Abstract
We retrospectively evaluated the prognostic significance of polypharmacy and inappropriate medication use among 150 patients >60 years of age receiving induction chemotherapy for acute myelogenous leukemia (AML). After adjustment for age and comorbidity, increased number of medications at diagnosis (≥4 vs. ≤1) was associated with increased 30-day mortality (OR=9.98, 95% CI=1.18–84.13), lower odds of complete remission status (OR=0.20, 95% CI=0.06–0.65), and higher overall mortality (HR=2.13, 95% CI=1.15–3.92). Inappropriate medication use (classified according to Beers criteria) was not significantly associated with clinical outcomes. Polypharmacy warrants further study as a modifiable marker of vulnerability among older adults with AML.
Keywords: leukemia, polypharmacy, medications, elderly, older, mortality
1. Introduction
Acute myeloid leukemia (AML) is predominantly a disease of older adults[1]. While some older adults benefit from curative therapies, overall survival (OS) is shorter and treatment-related toxicity remains higher for older adults compared with their younger counterparts [2–5]. There are many reasons for poor outcomes in older patients. Therapy is less effective because of the biology of the disease [6–11], while patient-specific factors such as comorbidity and poor functional status decrease treatment tolerance [2, 4, 5, 12–17]. There is a growing effort to identify modifiable patient-specific factors that may influence treatment tolerance among older adults receiving AML therapy [15, 18, 19].
One challenge faced when treating older patients is the use of multiple concomitant medications (polypharmacy). Polypharmacy is a problem of growing interest given the increase in drug consumption in recent years, particularly among people over the age of 65. Studies indicate that many older adults with cancer are taking more than five medications [20–24]. Longer life expectancy, co-morbidity and the implementation of evidence-based clinical practice guidelines in the setting of multi-morbidity all contribute to the presence of polypharmacy [25]. However, polypharmacy also has important negative consequences, such as a higher risk of adverse drug reactions. Among elders without cancer each new drug increases risk of adverse drug events by 10% [26]. Polypharmacy is associated with additional adverse outcomes such as risk of hospitalization and mortality among older adults with and without cancer [27–32]. Use of multiple medications, including potentially unnecessary or inappropriate medications1 (PIM) [33], at the time of chemotherapy initiation may place patients at a higher risk of toxicity due to adverse drug events or interactions and could represent a modifiable risk factor for toxicity among older adults receiving treatment for AML [34–38].
No study to date has evaluated the implications of polypharmacy in the setting of AML therapy. The risk of adverse drug events and their consequences may be particularly high in the setting of induction chemotherapy for AML which requires initiation of multiple treatment and supportive care medications. The objectives of this study were to evaluate the prevalence and prognostic importance of polypharmacy in older patients receiving treatment for AML. Investigating polypharmacy in this population may help improve patient assessment and provide an opportunity to design simple interventions to minimize unnecessary morbidity associated with treatment.
2. Methods
2.1 Study design and population
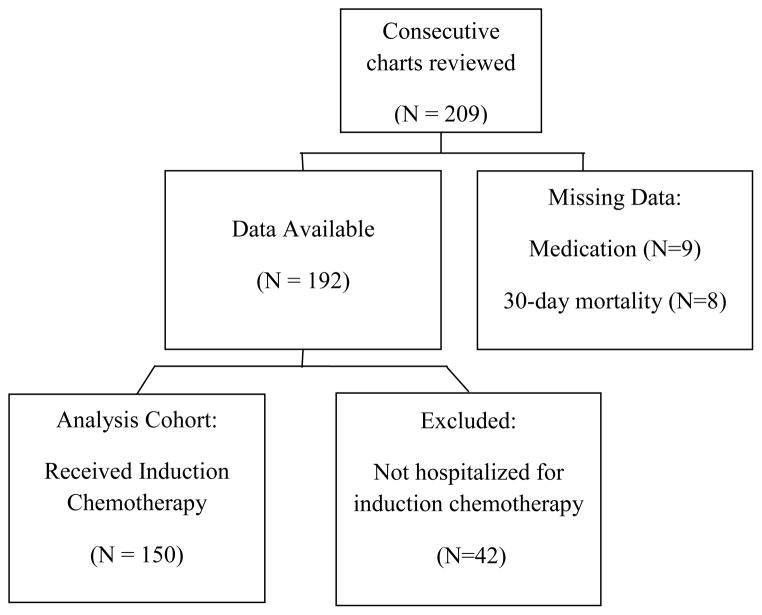
A retrospective chart review was conducted using the tumor registry and medical records at Wake Forest University Baptist Medical Center to identify older adults hospitalized with newly diagnosed AML who received induction chemotherapy between 2004 and 2009. Eligibility criteria included: 1) age >60 years at the time of diagnosis, 2) confirmed diagnosis of AML, and 3) received induction chemotherapy. AML diagnoses were confirmed using pathology reports. Patients for whom 30-day mortality or medication information was unavailable were excluded from the study (N=23). For consistency with existing literature age 60 was used to define the older adult cohort for this study [2].
2.2 Measures
Prescription medications (scheduled and as needed) were assessed at the time of hospital admission and discharge for AML induction chemotherapy. Medication data was obtained from the electronic medical record. Prescribed eye-drops, mouthwash, and topical creams were excluded as were non-prescription medications including vitamins and supplements. Three classes of prescription drugs were categorized for exploratory analyses: 1) medications used to treat cardiac conditions; 2) medications used to treat diabetes; and 3) medications having a clinically relevant association with CYP3A4 defined by the Drug Information Handbook and pharmacist review. Baseline medication data reflects pre-induction therapy medications. For prevalence estimates, polypharmacy was defined as ≥5 medications, a commonly used cutoff, to compare the AML population to existing literature in geriatric populations [22, 27, 39, 40]. To evaluate the prognostic significance of number of medications at diagnosis in the setting of AML therapy, number of prescription medications at baseline was evaluated as a categorical variable utilizing optimal cutpoints to predict outcomes in this setting (described in section 2.4). We chose this approach because optimal cutpoints differ depending upon clinical context [29, 32]. The use of inappropriate medications, defined according to the Beers list (2012 Update)[41], was collected at baseline and discharge and evaluated as a categorical variable. The Beers list, developed and updated by expert consensus, represents the most widely cited criteria to assess inappropriate drug prescribing for elders. The list includes medications considered inappropriate due to either ineffectiveness or high risk for adverse events in older patients and is a proxy for inappropriate prescribing of other medications [42].
The primary outcome for this analysis was 30-day mortality calculated from the date of initiation of induction chemotherapy. Secondary outcomes included OS from the date of initial treatment, achievement of complete remission (CR) post induction therapy, transfer to an intensive care unit during initial induction hospitalization, and length of hospitalization for induction chemotherapy. Length of induction hospitalization was calculated from the date of admission and considered as a categorical variable. To account for early mortality, length of hospitalization was evaluated in the subset of patients who survived 60 days. Complete remission was defined as less than 5% blasts on bone marrow biopsy and normalization of peripheral blood counts post induction chemotherapy.
2.3 Covariates
Demographic data (age, sex, race, and marital status) and health behaviors were collected from the electronic medical record. Age at cancer diagnosis was used as a continuous variable.
Comorbidity was evaluated using independent conditions and standardized comorbidity indices from data available in the medical record at the time of hospital admission. Specifically, we collected history of congestive heart failure, coronary artery disease, cerebrovascular disease (stroke or TIA), diabetes mellitus, chronic obstructive pulmonary disease, hypertension, renal dysfunction, depression, osteoporosis, rheumatologic disease, cognitive impairment, venous thrombosis, cardiac valve disease, myelodysplastic syndrome (MDS), or previous cancer. Previous cancer was defined as any cancer other than non-melanoma skin cancer. Comorbidity burden was categorized using the modified Charlson comorbidity index [12, 43] and the Hematopoietic Cell Transplantation comorbidity index (HCT-CI) [44].
Cytogenetic risk grouping was collected from the pathology report and categorized according to SWOG criteria [2]. Baseline laboratory values (white blood cell count [WBC]) and hematocrit were abstracted from the medical record for the date of induction admission.
2.4 Statistical Analysis
Descriptive statistics (frequencies, medians and ranges) were used to describe the characteristics of the study population. Both the Wilcoxon test and Cox proportional hazards regression was used to model the impact of number of medication category and receipt of Beers medications on overall survival. To examine the form of the total medications variable, the cumulative martingale residuals were plotted against the values of the total medications, and we examined the observed residual plot along with simulated realizations from the null distribution. Utilizing these residual plots, optimal cutpoints appeared to be at one and four medications; we also used these residuals to assess the proportional hazards assumption [45, 46]. Exploratory analysis evaluating number of medications as a continuous variable was also performed. The relationship between number of medications and outcomes was non-linear supporting the use of a categorical form of the variable. A Fisher’s exact test was used to compare the rates of 30-day mortality by prescription medication category and between remission and medication class. The Kaplan-Meier method was used to estimate OS and survival stratified by number of prescription medications and use of Beers medications. McNemar’s test was used to compare the prevalence of polypharmacy from baseline to follow-up. Pearson’s correlation was used to quantify the association between the number of medications used at baseline with the two comorbidity indices and age. ANOVAs were used to compare the number of prescription medications by gender, race, cytogenetic risk group, and chemotherapy type. A t-test was used to compare the mean number of medications by 30-day mortality. Univariable logistic regression models were used to evaluate the association between medication variables and 30-day mortality, complete remission attainment, transfer to ICU, and length of stay greater than 35 days; models for number of medications were also adjusted for age and Charlson score. To account for the effect of additional clinical characteristics known to influence outcomes for older adults with AML, additional multiple logistic regression and Cox proportional hazards models were conducted that included gender, antecedent MDS, white cell count, hematocrit, and cytogenetic risk group on the subset of patients with risk group data available (N=119).
Exploratory analyses were also conducted to investigate the associated between medication classes and clinical outcomes (30-day mortality, complete remission, overall survival). Categorical variables indicating use of one or more medications in each class were added to the multiple logistic regression and Cox proportional hazards models for each outcome adjusting for age, comorbidity (CCI) and total number of baseline medications. A two-sided alpha level of 0.05 was used to indicate statistical significance. All analysis was performed in SAS (version 9.3, Cary, NC).
3. Results
Among 209 older adults with newly diagnosed AML, 150 met criteria for inclusion into the study (Figure 1). The median patient age was 69 years (range 61–87 years). Sixty-one percent were male and 92% were white (Table 1). The median number of comorbid conditions was three (range from 0–10) with low prevalence of coronary artery disease (17%), diabetes (19%), chronic pulmonary disease (9%) and chronic renal disease (4%). Less than half met criteria of significant comorbidity burden using the Charlson comorbidity index ≥1 (45%) or HCT-CI>1 (45%). Approximately 13% had prior MDS and only 14% had favorable risk cytogenetics. A majority (63%) were treated with standard anthracycline + cytarabine ± etoposide induction chemotherapy.
Figure 1.
Table 1.
Baseline characteristics of older adults hospitalized for induction chemotherapy for newly diagnosed AML (N=150)
| Characteristic | Median (range) or % |
|---|---|
| Demographics | |
| Age (years) | 69 (61–87) |
| Female | 39 |
| White | 92 |
| Clinical Characteristics | |
| Number of Comorbidities | 3 (0–10) |
| Coronary Artery Disease | 17 |
| Diabetes | 19 |
| HCT-CI Score (>1) | 45 |
| Charlson Comorbidity Index Score (≥1) | 45 |
| Hematocrit | 28 (17–90) |
| White cell count (x103/mm3) | 4 (0.2–268) |
| Prior myelodysplastic syndrome | 13 |
| Cytogenetic risk group (N=120) | |
| Favorable | 14 |
| Intermediate | 61 |
| Poor | 25 |
| Prescription medications at admission | 4 (0–15) |
| Inappropriate meds (any Beers medication) at admission | 19 |
| Treatment received | |
| Anthracycline+cytarabine+etoposide | 26 |
| Anthracycline+cytarabine | 37 |
| Anthracycline+cytarabine+ATRA | 8 |
| Anthracycline+cytarabine+investigational drug | 7 |
| Anthracycline+cytarabine+bortezomib | 6 |
| Cytarabine+investigational drug | 4 |
| Hypomethylating agents | 3 |
| Other | 13 |
HCT-CI, Hematopoietic cell transplantation comorbidity index; ATRA, all-trans retinoic acid
At the time of admission for induction chemotherapy, 38% of AML patients were prescribed ≥ 5 medications. The median number of prescribed medications was four (range 0 –15). The prevalence of polypharmacy increased significantly to 68% (p<0.0001) among patients who survived induction and were discharged from the hospital and for whom we could abstract medication use (N=98). Also, 19% of patients were prescribed at least one “inappropriate medication” according to the Beers Criteria at the time of admission, which increased to 36% at the time of discharge. Anticholinergic medications and benzodiazepines were the most commonly prescribed category of Beers medications at diagnosis and discharge.
Forty-seven percent of the patients in this study achieved a CR. The median length of hospital stay was 35 days (range 4–132), and 20% of patients were admitted to the ICU during induction. Twenty-nine (19%) patients died within 30 days of induction chemotherapy, and 53 (35%) died within 60 days. Median overall survival was 6.4 months.
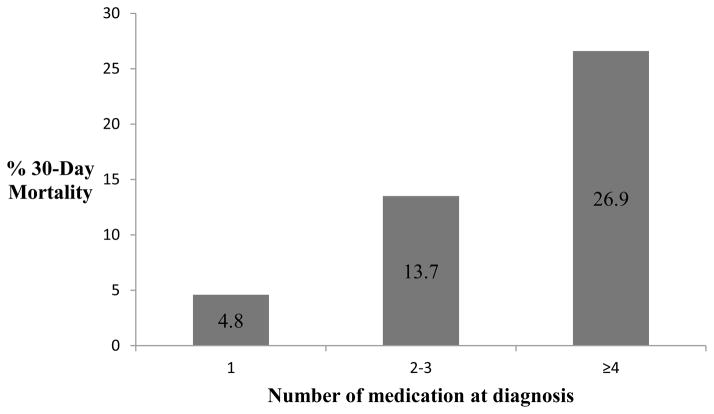
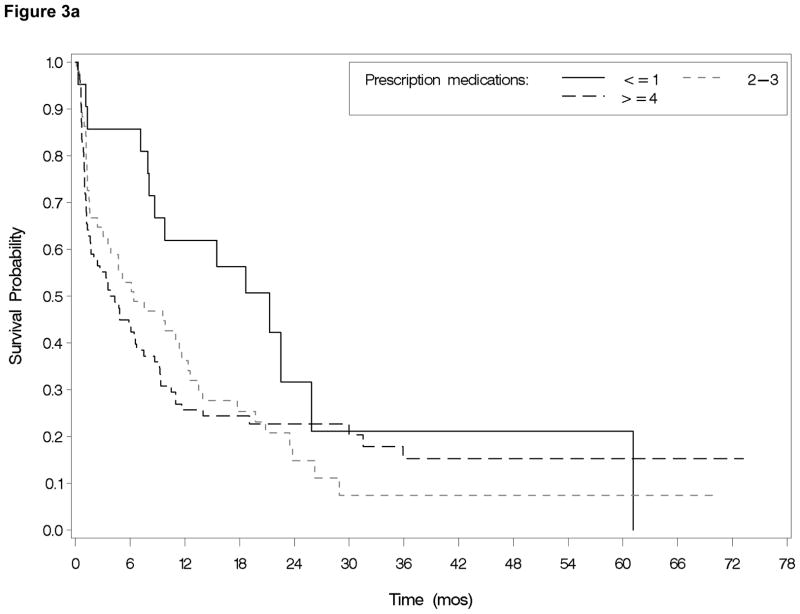
The number of medications at baseline was strongly correlated with comorbidity using either comorbidity index (Charlson, r=0.53, p<0.0001 or HCT-CI, r=0.35, p<0.0001). Number of medications was not associated with gender, race, age, tumor biology as measured by cytogenetic risk grouping, or treatment type. With respect to treatment outcomes, the total number of prescription medications at baseline was associated with increased 30-day mortality (p=0.02) (Figure 2) and achievement of CR in univariate analyses (Table 2). Patients who died within 30 days of chemotherapy initiation were on an average of 5.3 baseline medications versus 3.9 for those who survived for 30 days or longer (p=0.01). After adjusting for comorbidity and age in the logistic regression model, being on four or more baseline medications (vs. ≤1) was associated with a ten times greater odds of 30-day mortality (OR=9.98, 95% CI=1.18–84.13). Odds of achieving a CR were significantly lower for patients on ≥4 medications (vs. ≤1) in adjusted analyses (OR=0.20, 95% CI=0.07–0.63). Total number of medications at baseline was not associated with a hospital stay greater than 35 days or an ICU stay. Overall survival (Figure 3a) was significantly associated with total number of medications in unadjusted analyses (p= 0.01) and after adjusting for age and comorbidity (p=0.04), with those taking ≥4 medications at baseline and those taking 2–3 medications having risk of death two times greater than those taking ≤1 (HR=2.13; 95% CI=1.15–3.92 and HR 2.07; 95% CI=1.12 and 3.84 respectively). Results were similar in models which additionally controlled for gender, MDS, white cell count, hematocrit and cytogenetic risk group (30-day mortality [OR 5.6 (95% CI= 0.3–90.5) for 2–3 meds, OR 14.9 (95% CI =1.0–229 for ≥4 medications compared to ≤1 medication]; complete remission [OR 0.1 (95% CI=0.02–0.8) for 2–3 medications, OR 0.1 (95% CI=0.02–0.6) for ≥4 medications compared to ≤1 medication], and overall survival [HR 2.9 (95% CI = 1.3–6.6) for 2–3 medications, HR 3.1 (95% CI 1.4–7.1) for ≥4 medications compared to ≤1 medication].
Figure 2.
Table 2.
Relationship between baseline medication variables and clinical outcomes
| Outcomes
| ||||
|---|---|---|---|---|
| Number of medications OR (95% CI) | Beers medication | |||
|
| ||||
| <=1 (N=21) | 2–3 (N=51) | >=4 (N=78) | ||
| 30-day mortality | 1.00 | 3.18 (0.37–27.6) | 7.37 (0.93–58.38) | 0.89 (0.31–2.58) |
| 1.00 | 3.63 (0.40–32.71)a | 9.98 (1.18–84.13)a | ||
| Complete remission | 1.00 | 0.33 (0.11–1.06) | 0.21 (0.07–0.63) | 0.96 (0.42–2.19) |
| 1.00 | 0.32 (0.10–1.03)a | 0.20 (0.06–0.65)a | ||
| ICU stay | 1.00 | 6.15 (0.75–50.76) | 5.57 (0.70–44.57) | 0.42 (0.12–1.51) |
| 1.00 | 6.40 (0.77–52.97)a | 6.57 (0.80–53.72)a | ||
| LOS >35 days among those surviving 60 days (N=97) | 1.00 | 0.57 (0.18–1.81) | 1.15 (0.37–3.56) | 0.87 (0.32–2.34) |
| 1.00 | 0.53 (0.16–1.71)a | 0.94 (0.29–3.08)a | ||
CI, confidence interval; ICU, Intensive care unit; LOS, length of stay; OR, odds ratio
Adjusted for age and Charlson comorbidity score
Figure 3.
In exploratory analyses, evaluating number of medications as a continuous variable showed that each additional medication at baseline was associated with a 28% increased odds of 30-day mortality (OR=1.28; 95% CI=1.07–1.53) after adjustment. There was no statistically significant relationship between the three medication classes considered and any outcome (data not shown) with the exception of CYP3A4 medications and complete remission. Patients on no CYP3A4 medications at baseline were more likely to achieve remission than those taking at least one of these medications (63% versus 37%, p=0.002). When CYP3A4 medication use was included in a multiple logistic regression model it attenuated the effect of total number of medications on complete remission (p=0.19). Use of CYP3A4 medication was associated with lower odds of remission (OR 0.5, 95% CI=0.2–0.9) controlling for age, CCI, total number of medications (categorized <4 versus ≥4) and use of cardiac and diabetic meds. These results should be considered hypothesis generating only given the small sample size and exploratory nature of the anlaysis.
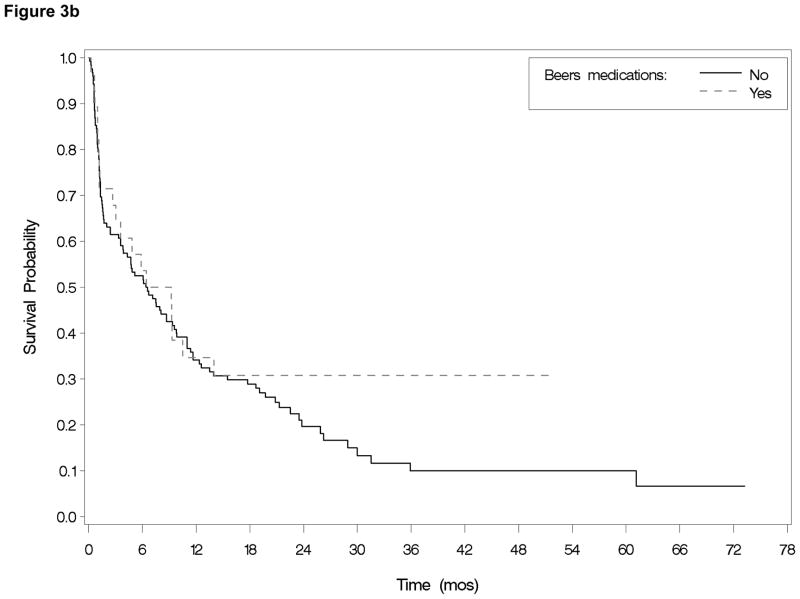
Use of Beers medication at baseline was not associated with any measured clinical outcomes in unadjusted or adjusted analyses (Table 2; Figure 3b). Among those who survived induction, neither the number of prescription medications nor the use of Beers medications at the time of discharge from induction hospitalization were associated with overall survival (HR=1.01, 95% CI=0.93–1.10 and HR=0.79, 95% CI=0.48–1.32 respectively).
4. Discussion
The aim of this study was to examine the prevalence and prognostic significance of polypharmacy and PIM use among older adults receiving induction chemotherapy for AML. We found a high prevalence of both polypharmacy and PIM use at baseline, which was further increased at the time of discharge from initial induction hospitalization. The key finding of this analysis is a positive association between a higher number of prescription medications at baseline and increased treatment-related mortality (30-day mortality). Patients taking more medications at baseline were also less likely to achieve remission and had shorter survival. Use of Beers medications was not associated with negative clinical outcomes in this population.
The prevalence of polypharmacy in our cohort is comparable to reports of other elderly cancer populations (average daily use of four to nine medications) [21–23]. Among ambulatory older cancer patients, reported rates of polypharmacy (defined as ≥ 5 prescription medications) ranges from 50% to 80% [22, 23, 30, 39, 40]. In an older inpatient cancer population, approximately one-third of patients were prescribed ≥ 9 medications prior to admission [21]. In a study of 108 patients with hematologic cancers 65% were taking ≥5 medications [40]. Prevalence estimates of PIM use, assessed by the Beers criteria, range from 21% to 41% among older cancer patients [21, 22].
Our study suggests that use of ≥4 prescription medication at the time of AML treatment is associated with an increased risk of death within 30 days of chemotherapy initiation with lower odds of achieving remission and shorter survival. This is consistent with observations in non-cancer elders that each additional medication incurs increased risk of adverse drug events, hospitalizations and mortality [27, 31, 32]. Several studies have investigated the prognostic significance of polypharmacy specifically among older cancer patients. Results in general have been mixed. Most common are studies that have utilized polypharmacy as a risk factor in the context of a geriatric assessment to better risk-stratify older cancer patients with respect to treatment toxicity and survival. In three such studies, no association was found between polypharmacy prior to chemotherapy treatment and toxicity or survival [40, 47, 48]. In each case the study cohorts included multiple different cancer types and varied treatments. In a study of older adults with advanced ovarian cancer treated with the same chemotherapy regimen, presence of ≥6 medications at the time of initiation of treatment was associated with decreased overall survival [29]. Similarly, among older women receiving chemotherapy for metastatic breast cancer, polypharmacy (≥5 medications) was independently associated with treatment toxicity [30]. Among older cancer patients requiring abdominal surgery, polypharmacy was associated with increased length of hospitalization post-surgery [28]. Taken together, current data suggests the implications of polypharmacy may be specific to the clinical scenario and most evident in patients at high risk for treatment-related toxicity.
Mechanistically, polypharmacy may directly increase the risk of toxicity related to treatment or is a surrogate marker for an unmeasured vulnerability. One explanation for an association between polypharmacy and adverse outcomes is an increased risk of adverse drug reactions and drug-drug interactions. Physiologic changes of aging can impact pharmacokinetics and pharmacodynamics of drugs significantly. In a study of older ambulatory cancer patients using software to identify potential drug problems, the majority (62%) had a potential drug problem of any severity identified, with approximately 50% of these rated as moderate to severe [23]. Increasing age and increased number of medications were independently associated with risk of moderate to severe potential drug problems. This has significant implications in the setting of AML therapy. Patients treated for AML are exposed to multiple new drugs including chemotherapy drugs, antibiotics, and supportive care medications over a short period of time. The complexity and intensity of treatment in combination with the acuity of illness can make it difficult to identify and manage consequences of potential drug interactions. Similarly, adverse events are likely increased when adding multiple new drugs to a standing regimen in the acutely ill setting. Exploratory analyses suggesting a negative relationship between use of medications associated with CYP3A4 and remission status could support this mechanism and warrants further study.
Alternatively, polypharmacy in our analysis may be a surrogate for unmeasured vulnerability in this patient population. Factors associated with increased number of prescription medications among older cancer patients include comorbidity, functional status, and PIM use [22]. Although we controlled for comorbidity burden, this may not adequately capture differences in severity of specific comorbid conditions. For example, an older adult with well-compensated congestive heart failure may be on fewer medications than an adult with poorly compensated disease. Alternatively, polypharmacy may be a surrogate measure of functional status in our patient population. Unfortunately, we were unable to account for performance status in this analysis as it was not routinely recorded in hospital charts. Further studies will need to account for the relationship between polypharmacy and performance status as it relates to early mortality. If, however, polypharmacy is found to be independently associated with treatment mortality in the setting of AML therapy, this could provide important insight into estimates of treatment tolerance and an opportunity for intervention to minimize associated risks.
In contrast to polypharmacy, we found no association between the use of potentially inappropriate medications, defined according to the Beers list, and clinical outcomes in this patient cohort. Although several studies of older cancer patients have documented high prevalence of the use of these medications, none have clearly documented clinical consequences. The most common types of Beers medications used in our cohort were anticholinergics and benzodiazepines, many of which were used as supportive care medications even at the time of diagnosis. As such, the potential benefits of these medications in the context of supportive care for AML may outweigh the risks. Alternatively, in this patient population with high competing morbidity and mortality, specific “inappropriate medications” may not independently influence outcomes.
This study has several limitations. This is a retrospective single institution cohort, which limits the generalizability of our findings. The sample size is relatively small, and may be underpowered to detect associations between predictors and secondary outcomes and to fully elucidate contributions of specific drug classes. Drug dosing is also not available. An important limitation is the lack of data on functional status. Such data will be needed to further explore the relationship between polypharmacy and early mortality. However, all patients in our cohort were considered fit enough by their treating oncologist to receive induction therapy for AML, with the majority receiving intensive treatments.
In conclusion, the number of medications at the time of initiation of induction chemotherapy was associated with early mortality independent of comorbidity burden in this analysis. Larger prospective studies are needed to validate these findings. If validated, this could potentially provide an additional marker of vulnerability for treatment toxicity which is readily available in routine clinical care. Importantly, this may also provide an additional opportunity to intervene upon vulnerability in the older adult population. Specifically, medication review with discontinuation of unnecessary medications and attention to potential drug-drug interactions might improve treatment tolerance for older adults receiving intensive therapy.
Highlights.
Polypharmacy is common among older adults receiving therapy for acute myeloid leukemia.
Polypharmacy is associated with higher 30-day mortality and shorter overall survival.
Inappropriate medication use (according to Beers criteria) is not associated with clinical outcomes.
Acknowledgments
This research was supported by the Doug Coley Foundation. Dr. Klepin was also supported by the American Society of Hematology, Atlantic Philanthropies, John A. Hartford Foundation, Association of Specialty Professors. Current support includes a Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by NIA, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies) and The Gabrielle’s Angel Foundation for Cancer Research. Statistical analyses for this study were provided by the Biostatistics Shared Resource of the Comprehensive Cancer Center of Wake Forest University (CCSG Grant P30CA012197). Editorial assistance was provided by Megan J. Whelen, MPH (Comprehensive Cancer Center of Wake Forest University).
Footnotes
PIM = potentially unnecessary or inappropriate medications
The authors have no conflicts of interest.
Contributions
Conception and study design [JA,RG,BP,HK]; Acquisition of data [RG, KE, HK, JA]; Analysis and interpretation of data [KE, HK, RG, JA, BP,TP, ER, LK, KC, HK]; Drafting and critically revising the manuscript [KE, HK, RG, JA, BP,TP,ER, LK, KC, HK]. All authors have approved the final version of the manuscript submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen Elliot, Email: kelliot@wakehealth.edu.
Janet A. Tooze, Email: jtooze@wakehealth.edu.
Rachel Geller, Email: rgeller@wakehealth.edu.
Bayard L. Powell, Email: bpowell@wakehealth.edu.
Timothy S. Pardee, Email: tspardee@wakehealth.edu.
Ellen Ritchie, Email: ritchie@med.cornell.edu.
LeAnne Kennedy, Email: lakenned@wakehealth.edu.
Kathryn E. Callahan, Email: kecallah@wakehealth.edu.
Heidi D. Klepin, Email: hklepin@wakehealth.edu.
Reference List
- 1.SEER Cancer Statistics Review. 1975–2009 http://seercancergov/publications/2012.
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–87. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, O’brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Ravandi F, O’brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–9. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farag SS, Archer KJ, Mrozek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 8.Rao AV, Valk PJ, Metzeler KH, Acharya CR, Tuchman SA, Stevenson MM, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:5580–6. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- 9.Krug U, Rollig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376:2000–8. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 10.Rollig C, Thiede C, Gramatzki M, Aulitzky W, Bodenstein H, Bornhauser M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–8. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 11.Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 12.Etienne A, Esterni B, Charbonnier A, Mozziconacci MJ, Arnoulet C, Coso D, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–83. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 13.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–24. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedding U, Rohrig B, Klippstein A, Fricke HJ, Sayer HG, Hoffken K. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–71. doi: 10.1007/s00432-006-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013 doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djunic I, Virijevic M, Novkovic A, Djurasinovic V, Colovic N, Vidovic A, et al. Pretreatment risk factors and importance of comorbidity for overall survival, complete remission, and early death in patients with acute myeloid leukemia. Hematology (Amsterdam, Netherlands) 2012;17:53–8. doi: 10.1179/102453312X13221316477651. [DOI] [PubMed] [Google Scholar]
- 17.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–7. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 18.Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98:208–16. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman AE, Motyckova G, Fega KR, Deangelo DJ, Abel GA, Steensma D, et al. Geriatric assessment in older patients with acute myeloid leukemia: a retrospective study of associated treatment and outcomes. Leukemia research. 2013;37:998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balducci L, Goetz-Parten D, Steinman MA. Polypharmacy and the management of the older cancer patient. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2013;24(Suppl 7):vii36–40. doi: 10.1093/annonc/mdt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flood KL, Carroll MB, Le CV, Brown CJ. Polypharmacy in hospitalized older adult cancer patients: experience from a prospective, observational study of an oncology-acute care for elders unit. Am J Geriatr Pharmacother. 2009;7:151–8. doi: 10.1016/j.amjopharm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Prithviraj GK, Koroukian S, Margevicius S, Berger NA, Bagai R, Owusu C. Patient Characteristics Associated with Polypharmacy and Inappropriate Prescribing of Medications among Older Adults with Cancer. J Geriatr Oncol. 2012;3:228–37. doi: 10.1016/j.jgo.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puts MT, Costa-Lima B, Monette J, Girre V, Wolfson C, Batist G, et al. Medication problems in older, newly diagnosed cancer patients in Canada: How common are they? A prospective pilot study. Drugs Aging. 2009;26:519–36. doi: 10.2165/00002512-200926060-00008. [DOI] [PubMed] [Google Scholar]
- 24.Turner JP, Shakib S, Singhal N, Hogan-Doran J, Prowse R, Johns S, et al. Prevalence and factors associated with polypharmacy in older people with cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014 doi: 10.1007/s00520-014-2171-x. [DOI] [PubMed] [Google Scholar]
- 25.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 26.Steinman MA, Lund BC, Miao Y, Boscardin WJ, Kaboli PJ. Geriatric conditions, medication use, and risk of adverse drug events in a predominantly male, older veteran population. J Am Geriatr Soc. 2011;59:615–21. doi: 10.1111/j.1532-5415.2011.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60:34–41. doi: 10.1111/j.1532-5415.2011.03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badgwell B, Stanley J, Chang GJ, Katz MH, Lin HY, Ning J, et al. Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. Journal of surgical oncology. 2013;108:182–6. doi: 10.1002/jso.23369. [DOI] [PubMed] [Google Scholar]
- 29.Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16:1795–800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 30.Hamaker ME, Seynaeve C, Wymenga AN, van Tinteren H, Nortier JW, Maartense E, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch Breast Cancer Trialists’ Group. Breast. 2014;23:81–7. doi: 10.1016/j.breast.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Jyrkka J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26:1039–48. doi: 10.2165/11319530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Sganga F, Landi F, Ruggiero C, Corsonello A, Vetrano DL, Lattanzio F, et al. Polypharmacy and health outcomes among older adults discharged from hospital: Results from the CRIME study. Geriatrics & gerontology international. 2014 doi: 10.1111/ggi.12241. [DOI] [PubMed] [Google Scholar]
- 33.Dedhiya SD, Hancock E, Craig BA, Doebbeling CC, Thomas J., III Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010;8:562–70. doi: 10.1016/S1543-5946(10)80005-4. [DOI] [PubMed] [Google Scholar]
- 34.Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56:286–90. doi: 10.1007/s00280-004-0998-4. [DOI] [PubMed] [Google Scholar]
- 35.Ko Y, Tan SL, Chan A, Wong YP, Yong WP, Ng RC, et al. Prevalence of the coprescription of clinically important interacting drug combinations involving oral anticancer agents in Singapore: a retrospective database study. Clin Ther. 2012;34:1696–704. doi: 10.1016/j.clinthera.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Puts MT, Monette J, Girre V, Costa-Lima B, Wolfson C, Batist G, et al. Potential medication problems in older newly diagnosed cancer patients in Canada during cancer treatment: a prospective pilot cohort study. Drugs Aging. 2010;27:559–72. doi: 10.2165/11537310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T, Fujita K, Sunakawa Y, Ishida H, Yamashita K, Miwa K, et al. Concomitant polypharmacy is associated with irinotecan-related adverse drug reactions in patients with cancer. International journal of clinical oncology. 2013;18:735–42. doi: 10.1007/s10147-012-0425-5. [DOI] [PubMed] [Google Scholar]
- 38.Sokol KC, Knudsen JF, Li MM. Polypharmacy in older oncology patients and the need for an interdisciplinary approach to side-effect management. J Clin Pharm Ther. 2007;32:169–75. doi: 10.1111/j.1365-2710.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 39.Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–42. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 40.Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Annals of hematology. 2014 doi: 10.1007/s00277-013-2001-0. [DOI] [PubMed] [Google Scholar]
- 41.American Geriatrics Society. Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund BC, Steinman MA, Chrischilles EA, Kaboli PJ. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. The Annals of pharmacotherapy. 2011;45:1363–70. doi: 10.1345/aph.1Q361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 44.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grambsch PM, Therneau TM, Fleming TR. Diagnostic Plots to Reveal Functional Form for Covariates in Multiplicative Intensity Models. Biometrics. 1995;51:1469–82. [PubMed] [Google Scholar]
- 46.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 47.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, Defelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2011;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 48.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–7. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]