and caused nociceptive and inflammatory changes similar to those observed after tibia fracture and casting, and that early mobilization after fracture with pinning inhibited these changes. Early limb mobilization after fracture may prevent the development of CRPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors do not have financial or other relationships that might lead to conflict of interest. This study was funded by National Institutes of Health grant NS072168, Department of Veterans Affairs Rehabilitation Research and Development Merit grant F7137R.
References
- 1.Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999;80:539–544. doi: 10.1016/S0304-3959(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 2.Bernateck M, Karst M, Gratz KF, Meyer GJ, Fischer MJ, Knapp WH, Koppert W, Brunkhorst T. The first scintigraphic detection of tumor necrosis factor-alpha in patients with complex regional pain syndrome type 1. Anesthesia and analgesia. 2010;110:211–215. doi: 10.1213/ANE.0b013e3181c4bab7. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia KP, Bhatt MH, Marsden CD. The causalgia-dystonia syndrome. Brain. 1993;116:843–851. doi: 10.1093/brain/116.4.843. [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM, Dawson LF, Clark JD, Kingery WS. Activation of cutaneous immune responses in complex regional pain syndrome. The journal of pain : official journal of the American Pain Society. 2014;15:485–495. doi: 10.1016/j.jpain.2014.01.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–2184. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 6.Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta Orthop Belg. 1998;64:448–451. [PubMed] [Google Scholar]
- 7.Caron AZ, Drouin G, Desrosiers J, Trensz F, Grenier G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J Appl Physiol. 2009;106:2049–2059. doi: 10.1152/japplphysiol.91505.2008. [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 9.Cottrell JA, Meyenhofer M, Medicherla S, Higgins L, O'Connor JP. Analgesic effects of p38 kinase inhibitor treatment on bone fracture healing. Pain. 2009;142:116–126. doi: 10.1016/j.pain.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 10.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesthesia and analgesia. 2003;96:1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. [DOI] [PubMed] [Google Scholar]
- 11.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Freeman KT, Koewler NJ, Jimenez-Andrade JM, Buus RJ, Herrera MB, Martin CD, Ghilardi JR, Kuskowski MA, Mantyh PW. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108:473–483. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 13.Gitter BD, Bruns RF, Howbert JJ, Waters DC, Threlkeld PG, Cox LM, Nixon JA, Lobb KL, Mason NR, Stengel PW, Cockerham SL, Silbaugh SA, Gehlert DR, Schober DA, Iyengar S, Calligaro DO, Regoli D, Hipskind PA. Pharmacological characterization of LY303870: a novel, potent and selective nonpeptide substance P (neurokinin 1) receptor antagonist. J Pharmacol Exp Therapeut. 1995;275:737–744. [PubMed] [Google Scholar]
- 14.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Molecular pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators of inflammation. 2006;2006:28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipskind PA, Howbert JJ, Bruns RF, Cho SSY, Crowell TA, Foreman MM, Gehlert DR, Iyengar S, Johnson KW, Krushinski JH, Li DL, Lobb KL, Mason NR, Muehl BS, Nixon JA, Phebus LA, Regoli D, Simmons RM, Threlkeld PG, Waters DC, Gitter BD. 3-Aryl-1,2,-diacetamidopropane derivatives as novel and potent NK-1 receptor antagonists. J Med Chem. 1996;39:736–748. doi: 10.1021/jm950616c. [DOI] [PubMed] [Google Scholar]
- 19.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators of inflammation. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004;91:147–154. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar S, Hipskind PA, Gehlert DR, Schober D, Lobb KL, Nixon JA, Helton DR, Kallman MJ, Boucher S, Couture R, Li DL, Simmons RMA. LY303870, a centrallyactive neurokinin-1 antagonist with a long duration of action. J Pharmacol Exp Ther. 1997;280:774–785. [PubMed] [Google Scholar]
- 22.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer HH, Eberle T, Uceyler N, Wagner I, Klonschinsky T, Muller LP, Sommer C, Birklein F. TNF-alpha in CRPS and ‘normal’ trauma--significant differences between tissue and serum. Pain. 2011;152:285–290. doi: 10.1016/j.pain.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Experimental neurology. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain. 2013;154:1224–1236. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–852. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–313. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molander C, Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience. 1986;19:297–312. doi: 10.1016/0306-4522(86)90023-0. [DOI] [PubMed] [Google Scholar]
- 29.Oerlemans HM, Oostendorp RA, de Boo T, Goris RJ. Pain and reduced mobility in complex regional pain syndrome I: outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain. 1999;83:77–83. doi: 10.1016/s0304-3959(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 30.Oerlemans HM, Oostendorp RA, de Boo T, van der Laan L, Severens JL, Goris JA. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Archives of physical medicine and rehabilitation. 2000;81:49–56. [PubMed] [Google Scholar]
- 31.Oyen WJ, Arntz IE, Claessens RM, Van der Meer JW, Corstens FH, Goris RJ. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response? Pain. 1993;55:151–157. doi: 10.1016/0304-3959(93)90144-E. [DOI] [PubMed] [Google Scholar]
- 32.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 33.Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- 34.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–519. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 37.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. The Clinical journal of pain. 2006;22:235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- 39.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept. 2013 doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurdsen UE, Reikeras O, Utvag SE. External fixation compared to intramedullary nailing of tibial fractures in the rat. Acta Orthop. 2009;80:375–379. doi: 10.3109/17453670903035567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tadano T, Namioka M, Nakagawasai O, Tan-No K, Matsushima K, Endo Y, Kisara K. Induction of nociceptive responses by intrathecal injection of interleukin-1 in mice. Life sciences. 1999;65:255–261. doi: 10.1016/s0024-3205(99)00244-1. [DOI] [PubMed] [Google Scholar]
- 42.Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology. 2008;109:297–307. doi: 10.1097/ALN.0b013e31817f4c9d. [DOI] [PubMed] [Google Scholar]
- 43.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in neurosciences. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 44.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 45.Wei T, Guo TZ, Li WW, Hou S, Kingery W, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. Journal of neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–286. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain. 2009;13:253–262. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS. Acute effects of substance P and calcitonin gene-related peptide in human skin-a microdialysis study. The Journal of investigative dermatology. 2000;115:1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]