Abstract
The stress response in Drosophila melanogaster reveals sex differences in behavior, similar to what has been observed in mammals. However, unlike mammals, the sex determination pathway in Drosophila is well established, making this an ideal system to identify factors involved in the modulation of sex-specific responses to stress. In this study, we show that the Drosophila fat body, which has been shown to be important for energy homeostasis and sex determination, is a dynamic tissue that is altered in response to stress in a sex and time-dependent manner. We manipulated the sex determination pathway in the fat body via targeted expression of transformer and transformer-2 and analyzed these animals for changes in their response to stress. In the majority of cases, manipulation of transformer or transformer-2 was able to change the physiological output in response to starvation and oxidative stress to that of the opposite sex. Our data also uncover the possibility of additional downstream targets for transformer and transformer-2 that are separate from the sex determination pathway and can influence behavioral and physiological responses.
Keywords: transformer, transformer-2, behavior, stress, sex differences
sex differences have been observed in the stress response in Drosophila (38, 39), analogous to what has been described in mammals. Additional studies have suggested that underlying the different behavioral responses are distinct neuronal stress-response circuits for males and females (2). These include changes in heart rate and several parameters of locomotor behavior (centrophobism, escape behavior, freezing, and changes in patterns of movement, which are analogous to stress-response behaviors described in mammals), which were assessed after starvation or oxidative stress, two stressors that have been linked to the development of psychiatric illnesses (34, 46). It is likely that the mammalian stress response circuitry is also sex specific, based on differences in stress responses and susceptibility to stress-related pathologies (15, 45). Drosophila thus provides an ideal model for the elucidation of factors modulating sexual dimorphism in the stress response.
We used the genetic tractability of Drosophila to manipulate the sex determination pathway and assess the effect of changing the sexual milieu of the brain on the response to stress. Sex determination is Drosophila is a cell autonomous process, meaning that each cell “decides” whether it will be male or female based on the ratio of X-linked numerator genes [sisterless-a (sisA), sisterless-b (sisB), sisterless-c (sisC), and runt (run)] to an autosomal denominator gene [deadpan (dpn)] (6, 14, 17, 49, 54; reviewed in 44). In females, where the numerator to denominator ratio is 2:2, the resulting gene products are transcription factors that produce functional sex-lethal (SXL) protein (14, 17, 49, 54). In females SXL produces a functional transformer (TRA) protein, whereas in males there is no functional SXL and therefore no TRA (9, 13). tra encodes an RNA-binding protein that mediates alternative splicing of doublesex (dsx), which encodes a transcription factor that regulates downstream male- and female-specific genes (7, 28, 29, 36). Presence or absence of functional TRA results in either a female- or male-specific isoform of DSX (DSXF or DSXM, respectively). Transformer-2 (TRA2) is a TRA cofactor that is active in both sexes but only required in females to prevent male sex determination (8, 23). An example of how this sex determination pathway can result in sex-specific centrally controlled behavior is that sex-appropriate expression of TRA and TRA2 regulates expression of fruitless (fru), the products of which are expressed in neurons in the male brain that are critical for male courtship behavior (27, 47). Previous work has shown that females mutant for tra2 develop as pseudo-males (8), and males expressing functional TRA develop as pseudo-females (37).
tra and tra2 expression (where traF and tra2 are female isoform of transformer and transformer-2, respectively) were manipulated using the Gal4/UAS system for targeting a transgene (16) to alter the sexual identity of the fat body in an otherwise unaltered individual. Fat body is known to control regulation of body size and energy stores (30, 42, 51), but other studies have suggested that the fat body surrounding the brain is likely to have important modulatory functions in behavior (19). Many genes that are important for sex-specific behaviors are not expressed within the brain (31). Recent work suggests that the fat body surrounding the head houses sex-biased transcripts (such as those involved in the sex determination pathway), the products of which are then secreted to act on the brain (24).
Our results demonstrate that the sex of the fat body mediates behavioral and physiological outcomes in response to stress and may be largely responsible for the observed sexual dimorphism. Furthermore, our results suggest that TRA and TRA2 may function to influence the stress response in pathways distinct from those for sex determination.
MATERIALS AND METHODS
Fly culture.
Flies were maintained in glass pint bottles containing standard agar-cornmeal-yeast food at 25°C on a 12-h light-dark cycle. Male and female progeny were collected immediately after eclosion and maintained separately in groups of <20 under identical conditions until needed for analysis.
Fly strains.
All stocks were obtained from the Bloomington Stock Center (University of Indiana, Bloomington, IN) unless otherwise noted. w1118 is the parental strain for both UAStraF and UAStra2IR (where tra2IR is transformer 2 RNAi) and was used as a control for the genetic background of both these transgenes. w1118; P{Cg-GAL4.A}2 (Cg-Gal4) was generated using the promoter sequences from the collagen type IV (collagen 25c) gene and drives expression in the fat body, hemocytes, and lymph gland. Cg-Gal4 was chosen because it expresses equally strongly in both male and female adult heads at all ages examined without sexual dimorphism when crossed to a membrane tethered green fluorescent protein reporter (data not shown). y1w1;UAS2XEYFP (two times enhanced yellow fluorescent protein, which expresses fluorescent protein under UAS control) and w*;UAScytoβ-galactosidase (UAS β-galalactosidase) were gifts from Enrique Masa (Texas A&M University, Kingsville, TX). w1118;P{UAS-tra F}20J7 (traF) expresses the female isoform of transformer under UAS control (21). w1118,UAStra2IR;+;UAStra2IR (tra2IR) expresses 2 copies (1 on the X and 1 on the 3rd chromosome) of an inverted repeat for transformer 2 under UAS control and was a gift from Brigitte Dauwalder (University of Houston, Houston, TX) (35). Because the tra2IR transgenic line contained a copy on the X chromosome, all crosses were set up using tra2IR females and Cg-Gal4 males to keep the dose consistent.
Stress paradigms.
On either the same day as collection (sexually immature, 1 day) or after 5 days (sexually mature), males and females were placed in vials containing either 2% yeast, 5% sucrose dissolved in 1 ml deionized water (control for stress), water only (starvation stress), or 2% yeast, 5% sucrose, 30 mM methyl viologen dichloride hydrate (paraquat) (Sigma-Aldrich, St. Louis, MO) dissolved in 1 ml deionized water (oxidative stress) on a 2.1-cm glass fiber filter circle (Fisher Scientific, Waltham, MA). Animals were maintained under these conditions for 24 h at 25°C on a 12-h light-dark cycle before imaging, freezing, or behavioral analyses.
Quantification of β-galactosidase.
Male and female Cg-Gal4>UAS β-galactosidase flies were collected immediately after eclosion, stressed as previously described, then quick frozen in liquid nitrogen. Heads were separated from the bodies by vortexing, and crude protein was prepared from the head tissue. Protein extract was incubated with 50 mM potassium phosphate, 1 mM MgCl2, 1 mM chlorophenol red-β-d-galactoparanaside (Sigma-Aldrich), pH 7.5 for 20 min at 37°C, and absorbance at 574 nm was determined. Nine to ten flies were used for each population and condition.
Heart rate.
Flies were anesthetized using FlyNap (Carolina Biological Supply), placed dorsal side up on a piece of sticky tap with the wings extended, and viewed under ×200 as previously described (38). Forty individuals for each population (sexually immature and mature males and females), for each stress (starvation and oxidative stress), and control for stress condition were used for this task.
Locomotion.
The EthoVision XT tracking system (Noldus Information Technology, Leesburg, VA) was used to assess locomotor behaviors. Flies were individually aspirated into a 60-mm Petri dish in which the bottom portion of the dish was painted white to increase color contrast and reduce glare. Arenas were set up separating between a 30-mm diameter center zone and a 10-mm diameter outer zone around the outer edge of the Petri dish to allow for determination of centrophobism. Tracking videos were 20 min long. After an initial 5-min setup period, the following 2 min was designated as exploratory locomotion, followed by an 8-min period during which no tracking occurred. The last 5 min was designated as basal locomotion. Threshold velocities for the degree of mobility (stopping, walking, highly mobile) were determined using velocities from Cg-Gal4>w1118 animals: <3.3 mm/s was the threshold to stop, >3.4 mm/s was the threshold to start walking, and >26 mm/s was the threshold for high mobility. The threshold for stopped was not 0 mm/s because the flies display a small degree of wing vibration and twitching that is detected by the EthoVision software as having a velocity between 0 and 3.3 mm/s even when no movement is apparent to an observer. Thirty-nine to forty-five individuals for each population (sexually immature and mature males and females) were used for each condition (control, starvation stress, and paraquat stress).
Statistical analysis.
All statistical analyses were performed using SPSS for Windows from IBM (Armonk, NY) and represented visually in graphs made using GraphPad Prism (GraphPad Softward, La Jolla, CA). For all analyses, a significance level of P = 0.05 was used. All error bars represent the mean ± SE.
RESULTS
The fat body surrounding the adult brain is a dynamic tissue that is altered in response to stress.
We have previously demonstrated that the stress response in Drosophila is sex specific (2, 38, 39), and it has been suggested that the fat body in the head could modulate sexually dimorphic circuits in the brain (24). Here we assessed whether this apparently amorphous tissue could be directly affected by stress. To quantify changes in the fat body following a stress exposure, Escherichia coli β-galactosidase was targeted to this tissue using the Cg-Gal4 driver, and male and female progeny from this cross were exposed to starvation or oxidative stress (paraquat) for 24 h before quick freezing for analysis of β-galactosidase activity (Fig. 1). Cg-Gal4 has been previously used to target expression to the Drosophila fat body in both larval and adult males and females (4, 26, 48, 50, 52) and was chosen for our studies because of its strong expression in adults. This driver was generated using the promoter sequences from the collagen type IV gene, which is also expressed in hemocytes and lymph gland in addition to fat body; however, these tissues have not been shown to be sexually dimorphic or to modulate centrally controlled behaviors. In this assay, a change in β-galactosidase activity directly correlates to a change in collagen type IV expression, which could be indicative of a change in the structure or volume of the fat body tissue. For this experiment, β-galactosidase activity was analyzed only in heads to specifically assay the fat body surrounding the brain.
Fig. 1.

Fat body surrounding the adult brain could respond to stress in a sex- and time-dependent manner. A–D: fluorescently labeled fat body surrounding the brain for 1-day-old (A and B) and 5-day-old (C and D) female (A and C) and males (B and D). E–H: Cg-Gal4>UAS-β-galactosidase animals were exposed to starvation or paraquat for 24 h either immediately after eclosion (1 day, E and F) or 5 days posteclosion (G and H). A574 was determined in crude protein from head extracts for females (E and G) and males (B and H) after a 20-min incubation in 1 mM chlorophenol red-β-d-galactoparanaside. *P < 0.05, n = 9–10, ANOVA with Dunnett's post test.
Sexually immature females displayed no significant changes in enzymatic activity in response to either starvation or oxidative stress (Fig. 1E), but sexually mature females displayed a significant decrease in β-galactosidase activity (F2,27 = 3.84, P < 0.05) after starvation stress. β-Galactosidase activity decreased in immature males exposed to paraquat (F2,24 = 3.377, P < 0.05, Fig. 1F), whereas stress did not affect this tissue in mature males. While the fat body could be altered in response to starvation stress due to its known involvement in energy storage, changes in the fat body volume with oxidative stress indicate that it is involved in the stress response in a more generalized manner. Changes in collagen type IV surrounding the brain, measured by the differences in β-galactosidase activity, revealed that the fat body tissue could be dynamic in its response to stress.
Manipulation of the sex determination pathway in the fat body affects sex-specific behaviors in response to stress.
We next assessed whether manipulation of the sexual identity of fat body tissue could alter the behavioral and physiological response to stress. After exposure to the stress paradigms described above, sexually immature and mature males and females were assayed for physiological and behavioral parameters (heart rate and patterns of movement, which included time spent in center zone, distance moved, meander, velocity, freezing, and escape behaviors). The center zone consists of a 30-mm diameter area surrounded by a 10-mm outer zone; Drosophila, like many other species, have a tendency to spend more time in close proximity to the boundaries of an arena, a behavior termed centrophobism or thigmotaxis (6). Meandering describes whether the animal's trajectory is a straight or curved line. Meandering, distance moved, and velocity all describe an animal's general locomotor pattern, as opposed to freezing and escape behaviors, which are responses to predation stress across a variety of species (12). In this case, freezing was defined as the time spent relatively immobile, and escape as the times during which the animals attempted short bursts of attempted flight. Each locomotor parameter was assessed during an exploratory as well as an adapted phase (the first 2 and last 5 min, respectively, of the 15-min observation period). We assayed behavioral and physiological outputs for Cg-Gal4>w1118 flies (a control for the genetic background since both of the transgenic lines used for manipulation for the sex determination pathway in the following experiments were generated in w1118), Cg-Gal4>traF, [the Cg-Gal4 driver is used to express the female isoform of the sex determination factor transformer (traF)], or Cg-Gal4>tra2IR [the Cg-Gal4 driver is used to express an RNAi corresponding to transformer2 (tra2IR)]. Table 1 shows the results of a three-way ANOVA comparing the interaction between genotype (Cg-Gal4>w1118, Cg-Gal4>traF, or Cg-Gal4>tra2IR), sex, and stress. The behaviors that displayed a significant interaction in this assay were then analyzed by a two-way ANOVA to test for a significant sex × stress interaction in the control genotype Cg-Gal4>w1118 (see Table 2).
Table 1.
Cg-Gal4>w1118, Cg-Gal4>traF, and Cg-Gal4>tra2IR sexually immature (1 day) and sexually mature (5 day) males and females were exposed to starvation or paraquat for 24 h before analysis of heart rate and locomotor behaviors
| Output | Starve Genotype × Sex × Stress | Paraquat Genotype × Sex × Stress | Starve Exploratory Genotype × Sex × Stress | Starve Basal Genotype × Sex × Stress | Paraquat Exploratory Genotype × Sex × Stress | Paraquat Basal Genotype × Sex × Stress |
|---|---|---|---|---|---|---|
| 1 Day old | ||||||
| Heart rate | F(479,11) = 24.043, P = 0.000 | F(472,11) = 8.105, P = 0.000 | ||||
| Total distance moved | F(538,11) = 6.433, P = 0.002 | F(534,11) = 0.941, P = 0.391 | F(538,11) = 1.869, P = 0.155 | F(533,11) = 0.783, P = 0.457 | ||
| In-zone duration | F(538,11) = 0.015, P = 0.985 | F(534,11) = 0.031, P = 0.31 | F(538,11) = 4.254, P = 0.015 | F(533,11) = 1.484, P = 0.228 | ||
| In-zone frequency | F(538,11) = 0.399, P = 0.671 | F(534,11) = 0.918, P = 0.4 | F(538,11) = 0.369, P = 0.692 | F(533,11) = 4.739, P = 0.009 | ||
| Meander mean | F(538,11) = 0.044, P = 0.957 | F(534,11) = 2.099, P = 0.124 | F(538,11) = 0.328, P = 0.72 | F(533,11) = 2.972, P = 0.052 | ||
| Walking frequency | F(538,11) = 0.618, P = 0.54 | F(534,11) = 0.849, P = 0.428 | F(538,11) = 0.78, P = 0.459 | F(533,11) = 0.19, P = 0.827 | ||
| Stopped duration | F(538,11) = 4.347, P = 0.013 | F(534,11) = 1.21, P = 0.299 | F(538,11) = 1.67, P = 0.189 | F(533,11) = 0.876, P = 0.417 | ||
| Stopped frequency | F(538,11) = 0.624, P = 0.536 | F(534,11) = 0.851, P = 0.428 | F(538,11) = 0.785, P = 0.456 | F(533,11) = 0.189, P = 0.828 | ||
| Highly mobile duration | F(538,11) = 4.526, P = 0.011 | F(534,11) = 0.443, P = 0.642 | F(538,11) = 0.268, P = 0.268 | F(533,11) = 4.63, P = 0.01 | ||
| Highly mobile frequency | F(538,11) = 1.932, P = 0.146 | F(534,11) = 0.452, P = 0.637 | F(538,11) = 2.204, P = 0.111 | F(533,11) = 3.195, P = 0.042 | ||
| Mean velocity | F(538,11) = 2.175, P = 0.115 | F(534,11) = 0.402, P = 0.669 | F(538,11) = 1.469, P = 0.231 | F(533,11) = 1.168, P = 0.312 | ||
| 5 Day old | ||||||
| Heart rate | F(479,11) = 2.785, P = 0.063 | F(449,11) = 10.401, P = 0.000 | ||||
| Total distance | F(539,11) = 7.82, P = 0.000 | F(539,11) = 4.866, P = 0.008 | F(539,11) = 15.024, P = 0.000 | F(539,11) = 4.563, P = 0.011 | ||
| Moved in-zone duration | F(539,11) = 0.752, P = 0.472 | F(539,11) = 2.349, P = 0.096 | F(539,11) = 0.788, P = 0.455 | F(539,11) = 1.976, P = 0.14 | ||
| In-zone frequency | F(539,11) = 1.688, P = 0.186 | F(539,11) = 0.804, P = 0.448 | F(539,11) = 3.318, P = 0.037 | F(539,11) = 1.174, P = 0.31 | ||
| Meander mean | F(539,11) = 1.356, P = 0.259 | F(539,11) = 2.133, P = 0.119 | F(539,11) = 1.507, P = 0.222 | F(539,11) = 1.439, P = 0.238 | ||
| Walking frequency | F(539,11) = 2.032, P = 0.132 | F(539,11) = 2.67, P = 0.07 | F(539,11) = 2.547, P = 0.079 | F(539,11) = 2.95, P = 0.053 | ||
| Stopped duration | F(539,11) = 3.287, P = 0.038 | F(539,11) = 3.367, P = 0.035 | F(539,11) = 3.083, P = 0.047 | F(539,11) = 0.377, P = 0.686 | ||
| Stopped frequency | F(539,11) = 2.051, P = 0.13 | F(539,11) = 2.67, P = 0.07 | F(539,11) = 2.56, P = 0.078 | F(539,11) = 2.954, P = 0.053 | ||
| Highly mobile duration | (F539,11) = 6.879, P = 0.001 | F(539,11) = 5.948, P = 0.003 | F(539,11) = 9.478, P = 0.000 | F(539,11) = 2.186, P = 0.113 | ||
| Highly mobile frequency | F(539,11) = 2.287, P = 0.103 | F(539,11) = 2.578, P = 0.077 | F(539,11) = 3.484, P = 0.031 | F(539,11) = 1.387, P = 0.251 | ||
| Mean velocity | F(539,11) = 6.68, P = 0.001 | F(539,11) = 4.219, P = 0.015 | F(539,11) = 8.047, P = 0.000 | F(539,11) = 0.76, P = 0.468 | ||
Exploratory and basal locomotion refer to the first 2 and last 5 min of a 15-min observation period, respectively. Three-way ANOVA (genotype × sex × stress), significant interactions are indicated in bold type.
n = 39–45 flies.
Table 2.
Cg-Gal4>w1118 sexually immature (1 day) and sexually mature (5 day) males and females were exposed to starvation or paraquat stress for 24 h before analysis of heart rate and locomotor behaviors
| Stress | Heart Rate | Exploratory or Basal | Total Distance Moved | In Zone Duration | In Zone Frequency | Stopped Duration | Highly Mobile Duration | Highly Mobile Frequency | Mean Velocity |
|---|---|---|---|---|---|---|---|---|---|
| 1 Day old | |||||||||
| Starve | F(159,3) = 10.048, P = 0.000 | Exploratory | F(179,3) = 6.256, P = −0.013 | F(179,3) = 0.22, P = 0.64 | F(179,3) = 5.688, P = −0.018 | F(179,3) = 5.343, P = 0.022 | |||
| Paraquat | F(159,3) = 1.245, P = 0.266 | Exploratory | |||||||
| Basal | F(179,3) = 3.161, P = 0.077 | F(179,3) = 3.308, P = 0.071 | F(179,3) = 0.899, P = 0.344 | ||||||
| 5 Day old | |||||||||
| Starve | Exploratory | F(179,3) = 9.407, P = 0.003 | F(179,3) = 2.418, P = 0.122 | F(179,3) = 10.997, P = 0.001 | F(179,3) = 8.264, P = 0.005 | ||||
| Basal | F(179,3) = 3.387, P = 0.067 | F(179,3) = 0.06, P = 0.807 | F(179,3) = 11.005, P = 0.001 | F(179,3) = 2.256, P = 0.135 | |||||
| Paraquat | F(179,3) = 0.025, P = 0.874 | Exploratory | F(179,3) = 1.394, P = 0.239 | F(179,3) = 2.419, P = 0.122 | F(179,3) = 0.123, P = 0.726 | F(179,3) = 2.191, P = 0.141 | F(179,3) = 1.519, P = 0.219 | F(179,3) = 1.669, P = 0.198 | |
| Basal | F(179,3) = 2.045, P = 0.155 | ||||||||
Exploratory and basal locomotion refer to the first 2 and last 5 min of a 15-min observation period, respectively. The results of a two-way ANOVA (sex × stress) are shown. Significant interactions are indicated in bold type. n = 39–45 flies.
Parameters with significant sex differences in the control genotype were subjected to further analysis in which Cg-Gal4>traF males, which would have feminized fat body, and Cg-Gal4>tra2IR females, which would have masculinized fat body, were compared back to sex-matched controls to determine whether feminization or masculinization of the fat body is sufficient to switch the behavioral response to stress to that of the opposite sex. Table 3 summarizes whether feminization of the fat body tissue with traF or masculinization of the fat body tissue with tra2IR resulted in a full reversal of the response to that of the opposite sex, a partial reversal, or a novel response. A full reversal was determined based on the lack of a statistically significant difference in the stress response of animals with feminized or masculinized fat body compared with control animals of the opposite sex. A partial reversal refers to instances in which there was still a significant statistical difference between the stress response of animals with feminized or masculinized fat body compared with control animals of the opposite sex, but the difference was less than what was observed when comparing control animals to those of the opposite sex. A novel response describes the times when comparison of animals with feminized or masculinized fat body to control animals resulted in a different statistical result than what was observed when comparing control animals to those of the opposite sex, but not in a direction that was similar to the response of the opposite sex. An example of a novel response would be if control males displayed an increase in response to stress for a behavior in which control females displayed no change and feminization/masculinization of the fat body resulted in a decrease in the behavioral parameter in response to stress.
Table 3.
Cg-Gal4>w1118, Cg-Gal4>traF, and Cg-Gal4>tra2IR sexually immature (1 day) and sexually mature (5 day) males and females were exposed to starvation or paraquat stress for 24 h before analysis of heart rate and locomotor behaviors
| Behavior | Exploratory or Basal Locomotion | Age | Stressor | Feminization | Masculinization |
|---|---|---|---|---|---|
| Heart rate | N/A | 1 Day | Starve | Full reversal | Full reversal |
| Total distance moved | Exploratory | 1 Day | Starve | Novel effect | Partial reversal |
| Total distance moved | Exploratory | 5 Day | Starve | Partial reversal | Full reversal |
| Stopped duration | Exploratory | 1 Day | Starve | Novel effect | Partial reversal |
| Highly mobile duration | Exploratory | 1 Day | Starve | Novel effect | Full reversal |
| Highly mobile duration | Exploratory | 5 Day | Starve | Partial reversal | Full reversal |
| Highly mobile duration | Basal | 5 Day | Starve | Full reversal | Full reversal |
| Highly mobile duration | Exploratory | 5 Day | Paraquat | Full reversal | Novel effect |
| Mean velocity | Exploratory | 5 Day | Starve | Partial reversal | Full reversal |
Exploratory and basal locomotion refer to the first 2 and last 5 min of a 15-min observation period, respectively. Cg-Gal4>traF males were compared with Cg-Gal4>w1118 males and Cg-Gal4>tra2IR females were compared with Cg-Gal4>w1118 females to determine the effects of feminization or masculinization, respectively. The results of these two-way ANOVAs (genotype × stress) were classified as either a full reversal if there was no statistically significant difference when compared with controls of the opposite sex, a partial reversal if there was a significant difference when compared with controls of the opposite sex that was smaller than the original sex difference shown in Table 1, or novel effect if the behavior changed, but in an unexpected way from what would be expected for feminization or masculinization. n = 39–45 flies
N/A, not applicable.
The response to stress was completely switched to that of the opposite sex for 50% of the outcomes. Percentages for these analyses were calculated by adding the number of responses that were switched with expression of traF in otherwise male flies plus the number of responses that were switched with expression of tra2IR in otherwise female flies, then dividing that total by the number of responses with sex differences multiplied by two to account for males and females. In this way, we were able to account for instances where there was a reversal with traF but not with tra2IR and vice versa. Of the outcomes with complete reversals, 33% were with traF and 67% with tra2IR. One such example was observed in sexually immature animals assayed for heart rate in response to starvation stress. Cg-Gal4>w1118 sexually immature females decreased and sexually immature males increased their heart rate in response to starvation stress (F79,1 = 3.974, P = 0.05 and F79,1 = 11.573, P = 0.001, respectively, Fig. 2). Cg-Gal4>traF males decreased their heart rate in response to starvation stress (F79,1 = 14.585, P = 0.000) and displayed a significant two-way interaction compared with Cg-Gal4>w1118 males (genotype × stress) (F159,1 = 23.113, P = 0.000) but not compared with Cg-Gal4>w1118 females (F159,1 = 0.023, P = 0.88). Cg-Gal4>tra2IR females increased their heart rate in response to starvation stress (F79,1 = 15.625, P = 0.000) and displayed a significant two-way interaction compared with Cg-Gal4>w1118 females (F159,1 = 10.765, P = 0.00) but not compared with Cg-Gal4>w1118 males (F159,3 = 2.383, P = 0.125).
Fig. 2.
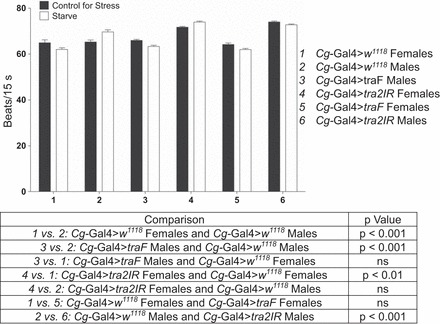
Altering the sex of the fat body reversed heart rate to that of the opposite sex after starvation stress. Sexually immature Cg-Gal4>w1118, Cg-Gal4>traF, and Cg-Gal4>tra2IR males and females were collected immediately after eclosion and assayed for heart rate in response to a 24-h exposure to starvation stress. Heart rate was assessed for 5 intervals of 15 s each. Two-way ANOVAs (genotype × stress), n = 40.
To assess any potential actions of traF and tra2 outside of the sex determination pathway, Cg-Gal4>traF females and Cg-Gal4>tra2IR males were compared with the Cg-Gal4>w1118 females and males, respectively (Table 4). Expression of traF in females and decreased expression of tra2 in males should not affect sexual differentiation; however, response differences were sometimes observed compared with controls. In the case of the heart rate response to starvation stress, there was no significant difference between Cg-Gal4>w1118 females and Cg-Gal4>traF females (F159,3 = 0.17, P = 0.681), but there was a significant difference between Cg-Gal4>w1118 males and Cg-Gal4>tra2IR males (F159,3 = 32.873, P = 0.000). For the males, the significant differences appeared to be due to an increase in heart rate under control for stress conditions in the Cg-Gal4>tra2IR males rather than a difference in heart rate in response to stress. In 40% of the outcomes that displayed significant three-way interactions in Table 1, we observed that in both sexually immature and mature flies, overexpression of traF in females and reduced expression of tra2 in males resulted in a significant two-way interaction (genotype × stress) in a comparison with sex-matched controls. In these instances, changes in the control response, the stress response, or both, in the traF females or tra2IR males resulted in a significant interaction and were included in the 40%.
Table 4.
Cg-Gal4>w1118, Cg-Gal4>traF, and Cg-Gal4>tra2IR sexually immature (1 day) and sexually mature (5 day) males and females were exposed to starvation or paraquat stress for 24 h before analysis of heart rate and locomotor behaviors
| Output | Stress | Exploratory or Basal | Age, days old | Cg-Gal4>w1118 vs. Cg-Gal4>traF Females | Cg-Gal4>w1118 vs. Cg-Gal4>tra2IR Males |
|---|---|---|---|---|---|
| Heart rate | Starve | N/A | 1 | F(159,3) = 0.17, P = 0.681 | F(159,3) = 32.873, P = 0.000 |
| Paraquat | N/A | 1 | F(159,3) = 4.457, P = 0.036 | F(152,3) = 5.909, P = 0.016 | |
| 5 | F(159,3) = 3.716, P = 0.056 | F(159,3) = 10.063, P = 0.002 | |||
| Total distance moved | Starve | Exploratory | 1 | F(179,3) = 9.512, P = 0.002 | F(178,3) = 17.226, P = 0.000 |
| 5 | F(179,3) −5.054, P = 0.026 | F(179,3) = 1.463, P = 0.228 | |||
| Basal | 5 | F(179,3) = 12.669, P = 0.000 | F(179,3) = 0.000, P = 0.984 | ||
| Paraquat | Exploratory | 5 | F(179,3) = 3.495, P = 0.063 | F(179,3) = 2.69, P = 0.103 | |
| Basal | 5 | F(179,3) = 2.093, P = 0.15 | F(179,3) = 1.17, P = 0.281 | ||
| In-zone duration | Paraquat | Exploratory | 1 | F(179,3) = 3.285, P = 0.072 | F(178,3) = 0.157, P = 0.692 |
| In-zone frequency | Paraquat | Exploratory | 5 | F(179,3) = 10.637, P = 0.001 | F(179,3) = 1.279, P = 0.26 |
| Basal | 1 | F(179,3) = 0.248, P = 0.619 | F(173,3) = 9.167, P = 0.003 | ||
| Stopped duration | Starve | Exploratory | 1 | F(179,3) = 5.975, P = 0.015 | F(178,3) = 1.7, P = 0.194 |
| 5 | F(179,3) = 1,783, P = 0.184 | F(179,3) = 1.016, P = 0.315 | |||
| Basal | 5 | F(179,3) = 7.529, P = 0.007 | F(179,3) = 0.355, P = 0.552 | ||
| Paraquat | Exploratory | 5 | F(179,3) = 0.22, P = 0.64 | F(179,3) = 0.795, P = 0.374 | |
| Highly mobile duration | Starve | Exploratory | 1 | F(179,3) = 3.392, P = 0.067 | F(178,3) = 5.252, P = 0.023 |
| 5 | F(179,3) = 10.573, P = 0.001 | F(179,3) = 0.774, P = 0.38 | |||
| Basal | 5 | F(179,3) = 18.449, P = 0.000 | F(179,3) = 0.51, P = 0.476 | ||
| Paraquat | Exploratory | 5 | F(179,3) = 8.316, P = 0.004 | F(179,3) = 0.000, P = 0.993 | |
| Basal | 1 | F(179,3) = 11.454, P = 0.001 | F(173,3) = 7.512, P = 0.007 | ||
| Highly mobile frequency | Paraquat | Exploratory | 5 | F(179,3) = 0.86, P = 0.355 | F(179,3) = 0.343, P = 0.559 |
| Basal | 1 | F(179,3) = 11.307, P = 0.001 | F(173,3) = 3.511, P = 0.063 | ||
| Mean velocity | Starve | Exploratory | 5 | F(179,3) = 3.811, P = 0.052 | F(179,3) = 2.013, P = 0.158 |
| Basal | 5 | F(179,3) −11.756, P = 0.001 | F(179,3) = 0.119, P = 0.731 | ||
| Paraquat | Exploratory | 5 | F(179,3) = 2.816, P = 0.095 | F(179,3) = 0.781, P = 0.378 |
Exploratory and basal locomotion refer to the first 2 and last 5 min of a 15-min observation period, respectively. Cg-Gal4>traF females and Cg-Gal4>tra2IR males were compared with sex-matched Cg-Gal4>w1118 animals. The results of a two-way ANOVA (genotype × stress) are shown. Significant intereactions are indicated in bold type. n = 39–45 flies. N/A, not applicable.
A partial reversal of the response was observed in 28% of the analyses and in many instances was observed for either the traF males or the tra2IR females for a given response, but not both. This effect was seen for sexually mature flies assayed for time spent highly mobile during exploratory locomotion in response to starvation stress (Fig. 3). Cg-Gal4>w1118 sexually mature females increased their time spent highly mobile in response to this stress (F89,1 = 46,785, P = 0.000), whereas sexually mature males did not display a significant effect (F89,1 = 0.802, P = 0.373). Cg-Gal4>traF males increased their time spent highly mobile in response to stress (F89,1 = 5.353, P = 0.023) [t(5) = 1.389, P < 0.05]; there was not a significant two-way interaction compared with Cg-Gal4>w1118 males (F179,3 = 0.715, P = 0.399), and there was still a significant two-way interaction compared with Cg-Gal4>w1118 females even though they both displayed an increase (F179,1 = 6.891, P = 0.009). Cg-Gal4>tra2IR females displayed a full reversal and did not change their time spent highly mobile (F89,1 = 0.003, P = 0.955), which was significantly different from Cg-Gal4>w1118 females (F179,1 = 17.677, P = 0< .000) and comparable to Cg-Gal4>w1118 males (F179,3 = 0.384, P = 0.536). In the analysis between sex-matched animals, Cg-Gal4>w1118 females and Cg-Gal4>traF females were significantly different (F179,3 = 10.573, P = 0.001), whereas Cg-Gal4>w1118 males and Cg-Gal4>tra2IR males were comparable (F179,3 = 0.774, P = 0.38). Whereas the difference between sex-matched flies in Fig. 2 was due to a difference in the control for stress response, the difference between the sex-matched flies in Fig. 3 was attributed to differences in time spent highly mobile in response to starvation for Cg-Gal4>traF females compared with Cg-Gal4>w1118 females.
Fig. 3.
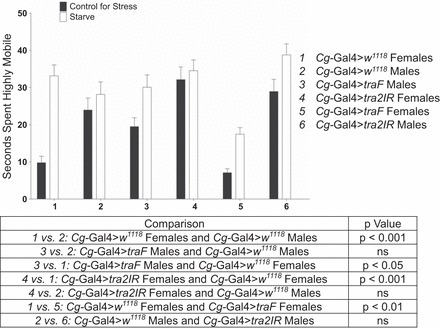
Altering the sex of the fat body partially reversed time spent highly mobile to that of the opposite sex after starvation stress. Sexually mature Cg-Gal4>w1118, Cg-Gal4>traF, and Cg-Gal4>tra2IR males and females were collected immediately after eclosion, aged for 5 days, and assayed for time spent highly mobile during exploratory locomotion in response to starvation stress. Two-way ANOVAs (genotype × stress), n = 39–45.
In 22% of the outcomes, a novel response was observed following manipulation of the fat body. This was seen with sexually immature flies assayed for total distance moved during exploratory locomotion in response to starvation stress (Fig. 4). Cg-Gal4>w1118 sexually immature females and males increased their total distance moved in response to starvation stress (F89,1 = 17.038, P = 0.001 and F89,1 = 32.588, P = 0.000, respectively). Cg-Gal4>traF males did not alter their total distance moved (F89,1 = 1.354, P = 0.248) and displayed significant two-way interaction compared with Cg-Gal4>w1118 males and females (F179,3 = 20.932, P = 0.000 and F179,3 = 9.142, P = 0.003, respectively). Cg-Gal4>tra2IR females displayed a partial reversal and displayed an increase in their total distance moved in response to starvation (F89,1 = 9.671, P = 0.003), resulting in no significant difference compared with Cg-Gal4>w1118 females or males (F179,3 = 0.108, P = 0.742 and F179,3 = 3.38, P = 0.068). For this behavior Cg-Gal4>traF females did not display a significant change (F89,1 = 0.045, P = 0.832) and were significantly different from sex-matching controls (F179,3 = 9.512, p 0 0.002). Cg-Gal4>tra2IR males did not display a significant change and were also significantly different from sex-matched controls (F178,3 = 17.226, P = 0.000).
Fig. 4.

Analysis of total distance moved following manipulation of the sex determination pathway showed a novel response, unlike controls of either sex. Sexually immature Cg-Gal4>w1118 males and females and Cg-Gal4>traF males and Cg-Gal4>tra2IR females were collected immediately after eclosion and assayed for total distance moved during exploratory locomotion in response to starvation. Statistical results are shown in the table. Two-way ANOVAs (genotype × stress), n = 45.
DISCUSSION
Neuronal control of behavioral responses to stress in Drosophila has been well established. Previous data from our lab has shown that tyrosine hydroxylase activity in the brain was altered in a sex- and time-dependent manner following starvation and oxidative stress, consistent with observations that dopamine signaling pathways are altered in response to stress (39). In addition, heart rate and locomotor parameters are also affected in response to stress in Drosophila (2, 3, 38) and mammalian species (18, 32, 41, 53). Our current results provide evidence for a role for fat body in the stress response in Drosophila. While most studies have focused on the abdominal fat body and its role as the analog of the mammalian liver, recent studies have suggested the fat body surrounding the brain functions in housing factors that help to establish the sexual identity of the brain (24). In mammals, it is known that the hypothalamic-pituitary-adrenal axis (HPA), which responds to stress, is influenced by sex hormones such as testosterone and estrogen (55). In one study, researchers found that feminization of the androgen receptor can cause higher HPA activity, which is more similar to what naturally occurs in females (56). In this case, the stress response to a novel object was not completely feminized, but the results do suggest, similar to what we observed in Drosophila, that the sexual environment surrounding and within the brain modulates behavioral and physiological responses to stress, consistent with a role for fat body in maintaining neuronal homeostasis.
The majority of studies in the literature have dealt either entirely with the larval fat body or with changes in the fat body as metamorphosis occurs; few studies have addressed the function of this tissue in mature adults. Distinguishing between larval and adult fat body is important since the two tissues are of different lineages. During metamorphosis the larval fat body gradually decreases in cell number as energy stores are depleted while the animal is not eating (10). When a newly eclosed adult emerges from its pupal case, it contains freely floating larval fat cells, which die and are replaced by adult fat cells by 3–4 days posteclosion (1). By using the Cg-Gal4 line, which expresses in both larval and adult fat body, we ensured that we were able to target the fat body in flies both 1 day and 5 days posteclosion. Studies in adult fat body have focused on the role of the regulation of lifespan by insulin-like peptides expressed in this tissue, on carbohydrate and fat storage, and on improved resistance to oxidative stress (5). These functions in energy metabolism are largely shared by larval fat body (1). Additional studies have established roles for the fat body in immunity (20) and sex determination (24). In adults, there is also a precedent for the importance of this tissue in affecting behavior. Lazareva et al. (31) demonstrated that the fat body surrounding the brain expresses genes involved in courtship that are not expressed within the brain and that feminization of the fat body greatly reduced male courtship. The authors proposed that feminization of the fat body resulted in changes in the synthesis of male-specific secreted circulating proteins that act on the brain. Ellis and Carney (19) analyzed gene expression profiles in 5-day-old mated males and found that several of the changes were localized not to the brain, but rather, to the fat body, further evidence that the fat body can modulate neuronal function and centrally modulated behaviors. These studies focused specifically on the fat body surrounding the head, which has been shown to contain sex-specific genes that are not present in the fat body in the abdomen, suggesting that there could be two distinct types of fat body tissue with specific functions (24). Our studies investigate the hypothesis that in addition to these roles, the fat body and its secreted factors are critical for the establishment and maintenance of sexually dimorphic neuronal response circuits.
The sex-specific changes in the fat body in response to stress are of particular interest because of demonstrated differences between males and females in susceptibility to stress-related pathologies (15). We observed that manipulation of tra or tra2 in fat body resulted in a complete switch of the response to stress to that of the opposite sex for the majority of responses that were found to display sex differences. For the remaining responses, there was a partial shift to be more similar to that of the opposite sex, and there was only a small proportion of instances where the response in the manipulated flies did not resemble that of the opposite sex. Interestingly, we observed reversals to that of the opposite sex in both sexually immature individuals that would contain larval fat body as well as sexually mature flies, which would have entirely adult fat body, indicating that both larval and adult fat body share a common function in mediating sex-specific centrally controlled behaviors. Previous work has shown that the larval fat body is not involved in mating, making this the first demonstration for a role of the larval fat body in modulation of sexually dimorphic behavior (31). This implication that feminization and masculinization of the stress response circuitry may be different from that of reproductive circuitry is an interesting question for future research.
After traF overexpression in males and reduced tra2 expression in females, the flies displayed no outward changes in genetic sex, yet the percentage of occurrences in which there was a complete reversal of the response was highly significant. These results confirm that, for most of the responses we tested, manipulation of the sex determination pathway in the fat body is sufficient to alter the sex-specific stress response. This supports the hypothesis that sexually dimorphic actions of the brain can also arise from effectors from the fat body in addition to the brain transcriptome (24).
Since tra and tra2 are both involved in mRNA splicing, their target sequences could have diverse actions in addition to that of sex determination. We showed that overexpression of traF in females and reduction of tra2 in males altered a variety of behavioral parameters and heart rate. Interestingly, these changes were not simply in the stress-treated groups, but could occasionally be seen under control for stress conditions and in both sexually immature and mature populations. These results are suggestive of actions for tra and tra2 distinct from their roles in sex determination. In support of this interpretation, studies by others have identified additional targets beyond those involved in sex determination via coimmunoprecipitation experiments (25); in many cases the functions of these targets are still unknown, and the list of interactions may not be comprehensive. One downstream target of tra2 of particular interest is CG6937, which was also identified in a screen for factors involved in neurogenesis (40), providing a potential mechanism for tra2 in the modulation of centrally controlled behaviors, as well as in the response to stress.
The expression data for tra and tra2 are also suggestive of pleiotropic actions. tra is moderately expressed in 20-day-old females and is higher in 20-day-old males (11); although it has not been determined whether a functional protein is made, the possibility is suggestive of actions that extend beyond sex determination, which occurs early in adult development. Both tra and tra2 are expressed in the larval central nervous system, larval and adult digestive tracts, and larval and adult dorsal vessels (11, 12), as well as in the ovaries and testis; however, tra expression is higher in the ovaries compared with tra2, and the opposite is true for the testis (11). While expression within the gonads is likely important for sex determination, there is evidence to suggest that gonadal sex determination is effected by the fat body in the abdomen, analogous to the role the fat body in head plays in determining the sexual identity of the brain (24). It should, be noted, however, that Cg-Gal4 does target expression to the thoracic and abdominal fat body as well as that in head, so we cannot entirely rule out a potential role for the abdominal fat body in stress behavior. The only other cells or tissues targeted by Cg-Gal4 are hemocytes and lympth gland, neither of which have been shown to express tra or tra2 (11). Interestingly, other factors from the sex determination pathway [i.e., sex lethal (sxl), dsx, and female-specific independent of transformer (fit)] are also expressed in older animals and other tissues, such as the dorsal vessel, that are not thought of as important for sex determination (11, 12), suggesting that many of the factors involved in sex determination have pleitropic actions. Furthermore, expression data for tra identified changes between virgin and mated females (11), populations that have also been noted as having a different survival rate in response to stress (39).
It is possible that some of the effects of overexpression of tra2 in males could be due to off-target effects. There are two off-target genes predicted for tra2 RNAi, tay bridge (tay) and Paramyosin (Prm) (22). Analysis of tay mutants has revealed a role for this gene in locomotor behavior, making it a potential candidate for some of the effects we observed (43). Analysis of Prm reveals a role in muscle development, specifically of the flight muscles, which are not necessary for any of the behavioral parameters we assayed, with the exception of high mobility (33). Although these off-target predictions could be involved in some of our observations, they cannot account for any of the effects observed with overexpression of traF. However, since the precise mechanism of action for tra and tra2 within the sex-specific stress response is not yet understood, it is impossible to definitively state whether the effects observed are conclusive evidence of roles outside of the sex determination pathway.
Perspectives and Significance
Analyses of flies with feminized or masculinized fat body in an otherwise unaltered individual provides support for sexual dimorphism of the brain via sex determination factors localized in the fat body. Using the genetic tractability of Drosophila, we have also been able to uncover a role for the fat body in the sex- and time-dependent responses to stress. These data suggest that the fat body in head tissue may serve as the functional analogue of the pituitary gland. Additionally, data showing that overexpression of traF in females and reduced expression of tra2 in males can alter not only the stress response, but also modulate other behaviors, demonstrated potential roles for tra and tra2 that are distinct from actions in sex determination. Further analysis of these factors will be useful for the elucidation of mechanisms for sexual dimorphism of the brain and for identification of factors important for the modulation of sex-specific, centrally controlled behaviors.
GRANTS
This work was funded by the National Institutes of Health NIMH RO1MH083771.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.J.A. and W.S.N. conception and design of research; K.J.A. performed experiments; K.J.A. analyzed data; K.J.A. interpreted results of experiments; K.J.A. prepared figures; K.J.A. drafted manuscript; K.J.A. and W.S.N. edited and revised manuscript; K.J.A. and W.S.N. approved final version of manuscript.
REFERENCES
- 1.Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK. The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol 210: 956–963, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Argue KJ, Neckameyer WS. Sexually dimorphic recruitment of dopamine neurons into the stress response circuitry. Behav Neurosci 127: 734–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argue KJ, Neckameyer WS. Temporally dimorphic recruitment of dopamine neurons into stress response circuitry in Drosophila. Behav Neurosci 127: 725–733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosphila hemocytes. Genetics 163: 203–215, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai H, Kang P, Tatar M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11: 978–985, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94: 383–423, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker BS, Wolfner MF. A molecular analysis of doublesex, a bifunctional gene that control both male and female sexual differentiation in Drosophila melanogaster. Genes Dev 2: 477–489, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Belote JM, Baker BS. Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proc Natl Acad Sci USA 79: 1568–1572, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50: 739–747, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Butterworth FM, Emerson L, Rasch EM. Maturation and degeneration of the fat body in the Drosophila larva and pupa as revealed by morphometric analysis. Tissue Cell 20: 255–268, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, Micklem G, Piano F, Snyder M, Stein L, White KP, Waterston RH, modENCODE Consortium. Unlocking the secrets of the genome. Nature 459: 927–930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39: 715–720, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Cline TW. Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90: 683–698, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cline TW. Evidence that sisterless-a and sisterless-b are two of several discrete “numerator elements” of the X/A sex determination signal in Drosophila that switch Sxl between two alternative stable expression states. Genetics 119: 829–862, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dedovic K, Wadiwalla M, Engert V, Pruessner JC. The role of sex and gender socialization in stress reactivity. Dev Psychol 45: 45–55, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Duffy JB. GAL4 system in Drosophila: a fly geneticists's Swiss army knife. Genesis 34: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Duffy JB, Gergen JP. The Drosophila segmentation gene runt acts as a position-specific numerator element necessary for the uniform expression of the sex-determining gene Sex-lethal. Genes Dev 5: 2176–2187, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Edut S, Eilam D. Rodents in open space adjust their behavioral response to the different risk levels during barn-owl attack. BMC Ecol 3: 10, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis LL, Carney GE. Mating alters expression patterns in Drosophila melanogaster male heads. BMC Genomics 11: 558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signaling during bacterial and fungal infections. Nat Rev Immunol 7: 862–874, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ferveur JF, Störtkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 267: 902–905, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Flockhart I, Booker M, Kiger A, Boutros M, Armknecht S, Ramadan N, Richardson K, Xu A, Perrimon N, Mathey-Prevot B. FlyRNAi: the Drosophila RNAi screening center database. Nucleic Acids Res 34: D489–D494, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujihara T, Kawabe M, Oishi K. A sex-transformation gene in Drosophila melanogaster. J Hered 69: 229–236, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Fujii S, Amrein H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J 21: 5353–5363, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Beekman C, Wong C, Rhee DY, Cenaj O, McKillip E, Shah S, Stapleton M, Wan KH, Yu C, Parsa B, Carlson JW, Chen X, Kapadia B, VijayRaghavan K, Gygi SP, Celniker SE, Obar RA, Artavanis-Tsakonas S. A protein complex network of Drosophila melanogaster. Cell 14: 690–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28: 464–476, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Heinrichs V, Ryner LC, Baker BS. Regulation of sex-specific selection of fruitless 5′ splice sites by transformer and transformer-2. Mol Cell Biol 18: 450–458, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildreth PE. Doublesex, recessive gene that transforms both males and females of Drosophila into intersexes. Genetics 51: 659–678, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 252: 833–836, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Kohyama-Koganeya A, Nabetani T, Miura M, Hirabayashi Y. Glucosylceramide synthesis in the fat body controls energy metabolism in Drosophila. J Lipid Res 52: 1392–1399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazareva AA, Roman G, Mattox W, Hardin PE, Dauwalder B. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet 3: e16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lester D. The effect of fear and anxiety on exploration and curiosity: toward a theory of exploration. J Gen Psychol 79: 105–120, 1968 [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Miller MS, Swank DM, Kronert WA, Maughan DW, Bernstein SI. Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster. Proc Natl Acad Sci USA 102: 10522–10527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, Catena Dell'Osso M. Psychiatric disorders and mitochondrial dysfunctions. Eur Rev Med Pharmacol Sci 16: 270–275, 2012 [PubMed] [Google Scholar]
- 35.McGuffin ME, Chandler D, Somaiya D, Sauwalder B, Mattox W. Autoregulation of transformer-2 alternative splicing is necessary for normal male fertility in Drosophila. Genetics 149: 1477–1486, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeown M, Belote JM, Baker BS. A molecular analysis of transformer, a gene in Drosophila melanogaster that control female sexual differentiation. Cell 48: 489–499, 1987 [DOI] [PubMed] [Google Scholar]
- 37.McKeown M, Belote JM, Boggs RT. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell 53: 887–895, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Neckameyer WS, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience 156: 841–856, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Neckameyer WS, Weinstein J. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress 8: 117–132, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Neumüller RA, Richter C, Fischer A, Navatchkova M, Neumüller KG, Knoblich JA. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell 8: 580–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nosaka S. Modifications of arterial baroreflexes: obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn J Physiol 46: 271–288, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Okamoto N, Yamanaka M, Yagi Y, Nashida Y, Kataoka H, O'Connor MB, Mizoguchi A. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17: 885–891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poeck B, Triphan T, Neuser K, Strauss R. Locomotor control by the central complex in Drosophila. An analysis of the tay bridge mutant. Dev Neurobiol 68: 1046–1058, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Pomiankowski A, Nothiger R, Wilkins A. The evolution of Drosophila sex determination pathway. Genetics 166: 1761–1773, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes ME, Rubin RT. Functional sex differences ('sexual diergism') of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev 30: 135–152, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Rooseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, Rooij SR. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Muturitas 70: 141–145, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87: 1079–1089, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Sassu ED, McDermott JE, Keys BJ, Esmaeili M, Keene AC, Birnbaum MJ, DiAngelo JR. Mio/dCHREBP coordinately increases fat mass by regulating lipid synthesis and feeding behavior in Drosophila. Biochem Biophys Res Commun 14: 43–48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sefton L, Timmer JR, Zhang Y, Béranger F, Cline TW. An extracellular activator of the Drosophila JAK/STAT pathway is a sex-determination signal element. Nature 405: 970–973, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sieber MH, Thummel CS. The DHR96 nuclear receptor controls tiacylglycerol homeostasis in Drosophila. Cell 10: 481–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Søndergaard L. Homology between the mammalian liver and the Drosophila fat body. Trends Genet 9: 193, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Takata K, Yoshida H, Yamaguchi M, Sakaguchi K. Drosophila damaged DNA-binding protein 1 is an essential factor for development. Genetics 168: 855–865, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treit D, Fundytus M. Thigmotaxis as a test of anxiolytic activity in rats. Pharmacol Biochem Behav 31: 959–962, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Younger-Shepherd S, Vaessin H, Bier E, Jan LY, Jan YN. Deadpan, an essential pan-neural gene encoding an HLH protein, acts as a denominator in Drosophila sex determination. Cell 70: 911–922, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Zou S, Chang J, LaFever L, Tang W, Johnson EL, Hu J, Wilk R, Krause HM, Drummond-Barbosa D, Irusta PM. Identification of dAven, a Drosophila melanogaster ortholog of the cell cycle regulator Aven. Cell Cycle 10: 989–998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuloaga DG, Poort JE, Jordan CL, Breedlove SM. Male rats with the testicular feminization mutation of the androgen receptor display elevated anxiety-related behavior and corticosterone response to mild stress. Horm Behav 60: 380–388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


