Abstract
The anti-apoptotic protein Bcl-2 is a well-known and attractive therapeutic target for cancer. In the present study the solution-phase T3P-DMSO mediated efficient synthesis of 2-amino-chromene-3-carbonitriles from alcohols, malanonitrile and phenols is reported. These novel 2-amino-chromene-3-carbonitriles showed cytotoxicity in human acute myeloid leukemia (AML) cell lines. Compound 4g was found to be the most bioactive, decreasing growth and increasing apoptosis of AML cells. Moreover, compound 4g (at a concentration of 5 µM) increased the G2/M and sub-G1 (apoptosis) phases of AML cells. The AML cells treated with compound 4g exhibited decreased levels of Bcl-2 and increased levels of caspase-9. In silico molecular interaction analysis showed that compound 4g shared a similar global binding motif with navitoclax (another small molecule that binds Bcl-2), however compound 4g occupies a smaller volume within the P2 hot spot of Bcl-2. The intermolecular π-stacking interaction, direct electrostatic interactions, and docking energy predicted for 4g in complex with Bcl-2 suggest a strong affinity of the complex, rendering 4g as a promising Bcl-2 inhibitor for evaluation as a new anticancer agent.
Introduction
Programmed cell death, or apoptosis, is the primary mechanism for the removal of aged and damaged cells. Cancer cells can gain a growth advantage over their normal counterpart by either dividing more quickly, not undergoing terminal differentiation and thus remaining in the proliferative pool, or not undergoing apoptosis [1]. On the functional level, interactions between pro-apoptotic proteins such as Bax, Bak, Bad, Bim, Noxa, Puma, and pro-survival proteins such as Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bfl-1 control the regulation of programmed cell death. Cancer cells alter the balance among these opposing factions to undermine normal apoptosis, and thus gain a survival advantage [2], [3]. The first identified apoptotic regulator, Bcl-2, was cloned from human follicular B cell lymphoma cells which nearly invariably have a chromosomal t(14;18) translocation, placing the Bcl-2 gene under the control of the powerful IgG heavy chain promoter [4], [5] with the consequence of elevated levels of Bcl-2 promoting increased cell survival [6]. A common feature in many human tumors is overexpression of the pro-survival Bcl-2 family members Bcl-2 and Bcl-xL, which make tumor cells resistant to conventional cancer therapeutic agents.
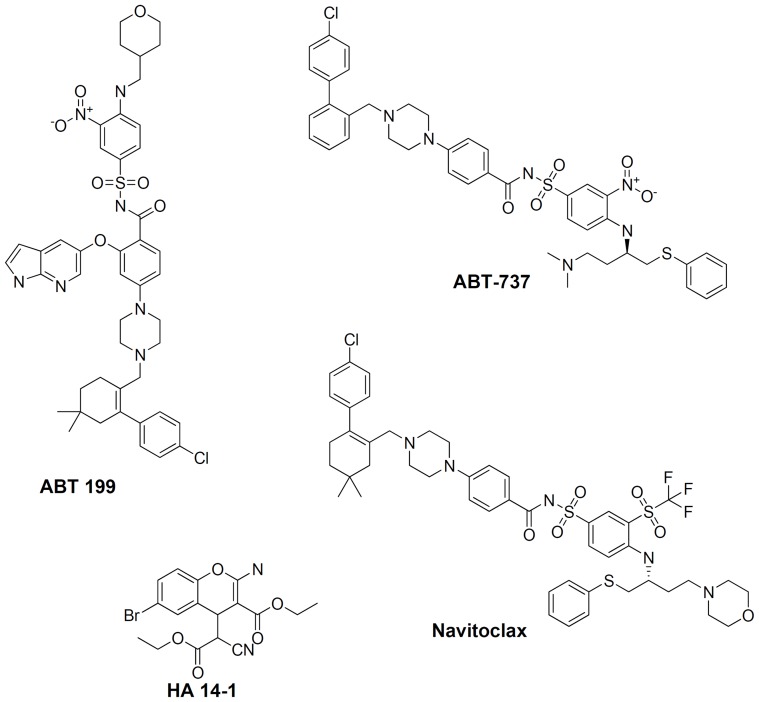
Numerous synthetic small molecules targeting Bcl-2 protein have been studied extensively and few of them have advanced to clinical trials ( Figure 1 ). Structure-based drug design approaches have previously yielded small molecules that bind to Bcl-2 such as navitoclax (ABT-263) [7]. This molecule binds to Bcl-2 and Bcl-xL; unfortunately in clinical trials it caused severe thrombocytopenia due to binding and inhibiting Bcl-xL [8]. Another structure-based synthesis has produced BM-957, a potent small-molecule inhibitor of Bcl-2 and Bcl-xL, which was capable of achieving complete tumor regression in a small lung cancer xenograft model [9]. Similarly, the co-crystal structure of Bcl-2 resulted in identification of a small molecule called ABT-199; a Bcl-2–selective inhibitor approved by the FDA for cancer therapy [10]. The above study strongly suggested that an indole based carbinol inhibited the growth of prostate cancer cells by arresting them in the G1 phase of the cell cycle, leading to apoptosis via down-regulation of Bcl-2.
Figure 1. Known small molecules that target Bcl-2.
As can be seen, different bioactive scaffolds have been established, however both efficacy and avoiding off-target effects of this class of compounds still remains a challenge.
Chromene-based natural and synthetic compounds have contributed substantially to the development of therapeutics as anti-neoplastic agents against various human malignancies [11], [12]. Sesilin, tephrosin, calanone and acronycine are some of the naturally occurring chromene derivatives with a very good anti-cancer activity. An important breakthrough in the development of 4H-chromenes as anti-cancer agents was given by the discovery of HA 14-1, which targets the Bcl-2 protein. It is reported to inhibit Bcl-2 by abrogating the interaction of Bax/Bcl-2, and it has also shown synergistic effect when combined with flavopiridol [13]–[15]. Therefore, the design of new chromene derivatives against anti-apoptotic members of the Bcl-2 family provides a plausible target in cancer therapeutics [16]. We recently reported the synthesis and explored the biological property of chromene-containing benzoxazines and other biologically important heterocyclic libraries [17], [18].
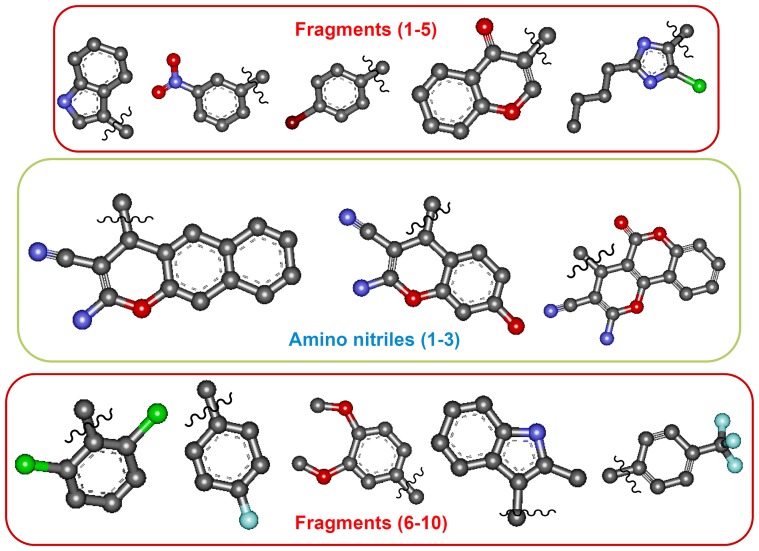
In order to improve the efficacy of chromene-based small molecules as selective inhibitors of Bcl-2, we have in the current work synthesized a library of 2-amino chromene-3-carbonitriles to quantify and compare the molecular diversity between different types of libraries that would interact with Bcl-2 ( Figure 2 ). We also outline the simple graphical approach for describing and comparing molecular diversity within the library of amino-nitriles.
Figure 2. Molecular diversity of amino-nitrils.
Structural representation (ball and stick model) of the combinatorial libraries of Bcl-2 inhibitors that depicts top and bottom row side chains, which are incorporate to three amino nitrile scaffolds.
Materials and Methods
Synthesis and characterization of various 2-amino-chromene-nitriles were provided as supplementary data (Data S1 and Table S1)
Cell Culture
Myeloid leukemia cell lines; MOLM13 and MV4-11 were kind gifts by Dr. Martin Grundy (Department of Academic Haematology, University of Nottingham) [28]. MOLM14 cells were generously provided by Dr. Didier Bouscary (Department of Hematology-Immunology, Institut Cochin, Paris, France) [29]. The HL-60 cell line was purchased from ATCC. Cell lines were cultured and maintained in RPMI medium containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere with 5% CO2. All cell lines were regularly screened for absence of mycoplasma. Bone marrow cells from C57BL/6 mice were cultured in IMDM supplemented with 20% FBS and cytokines (10 ng/ml recombinant mouse IL-3, 10 ng/ml recombinant mouse IL-6 and 50 ng/ml recombinant mouse Stem Cell Factor (SCF). Antibodies against Bcl-2, Bcl-xL, Cleaved caspase-9 and GAPDH were purchased from Cell Signalling. ABT-737 was used as a reference compound for all the experiments.
Cell proliferation assay
Cellular proliferation of AML cell lines was measured by using methylthiazolyl-diphenyl-tetrazolium bromide (MTT; sigma-aldrich). 8,000 cells per well were seeded in triplicate, 96-well plates (Corning, Lowell, MA, USA) in 100 µl of medium having 2-amino-chromene-nitriles derivatives. Cells were incubated overnight at 37°C in a CO2 incubator. At the end of the culture duration, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added into each well, and the final concentration of MTT in each well was 0.5 mg/ml. MTT plates were incubated at 37°C in a CO2 incubator for 3 hr. After incubation, Formazan crystals were dissolved in 100 µl of stop solution (SDS-HCl). Absorbance was measured at 570 nm using a Tecan Infinite 200 PRO spectrophotometer (Mannedorf, Switzerland).
Annexin V and propidium iodide (Annexin V–PI) apoptosis assays
HL-60, MOLM13, MOLM14 and MV4-11 (1×105) cells were seeded into each well of a 6-well plate and treated with 4g at the IC50 concentration for 48 and 72 hours. Staining was performed using FITC Annexin V Apoptosis Detection Kit II, BD Pharmingen (BD Biosciences, USA) according to the manufacturer protocol. Briefly, cells were washed with PBS at least three times, re-suspended in 1× binding buffer, and FITC-conjugated annexin V and propidium iodide (PI) was added for 15 min in the dark. Samples were analyzed by a LSR-II flow cytometer (Becton-Dickinson, San Jose, CA, USA).
Immunoblot analysis
Cell lysates were prepared using the ProteoJET Mammalian Cell lysis reagent (Fermentas) and the 1× protease inhibitor mixture (Roche Molecular Biochemicals, Pleasanton, CA). Immunoblotting was performed as described earlier [30]. Monoclonal antibodies against Bcl-2, GAPDH and cleaved caspase-9 (Cell Signalling, Boston, MA, USA) were used.
In Vitro analysis of the effect of amino-nitriles against Bcl-2
Zymed Bcl-2 ELISA kit was used for the evaluation of the binding of small molecules to Bcl-2. Initially various concentrations of small molecules and the human Bcl-2 was incubated for 5 minutes and transferred the mixture to the mAb coated 96-well plate. The bound Bcl-2 was tagged with anti-Bcl-2 that conjugated with biotin. The biotin conjugate was bound with streptauvidin-HRP. The Streptavidin-HRP was reacted with TM and the absorbance is measured at 450 nm. A standard curve is prepared to determine the Bcl-2 concentration and% inhibition of the Bcl-2 binding to its antibody was presented.
Molecular docking analysis
The molecular modeling was achieved with commercially available InsightII, Discovery Studio (DS) Version 2.5 software packages. Initially, the 3D structure of Bcl-2 was cleaned and the navitoclax binding site was considered for further analysis. All of the calculations were performed using the CHARMM force field as reported previously [31]. Each energy-minimized final docking position of the individual apigenin structural analogues was evaluated using the interaction score function in the CDOCKER module of DS version 2.5 as reported previously from our group [32]. Based on the low CDOCKER energy value, compound 4g was selected for further studies.
Results and Discussion
Synthesis of novel 2-amino chromene-3-carbonitriles derivatives
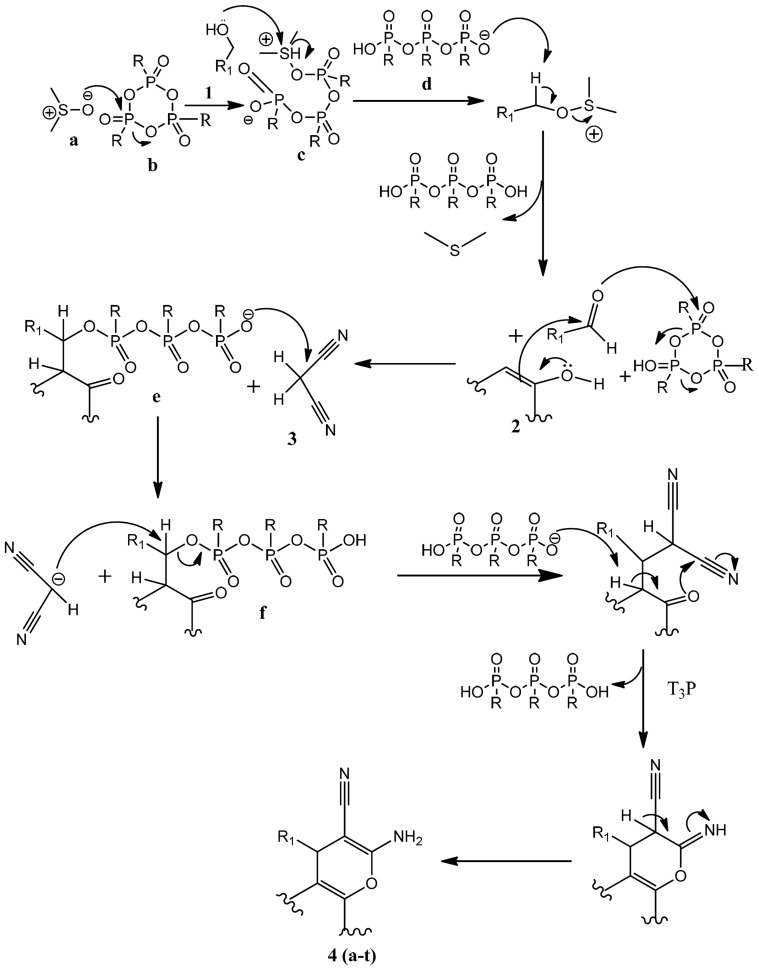
Naturally occurring 2-amino-carbonitriles containing pyrano[3,2-c]pyridine-based structural motifs are commonly found in alkaloids and exhibit diverse biological activities, including anti-cancer activity [19]. A series of pyrano[3,2-c]pyridine based flavone and coumarin isosteres containing a 2-amino carbonitrile moiety have also exhibited a broad spectrum of antitumor activity against breast cancer cells [20]. In continuation of our efforts to develop efficient solution phase synthesis of novel small molecules with anti-cancer agents, we reported before the synthesis of 2-amino-chromene-3-carbonitriles [21], [22], where solution phase T3P-DMSO mediated the efficient synthesis using alcohols, malanonitrile and phenols ( Figure 3 ). The possible mechanism involves the reaction of DMSO with T3P to give an electrophilic sulphur species (c), followed by a substitution reaction of alcohol to form an arylsulphonium salt. This is followed by a hydrogen abstraction of the arylsulphonium salt by hydrolyzed T3P (d) to create a carbonyl compound after elimination of dimethyl sulfide. Next, the phenolic hydroxyl group (2) attacks the carbonyl carbon, and the oxygen on the carbonyl group attacks one more T3P to form an intermediate (e), which in turn attacks malanonitrile (3) to give an anion. This anion attacks intermediate (f), which upon further cyclization converts to compound 4(a-t) ( Figure 4 ). In addition, a variety of aromatic and heterocyclic alcohols, β-naphthol/resorcinol/4-hydroxycoumarin and malanonitrile were added to T3P (50% solution in ethylacetate), DMSO and ethyl acetate as a solvent medium, in order to obtain compounds 4a-t. The reaction between 1, 2 and 3 in the presence of T3P (2.5 equiv) DMSO generally resulted in a product of appreciable yield (see Table 1 ) as well as being rather versatile with regard to the choice of substrates. It was observed during the reaction that an increase in temperature resulted in the gradual decrease in yield of the product.
Figure 3. Synthesis of a library of 2-amino-chromene-3-carbonitriles from alcohols.
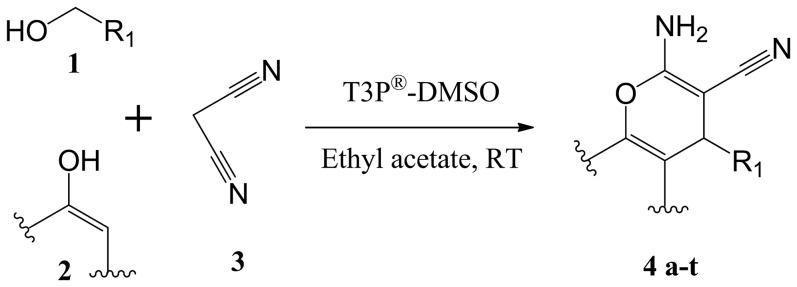
Figure 4. Plausible mechanism of the T3P-DMSO mediated synthesis of title compounds.
Table 1. Physical characteristics of the synthesized 2-amino-chromene-3-carbonitriles.
| Entry | 1 (Alcohol) | 3 (Phenols) | Title compounds (4a-t) | Time (Hr) | Yield (%) | MP (OC) |
| 4a | (3-nitrophenyl)methanol | naphthalen-2-ol | 2-amino-4-(3-nitrophenyl)-4H-benzo[g]chromene-3-carbonitrile | 3–4 | 95a | 179 –182 |
| 4b | (4-bromophenyl)methanol | naphthalen-2-ol | 2-amino-4-(4-bromophenyl)-4H-benzo[g]chromene-3-carbonitrile | 4.5 | 96a | 138–140 |
| 4c | (1H-indol-3-yl)methanol | naphthalen-2-ol | 2-amino-4-(1H-indol-3-yl)-4H-benzo[g]chromene-3-carbonitrile | 5 | 92a | 198–200 |
| 4d | 3-(hydroxymethyl)-4H-chromen-4-one | naphthalen-2-ol | 2-amino-4-(4-oxo-4H-chromen-3-yl)-4H-benzo[g]chromene-3-carbonitrile | 9 | 84 | - |
| 4e | (2-butyl-4-chloro-1H-imidazol-5-yl)methanol | naphthalen-2-ol | 2-amino-4-(2-butyl-4-chloro-1H-imidazol-5-yl)-4H-benzo[g]chromene-3-carbonitrile | 12 | 79 | - |
| 4f | (2-nitrophenyl)methanol | naphthalen-2-ol | 2-amino-4-(2-nitrophenyl)-4H-benzo[g]chromene-3-carbonitrile | 4-5 | 89a | - |
| 4g | (2,6-dichlorophenyl)methanol | naphthalen-2-ol | 2-amino-4-(2,6-dichlorophenyl)-4H-benzo[g]chromene-3-carbonitrile | 8 | 91 | - |
| 4h | (4-fluorophenyl)methanol | naphthalen-2-ol | 2-amino-4-(4-fluorophenyl)-4H-benzo[g]chromene-3-carbonitrile | 4.5 | 93a | 187–189 |
| 4i | (4-fluorophenyl)methanol | resorcinol | 2-amino-4-(4-fluorophenyl)-7-hydroxy-4H-chromene-3-carbonitrile | 6 | 96a | 218–220 |
| 4j | (1H-indol-3-yl)methanol | resorcinol | 2-amino-7-hydroxy-4-(1H-indol-3-yl)-4H-chromene-3-carbonitrile | 5 | 92a | - |
| 4k | 3-(hydroxymethyl)-4H-chromen-4-one | resorcinol | 2′-amino-7′-hydroxy-4-oxo-4H,4′H-[3,4′-bichromene]-3′-carbonitrile | 9.5 | 82 | - |
| 4l | (2-butyl-4-chloro-1H-imidazol-5-yl)methanol | resorcinol | 2-amino-4-(2-butyl-4-chloro-1H-imidazol-5-yl)-7-hydroxy-4H-chromene-3-carbonitrile | 14 | 74 | - |
| 4m | (4-bromophenyl)methanol | 4-hydroxy-2H-chromen-2-one | 2-amino-4-(4-bromophenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 3.5 | 97a | 254–256 |
| 4n | (1H-indol-3-yl)methanol | 4-hydroxy-2H-chromen-2-one | 2-amino-4-(1H-indol-3-yl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 5.5 | 86a | 215–217 |
| 4o | 3-(hydroxymethyl)-4H-chromen-4-one | 4-hydroxy-2H-chromen-2-one | 2-amino-5-oxo-4-(4-oxo-4H-chromen-3-yl)-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 10 | 72 | - |
| 4p | (2-butyl-4-chloro-1H-imidazol-5-yl)methanol | 4-hydroxy-2H-chromen-2-one | 2-amino-4-(2-butyl-4-chloro-1H-imidazol-5-yl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 9 | 81 | - |
| 4q | (3,4-dimethoxyphenyl)methanol | 4-hydroxy-2H-chromen-2-one | 2-amino-4-(3,4-dimethoxyphenyl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 2.5 | 82a | 228–230 |
| 4r | (2-methyl-1H-indol-3-yl)methanol | 4-hydroxy-2H-chromen-2-one | 2-amino-4-(2-methyl-1H-indol-3-yl)-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 6 | 69 | - |
| 4s | (3,4-dimethoxyphenyl)methanol | 4-hydroxy-6,7-dimethyl-2H-chromen-2-one | 2-amino-4-(3,4-dimethoxyphenyl)-8,9-dimethyl-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 3.5 | 84 | - |
| 4t | (4-(trifluoromethyl)phenyl)methanol | 4-hydroxy-6,7-dimethyl-2H-chromen-2-one | 2-amino-8,9-dimethyl-5-oxo-4-(4-(trifluoromethyl)phenyl)-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile | 4 | 89 | - |
Isolated yield.
MP- Melting point.
2-amino chromene-3-carbonitrile derivatives elicit an anti-proliferative effect against AML cells
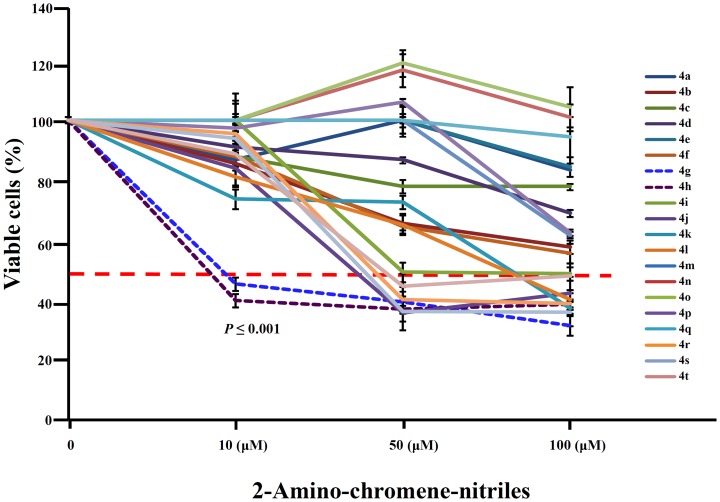
4H-chromenes are potent cytotoxic agents which have been tested against a panel of human cancer cell lines, and a chromene analog, Crolibulin (EPC2407) is currently in Phase I/II clinical trials for the treatment of advanced solid tumors [23]. We initially tested a series of 20 chromene derivatives 4(a-t) for their cytotoxic effects against a human acute myeloid leukemia (AML) cell line (HL-60), using three different concentrations (10, 50 and 100 µM) of 2-amino-chromene-carbonitriles. Among the tested compounds, 4g significantly (P ≤0.001) decreased proliferation of the AML cells, even at 10 µM concentrations. Other derivatives of these compounds showed either no or very low effect at 10 µM concentrations ( Figure 5 ).
Figure 5. Screening of active compounds affecting the proliferation of HL-60 AML cells from a library of 2-amino-chromene-nitriles derivatives.
MTT assays were performed after incubation of HL-60 cells with indicated concentrations of chromene derivatives 4 (a-y) for 72 hr. For each concentration, percent inhibition values were calculated and data was normalized to diluent controls. The scale X-axis is non-linear and the data represent mean ±SD from three independent experiments done in quadruplicates. ** P≤0.001 (Student's t-test).
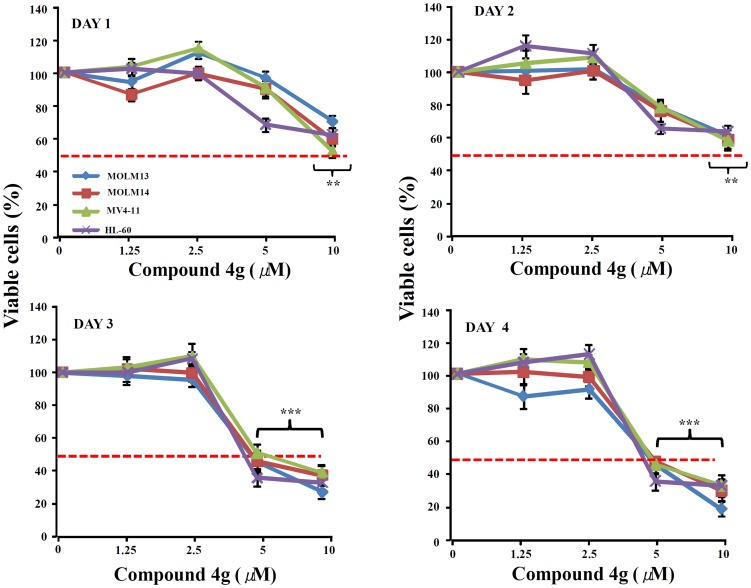
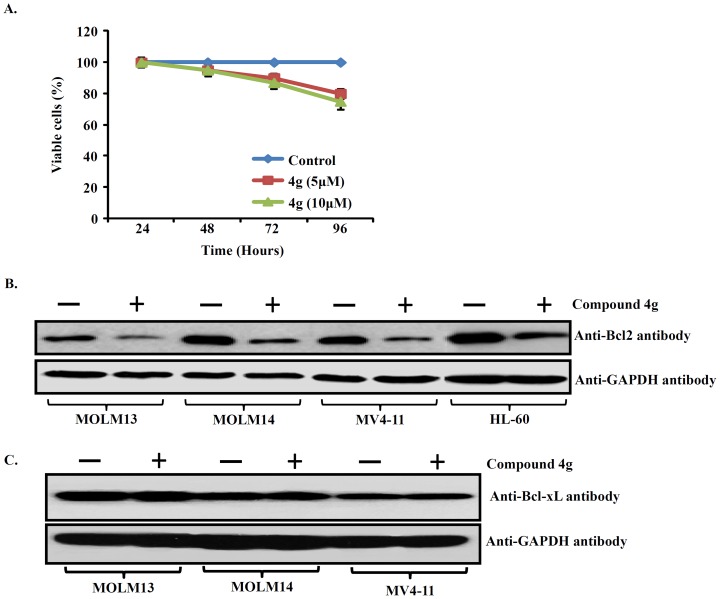
Additional studies were performed for compound 4g, which was the most active among the structurally related compounds tested in culture. The anti-proliferative activity of compound 4g against three additional AML cell lines (MOLM13, MOLM14 and MV4-11) was examined. Again, compound 4g significantly decreased the proliferation of the AML cells with an ED50 of 5 to 10 µM after 48, 72 and 96 hours of culture ( Figures 6 ). Compound 4g inhibited proliferation of the AML cells with an IC50 of 5 µM. Further, C57BL/6 mouse bone marrow cells were treated with 5, 10 µM compound 4g to assess the effect on normal hematopoietic cells. As shown in Figure 7A , compound 4g (5, 10 µM) treatment displayed minimal anti-proliferative response against the normal bone cells suggesting that compound 4g decreases proliferation of the AML cells, and has little effect on proliferation of normal bone marrow cells.
Figure 6. Anti-proliferative effect of 4g tested against AML cell lines in liquid culture.
Panels (A–D): MTT assay determined cell viability of AML cells. 8,000 cells per well were seeded for MOLM13, MOLM14, MV4-11 and HL-60 cells in 96 well plates in quadruplicates. A series of dilutions (starting from 1.25 µm to 10 µm) of 4g were added into the wells. Cell proliferation was measured after compound 4g treatment relative to diluents controls. Results represent the mean ±SD of three independent experiments with quadruplicate wells per experiment point. ** P≤0.001; *** P≤0.0001 (Student's t-test).
Figure 7. Expression of Bcl-2 proteins in human AML cell lines and anti-proliferative effect of compound 4g (5, 10 µm against C57BL/6 mouse bone marrow cells in liquid culture.
A. MTT assay determined cell viability of C57BL/6 mouse total bone marrow cells. Results represent the mean ±SD of three independent experiments with quadruplicate wells per experiment point. B. MOLM13, MOLM14, MV4-11 and HL-60 AML cells were cultured either with compound 4g (5 µM, 24 hr) or diluents control, and levels of Bcl-2 and Bcl-xL were examined by western blot. GAPDH was used as an internal loading control.
Compound 4g inhibits Bcl-2 protein and triggers cell cycle arrest at G2/M phase in AML cells
HA14-1 is a cell permeable Bcl-2 inhibitor, and acts by binding to the surface pocket of Bcl-2 [24] and disrupts the Bax/Bcl-2 interaction, resulting in induction of apoptosis of tumour cells. Interestingly, treatment of HL-60, MOLM13, MOLM14, MV4-11 cell lines with compound 4g (5 µM, 24 hr) decreased levels of Bcl-2 ( Figure 7B ). Moreover, we checked the effect of compound 4g on the expression of the Bcl-xL. Our western blotting results showed that compound 4g does not inhibit the expression of Bcl-xL protein ( Figure 7C ).
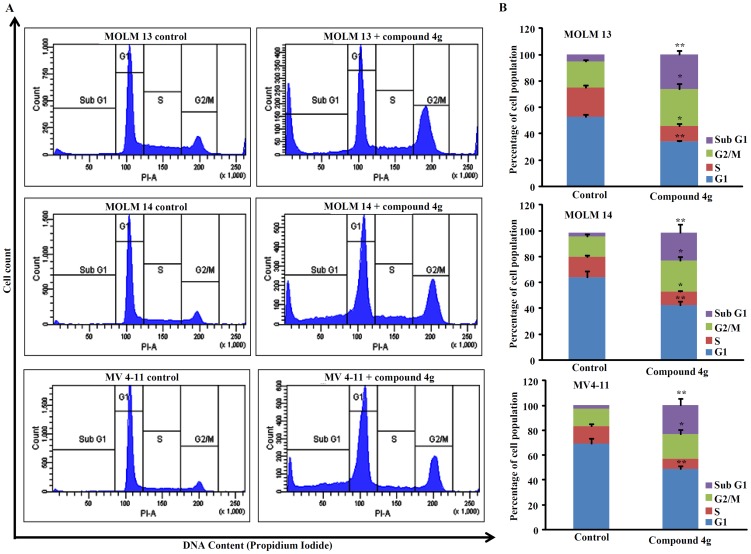
The effect of compound 4g (5 µM, 72 hr) on the cell cycle analysis of MOLM13, MOLM14, and MV4-11 AML cells using flow cytometry showed significant increase in the G2/M and sub-G1 phases (apoptosis), as compared to the diluents control cells ( Figures 8A and 8B ). The cell population in the G1 phase of the cell cycle decreased.
Figure 8. Cell cycle analysis of AML cell lines treated with compound 4g (5 µM, 72 hr).
Panel (A), fluorescent activated cell sorter analyzed the percent cells in each phase of the cell cycle. Cell cycle analysis of three cell lines (MOLM14, MOLM14 and MV4-11) treated with diluents control and compound 4g (5 µM) for 72 h. The figures are representative of three independent experiments. Data are presented in histograms as mean ±SD of three independent experiments. * P≤0.005; ** P≤0.001.
Compound 4g induces apoptosis of AML cells
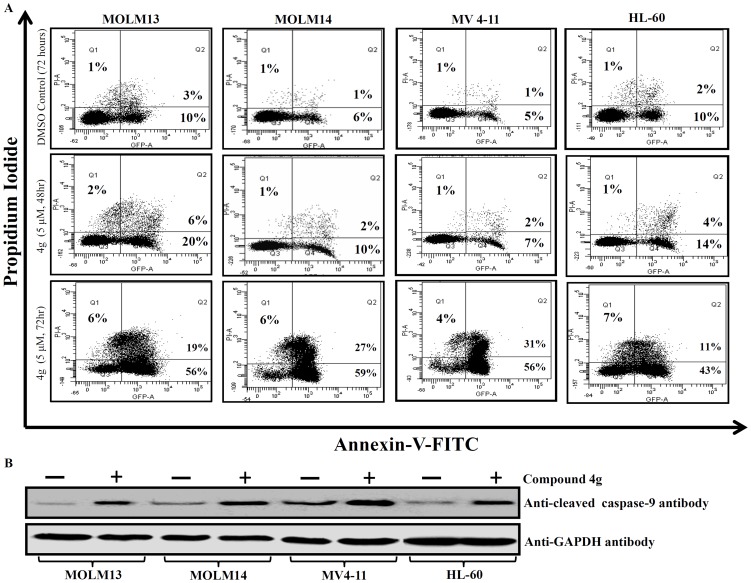
Core scaffolds such as flavones or chromenes induce apoptosis in human myeloid tumor cells [25]. Therefore, the HL-60, MOLM13, MOLM14, and MV4-11 cells were treated with compound 4g (5 µM for 48 and 72 hr) and analysed for the induction of apoptosis using FITC-conjugated annexin V (AV) and propidium iodide (PI). Both early (AV+PI−), as well as, late (AV+PI+) apoptosis occurred in AML cells ( Figure 9A ). Detailed percentage of apoptotic cells in cell lines are summarized in supplementary data (Table S1). This was associated with increased levels of cleaved caspase-9 in these cell lines ( Figure 9B ), which is an enzyme known to be activated during programmed cell death.
Figure 9. Compound 4g induces apoptosis of AML cells in a time-dependent manner.
A. Flow cytometry profile represents Annexin V-FITC staining on the X-axis and PI staining on the Y-axis. The upper left quadrants display the necrotic cells, upper right quadrants show the late apoptotic cells, lower left quadrants display the live cells and lower right quadrants show the early apoptotic cells. B. Western blot showed increased expression of cleaved caspase-9 protein in compound 4g treated AML cells compared to control cells.
In Vitro analysis of the effect of amino-nitriles against Bcl-2
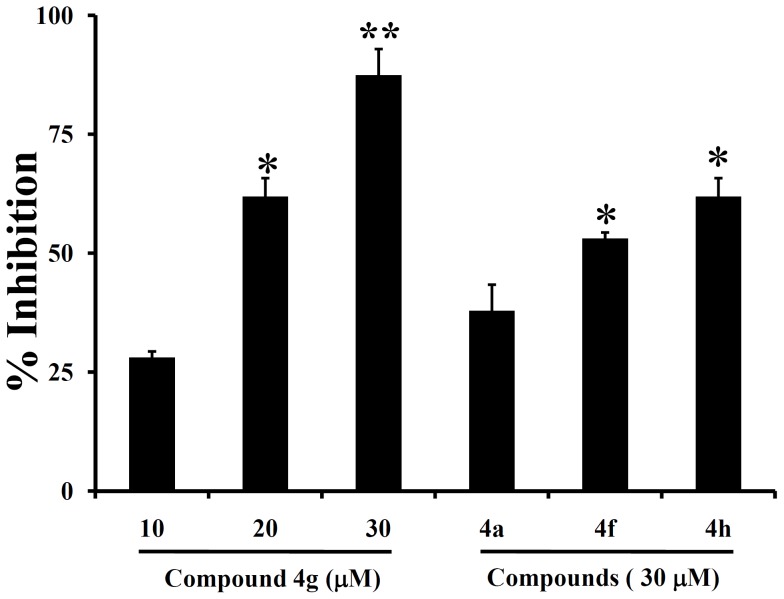
Bcl-2 ELISA kit from Zymed was used to determine the inhibitory activity of most active of small molecule towards Bcl-2. Among the four tested compounds, 4a, 4h, and 4f at 30 µM concentration, inhibited the Bcl-2 binding to its antibody by 37.8, 53.0, 61.9% respectively ( Figure 10 ). The most active compound 4g inhibited the Bcl-2 binding to its antibody effectively with an IC50 value of 17.8 µM, indicate its strong affinity towards Bcl-2 compared to structurally related chromenes. Among the tested small molecules, 2,6- dichlorophenyl, 2-nitrophenyl, 4-fluorophenyl and 3-nitrophenyl side chain containing amino-nitrils displayed significant binding affinity towards Bcl-2 indicating that electron withdrawing group favors the effective binding to Bcl-2.
Figure 10. Amino-nitriles inhibit Bcl-2 in vitro.
Most active small molecules 4a, 4f, 4g and 4h were analysed for their Bcl-2 inhibitory activity. 4g displayed higher percentage of Bcl-2 inhibition in a dose dependent manner and presented as the most potent inhibitor of Bcl-2.
In silico docking analysis of chromene based small molecule (4g) that targets Bcl-2
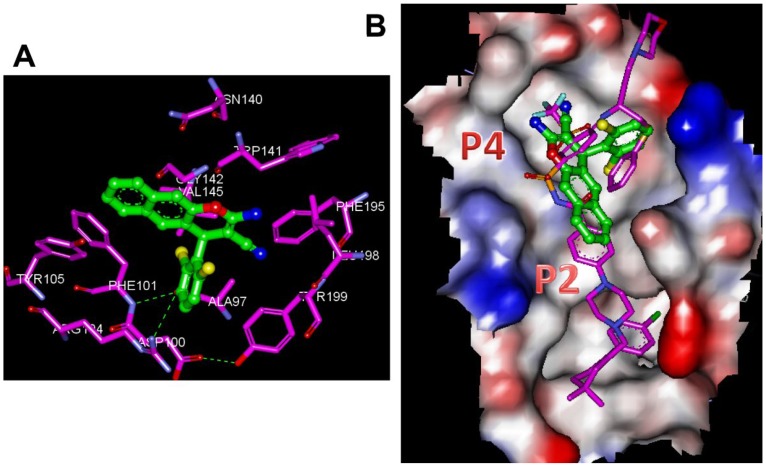
Bcl-2 appears to be a possible target for compound 4g as it induced the apoptosis by decreasing the level of anti-apoptotic protein Bcl-2 in AML cell lines. Hence, we performed in silico molecular docking using the three-dimensional structures of Bcl-2, which consists of two central primarily hydrophobic α-helices surrounded by amphipathic helices. Our study was aided by the recent definition of the X-ray crystal structure of Bcl-2–navitoclax (PDB ID: 4LVT) [10]. Navitoclax engages the two hydrophobic ‘hot spots’ regions termed P2 and P4 that are known to show a high-affinity binding by proapoptotic peptides [26], [27]. All docking calculations were performed using Accelrys Discovery Studio Version 2.5 and the automated docking program CDOCKER, which utilises a molecular dynamics-based simulated annealing algorithm. The active site of the enzyme was defined, and CDOCKER docked the most active compound against the navitoclax binding hotspot of Bcl-2. Figure 10 shows the best-docked conformation of compound 4g and navitoclox in Bcl-2 obtained from the CDOCKER program. Docking energies of the best configuration of compound 4g and navitoclax against Bcl-2 were 4.55 and 5.45 kcal/mol, respectively. In addition, we also carried out the molecular docking analysis for the other bioactive amino nitriles with Bcl-2 and its CDOCKER energies are provided in the supplementary data (Table S2). The 3-dimensional illustrations of the interaction map of Bcl-2 with compound 4g, and navitoclax, were produced using the Accelrys visualization tool ( Figure 11A ), and an intermolecular π-stacking interaction of napthyl group with Phe101 and Tyr105 of P4 host spot of Bcl-2 was observed. The 2,6-dichlorophenyl group of compound 4g showed electrostatic interactions with the conserved arginine (Arg104) and in turn made a unique contact with the intra-molecular hydrogen bond formed by Ala97, Asp100, Phe101, and Tyr199 ( Figure 11A ). In addition to this, the amino-chromene scaffold was buried in the hydrophobic pocket of Gly142, Val145, Trp141, Asn140, Phe195, Leu198, and Tyr199. The analysis of compound 4g bound to the novitoclox binding site showed that it shares a similar global binding motif with navitoclax, but occupies a smaller volume within the P2 hot spot ( Figure 11B ).
Figure 11. Molecular docking analysis of the compound 4g in the navitoclax binding site of Bcl-2.
A. The 3-dimensional orientation of compound 4g in the navitoclax binding site of Bcl-2. Amino acid side chains are shown as stick (elements are purple color except for carbon-pink), the inhibitor is shown as a ball and stick (elements are green color except for carbon-green). The hydrogen bonding is represented as a green dotted line. B. Navitoclax and compound 4g bound surface view of the Bcl-2, showing the interaction at the P2 and P4 hotspot of the protein. The electrostatic potential of the key amino acids is shown. The bound ball and stick version of the compound 4g and navitoclax are represented.
Conclusions
We report for the first time the solution phase, T3P-DMSO mediated, efficient synthesis of 2-amino-chromene-3-carbonitriles from alcohols, malanonitrile and phenols. These 2-amino-chromene-3-carbonitriles were tested for their cytotoxicity using human AML cell lines. Compound 4g was the most bioactive among the structurally related compounds. It decreased proliferation, increased caspase-9 mediated apoptosis, and caused G2/M arrest of the MOLM13, MOLM14, and MV4-11 AML cells. Our docking analysis of 4g suggests Bcl-2 as a reasonable target, hence rendering compound 4g as a promising Bcl-2 inhibitor for future studies as a new anticancer agent.
Supporting Information
Quantification of early apoptotic and late apoptotic cells (%). AML cells were cultured with 4g (5 µM) for either 48 or 72 hours and propidium iodide (PI) and FITC conjugated Annexin V positive cells were enumerated. Results are a representative experiment. The experiments were done three times in triplicates.
(DOCX)
Computational analysis of the binding of amino nitriles against Bcl-2.
(DOCX)
General procedure for one pot synthesis of 2-amino chromene-3-carbonitriles.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from USA National Institutes of Health (R01CA02603830); the Singapore Ministry of Health's National Medical Research Council (NMRC) under its Singapore Translational Research (STaR) Investigator Award; and the Singapore Ministry of Education (to HPK.). This research was also supported by University Grants Commission (41-257-2012-SR), Vision Group Science and Technology, Department of Science and Technology (NO. SR/FT/LS-142/2012) to B. KSR thanks Department of Science and Technology (F.NO.SR/SO/HS-006/2010 [G]) and University Grant Commission (F.No.39-106/2010 [SR Dated 24-12-2010]) for funding. HKK, CDM, and B are thankful to UGC, DST-INSPIRE, and PAVATE foundation for providing fellowships respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2. Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59. [DOI] [PubMed] [Google Scholar]
- 4. Tsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228: 1440–1443. [DOI] [PubMed] [Google Scholar]
- 5. Cleary ML, Smith SD, Sklar J (1986) Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t (14;18) translocation. Cell 47: 19–28. [DOI] [PubMed] [Google Scholar]
- 6. Vaux DL, Cory S, Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335: 440–442. [DOI] [PubMed] [Google Scholar]
- 7. Park CM, Bruncko M, Adickes J, Bauch J, Ding H, et al. (2008) Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem 51: 6902–6915. [DOI] [PubMed] [Google Scholar]
- 8.Schoenwaelder SM, Jackson SP (2012) Bcl-xL-inhibitory BH3 mimetics (ABT-737 or ABT-263) and the modulation of cytosolic calcium flux and platelet function. Blood 119: : 1320–1321; author reply 1321–1322. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Zhou H, Aguilar A, Liu L, Bai L, et al. (2012) Structure-based discovery of BM-957 as a potent small-molecule inhibitor of Bcl-2 and Bcl-xL capable of achieving complete tumor regression. J Med Chem 55: 8502–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19: 202–208. [DOI] [PubMed] [Google Scholar]
- 11. Kemnitzer W, Drewe J, Jiang S, Zhang H, Zhao J, et al. (2007) Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure-activity relationships of fused rings at the 7,8-positions. J Med Chem 50: 2858–2864. [DOI] [PubMed] [Google Scholar]
- 12. Gourdeau H, Leblond L, Hamelin B, Desputeau C, Dong K, et al. (2004) Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol Cancer Ther 3: 1375–1384. [PubMed] [Google Scholar]
- 13. Pei XY, Dai Y, Grant S (2004) The small-molecule Bcl-2 inhibitor HA14-1 interacts synergistically with flavopiridol to induce mitochondrial injury and apoptosis in human myeloma cells through a free radical-dependent and Jun NH2-terminal kinase-dependent mechanism. Mol Cancer Ther 3: 1513–1524. [PubMed] [Google Scholar]
- 14. Fischer U, Schulze-Osthoff K (2005) Apoptosis-based therapies and drug targets. Cell Death Differ 12 Suppl 1942–961. [DOI] [PubMed] [Google Scholar]
- 15. Doshi JM, Tian D, Xing C (2006) Structure-activity relationship studies of ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14-1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer. J Med Chem 49: 7731–7739. [DOI] [PubMed] [Google Scholar]
- 16. Kirkin V, Joos S, Zornig M (2004) The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta 1644: 229–249. [DOI] [PubMed] [Google Scholar]
- 17. Bharathkumar H, Sundaram MS, Jagadish S, Paricharak S, Hemshekhar M, et al. (2014) Novel benzoxazine-based aglycones block glucose uptake in vivo by inhibiting glycosidases. PLoS One 9: e102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rangappa KS (2005) New cholinesterase inhibitors: synthesis and structure–activity relationship studies of 1, 2-benzisoxazole series and novel imidazolyl-d2-isoxazolines. Journal of physical organic chemistry 18: 773–778. [Google Scholar]
- 19. Magedov IV, Manpadi M, Ogasawara MA, Dhawan AS, Rogelj S, et al. (2008) Structural simplification of bioactive natural products with multicomponent synthesis. 2. antiproliferative and antitubulin activities of pyrano[3,2-c]pyridones and pyrano[3,2-c]quinolones. J Med Chem 51: 2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Subbagh HI, Abu-Zaid SM, Mahran MA, Badria FA, Al-Obaid AM (2000) Synthesis and biological evaluation of certain alpha, beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 43: 2915–2921. [DOI] [PubMed] [Google Scholar]
- 21. Basappa, Sadashiva MP, Mantelingu K, Swamy SN, Rangappa KS (2003) Solution-phase synthesis of novel delta2-isoxazoline libraries via 1,3-dipolar cycloaddition and their antifungal properties. Bioorg Med Chem 11: 4539–4544. [DOI] [PubMed] [Google Scholar]
- 22. Basappa, Murugan S, Kavitha CV, Purushothaman A, Nevin KG, et al. (2010) A small oxazine compound as an anti-tumor agent: a novel pyranoside mimetic that binds to VEGF, HB-EGF, and TNF-alpha. Cancer Lett 297: 231–243. [DOI] [PubMed] [Google Scholar]
- 23. Patil SA, Patil R, Pfeffer LM, Miller DD (2013) Chromenes: potential new chemotherapeutic agents for cancer. Future Med Chem 5: 1647–1660. [DOI] [PubMed] [Google Scholar]
- 24. Manero F, Gautier F, Gallenne T, Cauquil N, Gree D, et al. (2006) The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res 66: 2757–2764. [DOI] [PubMed] [Google Scholar]
- 25. Piedfer M, Bouchet S, Tang R, Billard C, Dauzonne D, et al. (2013) p70S6 kinase is a target of the novel proteasome inhibitor 3,3′-diamino-4′-methoxyflavone during apoptosis in human myeloid tumor cells. Biochim Biophys Acta 1833: 1316–1328. [DOI] [PubMed] [Google Scholar]
- 26. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, et al. (1997) Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986. [DOI] [PubMed] [Google Scholar]
- 27. Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, et al. (2007) Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ 14: 1711–1713. [DOI] [PubMed] [Google Scholar]
- 28. Grundy M, Seedhouse C, Shang S, Richardson J, Russell N, et al. (2010) The FLT3 internal tandem duplication mutation is a secondary target of the aurora B kinase inhibitor AZD1152-HQPA in acute myelogenous leukemia cells. Mol Cancer Ther 9: 661–672. [DOI] [PubMed] [Google Scholar]
- 29. Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, et al. (2010) Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res 16: 5424–5435. [DOI] [PubMed] [Google Scholar]
- 30. Hayano T, Garg M, Yin D, Sudo M, Kawamata N, et al. (2013) SOX7 is down-regulated in lung cancer. J Exp Clin Cancer Res 32: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sukhorukov AY, Nirvanappa AC, Swamy J, Ioffe SL, Nanjunda Swamy S, et al. (2014) Synthesis and characterization of novel 1,2-oxazine-based small molecules that targets acetylcholinesterase. Bioorg Med Chem Lett 24: 3618–3621. [DOI] [PubMed] [Google Scholar]
- 32. Sugahara K, Thimmaiah KN, Bid HK, Houghton PJ, Rangappa KS (2012) Anti-tumor activity of a novel HS-mimetic-vascular endothelial growth factor binding small molecule. PLoS One 7: e39444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification of early apoptotic and late apoptotic cells (%). AML cells were cultured with 4g (5 µM) for either 48 or 72 hours and propidium iodide (PI) and FITC conjugated Annexin V positive cells were enumerated. Results are a representative experiment. The experiments were done three times in triplicates.
(DOCX)
Computational analysis of the binding of amino nitriles against Bcl-2.
(DOCX)
General procedure for one pot synthesis of 2-amino chromene-3-carbonitriles.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.