Abstract
The introduction of site-specific fungicides almost 50 years ago has revolutionized chemical plant protection, providing highly efficient, low toxicity compounds for control of fungal diseases. However, it was soon discovered that plant pathogenic fungi can adapt to fungicide treatments by mutations leading to resistance and loss of fungicide efficacy. The grey mould fungus Botrytis cinerea, a major cause of pre- and post-harvest losses in fruit and vegetable production, is notorious as a ‘high risk’ organism for rapid resistance development. In this review, the mechanisms and the history of fungicide resistance in Botrytis are outlined. The introduction of new fungicide classes for grey mould control was always followed by the appearance of resistance in field populations. In addition to target site resistance, B. cinerea has also developed a resistance mechanism based on drug efflux transport. Excessive spraying programmes have resulted in the selection of multiresistant strains in several countries, in particular in strawberry fields. The rapid erosion of fungicide activity against these strains represents a major challenge for the future of fungicides against Botrytis. To maintain adequate protection of intensive cultures against grey mould, strict implementation of resistance management measures are required as well as alternative strategies with non-chemical products.
Keywords: Fungicide resistance, Multiresistant, Drug efflux transporter, Resistance mutation
Introduction
To achieve the production of high-quality crops with optimal yields, farmers have to provide optimal growth conditions for the plants under the existing field conditions and to protect them from damage by pests including weeds, insects and fungi pathogenic microorganisms. The use of synthetic fertilizer and pesticides has become an integral part of modern agriculture and is one of the main reasons for the dramatic increase in productivity. However, as will be discussed in several articles of this issue of the Journal of Chemical Biology, the use of herbicides, insecticides and fungicides suffers from increasing problems of resistance in the target organisms. In this review, I will provide a short historical overview of chemical control of plant diseases caused by fungi and then focus on the grey mould fungus Botrytis cinerea to illustrate the problems that have been encountered with fungicide resistance.
History of plant disease control and fungicides; fungicide resistance mechanisms
Long before knowing the causal agents of plant diseases, farmers discovered cultivation techniques that provided disease control. In the eleventh century, three-field crop rotation was a common form of cultivation in Europe that reduced the disease pressure resulting from repeated cultivation of a crop in the same field. A readable history of fungicides has been written by Morton and Staub [46] and is summarized here. The earliest fungicidal treatments were performed with inorganic compounds, such as salt water which was used already in the seventeenth century for treatment of grain followed by liming to control rotting. They were followed by the discoveries of dusted sulphur against powdery mildew and other fungi, and the famous Bordeaux mixture, a mixture of copper sulphate and hydrated lime, which was found to be effective against downy mildew of grapes and other pathogens. Both sulphur and copper are used until today in organic and integrated farming. Between 1940 and 1970, organic antifungal compounds with broad-spectrum activity were developed by the newly emerging plant protection industry. Most of these fungicides are multisite inhibitors, for example, captan and folpet, which are active on thiol groups of glutathione and proteins [13]. A new generation of fungicides was developed in the 1960s, starting with the benzimidazoles. They have a specific mode of action towards a target protein in the fungal pathogens, are highly active and show low phytotoxicity. Most of these site-specific fungicides are systemic, i.e. they can penetrate the cuticle and are distributed within the plant, which increases their activity. Until today, several classes of fungicides with specific modes of action have been released for use in agriculture.
Only few years after the introduction of site-specific fungicides, resistance development in the pathogen populations and the loss of fungicide activity were observed, and B. cinerea was one of the first fungi for which resistance was described. Since then, the awareness of the resistance problem has increased, and it became a major focus of fungicide research. It was soon discovered that resistance development depends on several factors, in particular, the chemistry and mode of action of the fungicidal compound, the biology and reproductive ability of the target fungus, and the frequency of use of the fungicide [16]. In fungi, the most important resistance mechanism is modification of the fungicide target, caused by mutations in the encoding gene. However, this can occur only if the activity of the target protein is maintained at a level which allows the cell to survive without major losses in fitness. As discussed below, target site resistance has been observed against all site-specific fungicides and often leads to high levels of resistance against fungicides with the same mode of action (cross-resistance). However, there are exceptions to this rule. For example, resistance of the wheat pathogen Mycosphaerella graminicola against azole fungicides could be overcome by newer azoles which bind differently to the target protein, the cyp51-encoded lanosterol 14α-demethylase [19]. Another resistance mechanism, target site overexpression, is caused by mutations that increase transcription of the target gene. There are examples for overexpression of cyp51 in several fungi, such as the citrus pathogen Penicillium digitatum and the apple scab fungus Venturia inaequalis [43]. Resistance based on increased fungicide efflux has been identified in B. cinerea and will be described below. Other possible resistance mechanisms, such as decreased uptake or fungicide detoxification, do not seem to play a major role in plant pathogenic fungi.
Economic importance and biology of B. cinerea
B. cinerea causes grey mould on more than 240 plant species and is a major pathogen of cultivated fruits, vegetables and ornamental flowers. Economic damage attributed to Botrytis is enormous. It includes pre- and post-harvest losses in quantity and quality, expenses for plant protection measures in the fields and greenhouses, and direct and indirect costs to retailers and consumers for cooling facilities and for losses suffered by rotten plants [20]. The fungus is known by its ability to produce abundant amounts of conidia (asexual spores) that serve as the primary inoculum for disease initiation. Sexual reproduction occurs by sclerotia, large melanized hyphal aggregates which serve as long-term survival structures and as female parents and by microconidia which function as spermatia. Although sexual structures are rarely observed, sexual recombination seems to be important to maintain the high genetic variability that is observed in the fields. In addition to B. cinerea, more than 25 Botrytis species have been described until today, some are also economically important, for example, B. allii on onions and B. fabae on faba beans. Recently, grapes and strawberries, two of the most important crops attacked by grey mould, have been shown to be infected not only by B. cinerea but also by the related species B. pseudocinerea [38, 55].
Fields at high humidity, green houses and storage facilities provide ideal conditions for grey mould infections and often require repeated fungicide sprayings to protect the crops. Its short life cycle and ability for abundant sporulation make B. cinerea a classical high-risk pathogen with regard to fungicide resistance [17]. Therefore, and because B. cinerea is one of the best studied fungus in this respect worldwide, it is an excellent example to illustrate increasing resistance problems as a consequence of intensive use of fungicides.
Fungicides used against Botrytis
In Table 1 and the following text, short descriptions of the major anti-Botrytis fungicides, their mode of action and the resistance mechanisms in B. cinerea are provided. More detailed information are found in the reviews by Leroux et al. [41] and Walker et al. [56].
Table 1.
Fungicides used against Botrytis
| Fungicide class (major examples) | Year of first use | Target site/mode of action | Resistance riska | Target site mutations | |
|---|---|---|---|---|---|
| Site changes in target proteinb | Resistance levels | ||||
| Dithiocarbamates (thiram, mancozeb) | 1942 | Multisite inhibitor; thiol groups | Low | – | – |
| Phthalimides (captan) | 1952 | Multisite inhibitor; thiol groups | Low | – | – |
| Benzimidazoles (carbendazim, thiophanate-methyl) | 1968 | β-Tubulin | High | E198A, E198X, F200Y | High |
| Dicarboximides (iprodione) | 1975 | Osmosensing histidine kinase | Medium to high | I365S/N, Q369P, N373S | Low to high |
| Anilinopyrimidines (cyprodinil, pyrimethanil) | 1994 | Unknown | Medium | Unknown | High |
| Phenylpyrroles (fludioxonil) | 1995 | Osmoregulation | Low | Unknown | Medium (rarely found) |
| QoIs/strobilurins | 1996 | Cytochrome bc1 complex (respiration) | High | G143A | High |
| Hydroxyanilides (fenhexamid) | 1999 | 3-Keto reductase (sterol biosynthesis) | Low to medium | F412S, F412X, T63I | High (sometimes low to medium)c |
| SDHIs (boscalid) | 2004 | Succinate dehydrogenase (respiration) | Medium to high | H272R/Y, H272L, N230I, P225X | Medium to high |
aAccording to FRAC (http://www.frac.info/); with Botrytis, the resistance risk is considerably higher, except for fludioxonil
bNot comprehensive; major changes are in italics; X indicates different amino acids
cSee Fillinger et al. [25]
Multisite inhibitors (dithiocarbamates, captan, chlorothalonil) have been used against grey mould for a long time. Today, they play a minor role compared to the more active site-specific compounds. Due to their non-specific mode of action, the resistance risk is low. However, there are reports about reduced sensitivity of B. cinerea against these multisite inhibitors [8].
Benzimidazoles (carbendazim, benomyl, thiophanate-methyl) target the fungal cytoskeleton by interfering with assembly of β-tubulin subunit [41]. A single-point mutation in the β-tubulin gene, leading to an amino acid exchange at position 198 (E198A), results in loss of binding and high resistance to the drugs (BenR1 phenotype; [39]). There are additional mutations in β-tubulin associated with resistance to MBCs (E198K, F200Y, [6]). Due to frequent benzimidazole applications, resistance development occurred very quickly in B. cinerea populations in Central Europe with its humid climate and high disease pressure by grey mould and in greenhouses in many parts of the world.
Dicarboximides, with the main compound iprodione, soon superseded the benzimidazoles after their introduction in the 1970s. Their mode of action remained obscure for many years, although it was observed early on that fungal mutants resistant to dicarboximides are often hypersensitive to hyperosmotic stress [11]. It is now established that dicarboximides interfere with osmoregulation mediated by an osmosensing histidine kinase and a downstream MAP kinase cascade, but their detailed mode of action remains to be revealed [24]. B. cinerea field strains with low, intermediate and high resistance levels to dicarboximides have been found to contain one or several mutations in bos1 encoding the osmosensing histidine kinase, the most frequent mutation being located in codon I365 [48]. Dicarboximides are no longer registered for use anymore in several countries, because of resistance problems and their relatively low activity compared to the newer anti-Botrytis fungicides.
The anilinopyrimidines (APs) cyprodinil, pyrimethanil and menapyrim represent the first of four important classes of fungicides introduced in the 1990s, which expanded considerably the spectrum of chemical control options against grey mould. APs are highly active against Botrytis and other fungi and sold either as solo compounds or as a mixture with fludioxonil. They interfere with amino acid synthesis and protein secretion, and are ineffective in rich medium, but their molecular mode of action has not yet been resolved. Two types of resistance against APs have been described in the field. Strains with high levels of AP resistance (AniR1) were found to result from mutations in single, yet unmapped, gene loci [31]. In addition to AniR1, two phenotypes with low level AP resistance, originally named AniR2 and AniR3, were identified [18]. Later, it was shown that they are caused by efflux-based mechanisms.
The strobilurins (quinone outside inhibitors or QoIs) are respiration inhibitors that interact with the Qo site of the mitochondrial cytochrome bc1 complex. They are highly active against a variety of fungi and oomycetes but considered to be less effective against B. cinerea which contains an alternative terminal oxidase that can bypass the inhibition of the respiratory chain [59]. However, crops threatened by grey mould often receive strobilurin treatments against other diseases such as powdery mildew or downy mildew. QoI resistance can occur in many fungi, including B. cinerea, and is most often correlated with a point mutation in the cytB gene leading to a G143A substitution in cytochrome B [7]. However, this mutation can only occur in the absence of an intron, which is located next to the codon for G143. In the presence of the intron, the mutation would interfere with splicing and correct expression of cytB. In rust fungi and Alternaria solani, the intron prevents the G143A mutation and drastically decreases the chance of QoI resistance development [30]. B. cinerea populations are divided into strains that contain the intron and others that do not. In Greece, QoI resistance was found only in isolates with no intron, and all of them carried the G143A mutation [50].
The only registered hydroxyanilid, fenhexamid, is a highly effective systemic fungicide against Botrytis and several related fungi. It is a sterol biosynthesis inhibitor, but in contrast to the common sterol biosynthesis inhibitors, such as the azoles (which are not very active against Botrytis), fenhexamid does not block the lanosterol 14-α demethylase, but the 3-ketoreductase encoded by erg27, a later step in ergosterol biosynthesis [41]. Target site mutations leading to high levels of resistance (HydR3+) are located in erg27, most frequently in codon F412 [22, 25, 29]. Low to medium levels of fenhexamid resistance have been shown to be correlated with erg27 mutations (HydR-; [25]) or with a drug efflux mechanism (MDR2 phenotype).
Phenylpyrroles are derived from the antifungal antibiotic pyrrolnitrin, a tryptophan derivate produced by Pseudomonas species. The major phenylpyrrole, fludioxonil, is used as a seed treatment or sprayed against foliar diseases or as post-harvest fungicide against a broad variety of fungi. Fludioxonil is usually applied as a mixture with the AP cyprodinil, which is considered the most effective fungicide against Botrytis. Similar to the dicarboximides, fludioxonil interferes with the Bos1- and MAP kinase-dependent osmoregulation pathway, but cross-resistance between these fungicides is rarely observed in the field [54]. In sensitive cells, fludioxonil hyperactivates the pathway, which leads to a futile hyperosmolarity response, followed by glycerol accumulation and growth inhibition [34]. Laboratory-induced mutations in the Bos1 histidine kinase confer high levels of resistance against both fludioxonil and iprodione; however, these mutants show poor growth and are hypersensitive to high osmolarity, and such strains usually do not survive in the field. In fact, fludioxonil is exceptional among the site-specific fungicides used against Botrytis because highly resistant field strains are very rarely found. In contrast, strains with resistance to fludioxonil and iprodione have been observed in field populations of Alternaria brassicicola [5] and Penicillium spp. [33]. However, B. cinerea strains with low to medium fludioxonil resistance, due to a drug efflux mechanism, are common in many field populations.
The succinate dehydrogenase inhibitor (SDHI) boscalid, a carboxamide, was first registered for use in 2003. Usually sold in a mixture with pyraclostrobin, the fungicide is highly active against Botrytis, Sclerotinia and related fungi. Although it was not the first SDHI fungicide (this was carboxin, used since 1966 as a seed treatment), the success of boscalid triggered the development in different companies of various new SDHIs with a wide range of specificities that are currently entering the fungicide market. Resistance against different SDHIs has been intensively studied in several fungi. It is caused by several mutations in the SDH subunits SdhB, SdhC and SdhD, which form the binding pocket for ubiquinone and the SDHIs [51]. Mutations conferring boscalid resistance have appeared in B. cinerea populations few years after its introduction and are all located in SdhB [3, 53]. Interestingly, the two most common sdhB mutations in B. cinerea field strains, H272R and H272Y, confer medium resistance levels against boscalid but are ineffective against other SDHIs, such as the newly released fluopyram. Other mutations (e.g. in P225) confer high resistance against both boscalid and fluopyram [36, 53].
Efflux-mediated resistance mechanisms in Botrytis
A new type of non-specific fungicide resistance appeared in B. cinerea populations in French vineyards in the mid-1990s. Strains showing low to medium levels of resistance to structurally and functionally different fungicides were observed. Originally called AniR2 (conferring partial resistance to cyprodinil and fludioxonil) and AniR3 (conferring partial resistance to cyprodinil, fenhexamid and iprodione), they were renamed MDR1 and MDR2, respectively, because of their similarity to efflux-based multidrug resistance phenotypes previously described for cancer cells and human pathogenic microbes [1, 45]. Both multidrug resistance (MDR) types are caused by overexpression of drug efflux transporters, namely, the ABC transporter AtrB (MDR1) and the MFS transporter MfsM2 (MDR2). The MDR1 phenotype was caused by point mutations in a gene encoding a transcriptional regulator (Mrr1), resulting in its activation and constitutive induction of atrB. In contrast, MDR2 strains contain rearrangements in the mfsM2 promoter, which lead to constitutive expression of mfsM2 [35]. The frequencies of MDR strains in French vineyards increased steadily, up to more than 50 % (all MDR types) of the whole population in the Champagne in 2010. A survey in German vineyards also revealed high MDR frequencies, in particular for MDR1 [37]. Up to now, MDR2 and MDR3 (a combination of MDR1 and MDR2) strains have been unambiguously identified only in French and German vineyards. To what extent these phenotypes can reduce fungicide efficiency in the field is unclear [56].
A field’s race: fungicide treatments and resistance development in Botrytis
Until the end of the 1960s, inorganic or organic fungicides with low specific toxicity were used against plant pathogenic fungi, and resistance was unknown. The benzimidazoles were not only the first generation of modern fungicides, with high activity and systemic properties, but they also had a high inherent risk of resistance in the target organisms. The first publication about benzimidazole resistance in B. cinerea dates back to 1971 [15]. Strains carrying the E198A mutation (BenR1) in β-tubulin were highly resistant and seemed to have no impaired fitness when compared to sensitive field strains [32]. Because of the high spraying frequencies in the first years after their introduction, the benzimidazoles rapidly lost activity in the field, and their use was restricted. The discovery that BenR1 strains were hypersensitive to the N-phenylcarbamate diethofencarb (negative cross-resistance) lead to the first attempt of resistance management, by applying mixtures of both fungicides. However, another mutation in the β-tubulin gene (BenR2; F200Y) conferring resistance against both compounds was rapidly selected and made the mixture ineffective [41]. Although benzimidazoles have not been used anymore against grey mould in most European countries for many years, a significant proportion of B. cinerea populations from commercial vineyards and soft fruit fields remained resistant to benzimidazoles [38] [56], which further confirmed their high fitness under field conditions. After their release in the late 1970s, the dicarboximides rapidly replaced the benzimidazoles. However, again their excessive use resulted in high frequencies of resistance and failures in protection [12]. A well-documented case is from the Champagne-winegrowing region, where dicarboximides have been sprayed in the first years four to five times per season until widespread resistance in the grey mould populations resulted in the loss of their protective activity. The use of dicarboximides was therefore interrupted and later resumed with applications restricted to one per season and long rotations. This measure led to a decline in resistance frequencies and the recovery of dicarboximide activity [40, 41]. Similarly, B. cinerea isolates from Greece were found to lose dicarboximide resistance after interruption of treatments, whereas benzimidazole resistance remained stable [49]. This is explained by reduced fitness of dicarboximide-resistant strains and their replacement by sensitive strains in the absence of selection. In greenhouses in Israel, B. cinerea strains with multiple resistance to carbendazim, diethofencarb and dicarboximides were observed as early as 1989 [21].
After these experiences, evaluation of the risk of resistance to newly developed compounds became routine practice in the laboratories of plant protection industry. Another consequence was the foundation of Fungicide Resistance Action Committee (FRAC) in 1981, by members of the major agrochemical companies. The goal of FRAC is to prolong the effectiveness of fungicides that are likely to encounter resistance problems, by applying appropriate resistance management strategies. FRAC (www.frac.info) releases recommendations for restrictions of the number of their application in field and greenhouse cultures [17].
In the mid-1990s, the spectrum of effective anti-Botrytis fungicides was greatly expanded by introduction of the APs, fludioxonil, and a few years later, fenhexamid. The choice of powerful compounds with different modes of action allowed the farmers to apply effective resistance management strategies, by rotating between and mixing of fungicides with different modes of action. Nevertheless, grey mould strains resistant against these fungicides quickly appeared. Analysis of Greek kiwi fruits showing post-harvest storage rot, after being sprayed during flowering and before harvest, revealed high-resistance frequencies of the B. cinerea isolates and for the first-time isolates with multiple resistance against up to five fungicide classes [10]. In French vineyards, which are sprayed up to two or rarely three times against Botrytis, resistance frequencies remained at low to moderate levels which did not seem to reduce fungicide efficacy. It was concluded that chemical control of grey mould remained effective, if adequate resistance management strategies are followed, in particular, the restriction of applications of one class of fungicides to one per season [56]. However, in vineyards in Southern Italy which had received many fungicide sprayings, high frequencies of resistance were observed in recent years, and low efficacy of treatments was reported in some fields [4].
Strawberries are among the most important crops that are attacked by grey mould. Protection against grey mould usually demands weekly fungicide sprayings during flowering time, which exerts strong selection on Botrytis, and has led to increasing resistance problems in recent years. A survey of 353 isolates collected in 2010 from strawberries and other small fruits in Northern Germany revealed medium to high frequencies of resistance against thiophanate-methyl (40.5 %), iprodione (64.0 %), fenhexamid (45.0 %), QoIs (76.8 %), boscalid (21.5 %), cyprodinil (14.7 %) and fludioxonil (41.1 %) [58]. Similarly, isolates collected in different strawberry-growing regions in Germany between 2008 and 2011 revealed high-resistance frequencies to all site-specific fungicides and a large proportion of isolates that were multiresistant against several fungicides [38]. Many of these isolates showed higher levels of partial fludioxonil resistance than the previously characterized MDR1 isolates from vineyards and were therefore called MDR1h. While MDR1 isolates are 5- to 10-fold and 10- to 20-fold more tolerant than sensitive strains towards fludioxonil and cyprodinil, respectively, the tolerance of MDR1h strains against these fungicides is further increased 2- to 3-fold. All MDR1h strains analyzed carried a 3-bp deletion of amino acid L497 in Mrr1, which probably resulted in hyperactivation of Mrr1 and increased overexpression of atrB, compared to the point mutations found in MDR1 strains [38]. Monitoring of German strawberry fields in 2013 revealed further increased resistance frequencies (M. Hahn, unpublished). In addition, an increasing number of isolates showed multiple resistance to all registered fungicides used against Botrytis ([38]; M. Hahn and R. Weber, unpublished data). Also, in Florida, one of the major strawberry producers worldwide, resistance frequencies have reached threatening levels. In 2012, a severe grey mould epidemic was reported in several fields that could not be controlled anymore by the available fungicides. Of 392 B. cinerea isolates collected between 2010 and 2012, resistance frequencies were 85.4 % for boscalid, 86.5 % for pyraclostrobin, 44.4 % for fenhexamid, 52.7 % for cyprodinil and 17.8 % for fludioxonil (partial resistance, probably MDR1). Between 2010 and 2012, an increasing number of multiresistant isolates were obtained [2]. Multiresistant B. cinerea strains have been recently detected also in strawberry and blackberry fields in North and South Carolina [42], in strawberry fields and vineyards in Southern Italy [4], and in strawberry fields in Greece (G. Karaoglanidis, personal communication).
Diversity of B. cinerea populations
B. cinerea shows considerable phenotypic and genetic variability within a population. Genetic variability has been assessed by the analysis of genetic markers, such as PCR-RFLP, the presence or absence of transposable elements and microsatellites [27, 28, 47]. Recent studies indicated that B. cinerea genotypes show different host preferences [26]. In German strawberry fields, the majority of isolates could be distinguished from the common B. cinerea genotype and were assigned to a new subgroup of B. cinerea called group S [38]. MDR1h isolates containing the ΔL497 deletion all belonged to group S, and multiresistant (MR) isolates more frequently belonged to group S than to B. cinerea sensu stricto [38]. While group S isolates were dominant in strawberry fields, they were almost absent in vineyards. Similar to the situation in French vineyards, B. pseudocinerea was found as a minor sympatric species in strawberry fields together with B. cinerea [55]. B. pseudocinerea isolates are usually more abundant on rotten stems and leaves before the start of fungicide treatments and rarely found on fungicide-treated fruits (M. Hahn, unpublished data). Most B. pseudocinerea isolates were sensitive against all tested fungicides [38]. Thus, different genotypes of Botrytis differ in their ecological behaviour and in their ability to accumulate fungicide resistance. Therefore, knowledge of the genetic diversity in grey mould populations can provide important information for evaluation of the resistance situation.
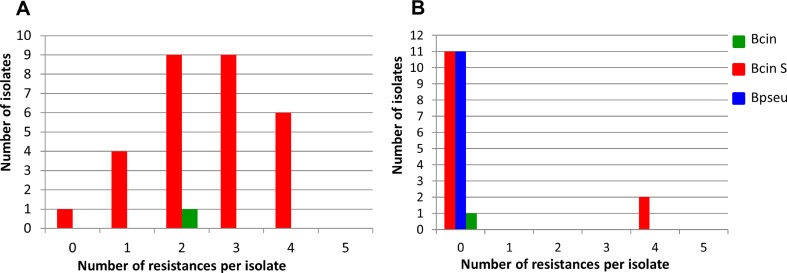
Because of the massive production of airborne conidia, resistant strains can migrate over long distances. This has been documented for B. cinerea MDR2 strains by a microsatellite-based population genetic study. The study indicated that an MDR2 founder strain originated in a French vineyard and gave rise to a population which spread in France and migrated into German winegrowing regions, over distances of several hundred kilometres [44]. Similarly, spread and migration of (multi-) resistant strains is expected to occur in strawberry-growing regions, if selection by fungicide treatments continues. Nevertheless, grey mould populations in closely adjacent fields can vary considerably. For example, Botrytis isolates from raspberries at two adjacent sites in Northern Germany, one site grown with wild plants and the other site being an intensively treated field, revealed striking differences (Fig. 1). Whereas most isolates from the wild-growing plants were sensitive, all but one isolate from the fungicide-treated field were resistant to one or several fungicides. In both fields, B. cinerea group S was dominating over B. cinerea, but B. pseudocinerea was present only in the untreated field. Two isolates from wild-grown raspberries with four resistances were likely immigrants from fungicide-treated fields. A similar observation was made in Greece, where isolates from cultivated strawberries showed high-resistance frequencies, whereas isolates from fruits of wild black blackberry plants growing adjacent to the strawberry field had almost no resistance (G. Karaoglanidis, personal communication).
Fig. 1.
Diversity of Botrytis populations on raspberries, depending on fungicide treatments. a Strains from a commercial raspberry field with intensive fungicide sprayings (n = 25). b Strains from a row of wild growing, untreated raspberries (n = 30). The distance between the two sampling sites in Northern Germany was less than 100 m. Fungicide resistance was tested for fenhexamid, cyprodinil, boscalid, azoxystrobin and fludioxonil. Bpseu, B. pseudocinerea; Bcin, B. cinerea; Bcin S, B. cinerea group S (M. Hahn and R. Weber, unpublished)
Perspectives
Since their introduction, fungicides have been powerful tools for grey mould control and are indispensable for cost-effective cultivation of crops such as strawberries in many regions. However, resistance of Botrytis in cultures with intensive treatments has resulted in a serious erosion of fungicide efficiency. The high frequencies of resistance observed in some fields, and laboratory data showing that resistant isolates often cannot be controlled anymore by standard fungicides applications, suggest that significant losses in fungicide efficacy occur. Quantification of these losses is difficult; however, because of inoculum density, the proportion of resistant isolates and the overall infection pressure are variable from site to site and difficult to measure.
To save the activity of the available fungicides for the future, strict application of resistance management strategies is required. These include good crop management and sanitation to reduce the risk of grey mould infection, such as limitation of nitrogen fertilizer, reduction of the humidity and removal of infected fruits and plant parts. To limit selection for resistance, rotation between fungicides of different classes and limitation of treatments with the same class of compounds to one per season should be mandatory, maybe except for fludioxonil which has a low risk of specific resistance. However, these rules are often not followed by advisors and farmers, and fungicides often are still being used excessively. One of the reasons is the maximum number of detectable residues of pesticides on fruits and vegetables that are imposed by national and international retailers, which limits the available options for rotations. Furthermore, fludioxonil and SDHIs are sold in many countries only as mixtures with other fungicides and seriously restrict the flexibility for optimization of spraying programmes.
The availability of quick cultivation-based and molecular tests allows rapid evaluation of resistance, including the prevalence of resistance mutations before and after the treatments [43]. This can help to devise resistance management programmes that are adapted to local situations. In Germany, the genetic diversity and different adaptation of grey mould populations in vineyards and strawberry fields appear to have prevented movements of resistances (MDR2 restricted to vineyards; MDR1h restricted to soft fruit fields; [38]) between the crops, so far. Despite the high overall resistance frequencies observed in grey mould populations, fields that have received no or only few fungicide treatments contain Botrytis populations with significantly lower resistance frequencies ([4]; Fig. 1; M. Hahn, unpublished). These observations give hope that resistance management will work despite the high-resistance frequencies observed in many fields.
Clearly, the appearance of multiresistant strains presents a great challenge for the future of fungicide control of Botrytis. Their control by fungicides is very poor, and they are selected by most or all fungicide treatment. A critical question is the long-term survival of multiresistant strains under field conditions. Studies with field strains of B. cinerea revealed no fitness defects associated with individual resistance to benzimidazoles, QoIs [52] and APs [9, 23]. Reduced survival in the field has been reported for strains resistant to dicarboximides and fenhexamid (R. Weber, personal communication). A detailed study with genetically defined fenhexamid resistant mutants carrying mutations in the target gene erg27, obtained by transformation of a sensitive laboratory strain, also indicated reduced fitness correlated with fenhexamid resistance [14]. For mutations associated with boscalid resistance, differential fitness defects were reported by Lalève et al. [36] and Veloukas et al. [52], while no major reductions in fitness were observed by Amiri et al. [3]. In German strawberry fields, multiresistant strains were found prior to the start of fungicide treatments in spring and in increased numbers after the treatments (unpublished data). These results indicate that they can survive in the field but are less competitive than sensitive strains in the absence of selection pressure.
At present, there are no equivalent alternatives for chemical protection against grey mould. Non-specific fungicides, such as captan and thiram, have inferior efficiency. Increasing interest is directed towards biological control, either by using non-synthetic compounds with antifungal or plant resistance inducing activity, or antagonistic microorganisms (biofungicides). Examples for products which are marketed are resistance-inducing extracts containing β-laminarin, or bacteria (e.g. Bacillus spp.), yeasts (e.g. Candida oleophila, Aureobasidium pullulans) and filamentous fungi (e.g. Trichoderma harzianum). These products, in particular, the biofungicides, often work well in controlled environments but are still unreliable in open-field cultures. Nevertheless, further development of these products is urgently needed as alternating treatments with chemical fungicides to maintain effective grey mould control in intensive cultures.
Acknowledgments
I am grateful to George Karaoglanidis for the helpful comments to the manuscript, and to him and Roland Weber for sharing unpublished data.
References
- 1.Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Amiri A, Heath SM, Peres NA. Phenotypic characterization of multifungicide resistance in Botrytis cinerea isolates from strawberry fields in Florida. Plant Dis. 2013;97:393–401. doi: 10.1094/PDIS-08-12-0748-RE. [DOI] [PubMed] [Google Scholar]
- 3.Amiri A, Heath SM, Peres NA. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2014;98:532–539. doi: 10.1094/PDIS-07-13-0753-RE. [DOI] [PubMed] [Google Scholar]
- 4.Angelini RM, Rotolo C, Masiello M, Gerin D, Pollastro S, Faretra F (2014) Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest Manag Sci. doi:10.1002/ps.3711 [DOI] [PubMed]
- 5.Avenot H, Simoneau P, Iacomi-Vasilescu B, Bataille-Simoneau N. Characterization of mutations in the two-component histidine kinase gene AbNIK1 from Alternaria brassicicola that confer high dicarboximide and phenylpyrrole resistance. Curr Genet. 2005;47:234–243. doi: 10.1007/s00294-005-0568-2. [DOI] [PubMed] [Google Scholar]
- 6.Banno S, Fukumori F, Ichiishi A, Okada K, Uekusa H, Kimura M, Fujimura M. Genotyping of benzimidazole-resistant and dicarboximide-resistant mutations in Botrytis cinerea using real-time polymerase chain reaction assays. Phytopathology. 2008;98:397–404. doi: 10.1094/PHYTO-98-4-0397. [DOI] [PubMed] [Google Scholar]
- 7.Banno S, et al. Characterization of QoI resistance in Botrytis cinerea and identification of two types of mitochondrial cytochrome b gene. Plant Pathol. 2009;58:120–129. doi: 10.1111/j.1365-3059.2008.01909.x. [DOI] [Google Scholar]
- 8.Barak E, Edgington LV. Cross-resistance of Botrytis cinerea to captan, thiram, chlorothalonil, and related fungicides. Can J Plant Pathol. 1984;6:318–320. doi: 10.1080/07060668409501536. [DOI] [Google Scholar]
- 9.Bardas GA, Myresiotis CK, Karaoglanidis GS. Stability and fitness of anilinopyrimidine-resistant strains of Botrytis cinerea. Phytopathology. 2008;98:443–450. doi: 10.1094/PHYTO-98-4-0443. [DOI] [PubMed] [Google Scholar]
- 10.Bardas GA, Veloukas T, Koutita O, Karaoglanidis GS. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag Sci. 2010;66:967–973. doi: 10.1002/ps.1968. [DOI] [PubMed] [Google Scholar]
- 11.Beever RE. Osmotic sensitivity of fungal variants resistant to dicarboximide fungicides. Trans Br Mycol Soc. 1983;80:327–331. doi: 10.1016/S0007-1536(83)80017-5. [DOI] [Google Scholar]
- 12.Beever RE, Brien HMR. A survey of resistance to the dicarboximide fungicides in Botrytis cinerea. N Z J Agric Res. 1983;26:391–400. doi: 10.1080/00288233.1983.10427048. [DOI] [Google Scholar]
- 13.Bernard BK, Gordon EB. An evaluation of the common mechanism approach to the Food Quality Protection Act: captan and four related fungicides, a practical example. Int J Toxicol. 2000;19:43–61. doi: 10.1080/109158100225033. [DOI] [Google Scholar]
- 14.Billard A, Fillinger S, Leroux P, Lachaise H, Beffa R, Debieu D. Strong resistance to the fungicide fenhexamid entails a fitness cost in Botrytis cinerea, as shown by comparisons of isogenic strains. Pest Manag Sci. 2012;68:684–691. doi: 10.1002/ps.2312. [DOI] [PubMed] [Google Scholar]
- 15.Bollen GJ, Scholten G. Acquired resistance to benomyl and some other systemic fungicides in a strain of Botrytis cinerea in cyclamen. Neth J Plant Pathol. 1971;77:83–90. doi: 10.1007/BF01981496. [DOI] [Google Scholar]
- 16.Brent KJ, Hollomon DW. Fungicide resistance: the assessment of risk. FRAC Monograph Nr. 2. Brussels: GCPF; 1998. [Google Scholar]
- 17.Brent KJ, Hollomon DW (1995) Fungicide resistance in crop pathogens: how can it be managed. FRAC Monograph No. 1 (second, revised edition). Fungicide Resistance Action Committee. Monogr. 1 GCPF, FRAC, Brussels, p 1–48
- 18.Chapeland F, Fritz R, Lanen C, Gredt M, Leroux P (1999) Inheritance and mechanisms of resistance to anilinopyrimidine fungicides in Botrytis cinerea (Botryotinia fuckeliana). Pestic Biochem Physiol 64:85–100
- 19.Cools HJ, Fraaije BA. Are azole fungicides losing ground against Septoria wheat disease? Resistance mechanisms in Mycosphaerella graminicola. Pest Manag Sci. 2008;64:681–684. doi: 10.1002/ps.1568. [DOI] [PubMed] [Google Scholar]
- 20.Dean R, et al. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elad Y, Yunis H, Katan T. Multiple fungicide resistance to benzimidazoles, dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol. 1992;41:41–46. doi: 10.1111/j.1365-3059.1992.tb02314.x. [DOI] [Google Scholar]
- 22.Esterio M, Ramos C, Walker AS, Fillinger S, Leroux P, Auger J. Phenotypic and genetic characterization of Chilean isolates of Botrytis cinerea with different levels of sensitivity to fenhexamid. Phytopathol Mediterr. 2011;50:414–420. [Google Scholar]
- 23.Fernandez-Ortuno D, Chen FP, Schnabel G. Resistance to cyprodinil and lack of fludioxonil resistance in Botrytis cinerea isolates from strawberry in North and South Carolina. Plant Dis. 2013;97:81–85. doi: 10.1094/PDIS-06-12-0539-RE. [DOI] [PubMed] [Google Scholar]
- 24.Fillinger S, Ajouz S, Nicot PC, Leroux P, Bardin M. Functional and structural comparison of pyrrolnitrin- and iprodione-induced modifications in the class III histidine-kinase Bos1 of Botrytis cinerea. PLoS One. 2012;7:e42520. doi: 10.1371/journal.pone.0042520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillinger S, Leroux P, Auclair C, Barreau C, Al HC, Debieu D. Genetic analysis of fenhexamid-resistant field isolates of the phytopathogenic fungus Botrytis cinerea. Antimicrob Agents Chemother. 2008;52:3933–3940. doi: 10.1128/AAC.00615-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier E, Giraud T. Sympatric genetic differentiation of a generalist pathogenic fungus, Botrytis cinerea, on two different host plants, grapevine and bramble. J Evol Biol. 2008;21:122–132. doi: 10.1111/j.1420-9101.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 27.Fournier E, et al. Characterization of nine polymorphic microsatellite loci in the fungus Botrytis cinerea (Ascomycota) Mol Ecol Notes. 2002;2:253–255. doi: 10.1046/j.1471-8286.2002.00207.x. [DOI] [Google Scholar]
- 28.Giraud T, Fortini D, Levis C, Lamarque C, Leroux P, LoBuglio K, Brygoo Y. Two sibling species of the Botrytis cinerea complex, transposa and vacuma, are found in sympatry on numerous host plants. Phytopathology. 1999;89:967–973. doi: 10.1094/PHYTO.1999.89.10.967. [DOI] [PubMed] [Google Scholar]
- 29.Grabke A, Fernandez-Ortuno D, Schnabel G. Fenhexamid resistance in Botrytis cinerea from strawberry fields in the Carolinas is associated with four target gene mutations. Plant Dis. 2013;97:271–276. doi: 10.1094/PDIS-06-12-0587-RE. [DOI] [PubMed] [Google Scholar]
- 30.Grasso V, Palermo S, Sierotzki H, Garibaldi A, Gisi U. Cytochrome b gene structure and consequences tor resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag Sci. 2006;62:465–472. doi: 10.1002/ps.1236. [DOI] [PubMed] [Google Scholar]
- 31.Hilber UW, Hilber-Bodmer M. Genetic basis and monitoring of resistance of Botryotinia fuckeliana to anilinopyrimidines. Plant Dis. 1998;82:496–500. doi: 10.1094/PDIS.1998.82.5.496. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KB, Sawyer TL, Powelson ML. Frequency of benzimidazole-resistant and dicarboximide-resistant strains of Botrytis cinerea in Western Oregon small fruit and snap bean plantings. Plant Dis. 1994;78:572–577. doi: 10.1094/PD-78-0572. [DOI] [Google Scholar]
- 33.Kanetis L, Forster H, Jones CA, Borkovich KA, Adaskaveg JE. Characterization of genetic and biochemical mechanisms of fludioxonil and pyrimethanil resistance in field isolates of Penicillium digitatum. Phytopathology. 2008;98:205–214. doi: 10.1094/PHYTO-98-2-0205. [DOI] [PubMed] [Google Scholar]
- 34.Kojima K, Takano Y, Yoshimi A, Tanaka C, Kikuchi T, Okuno T. Fungicide activity through activation of a fungal signalling pathway. Mol Microbiol. 2004;53:1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- 35.Kretschmer M, et al. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009;5:e1000696. doi: 10.1371/journal.ppat.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalève A, Gamet S, Walker AS, Debieu D, Toquin V, Fillinger S (2013) Site-directed mutagenesis of the P225, N230 and H272 residues of succinate dehydrogenase subunit B from Botrytis cinerea highlights different roles in enzyme activity and inhibitor binding. Environ Microbiol. doi:10.1111/1462-2920.12282 [DOI] [PubMed]
- 37.Leroch M, Kretschmer M, Hahn M (2011) Fungicide resistance phenotypes of Botrytis cinerea isolates from commercial vineyards in South West Germany. J Phytopathol 159:63–65
- 38.Leroch M, Plesken C, Weber RW, Kauff F, Scalliet G, Hahn M. Gray mold populations in german strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl Environ Microbiol. 2013;79:159–167. doi: 10.1128/AEM.02655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leroux P, Chapeland F, Desbrosses D, Gredt M. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Prot. 1999;18:687–697. doi: 10.1016/S0261-2194(99)00074-5. [DOI] [Google Scholar]
- 40.Leroux P, Clerjeau M. Resistance of Botrytis cinerea Pers. and Plasmopara viticola (Berk. & Curt.) Berl. and de Toni to fungicides in French vineyards. Crop Prot. 1985;4:137–160. doi: 10.1016/0261-2194(85)90014-6. [DOI] [Google Scholar]
- 41.Leroux P, et al. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag Sci. 2002;58:876–888. doi: 10.1002/ps.566. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Fernandez-Ortuno D, Chen S, Grabke A, Luo C-X, Bridges WC, Schnabel G (2014) Location-specific fungicide resistance profiles 1 and evidence for stepwise accumulation of resistance in Botrytis cinerea. Plant Dis. doi:10.1094/PDIS-10-13-1019-RE [DOI] [PubMed]
- 43.Ma ZH, Michailides TJ (2005) Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot 24:853–863
- 44.Mernke D, Dahm S, Walker AS, Lalève A, Fillinger S, Leroch M, Hahn M. Two promoter rearrangements in a drug efflux transporter gene are responsible for the appearance and spread of multidrug resistance phenotype MDR2 in Botrytis cinerea isolates in French and German vineyards. Phytopathology. 2011;101:1176–1183. doi: 10.1094/PHYTO-02-11-0046. [DOI] [PubMed] [Google Scholar]
- 45.Morschhauser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol. 2010;47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Morton V, Staub T (2008) A short history of fungicides. Online, APSnet Features. doi:10.1094/APSnetFeature2008-0308
- 47.Moyano C, Alfonso C, Gallego J, Raposo R, Melgarejo P (2003) Comparison of RAPD and AFLP marker analysis as a means to study the genetic structure of Botrytis cinerea populations. Eur J Plant Pathol 109:515–522
- 48.Oshima M, et al. Survey of mutations of a histidine kinase gene BcOS1 in dicarboximide-resistant field isolates of Botrytis cinerea. J Gen Plant Pathol. 2006;72:65–73. doi: 10.1007/s10327-005-0247-7. [DOI] [Google Scholar]
- 49.Pappas AC. Evolution of fungicide resistance in Botrytis cinerea in protected crops in Greece. Crop Prot. 1997;16:257–263. doi: 10.1016/S0261-2194(96)00096-8. [DOI] [Google Scholar]
- 50.Samuel S, Papayiannis LC, Leroch M, Veloukas T, Hahn M, Karaoglanidis GS. Evaluation of the incidence of the G143A mutation and cytb intron presence in the cytochrome bc-1 gene conferring QoI resistance in Botrytis cinerea populations from several hosts. Pest Manag Sci. 2011;67:1029–1036. doi: 10.1002/ps.2226. [DOI] [PubMed] [Google Scholar]
- 51.Sierotzki H, Scalliet G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology. 2013;103:880–887. doi: 10.1094/PHYTO-01-13-0009-RVW. [DOI] [PubMed] [Google Scholar]
- 52.Veloukas T, Kalogeropoulou P, Markoglou AN, Karaoglanidis GS. Fitness and competitive ability of Botrytis cinerea field isolates with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations. Phytopathology. 2014;104:347–356. doi: 10.1094/PHYTO-07-13-0208-R. [DOI] [PubMed] [Google Scholar]
- 53.Veloukas T, Markoglou AN, Karaoglanidis GS. Differential effect of sdhB gene mutations on the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Dis. 2013;97:118–122. doi: 10.1094/PDIS-03-12-0322-RE. [DOI] [PubMed] [Google Scholar]
- 54.Vignutelli A, Hilber-Bodmer M, Hilber UW. Genetic analysis of resistance to the phenylpyrrole fludioxonil and the dicarboximide vinclozolin in Botryotinia fuckeliana (Botrytis cinerea) Mycol Res. 2002;106:329–335. doi: 10.1017/S0953756202005683. [DOI] [Google Scholar]
- 55.Walker AS, Gautier A, Confais J, Martinho D, Viaud M, Le Pêcheur P, Dupont J, Fournier E. Botrytis pseudocinerea, a new cryptic species causing gray mold in French vineyards in sympatry with Botrytis cinerea. Phytopathology. 2011;101:1433–1445. doi: 10.1094/PHYTO-04-11-0104. [DOI] [PubMed] [Google Scholar]
- 56.Walker AS, Micoud A, Remuson F, Grosman J, Gredt M, Leroux P. French vineyards provide information that opens ways for effective resistance management of Botrytis cinerea (grey mould) Pest Manag Sci. 2013;69:667–678. doi: 10.1002/ps.3506. [DOI] [PubMed] [Google Scholar]
- 58.Weber RWS. Resistance of Botrytis cinerea to multiple fungicides in Northern German small-fruit production. Plant Dis. 2011;95:1263–1269. doi: 10.1094/PDIS-03-11-0209. [DOI] [PubMed] [Google Scholar]
- 59.Wood PM, Hollomon DW. A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Q(o) site of complex III. Pest Manag Sci. 2003;59:499–511. doi: 10.1002/ps.655. [DOI] [PubMed] [Google Scholar]