Abstract
Rationale
Findings from animal studies and human PET imaging indicate that nicotine and cigarette smoking affect glutamate (Glu) and related neurochemical markers in the brain, and imply that smoking reduces extracellular Glu. As Glu release is mediated by nicotinic acetylcholine receptors (nAChRs), which are present at high concentrations in the thalamus, we examined effects of smoking on thalamic Glu.
Objective
To determine the effects of tobacco smoking on thalamic glutamate levels.
Methods
Thalamic Glu levels were measured in vivo in 18 smokers and 16 nonsmokers using proton magnetic resonance spectroscopic imaging (1H MRSI) at 1.5 T.
Results
Mean Glu levels did not differ significantly between the subject groups. However, within smokers, Glu levels were negatively correlated with self-reports of both cigarettes/day over last 30 days (r = −0.64, p = 0.006) and pack-years of smoking (r = −0.66, p = 0.005).
Conclusions
Consistent with expectations based on preclinical studies, within smokers cigarettes/day and pack-years are associated with reduced Glu in thalamus, a brain region rich in nAchRs. These results encourage work on candidate glutamatergic therapies for smoking cessation and suggest a non-invasive metric for their action in the brain.
Keywords: Magnetic resonance spectroscopy (MRS), Smoking, Thalamus, Glutamate
Introduction
Despite well-known untoward effects of smoking and the fact that the number of smoking-attributable deaths in the Unites States has begun to decline, smoking remains a leading cause of mortality (Rostron 2013). Tobacco dependence, an important public health problem, is therefore a critical target for pharmacotherapy. The most useful approaches for smoking cessation involve direct interactions with nicotinic acetylcholine receptors (nAChRs), but dopaminergic and glutamatergic neurotransmission are also involved in tobacco dependence (D’Souza & Markou 2011). A more thorough understanding of how smoking interacts with these and other neurochemical systems may help identify new treatments to facilitate smoking cessation.
Glutamate (Glu) is the major excitatory neurotransmitter in the mature mammal brain. Using the novel positron emission tomography (PET) tracer [11C]ABP688, a ligand for metabotropic glutamate receptor 5 (mGluR5), Akkus et al. (2013) recently demonstrated markedly lower distribution volume ratio throughout the cerebral cortex and in subcortical gray-matter areas, including thalamus, in current chronic adult cigarette smokers compared to non-smokers (p < 0.0001-0.005), using mGluR5 binding in cerebellum as a reference value. In a third group of participants who were ex-smokers (mean 25 weeks abstinent), mGluR5 binding partially recovered. This small but rigorous study offers the first dramatic direct evidence of action of chronic smoking on central glutamatergic metabolism in living humans. It is preceded by multiple rodent studies documenting effects of nicotine self-administration on glutamate receptor markers in targeted brain regions. For example, nicotine self-administration upregulates N-methyl-D-aspartate receptor (NMDAR) subunit expression in the ventral tegmental area and the amygdala (Kenny et al. 2009), and withdrawal from nicotine self-administration down-regulates metabotropic glutamate 2/3 receptors (mGluR2/3) in the nucleus accumbens shell, ventral tegmental area, prefrontal cortex, hypothalamus and hippocampus (Liechti et al. 2007).
Importantly, rats self-administering nicotine showed decreased expression of the cysteine-glutamate exchanger (xCT) in the astrocyte membrane in the nucleus accumbens and the ventral tegmental area, and of the excitatory amino acid transporter (EAAT2 or GLT-1) in the nucleus accumbens (Knackstedt et al. 2009). These findings are fully analogous to earlier results with self-administered cocaine (reviewed in Kalivas 2009). Due to the functions of these proteins in transmembrane Glu transport, the reductions in xCT and EAAT2 are associated with decreased basal levels of extracellular extrasynaptic Glu during periods of drug withdrawal or active consumption (“relapse”) relative to the drug-naïve state. A complication is that, due to action of neuronal mGluR2/3s, extracellular synaptic levels of Glu in contrast are elevated during withdrawal and active consumption. For the case of cocaine (Baker et al. 2003; Madayag et al. 2007; McFarland et al. 2003), net (synaptic+extrasynaptic) extracellular Glu levels, measured by microdialysis, are ~50% below normal following chronic exposure. An analogous experiment with nicotine has yet to be done. However, in a small clinical trial, administration of the xCT activator N-acetyl-cysteine decreased the number of cigarettes smoked by nicotine-dependent individuals (Knackstedt et al. 2009), underlining the potential clinical relevance of glutamatergic mechanisms to smoking cessation.
At least in coarse fashion, proton magnetic resonance spectroscopy (MRS) and its multivoxel variant magnetic resonance spectroscopic imaging (MRSI) afford a safe, non-invasive assay of Glu in living humans. The signal measured thereby represents all MR-visible Glu in the voxel sampled, i.e., synaptic+extrasynaptic extracellular and intracellular Glu in neurons, glia, and any other proximal cells. Notwithstanding this limited specificity, it is plausible that a drop on the order of the above-mentioned 50% in the extracellular compartment might detectably reduce the aggregate MRS Glu signal. To investigate this question, we acquired MRSI in otherwise healthy, chronic adult smokers and healthy nonsmoker controls. Despite its prominence in the preclinical findings, we eschewed the nucleus accumbens as a target nucleus. Its small size and irregular geometry (Ballmaier et al. 2004) make it challenging to interrogate with MRS. Instead, we sampled the thalamus, which is comparatively large, smoothly ovoid in gestalt, and typically returns high-quality MR spectra. The choice of thalamus as target was also motivated by reasons relevant to the neurophysiology of smoking. The thalamus has a high density of nAChRs in humans (e.g., Horti et al. 1998), particularly α7 nAChRs (Rubboli et al. 1994a,b). Agonists of α7 nAChRs regulate Glu release in rodent and human in vitro preparations of cerebral cortex (Rousseau et al. 2005; Marchi et al. 2002; Livingstone et al. 2010). Nicotine stimulates the transient release of synaptic Glu via interactions at nAChRs of multiple subtypes, including α7 (Konradsson-Geuken et al. 2009). This effect may in part account for the acute ability of nicotine to enhance focused attention and other executive functions (Sarter et al. 2009). We considered the possibility that ongoing frequent exposure to nicotine in cigarette smoke might contribute to altered thalamic Glu levels in smokers.
Relatively few prior MRS investigations have examined smoking (Durazzo et al. 2004, 2006; Epperson et al. 2005; Gallinat et al. 2007; Gazdzinski et al. 2008; Mason et al. 2006; Wang et al. 2009); and of these, only one has reported on the thalamus, but apparently glutamatergic resonances were not included in the spectral analyses (Durazzo et al. 2004, 2006). Therefore, we used short echo-time (TE) MRSI to compare Glu levels between smokers and nonsmokers; and within smokers, we assessed their relationships to recent (cigarettes per day) and lifetime (pack-years) smoking. Short-TE facilitated quantitation of Glu; the high spatial resolution of MRSI enabled us to place voxels entirely or nearly entirely within the thalamus, to sample the thalamus bilaterally, and thus to test for potentially lateralized effects on Glu.
Methods
Participants
Participants were recruited via local print and radio advertisements, and were designated as smokers or nonsmokers. Participants in both groups were required to have at least moderate intelligence (score > 85 on the Shipley Institute of Living Scale; Zachary et al. 1985), to be right-handed (score > 20 on modified Edinburgh Handedness Test; Oldfield 1971), and to be taking no prescription medications or herbal products (e.g., Ginkgo biloba) expected to affect brain function. Any current Axis I psychiatric disorder, including a substance dependence disorder other than nicotine dependence (for smokers) was exclusionary. Past history of Axis I disorders was not exclusionary. One smoker had a history of THC abuse; one smoker had a lifetime diagnosis of alcohol dependence; and one nonsmoker had a lifetime diagnosis of anorexia nervosa. Thalamic Glu levels and cigarettes/day over last 30 days and pack-years for these three participants were in the mid-range for their respective groups. In-person screening for psychiatric disorders included the Structured Clinical Interview for DSM-IV (SCID; First et al. 2002). To test for possible sub-syndromal depression and/or anxiety in smokers (Peiper & Rodu 2013), the Beck Depression Inventory (BDI; Beck et al. 1996) and the Spielberger State Trait Anxiety Inventory (Spielberger 1983) were administered. Detailed drug-use data were obtained using the Addiction Severity Index (McClellan et al. 1980). Participants also underwent urine screening for use of cocaine, methamphetamine, opioids, Δ9-tetrahydrocannabinol and benzodiazepines. All participants with a history of notable head trauma, neurological disease, cardiovascular or pulmonary disease, or other significant major medical conditions, such as seropositivity for HIV, were excluded. Light marijuana consumption (≤ 1 joint per week) was permitted in both groups, but positive urine for other illicit drugs-of-abuse was exclusionary. Ethanol consumption was limited to ≤ 14 drinks per week for both groups and did not differ significantly between groups. After receiving a detailed explanation of the study, all subjects gave written informed consent. The study was approved by the UCLA Office for Protection of Human Subjects.
Assessment of tobacco use
Recent smoking was verified by carbon monoxide (CO) levels in expired air ≥ 10 ppm (Micro™smokerlyzer®; Bedfont Scientific Ltd, Kent, UK) and the presence of urinary cotinine (≥ 200 ng/ml by Accutest® NicAlert strips; JANT Pharmacal Corporation, Encino, CA). Nonsmokers were required to have less than 3 ppm CO and a negative test for cotinine. Smokers were asked about duration of tobacco abuse (years of smoking) and current level of tobacco abuse (cigarettes smoked per day over last 30 days before testing). Lifetime exposure to tobacco was computed as pack-years (the product of packs per day and years of smoking). Severity of current nicotine dependence was determined by the Fagerström Test for Nicotine Dependence (FTND; Fagerström et al. 1990).
Magnetic resonance acquisition
1H MRSI and prescriptive structural MRI were acquired together in a session lasting up to 2 h. Acquisitions were performed on a 1.5-T magnetic resonance scanner (Siemens Sonata) using a standard quadrature head coil. In addition to MRSI, scanning sequences included a localizer scout and a high-resolution T1-weighted whole-brain MRI. Water-suppressed PRESS 1H MRSI (TR 1500 ms, TE 30 ms, 8 averages, in-plane resolution 11 × 11 mm2, slab thickness 9 mm, and nominal voxel size 1.1 cc) was acquired from a two-dimensional axial-oblique slab (Fig. 1) oriented parallel to an imaginary line connecting the genu and splenium of the corpus callosum in the sagittal plane. This slab was centered dorsoventrally on the thalamus as seen sagitally. The slab was followed by an identical acquisition (only 1 average) with water-suppression turned off. The effective voxel size is larger than the nominal voxel size because the voxel is modestly smeared by the point-spread function of MRSI. The effective voxel size is not trivial to calculate; using factors obtained for other MRSI techniques (Théberge et al. 2005, Posse et al. 2007), we estimate roughly it roughly at 1.3-1.4 cc.
Fig. 1.
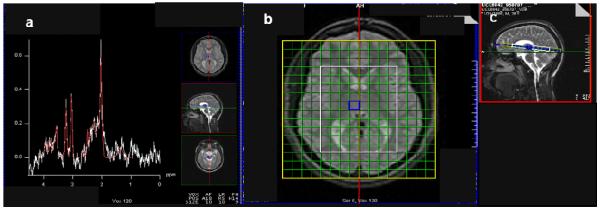
1H MRSI spectrum (a) acquired from right mesial thalamus of subject (PRESS, 1.5 T, repetition time/echo-time = 1500/30 ms). Glutamate and overlapping glutamine (together “Glx”) present as the shoulder in the 2.3-2.4 ppm range. Axial-oblique (b) and sagittal (c) MRI of the brain depicting 9-mm thick MRSI slab of 11×11-mm2 voxels. The PRESS volume (white; ~8×10 voxels) was aligned parallel to the genu-splenium line and fit to the subject. It sampled bilateral thalamus, and environs. The blue square in (b-c) denotes voxel yielding MR spectrum in (a).
Magnetic resonance post-processing
The T1-weighted volume of each subject was transformed into ICBM152 stereotaxic space (Mazziotta et al. 1995), skull-stripped, and segregated into gray-matter, white-matter, and CSF sub-volumes using the Statistical Parametric Mapping 5 (SPM5) software suite (Frackowiak et al. 1997). Using FSL FIRST version 2.1 (Patenaude et al. 2011), two regions-of-interest, representing left and right thalamus, were extracted automatically from the whole-brain T1-weighted volume, and then fine-edited by trained human operators. Each whole-brain tissue sub-volume and region-of-interest was converted into a binary mask and transformed back into native space.
MRSI spectra were fit automatically with LCModel (Provencher 2001), yielding fitted peaks with absolute metabolite levels referenced to the unsuppressed water resonance and expressed in institutional units (IU). Peaks fit included Glu as well as other metabolites, which were not evaluated in this study, and other compounds with weaker resonances. The MRSI Voxel Picker (MVP) software suite (Seese et al. 2011) was used for MRI/MRSI co-processing. The T1-weighted volume, and the gray-matter, white-matter, CSF, and thalamic binary masks were imported into MVP, as well as the MRSI raw-data file and LCModel output. For each subject’s MRSI slab, MVP reconstructed the T1-weighted volume and displayed it on a guided user interface (GUI) in register with the corresponding MRSI PRESS volume in its plane-of-acquisition. MVP similarly reconstructed each mask and performed two independent calculations of the tissue composition of each MRSI voxel. The first calculation determined the percentage of the 1.1-cc voxel volume that was occupied by gray matter of any kind, thalamic or otherwise (“whole-brain gray matter”), and similarly whole-brain white matter and CSF. These endpoints are referred to as “vol%” whole-brain gray matter, white matter, and CSF. The second calculation determined the percentage of the voxel volume that was occupied by left thalamus and the percentage occupied by right thalamus (vol% left thalamus, vol% right thalamus). (Note that a few midline voxels contained both left and right thalamus.) Then MVP corrected the LCModel-derived levels of each metabolite for voxel CSF content. Quality control of MRSI spectra was also implemented automatically by MVP, supplemented by operator inspection. Only those spectra that exhibited line-width ≤ 0.1 ppm and signal-to-noise ratio ≥ 5 were retained. Furthermore, within each voxel, only those Glu values that were considered reliable by LCModel (standard deviation of metabolite signal < 20%) were retained. Voxels were selected by a blinded operator on the MVP GUI in left and right hemisphere thalamus. Within the thalami, MVP averaged metabolite levels for all voxels that contained ≥ 70 vol% left and right thalamus, respectively, and that satisfied the other aforementioned quality-control criteria. Voxel tissue content did not differ significantly between the subject groups.
Statistical analysis
As Glu levels did not differ significantly between left and right thalamus, results were collapsed across the two, and the average of left and right thalamus was used for analysis.
Implementation of the aforementioned quality-control criteria resulted in rejection of Glu data from one smoker and one nonsmoker. Therefore, before analysis of metabolite levels, the subject groups were compared for potential imbalances in sex, age, and/or MRSI voxel tissue. Sex differences were compared using the χ2-test. Age and MRSI voxel tissue composition differences were compared using independent samples t-tests.
The potential between-group difference in thalamic Glu was assessed using univariate analysis of covariance (ANCOVA), with participant group (smoker and nonsmoker) as the between-subjects factor, the Glu metabolite level as the dependent measure, and age included as a covariate. Within the smoker group, separate correlations were examined between cigarettes per day over last 30 days and Glu and between pack-years and Glu (partialling age).
Results
Research participants
The final sample included 18 otherwise healthy, adult smokers and 16 healthy nonsmoker comparison subjects. The two groups did not differ in age [nonsmokers: mean (SEM) = 33.2 years (2.6); smokers: 35.4 years (2.1); t = −0.65, p = 0.52], sex (nonsmokers: 8 males, 8 females; smokers: 11 males, 7 females; χ2 = 0.42, p = 0.52), ethnicity [nonsmokers: 7 Caucasian, 3 African American, 4 Hispanic/Latino, 2 Asian American; smokers: 11 Caucasian, 6 African American, 1 Hispanic/Latino, 0 Asian American; χ2 = 5.59, p = 0.13], or years of education [nonsmokers: 13.8 years (0.5); smokers: 13.1 years (0.4); t = −1.2, p = 0.24]. The two groups did not differ significantly in BDI score [nonsmokers: 3.3 (1.0); smokers: 1.4 (0.5); t = −1.5, p = 0.14], nor in Spielberger Trait [nonsmokers: 25.9 (1.5); smokers: 26.5 (1.6); t = 0.3, p = 0.76] or State [nonsmokers: 28.3 (1.4); smokers: 27.6 (1.4); t = −0.4, p = 0.72] Anxiety. Within the smoker group (see Table 1), years of tobacco smoking ranged from 4.5 - 35 years, self-report of cigarettes per day over last 30 days ranged from 3.0 - 27.5, pack-years ranged from 0.75 - 35.0, and Fagerström scores ranged from 1 - 8.
Table 1.
Demographics and clinical variables for the two participant groups
| Smokers | Nonsmokers | |
|---|---|---|
| N(male/female) | 18(11/7) | 16(8/8) |
| Age, years | 35.4(2.1) | 33.2(2.6) |
| Ethnicity | 11 Caucasian | 7 Caucasian |
| 6 African | 3 African | |
| American | American | |
| 1 Hispanic/Latino | 4 Hispanic/Latino | |
| 0 Asian American | 2 Asian American | |
| Education, years | 13.1(0.4) | 13.9(0.5) |
| Beck Depression Inventory score | 1.4(0.5) | 3.3(1.0) |
| Spielberger Trait Anxiety | 26.5(1.6) | 25.9(1.5) |
| Spielberger State Anxiety | 28.3(1.4) | 27.6(1.0) |
| Years of Smoking | 16.9(2.1) | 0 |
| Cigarettes/day over last 30 days | 17.3(1.3) | 0 |
| Pack-years | 15.2(2.3) | 0 |
| Fagerström Score c | 4.7 (0.4) | 0 |
Data are means ± SEM.
Effect of smoking on thalamic Glu
A sample 1H MRSI spectrum and fit by LCModel are shown in Fig. 2. Thalamic Glu levels did not differ between groups significantly [nonsmokers (n = 15): mean (SEM) = 7.2 IU (0.3); smokers (n = 17): 6.89 IU (0.3); F1,29 = 0.60, p = 0.45). However, there was a significant correlation between Glu and self-reports of cigarettes per day over last 30 days (Spearman r = −0.64, p = 0.006; Fig. 3). There was also a significant negative correlation between Glu and pack-years, partialling age (r = −0.66, p = 0.005; Fig. 3). Similar negative relationships were observed between Glx (glutamate + glutamine) and cigarettes per day over last 30 days (r = −0.51, p = 0.029) and between Glx and pack-years (r = −0.55, p = 0.017). Age per se was not a significant predictor of thalamic Glu levels. Nor was FTND score related to thalamic Glu levels.
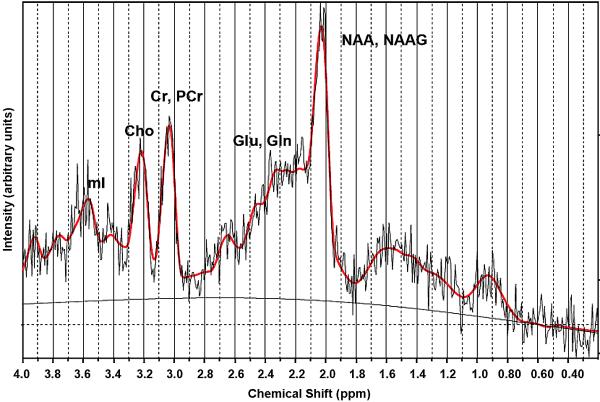
Fig. 2.
1H MRSI raw (light jagged black trace), LCModel fit (red), and baseline (smooth black) spectra for a representative voxel acquired as in Fig. 1. Principal resonances are labelled for N-acetyl-asparatate (NAA) and N-acetyl-aspartyl-glutamate (NAAG), glutamate (Glu) and glutamine (Gln), creatine (Cr) and phosphocreatine (PCr), choline-compounds (Cho), and myo-inositol (mI). Both Glu and Glx were fit by LCModel with < 20% standard deviation.
Fig. 3.
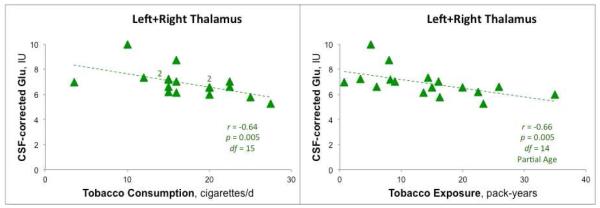
Left: Negative correlation (2-way Spearman) of glutamate (Glu) levels in thalamus (average of left and right) with cigarettes per day over last 30 days. Note: There are data overlapping from two participants at the points (15,7.2) and (20,6.6), each marked by “2”, for a total of 17 observations and 15 degrees-of-freedom. Right: Correlation with pack-years (2-way Spearman, participant age partialled out). Individual smokers represented by green triangles. Metabolite levels in Institutional Units (IU) corrected for voxel CSF content. Higher levels of tobacco use are associated with lower thalamic Glu. Similar results were observed for Glx (Glx = Glu + glutamine).
Discussion
In this study, both recent and cumulative cigarette smoking were correlated negatively with thalamic Glu level, as indexed by MRSI. When considered along with the effects of nicotine self-administration in rodents (e.g., Liechti et al. 2007; Kenny et al., 2009; Knackstedt et al. 2009), and reduced mGluR5 binding in the thalamus and other brain regions of smokers (Akkus et al. 2013), these data suggest that exposure to nicotine and perhaps other agents in cigarette smoke alters the glutamatergic biochemistry of the thalamus.
Glutamate and tobacco exposure
The negative association of thalamic Glu with recent and lifetime exposure to tobacco smoking was statistically significant, even when metabolite levels were age-corrected. With the exception of Mason et al. (2006) who, after adjusting for local N-acetyl-aspartate+N-acetyl-aspartyl-glutamate levels, found higher Glx in occipital cortex in smoking compared to non-smoking alcoholics, prior MRS investigations of smoking have not uncovered effects on glutamatergic metabolites (Gallinat & Schubert 2007). Other studies investigated regions such as the occipital cortex (Mason et al. 2006, Epperson et al. 2005), hippocampus (Gallinat et al. 2006; Gallinat & Schubert 2007; Gazdzinski et al. 2008), cingulate cortex (Gallinat & Schubert 2007), or white matter (Wang et al. 2009), where the relationship of Glu to smoking may differ from that presently reported for the thalamus. Since Durazzo et al. (2004, 2006) apparently did not include glutamatergic resonances in their spectral analyses, relationships between thalamic Glu and tobacco exposure in their sample are unknown.
Glu is a major neurotransmitter (Conti & Weinberg 1999) and endogenous excitotoxin (for review see Schousboe & Frandsen 1995). It is also a substrate for energy metabolism (Petroff et al. 2000) and is an osmolyte (Ross & Blüml 2001) that regulates the cell-water byproduct of energy catabolism. Therefore, the association of lower Glu with smoking in the present study may represent local changes in glutamatergic neurotransmission, energy metabolism, or osmotic conditions. The present Glu-related findings join other evidence (reviewed in Cryan et al. 2003; Jain et al. 2008) associating central glutamatergic systems, in particular N-methyl-D-aspartate (NMDA) receptors, with smoking, and inspiring potential pharmacotherapies that target glutamatergic neurotransmission to aid smoking cessation (D’Souza & Markou 2011).
In particular, the thalamus is one of several brain regions in which [11C]ABP688-PET demonstrated lower mGluR5 binding in smokers than in nonsmokers (Akkus et al. 2013). Our finding of diminished thalamic Glu with increased smoking is consistent with the notable drop in extracellular Glu measured or postulated in other brain regions (Baker et al. 2003; Madayag et al. 2007; McFarland et al. 2003) in rodents self-administering cocaine or nicotine, due to downregulation of xCT and EAAT2 (Knackstedt et al. 2009; Kalivas 2009). Although overall Glu levels in smokers in our study were within the normal range, it is possible that even small changes have functional significance. In order to address this question, a within-subject longitudinal study of involving measurements before and after smoking cessation and/or using a pro-glutamatergic drug such as N-acetyl-cysteine (Knackstedt et al. 2009) could determine whether increases in thalamic Glu are linked to behavioral measures. The present findings call for further exploration of glutamatergic mechanisms in nicotine dependence and smoking cessation.
Limitations
This study is limited by a small sample size. In addition, MRSI was acquired at 1.5 T; therefore, Glu quantitation was less reliable than at high-field strength (≥ 3 T). It has long been debated whether the spectral dispersion for Glu and glutamine (Gln) at 1.5T is sufficient to allow accurate quantitation of Glu (e.g., Hurd et al. 2004). We recently investigated this question systematically (Zhang 2013) and found, as expected, that under multiple pulse-sequences and conditions (including TE30 PRESS at 1.5 T), spectral fitting was much worse for Gln than for Glu, Gln often being unfittable due to broad linewidths. Considering that variance in Gln could translate into variance in Glx, we opted to report our results principally in terms of Glu. We thus take a position contrary to the frequent advocacy of Glx as the only reliable metric of the concentration of in vivo glutamatergic metabolites. In any case, findings were comparable for Glx and Glu. Future work at higher field strength is encouraged. Strengths of this study, on the other hand, include use of short echo-time MRSI to assay Glu at high spatial resolution, and determination of voxel tissue composition to mitigate partial volume effects. The findings we report here suggest that higher cigarettes/day and pack-years are related to lower glutamatergic neurometabolites in the thalamus, and point to possible involvement of nAChRs.
Acknowledgements
This work was supported by the National Institute on Drug Abuse (P20DA022539, R01DA020726, R03DA20512, and R21DA023192) (EDL and JON), NIMH grants T32MH073517 and K23MH094613 (ELN), the National Center for Research Resources (NIH M01RR00865, UCLA GCRC), and endowments from the Katherine K. and Thomas P. Pike Chair in Addiction Studies, and the Marjorie M. Greene Trust. Research support for projects other than the one reported here was supplied to Dr. Edythe D. London under UCLA Contract (number 20063287) with Philip Morris USA. All experimental procedures comply with the current laws of the United States of America.
Footnotes
Conflict of Interest
None of the authors has a financial relationship with any organization that sponsored this research and none of the sponsors had any involvement with the design, collection, analysis, writing of the manuscript or thedecision to submit the manuscript for publication. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
References
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovage J, Buck A, Hasler G. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A. 2013;110(2):737–742. doi: 10.1073/pnas.1210984110. doi:10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. doi:10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Siddarth P, Blanton RE, Levitt JG, Lee M, Caplan R. Thought disorder and nucleus accumbens in childhood: a structural MRI study. Psychiatry Res. 2004;130:43–55. doi: 10.1016/j.pscychresns.2003.10.001. doi:10.1016/j.pscychresns.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. 2nd Psychological Corporation, Harcourt, Brace; San Antonio, TX: 1996. Manual for the Beck Depression Inventory. [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trend Neurosci. 1999;22(10):451–458. doi: 10.1016/s0166-2236(99)01445-9. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Gasparini F, van Heeke G, Markou A. Non-nicotinic neuropharmacological strategies for nicotine dependence: beyond bupropion. Drug Discov Today. 2003;8(22):1025–1034. doi: 10.1016/s1359-6446(03)02890-3. doi: 10.1016/S1359- 6446(03)02890-3. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6(1):4–16. [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcoholinduced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28(12):1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. doi: 10.1097/01.ALC.0000148112.92525.AC. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30(3):539–551. doi: 10.1111/j.1530-0277.2006.00060.x. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57(1):44–48. doi: 10.1016/j.biopsych.2004.09.021. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69(11):763–765. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- Frackowiak R, Friston K, Frith C, Dolan R, Mazziotta J. Academic Press USA; New York: 1997. Human Brain Function. [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40(2):64–67. doi: 10.1055/s-2007-970144. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162(2):133–145. doi: 10.1016/j.pscychresns.2007.04.003. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horti AG, Scheffel U, Kimes AS, Musachio JL, Ravert HT, Mathews WB, Zhan Y, Finley PA, London ED, Dannals RF. Synthesis and evaluation of N-[11C]methylated analogues of epibatidine as tracers for positron emission tomographic studies of nicotinic acetylcholine receptors. J Med Chem. 1998;41(22):4199–4206. doi: 10.1021/jm980233p. doi: 10.1021/jm980233p. [DOI] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-Averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Jain R, Mukherjee K, Balhara YP. The role of NMDA receptor antagonists in nicotine tolerance, sensitization, and physical dependence: a preclinical review. Yonsei Med J. 2008;49(2):175–188. doi: 10.3349/ymj.2008.49.2.175. doi: 10.3349/ymj.2008.49.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA Receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: Role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34(2):266–281. doi: 10.1038/npp.2008.58. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. doi: 10.1016/j.biopsych.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63(12):1069–1082. doi: 10.1002/syn.20693. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Dickinson JA, Srinivasan J, Kew JN, Wonnacott S. Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU-120596. J Mol Neurosci. 2010;40:172–6. doi: 10.1007/s12031-009-9232-5. doi: 10.1007/s12031-009-9232-5. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–8. doi: 10.1046/j.0022-3042.2002.00805.x. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59(1):85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper N, Rodu B. Evidence of sex differences in the relationship between current tobacco use and past-year serious psychological distress: 2005–2008 National Survey on Drug Use and Health. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1261–1271. doi: 10.1007/s00127-012-0644-0. doi 10.1007/s00127-012-0644-0. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Mattson RH, Rothman DL, Proton MRS: GABA and glutamate. Adv Neurol. 2000;83:261–271. [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry P-G, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Uhurbil K, Lim KO, Alger JR. Proton Echo-Planar Spectroscopic Imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007;58:236–244. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. The Anatomical Record. 2001;265(2):54–84. doi: 10.1002/ar.1058. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Rostron B. Smoking-attributable mortality by cause in the United States: revising the CDC's data and estimates. Nicotine Tob Res. 2013;15(1):238–46. doi: 10.1093/ntr/nts120. doi: 10.1093/ntr/nts120. [DOI] [PubMed] [Google Scholar]
- Rousseau SJ, Jones IW, Pullar IA, Wonnacott S. Presynaptic alpha7 and nonalpha7 nicotinic acetylcholine receptors modulate [3H]D-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci. 1994a;6(10):1596–1604. doi: 10.1111/j.1460-9568.1994.tb00550.x. doi: 10.1111/j.1460- 9568.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Perry E, Clementi F. Distribution of neuronal nicotinic receptor subunits in human brain. Neurochem Int. 1994b;25(1):69–71. doi: 10.1016/0197-0186(94)90055-8. doi: 10.1016/0197-0186(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78(7):658–667. doi: 10.1016/j.bcp.2009.04.019. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Frandsen A. Glutamate receptors and neurotoxicity. In: Stone TW, editor. CNS Neurotransmitters and Neuromodulators: Glutamate. CRC Press; New York: 1995. pp. 239–251. [Google Scholar]
- Seese RR, O'Neill J, Hudkins M, Siddarth P, Levitt J, Tseng B, Wu KN, Caplan R. Proton magnetic resonance spectroscopy and thought disorder in childhood schizophrenia. Schizophr Res. 2011;133(1-3):82–90. doi: 10.1016/j.schres.2011.07.011. doi: 10.1016/j.schres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Consulting Psychology Press; Palo Alto: 1983. Manual for the State-Trait Anxiety Inventory (STAI-Form Y) [Google Scholar]
- Théberge J, Menon RS, Williamson PC, Drost DJ. Implementation issues of multivoxel STEAM-localize 1H spectroscopy. Magn Reson Med. 2005;53:713–718. doi: 10.1002/mrm.20350. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 2009;22(5):516–522. doi: 10.1002/nbm.1363. doi: 10.1002/nbm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA, Paulson MJ, Gorsuch RL. Estimating WAIS IQ from the Shipley Institute of Living Scale using continuously adjusted age norms. J Clin Psychol. 1985;41(6):820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. doi: 10.1002/1097-4679(198511)41:6. [DOI] [PubMed] [Google Scholar]
- Zhang JM. Human Brain Glutamate, Glutamine, γ-Aminobutyric Acid, Proton Magnetic Resonance, Spectral Quantification with the Fast Padé Transform. Doctoral dissertation. UCLA Department of Biomedical Physics. 2013 [Google Scholar]