Abstract
Gastric emptying, accommodation, and motility can be quantified with magnetic resonance imaging (MRI). The first step in image analysis entails segmenting the stomach from surrounding structures, usually by a time-consuming manual process. We have developed a semiautomated process to segment and measure gastric volumes with MRI. Gastric images were acquired with a three-dimensional gradient echo MRI sequence at 5, 10, 20, and 30 min after ingestion of a liquid nutrient (Ensure, 296 ml) labeled with gadolinium in 20 healthy volunteers and 29 patients with dyspeptic symptoms. The agreement between gastric volumes measured by manual segmentation and our new semiautomated algorithm was assessed with Lin's concordance correlation coefficient (CCC) and the Bland Altman test. At 5 min after a meal, food volumes measured by manual (352 ± 4 ml) and semiautomated (346 ± 4 ml) techniques were correlated {CCC[95% confidence interval (CI)] 0.70 (0.52, 0.81)}; air volumes measured by manual (88 ± 6 ml) and semiautomated (84 ± 6 ml) techniques were also correlated [CCC (95% CI) 0.89 (0.82, 0.94)]. Findings were similar at subsequent time points. The Bland Altman test was not significant. The time required for semiautomated segmentation ranged from an average of 204 s for the 5-min images to 233 s for the 20-min images. These times were appreciably smaller than the typical times of many tens of minutes, even hours, required for manual segmentation. To conclude, a semiautomated process can measure gastric food and air volume using MRI with comparable accuracy and far better efficiency than a manual process.
Keywords: emptying, motility, magnetic resonance imaging
the stomach relaxes or accommodates after a meal, which provides room for food to be broken down into smaller particles that are emptied into the duodenum. Disturbances of these motor functions contribute to upper gastrointestinal symptoms (i.e., indigestion) in common conditions such as dyspepsia and gastroparesis (23). Magnetic resonance imaging (MRI) is perhaps the only technique that can assess these processes [i.e., gastric volumes, emptying, and motility (8, 10–13, 18, 20, 27–29, 33)] without radiation exposure or an intraluminal catheter. “Static” MRI can reproducibly measure not only the volume of the entire stomach but also the volume of intragastric air and food (9). Gastric emptying can be measured from a time series of postprandial three-dimensional (3D) gastric volumes acquired at a relatively slow rate (i.e., static MRI) (11, 19); gastric motility can be evaluated by more rapid image acquisition sequences (i.e., “dynamic” MRI) (2).
Assessments of gastric motility by MRI demonstrated, for the first time, that the amplitude of gastric contractions in the entire stomach was greater in patients with functional dyspepsia and rapid, but not normal, gastric emptying compared with controls (2). Likewise, the ability to measure the postprandial increase in gastric volume is useful for identifying impaired gastric accommodation, which may explain early satiety in functional dyspepsia (4, 9, 17, 22). A particular advantage of measuring gastric volumes with MRI or other noninvasive techniques is that they do not, in contrast to a barostat, require an intragastric balloon, which distends the stomach, displaces food toward the antrum, and tends to accentuate accommodation (7, 21).
Despite these advantages, gastric accommodation and contractions are generally measured by scintigraphy, a barostat or single photon emission computed tomography, and manometry rather than by MRI (23). There is limited awareness of the ability of MRI to measure gastric motor functions and technical challenges with image analysis. Indeed, the use of MRI to evaluate gastric motor functions has lagged behind its application for other regions (e.g., brain, angiography) (26). One very significant challenge to using gastric MRI is the time required to segment the stomach from surrounding structures. Because of image noise, acquisition artifact, and possible low contrast between adjacent structures, this segmentation requires fine-grained demarcation of the stomach border. Moreover, identifying thin structures such as the diaphragm between the lungs and stomach can be difficult and require human expertise. Even experienced technicians require ∼3 h to segment the ∼100 images required to compute gastric volumes at each time point even when the nutrient meal is labeled with gadolinium, which is bright on T2-weighted sequences and hence can be discriminated from air within the lumen (9). Preliminary work in the development of a 3D image processing and analysis tool with minimal user intervention has been described (1). The tool enabled reconstruction of gastric 3D geometry with only 12–18 mouse clicks compared with 400 clicks for manual analysis; however, the time required for that analysis or a comparison of the data for gastric volumes computed by manual and semiautomated techniques was not studied.
We previously compared gastric volumes analyzed by MRI in 20 healthy subjects and 17 patients with functional dyspepsia (2, 9). After segmenting gastric images manually, fasting and postprandial gastric volumes were assessed. These volumes were reproducible within subjects and between days. The postprandial volume change was not significantly different between healthy subjects and dyspepsia patients. We adapted existing algorithms to develop a semiautomated process for segmenting the stomach and measuring gastric volumes with MRI. The objectives of this study were to: 1) measure postprandial volumes with a semiautomated analysis of MRIs; and 2) compare postprandial volumes measured by manual and semiautomated analysis in healthy subjects and patients with dyspepsia. We also measured the time required for semiautomated segmentation.
MATERIALS AND METHODS
Subjects.
Twenty healthy subjects [14 women, 35 ± (SE) yr, 26.0 ± 0.9 kg/m2] and 29 patients with upper gastrointestinal symptoms (23 women, 43 ± 4 yr, 25.1 ± 1.1 kg/m2) participated in this study. These studies were approved by the Institutional Review Board, conducted at Mayo Clinic, and analyzed by ANALYZE software. A gastroenterologist interviewed and examined all subjects. All subjects completed a questionnaire on functional gastroduodenal and bowel disorders framed to be consistent with Rome III criteria (31). None had previous gastrointestinal surgery (other than appendectomy), significant underlying illnesses [other than diabetes mellitus (DM)], or medication use, except for stable doses of birth control pill, l-thyroxine, or estrogen replacement therapy. In healthy subjects, functional gastrointestinal disorders, anxiety, and depression were excluded using validated screening questionnaires, a clinical interview, and a physical examination (32, 34). Healthy subjects were recruited from the local community by public advertisement, and patients were recruited from the clinical practice.
Gastric MRI.
With the use of a torso phased array coil and a 1.5 T magnet MRI (Twin Speed, GE Healthcare), fasting and postprandial images were acquired before and 5, 10, 20, and 30 min after ingestion of Ensure [296 ml, 308 kcal (64% carbohydrate, 22% fat, and 14% protein)] labeled with gadolinium [4 ml, 287 mg/ml, gadodiamide (Omniscan; GE Healthcare)]. Images were acquired with an axial 3D axial gradient echo (LAVA) sequence without fat saturation (i.e., 4-mm slices with 2-mm overlap, matrix size 256 × 160, typical left/right × anterior/posterior field of view of 40 cm × 32 cm, 1 excitation, parallel imaging acceleration factor of 2) that imaged the entire stomach in 13 s. The typical superior/inferior extent of the volume of interest, encompassing the stomach, was 20 cm, thus including ∼100 individual axial sections. Subjects were encouraged to hold their breath for this sequence.
Data analysis.
With the use of ANALYZE software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) (25), the volume of gastric air and fluids in MRIs was estimated by two approaches (manual tracing and a semiautomated segmentation). Investigators processing these images were blinded to the results of the other analysis. For the manual analysis, the results of which were detailed previously (9), a region of interest (ROI) was manually drawn around gastric food and air on each slice. Manual and automated segmentations were reviewed by a radiologist (Fidler) for accuracy. The sum of all pixels within each appropriate ROI for all slices was multiplied by voxel size to obtain the volume of gastric food and air.
The semiautomated program was implemented on a Windows PC (Intel Core 2 Quad CPU Q9300 at 2.5 GHz, 4.00 GB RAM) and incorporated the following steps. First, an automated multilevel threshold selection approach that minimizes variance between bright and dark objects (24) was applied to the histogram of the image data to identify threshold values for high-intensity (gadolinium-enhanced nutrient) and low-intensity (air) regions. These threshold values were not dependent on input from the user.
Next, the program identified coarse regions for gastric air and food using intensities below the air threshold and above the food threshold. The coarse region for food also included the surrounding structures, mostly perigastric adipose tissue. Therefore, manually selected seed points within food and air image regions were used to identify all regions connected to that seed point within food and air. Thereafter, a 9 × 9 × 3 pixel, 6.7 × 6.7 × 6 mm structuring element-based morphological opening was used to isolate gastric contents from surrounding organs (Fig. 1). This morphological opening process incorporated both erosion and dilation and was applied to an intensity thresholded binary mask. Erosion (i.e., removing a layer) eliminates thin connections between two objects and noise. In this instance, erosion demarcated the boundary between the ROI defined by food and air and surrounding organs. Dilation restores the layer without noise and thin connections. The structuring element size was selected so as to break connections to adjacent structures. User-identified seed points, typically placed in the middle of food and air structures, initiated a region growing algorithm that identified the desired food and air components. Seed selection is the only manual intervention used and is robust to initialization. Region growing occurs automatically, requiring <2 s/dataset. In some cases, regional variations in signal intensity within the air volume were heterogenous, which limited automated extraction by the process described above. In such cases, the program automatically repeated the extraction process after applying a grayscale minimum filter to the volume, which reduced the inhomogeneity of pixels within a region. Finally, the gap between food and air was closed by using a two-step process that entailed dilation followed by erosion. In the first step (i.e., dilation), symmetrical layers of 1 voxel thickness were simultaneously added to food and air object maps until the gap closed. Thereafter, the additional outer layers not contiguous to the gap were removed while the gaps were closed by reassigning them to air volume using a morphological close operation (dilation followed by erosion) (Fig. 2). Finally, the volume of air and food was computed.
Fig. 1.
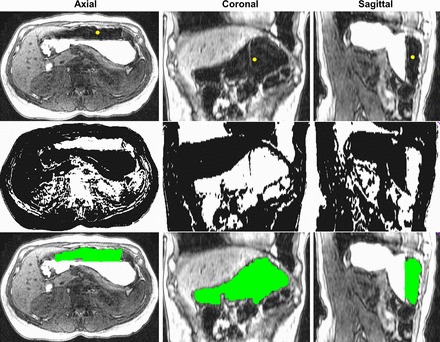
Representative example of stepwise semiautomated segmentation process for gastric air. Top: original images. All tissues identified by the automatically computed threshold for air using Reddi's method are shown in the middle. Next, morphology and region growing operations were applied to identify a region of interest for air, which is shaded green (bottom). A similar process was used to segment contents (data not shown).
Fig. 2.
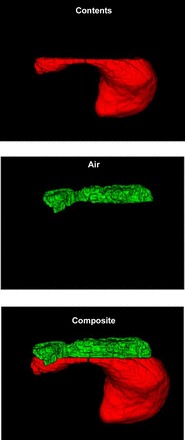
Rendered images of food (top), air (middle), and the composite (bottom) after morphological closing of the gap between air and food region of interest. Air and food are colored green and red, respectively. These images correspond to those in Fig. 1.
Statistical analysis.
The concordance between gastric volumes measured by manual and semiautomated sequences was assessed by Lin's concordance statistic [the concordance correlation coefficient (6)]. A Bland Altman assessment examined whether the magnitude of differences between manual and semiautomated techniques was correlated with the magnitude of the measured responses (i.e., the average value for both studies) using Pearson's correlation coefficient (3).
RESULTS
Clinical features.
Twenty three patients did not have any underlying explanation for their upper gastrointestinal symptoms. Five of six patients with DM had type 1 DM. Gastric emptying was measured by scintigraphy in 27 of 29 patients; 8 had normal, 11 had rapid, and 8 had delayed gastric emptying.
Fasting and postprandial gastric volumes.
After drinking Ensure (300 ml), gastric volumes increased by 403 ± 12 ml in healthy subjects, 442 ± 21 ml in patients with normal gastric emptying, and 383 ± 26 ml in patients with delayed gastric emptying as detailed previously (9). Because the semiautomated technique cannot reliably segment the empty stomach from surrounding structures, this study was limited to postprandial volumes.
Comparison of volumes measured by manual and semiautomated analysis.
The volume of gastric food at 5, 10, 20, and 30 min after a meal measured by manual and semiautomated techniques was significantly correlated (Table 1 and Fig. 3). Differences between manual and semiautomated volumes of gastric food were also small and ranged from 4.4 ml [95% confidence interval (CI) −1.7, 10.4] at 20 min to 7.1 ml (95% CI 0.7, 13.5) at 10 min. Moreover, these differences were not related to the average postprandial volumes measured by manual and semiautomated techniques (i.e., the Bland Altman test was not significant). The largest differences between manual and semiautomated techniques were observed in cases where the manually measured stomach content volume was >350 ml. However, differences between manual and semiautomated measurements exceeded 50 ml in only 4 of 196 images, i.e., 2%.
Table 1.
Comparison of gastric volumes measured by manual vs. semiautomated techniques
| Measurements | Manual | Semiautomated | CCC (95% CI) | Mean (95% CI) Difference (manual − semiautomated volume) |
|---|---|---|---|---|
| Food | ||||
| Postprandial 5 | 352 ± 4 | 346 ± 4 | 0.70 (0.52, 0.81) | 6.1 (0.1, 12.1) |
| Postprandial 10 | 343 ± 5 | 336 ± 5 | 0.79 (0.66–0.87) | 7.1 (0.7, 13.5) |
| Postprandial 20 | 328 ± 6 | 324 ± 5 | 0.85 (0.75–0.91) | 4.4 (−1.7, 10.4) |
| Postprandial 30 | 313 ± 7 | 309 ± 6 | 0.88 (0.80–0.93) | 5.4 (−1.3, 12.2) |
| Air | ||||
| Postprandial 5 | 88 ± 6 | 84 ± 6 | 0.89 (0.82, 0.94) | 4.8 (−0.8, 10.4) |
| Postprandial 10 | 86 ± 6 | 83 ± 7 | 0.91 (0.84, 0.94) | 2.4 (−3.1, 8.0) |
| Postprandial 20 | 85 ± 6 | 79 ± 6 | 0.89 (0.81, 0.93) | 5.9 (0.5, 11.3) |
| Postprandial 30 | 81 ± 6 | 78 ± 6 | 0.92 (0.86, 0.95) | 4.7 (0.2, 9.3) |
CCC, concordance correlaton coefficient; CI, confidence interval.
The nos. 5, 10, and 30 reflect imaging time, in min, relative to meal ingestion.
Fig. 3.
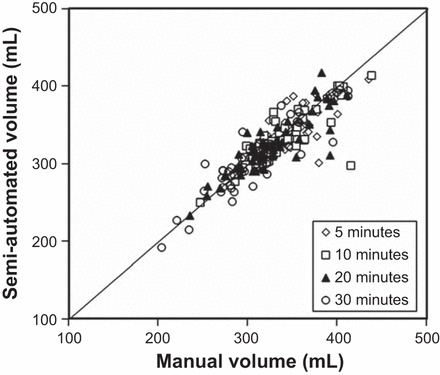
Comparison of gastric food volumes measured by manual and semiautomated techniques. The correlations between both techniques were excellent at all times (5, 10, 20, and 30 min) after a meal.
Postprandial air volumes measured by manual and semiautomated techniques were also significantly correlated (Table 1). For example, at 5 min, volumes were 88 ± 6 ml by manual and 84 ± 6 ml by semiautomated techniques with a concordance correlation coefficient of 0.89 (0.82, 0.94). Differences between manual and semiautomated gastric air volume were small and ranged from 2.4 ml (95% CI −3.1, 8.0) at 10 min to 5.9 ml (95% CI 0.5, 11.3) at 20 min after a meal. Moreover, the Bland Altman test was not significant.
Time required for semiautomated analysis.
The semiautomated analysis required 204 (95% CI 177–232) s for volume at 5 min, 216 (95% CI 171–261) s at 10 min, 233 (95% CI 189–276) s at 20 min, and 232 (95% CI 189–276) s at 30 min. This included the time required for analysis and for manually refining the ROI when necessary.
DISCUSSION
Previous studies that quantified gastric volumes and emptying with MRI manually segmented the stomach by a time-consuming process. This limits the widespread use of MRI for these applications (20). In this study, we demonstrate that a customized, semiautomated process can effectively measure the volume of gastric food and air in MRIs with similar accuracy and significantly improved efficiency compared with a manual process. This promising development provides, for the first time, an efficient and robust approach to analyzing gastric volumes and motility from MRI. It is particularly useful when numerous images need to be analyzed, as, for example, when assessing gastric emptying, which is not a linear process (19). Moreover, the ability to analyze the volume of not only food but also air is essential for evaluating postprandial gastric accommodation, which requires measuring the volume of the entire stomach (9).
As with any practical algorithm for image analysis, there is a trade-off between human expertise and automation. Some steps (e.g., automatic detection of thresholds) required no human intervention, and the outcome is very reproducible. By eliminating potential individual bias related to threshold perception, a consistent threshold can be identified at the onset of the processing. In contrast, human intervention is often necessary to identify anatomical interfaces, particularly in the presence of image noise and acquisition artifact. Although automated techniques could also be developed for these tasks, postalgorithm refinement by an expert would require substantially more effort.
A potential alternative is to segment the stomach with an atlas-based approach (14) that can effectively segment structures with a regular shape (e.g., the heart), which does not vary considerably among subjects. In contrast, the size and shape of the stomach varies considerably among subjects and is influenced, among other factors, by variations in body morphology, adjacent anatomy, surrounding fat, and extent of filling. Hence, model-based segmentation is impractical for the stomach. In the present technique, the stomach is located by a simple human click. By reducing the field of view to the region of the stomach, automated algorithms are able to overcome local inhomogeneity, noise, and partial volume effect. Morphological processing is an effective tool to deal with small gaps in signal that generally occur around thin structures (30). Accordingly, the size of the morphology kernels (i.e., structuring elements) used for this step is based on our understanding of the anatomy of the stomach and adjacent structures and is uniform for all subjects.
Whereas experienced technicians require ∼3 h to manually segment the images required to measure volume, this program did so in <5 min, which is over 30 times faster. Air and labeled fluid can be segmented using semiautomated threshold connectivity methods (25) but nearly always require limited manual correction to ensure the automatically extracted regions do not violate the stomach wall boundary. The semiautomated process used in this study relied on standard image processing techniques and in a minority of cases required only minimal refinement by an observer. Although the streamlined workflow for automatic segmentation of stomach volumes is not currently distributed with commercial versions of Analyze, the steps used to perform the segmentations can be reproduced using various programs in Analyze. Commercially available software packages include several functions (e.g., automatic thresholding, spatial filtering, region growing, and mathematical morphology) that were used in this project. However, to our knowledge, most commercially available products do not incorporate certain essential features that are necessary to manage the inherent variability of intensities in gastric MRIs. These features include, but are not limited to, structuring element size, inhomogeneity correction, and grayscale and automatic threshold selection (5, 24). Similar approaches are used to segment regions and estimate volumes using MRI in the brain and computed tomography in the lung (15, 16).
The image analysis algorithms developed for this semiautomated process were customized to analyze volume images acquired by a specific novel MRI technique. However, these algorithms can be readily modified for images acquired by different techniques or MR protocols. The 3D acquisition was designed to reduce respiratory misregistration and improve accuracy. Because patients successfully held their breath during the acquisition, breathing artifact was minimal. A bright intragastric signal is essential for semiautomated segmentation. However, native gastric fluid content, which is not labeled with gadolinium, is not distinctly visible in fasting images acquired by a T1-weighted sequence. Whereas baseline fluid content appears bright on T2-weighted images, to image the entire volume of interest in a single 20-s breath hold with a 3D T2-weighted turbo- or fast-spin-echo sequence would likely result in unacceptable loss of T2 contrast because of high turbo factors and loss of signal-to-noise ratio due to high acceleration factors.
In summary, the volume of gastric food and air in MRIs can be measured by a semiautomated process with comparable accuracy and far better efficiency than a manual process. These validated algorithms should facilitate increased utilization of MRI for evaluating gastric volumes, emptying, and motility, both in research and in clinical practice.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-DK-68055.
DISCLOSURES
As acknowledged in the paper, Dr. Robb, Mr. Camp, Mr. Karwoski and Mayo Clinic have a significant financial interest in technologies they have developed that are used in this research. This development and research have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are being conducted in compliance with Mayo Clinic Conflict of Interest policies.
AUTHOR CONTRIBUTIONS
Author contributions: A.E.B., J.L.F., S.J.R., and A.R.Z. conception and design of research; A.E.B. and J.L.F. performed experiments; A.E.B., R.A.K., J.L.F., D.R.H., and A.R.Z. analyzed data; A.E.B., J.L.F., and A.R.Z. interpreted results of experiments; A.E.B. and R.A.K. prepared figures; A.E.B. and R.A.K. drafted manuscript; A.E.B., R.A.K., J.L.F., D.R.H., R.A.R., S.J.R., and A.R.Z. edited and revised manuscript; A.E.B., R.A.K., J.L.F., D.R.H., R.A.R., S.J.R., and A.R.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the excellent technical support provided by Xin Ge and Jon Camp.
REFERENCES
- 1.Banerjee S, Fox MR, Schwizer W, Fried M, Pal A. W1152 a novel image analysis tool ‘MRI3D’ for detailed assessment of gastrointestinal structure in three dimensions. Gastroenterology 138: S1–S662, 2010 [Google Scholar]
- 2.Bharucha AE, Manduca A, Lake DS, Fidler J, Edwards P, Grimm RC, Zinsmeister AE, Riederer SJ. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil 23: 617-e252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Comparing two methods of clinical measurement: a personal history. Int J Epidemiol 24 S7–S14, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol 1: 264–272, 2003 [PubMed] [Google Scholar]
- 5.Brinkmann BH, Manduca A, Robb RA. Optimized homomorphic unsharp masking for MR grayscale inhomogeneity correction. IEEE Trans Med Imaging 17: 161–171, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics 59: 849–858, 2003 [DOI] [PubMed] [Google Scholar]
- 7.de Zwart IM, Haans JJL, Verbeek P, Eilers PH, de Roos A, Masclee AA. Gastric accommodation and motility are influenced by the barostat device: assessment with magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol 292: G208–G214, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Feinle C, Kunz P, Boesiger P, Fried M, Schwizer W. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut 44: 106–111, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler J, Bharucha AE, Camilleri M, Camp J, Burton D, Grimm R, Riederer SJ, Robb R, Zinsmeister AR. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil 21: 42–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruehauf H, Goetze O, Steingoetter A, Kwiatek M, Boesiger P, Thumshirn M, Schwizer W, Fried M. Intersubject and intrasubject variability of gastric volumes in response to isocaloric liquid meals in functional dyspepsia and health. Neurogastroenterol Motil 19: 553–561, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fruehauf H, Steingoetter A, Fox MR, Kwiatek MA, Boesiger P, Schwizer W, Fried M, Thumshirn M, Goetze O. Characterization of gastric volume responses and liquid emptying in functional dyspepsia and health by MRI or barostat and simultaneous C-acetate breath test. Neurogastroenterol Motil 21: 697-e637, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Goetze O, Steingoetter A, Menne D, van der Voort IR, Kwiatek MA, Boesiger P, Weishaupt D, Thumshirn M, Fried M, Schwizer W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am J Physiol Gastrointest Liver Physiol 292: G11–G17, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Goetze O, Treier R, Fox M, Steingoetter A, Fried M, Boesiger P, Schwizer W. The effect of gastric secretion on gastric physiology and emptying in the fasted and fed state assessed by magnetic resonance imaging. Neurogastroenterol Motil 21: 725-e742, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Heimann T, Meinzer HP. Statistical shape models for 3D medical image segmentation: a review. Med Image Anal 13: 543–563, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hohne KH, Hanson WA. Interactive 3D segmentation of MRI and CT volumes using morphological operations. J Comput Assist Tomogr 16: 285–294, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 20: 490–498, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology 130: 296–303, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kunz P, Crelier GR, Schwizer W, Borovicka J, Kreiss C, Fried M, Boesiger P. Gastric emptying and motility: assessment with MR imaging–preliminary observations. Radiology 207: 33–40, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, Fruehauf H, Boesiger P, Fried M, Schwizer W, Fox MR. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol 297: G894–G901, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Marciani L. Assessment of gastrointestinal motor functions by MRI: a comprehensive review. Neurogastroenterol Motil 23: 399–407, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Mundt MW, Hausken T, Samsom M. Effect of intragastric barostat bag on proximal and distal gastric accommodation in response to liquid meal. Am J Physiol Gastrointest Liver Physiol 283: G681–G686, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Mundt MW, Samsom M. Fundal dysaccommodation in functional dyspepsia: head-to-head comparison between the barostat and three-dimensional ultrasonographic technique. Gut 55: 1725–1730, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkman HP, Camilleri M, Farrugia G, McCallum RW, Bharucha AE, Mayer EA, Tack JF, Spiller R, Horowitz M, Vinik AI, Galligan JJ, Pasricha PJ, Kuo B, Szarka LA, Marciani L, Jones K, Parrish CR, Sandroni P, Abell T, Ordog T, Hasler W, Koch KL, Sanders K, Norton NJ, Hamilton F. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil 22: 113–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddi SS, Rudin SF, Keshavan HR. An optimal multiple threshold scheme for image segmentation. IEEE Trans Syst Man Cybern 14: 661–665, 1984 [Google Scholar]
- 25.Robb RA. Biomedical Imaging, Visualization and Analysis. New York, NY: Wiley, 1999 [Google Scholar]
- 26.Runge VMMD. Current technological advances in magnetic resonance with critical impact for clinical diagnosis and therapy. Invest Radiol 48: 869–877, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Schwizer W, Fraser R, Borovicka J, Crelier G, Boesiger P, Fried M. Measurement of gastric emptying and gastric motility by magnetic resonance imaging (MRI). Dig Dis Sci 39: 101S–103S, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Schwizer W, Maecke H, Fried M. Measurement of gastric emptying by magnetic resonance imaging in humans. Gastroenterology 103: 369–376, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Schwizer W, Steingotter A, Fox M, Zur T, Thumshirn M, Bosiger P, Fried M. Non-invasive measurement of gastric accommodation in humans. Gut 51: i59–i62, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soille P. Morphological Image Analysis; Principles and Applications. New York, NY: Springer, 2010 [Google Scholar]
- 31.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology 130: 1466–1479, 2006. [erratum appears in Gastroenterology 131: 336, 2006] [DOI] [PubMed] [Google Scholar]
- 32.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 65: 1456–1479, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Treier R, Steingoetter A, Weishaupt D, Goetze O, Boesiger P, Fried M, Schwizer W. Gastric motor function and emptying in the right decubitus and seated body position as assessed by magnetic resonance imaging. J Magn Reson Imaging 23: 331–338, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983 [DOI] [PubMed] [Google Scholar]


