Abstract
Fungal diseases are an increasing global burden. Fungi are now recognised to kill more people annually than malaria, whilst in agriculture, fungi threaten crop yields and food security. Azole resistance, mediated by several mechanisms including point mutations in the target enzyme (CYP51), is increasing through selection pressure as a result of widespread use of triazole fungicides in agriculture and triazole antifungal drugs in the clinic. Mutations similar to those seen in clinical isolates as long ago as the 1990s in Candida albicans and later in Aspergillus fumigatus have been identified in agriculturally important fungal species and also wider combinations of point mutations. Recently, evidence that mutations originate in the field and now appear in clinical infections has been suggested. This situation is likely to increase in prevalence as triazole fungicide use continues to rise. Here, we review the progress made in understanding azole resistance found amongst clinically and agriculturally important fungal species focussing on resistance mechanisms associated with CYP51. Biochemical characterisation of wild-type and mutant CYP51 enzymes through ligand binding studies and azole IC50 determinations is an important tool for understanding azole susceptibility and can be used in conjunction with microbiological methods (MIC50 values), molecular biological studies (site-directed mutagenesis) and protein modelling studies to inform future antifungal development with increased specificity for the target enzyme over the host homologue.
Keywords: CYP51, Sterol 14-demethylase, Point mutations, Azole resistance, Antifungals, Fungicides
Introduction
Most fungi are saprophytic; however, there are several fungal genera including species that can cause disease in plants, animals and man. Some are primary pathogens, capable of causing disease in a healthy, uncompromised host, whilst others are opportunistic (or secondary) pathogens. These generally only cause disease when host defences are impaired, e.g. when a patient is immunocompromised or immunosuppressed. Many emerging fungal pathogens have appeared and their increase is of concern. Opportunistic fungal pathogens now account for around 10–15 % of nosocomial infections [1].
Worldwide, over 300 million individuals each year contract a serious fungal infection [2]. These infections range from the relatively minor such as a single episode of vulvovaginal thrush to life-threatening conditions such as chronic pulmonary aspergillosis. Global mortality from fungal infection is over 1.35 million people per annum [2], far more than are killed by malaria (~0.63 million) [3]. In addition, fungal infections of hair, nails and skin affect approximately 25 % of the world’s population [4].
Oral candidosis affects an estimated 9.5 million people per annum [5], and Candida infection of the oesophagus affects a further two million people [6]. Recurrent bouts of vulvovaginal candidosis (thrush) affect at least 75 million women annually [7], and the annual incidence of candidaemia has been estimated at 300,000 cases worldwide with a mortality rate of 30–55 % [8].
Meanwhile, over ten million patients are at risk of invasive aspergillosis (IA) each year through corticosteroid and anticancer therapies with over 200,000 patients developing IA annually [9, 10] and mortality rates of over 50 % even with treatment. Approximately 4 million of the 193 million asthmatics worldwide develop allergic bronchopulmonary aspergillosis (ABPA), and a further 3 to 13 million adults suffer from severe asthma with fungal sensitisation (SAFS) [11]. In addition, chronic pulmonary aspergillosis (CPA) is estimated to affect more than three million people, with 1.2 million cases following tuberculosis [11–13].
Over one million people, mainly HIV-AIDS patients, develop cryptococcal meningitis each year, caused by the basidiomycete yeast Cryptococcus neoformans, which gives rise to a high mortality rate of over 60 % [14]. In addition, around 100,000 to 300,000 cases of coccidioidomycosis occur each year in the USA, mainly amongst immunocompetent individuals [15] with additional outbreaks reported in Central and South America.
Filamentous fungi such as Aspergillus sp. and Fusarium sp. are also responsible for over one million eye infections in Asia and Africa annually accounting for approximately 10 % of the 284 million visually impaired people worldwide [16]. The main etiological agents of fungal infections of the hair, nails and skin are the basidiomycete Malassezia yeasts Malassezia globosa and Malassezia restricta associated with dandruff, seborrheic dermatitis, pityriasis versicolor and malassezia folliculitis (and may also exacerbate atopic dermatitis and psoriasis) [17–19] and the dermatophytes such as Trichophyton rubrum, an important etiological agent in athlete’s foot (tinea pedis), ringworm (tinea), nail infections (onychomycosis) and groin infections (tinea cruris) [20, 21]. Azole antifungal agents are an important treatment therapy against all these fungal infections, although there are alternative therapies with the squalene epoxidase inhibitor terbinafine important in treating athlete’s foot.
Numerous fungal species are responsible for diseases affecting arable crops. The causative agents of septoria wheat blotch, wheat headblight, eyespot disease in wheat and barley, powdery mildew in wheat, barley and grapes, brown rust in wheat, black sigatoka in bananas and plantain, leaf scald in cereals, leaf spot in sugar beet, apple scab and the spoilage of citrus fruit threaten crops and must be controlled to protect yields. In addition, some phytopathogenic fungi including Aspergillus, predominantly Aspergillus flavus, and some Fusarium and Penicillium species also synthesise mycotoxins, e.g. aflatoxins, and must be controlled in agriculture to ensure food safety.
Control of both plant and animal fungal pathogens is clearly desirable, and for several decades, the use of azole antifungal drugs and fungicides has been extensively used to maintain the health and food security of the world [22]. Should azole antifungal agents lose their effectiveness, arable crop yields will fall [23] and mortality rates amongst patients with fungal infections will rise [22]. For example, if the use of azole fungicides ceased, there would be a fall in European wheat production of ~7 % (9.8 million tons) in the short term and ~12 % (18.6 million tons) by 2020 [23]. This decreased productivity would represent a financial loss of 2.4 billion euros in the short term and 4.6 billion euros by 2020. It would also affect the ability of Europe to be self-sufficient in terms of wheat production [23]. Azole use in agriculture extends to animal husbandry; both domestic and agricultural animals have implications for the food chain beyond their use to control fungal infection of food crops [24]. Furthermore, azoles are used to stop fungal growth on materials, e.g. they are included in wood preservatives such as ‘Copper azole type B’ (96 % copper, 4 % tebuconazole) [25], antimold additives to paints [26] and as copper corrosion inhibitors (tolyltriazole and benzotriazole) added to aqueous coolant systems [27]. It is clear that development of resistance to azole antifungals has the potential to affect health, food supply and quality of life.
Azoles inhibit the synthesis of ergosterol in fungi through direct binding to sterol 14α-demethylase (CYP51). CYP51 is a member of the superfamily of haem-containing enzymes, cytochrome P450. CYP51 is located in the outer membrane of the endoplasmic reticulum and catalyses the removal of the methyl group at carbon 14 [28]. A lone pair of electrons on the nitrogen of the azole ring binds to the iron atom in the haem prosthetic group of CYP51, located in the active site of the enzyme, whilst the N-1 substituent group interacts with amino acids that line the active site pocket [29]. Specificity of azole compounds depends upon the interaction between side groups of the azole compound and the CYP. Azole binding to CYP51 is non-competitive and results in a depletion of the final fungal sterol (usually ergosterol) and a concomitant accumulation of 14-methylated sterols which inhibit fungal growth by disrupting the cell membrane [30]. In some species, including Candida albicans, 14α-methyl-ergosta-8,24(28)-dien-3β-6α-diol is accumulated, which is known to disrupt the cell membrane [30, 31] whilst other species, such as C. neoformans, accumulate 14α-methyl products and the 3-ketosteroid obtusifolione [32].
Clinical azole fungicides
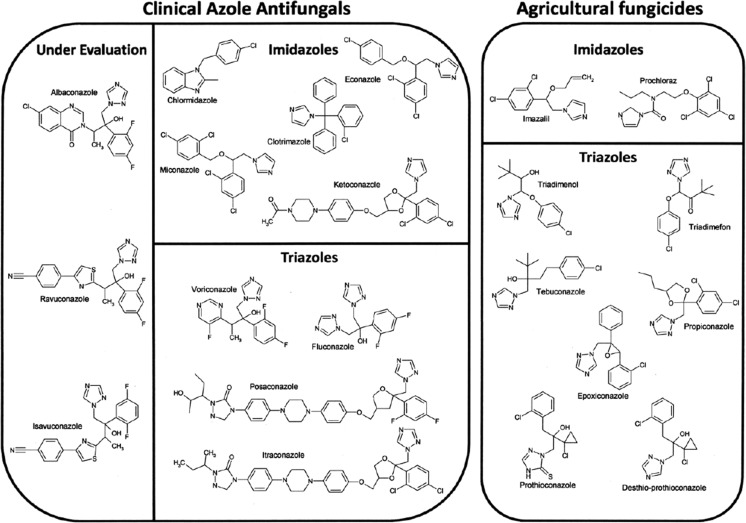
The antifungal action of azole compounds was first reported in 1944 [33, 34]. Various compounds have been introduced over the years; firstly, there were imidazoles (two nitrogen azole ring) which were followed by triazoles (three nitrogen azole ring, see Fig. 1). In 1958, chlormidazole became the first azole antifungal available for topical use in the clinic. Later, in 1969, other imidazole-based azole antifungals, clotrimazole and miconazole, were introduced for topical use followed by econazole in 1974 [35]. The first oral treatment for systemic fungal infections was ketoconazole [36] though its use was limited by its toxicity [37–40]. During the 1990s, the first systemic triazoles, firstly fluconazole and later itraconazole, were introduced in the USA. Both fluconazole and itraconazole have good antifungal activity and are less toxic than ketoconazole [41]. In the late 1990s and 2000s, second-generation triazoles were introduced, including voriconazole and posaconazole. They are now used to combat fluconazole-resistant strains and species [42] and have proved especially to be effective against Aspergillus fumigatus [43, 44]. Triazole antifungal agents currently undergoing clinical evaluation include albaconazole [45–47], ravuconazole [48, 49], isavuconazole [50, 51] and pramiconazole [52–55].
Fig. 1.
Structures of the clinical and agricultural azole compounds discussed in this review
Agricultural azole fungicides
The first azole fungicides introduced for agricultural use were both imidazole and triazole compounds. The first imidazole fungicides included imazalil (1973) and prochloraz (1977) with imazalil used as a seed treatment and post-harvest treatment, whilst prochloraz was used on cereals. The first triazole fungicides included triadimefon (1973), triadimenol (1977) and propiconazole (1979) [33, 34]. Triazoles have become the most widely used class of fungicide accounting for over 20 % market share with other azole fungicides accounting for a further 5 % [56]. Numerous other triazoles have subsequently been released including tebuconazole (1986), epoxiconazole (1990) and prothioconazole (2002). The three most commonly used fungicides in the UK, the Netherlands and Denmark in 2008 were prothioconazole, epoxiconazole and tebuconazole [57]. Azole fungicides have persisted through the decades because they are high-efficiency broad-spectrum fungicides where resistance has occurred slowly over time to a specific fungicide. Progressive introduction of newer triazoles that are intrinsically more active assists in maintaining the effectiveness (ED50 values) of azole fungicides.
Resistance mechanisms
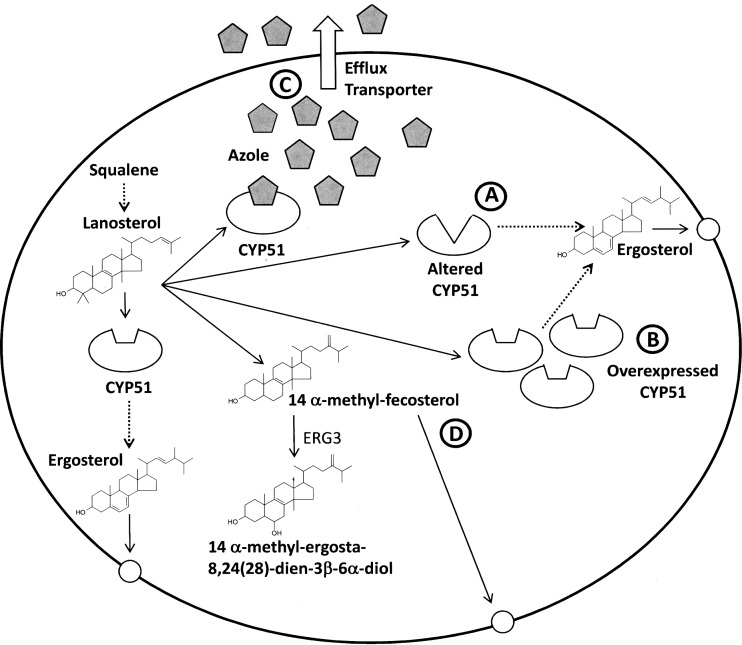
The increasing emergence of azole resistance in yeast and fungi can be attributed to the prophylactic use of azole drugs and prolonged treatment regimens in the clinic and usage of agricultural azole fungicides in crop protection [58–63]. Resistance to azoles can arise through four main mechanisms (see Fig. 2). The affinity for the target enzyme (CYP51) may be reduced through point mutations. The amount of target enzyme present may be increased due to upregulation of the gene, and the efflux of azole from the cell may be increased due to the overexpression of transporters. In addition, it is possible for secondary mutations to occur which confer resistance. This has been observed in Candida spp. where an ERG3 null mutant is resistant to azoles. A lack of ERG3 activity results in the production of 14-methyl fecosterol when treated which is capable of supporting membrane function; thus, the fungistatic effect of the accumulation of other 14-methylated sterols is circumvented [31, 64–66].
Fig. 2.
Azole resistance mechanisms in C. albicans. CYP51 is an essential step in the biosynthesis of ergosterol, which is required for membrane stability and functionality. Azoles inhibit CYP51 causing the accumulation of 14 α-methyl-ergosta-8,24(28)-dien-3β-6α-diol. Resistance to azoles can occur through a an altered CYP51 (point mutations), b overexpression of CYP51, c overexpression of efflux transporters and d null mutation of ERG3 which blocks the synthesis of 14 α-methyl-ergosta-8,24(28)-dien-3β-6α-diol, resulting in the accumulation of 14 α-methyl-fecosterol which is capable of supporting membrane function
The complex nature of resistance mechanisms in fungal species is still not fully understood. In some species, there are multiple copies of CYP51 (e.g. Aspergillus and Fusarium spp.). In addition, some resistant strains appear to contain adaptations to more than one resistance mechanism. However, mutations that affect the binding of azoles to CYP51 are commonly found in azole-resistant fungal strains. CYP51 mutations have been extensively studied and often correlate directly with observed azole resistance. Understanding the effects mutations have on the interaction between CYP51 and azoles and the inhibition of CYP51 activity is necessary in order to design new improved drugs that combat present and future azole resistance.
CYP51 mediated resistance in the clinic
C. albicans
C. albicans is a dimorphic diploid asexual yeast and the most common fungal opportunistic pathogen of humans [67]. C. albicans causes a wide range of clinical infections in humans from mucosal infections such as thrush and oral candidosis to potentially life-threatening systemic candidiasis and candidaemia. Candida bloodstream infections cause significant morbidity and mortality, particularly in intensive care patients [68, 69]. The incidence of fungal infections by C. albicans and non-albicans Candida species has steadily been increasing over the last two decades not only partially as a result of HIV-AIDS but also due to the growing number of patients who are immunodeficient as a result of organ and bone marrow transplants and cancer treatments [70]. Predisposing factors for invasive candidiasis include immunosuppressive and cytotoxic therapies, treatment with broad-spectrum antibiotics, HIV-AIDS or diabetes and in situ central venous catheters and urinary tract catheters [71–73]. Controlling invasive fungal infections amongst haematology, oncology and intensive care patients is now a major challenge [61]. Azole antifungals are relatively safe, easy to administer (often by mouth) and have a high therapeutic index leading to triazole antifungals becoming the standard first-line therapy to combat fungal infections. However, prophylactic use of azole drugs and prolonged treatment regimens in the clinic has led to increasing emergence of azole-resistant yeast and fungal strains. Presently, there are four known mechanisms that confer azole resistance in C. albicans [74, 75]. These include mutations that alter the amino acid sequence of CYP51 [76–78], overexpression of efflux transporters (CaCDR1, CaCDR2 and CaMDR1) [79, 80], overexpression of CYP51 and alterations in the ergosterol biosynthetic pathway such as mutations in ERG3. These resistance mechanisms are often combined in clinical isolates [66, 81–85].
More than 140 different amino acid substitution mutations have been reported in the CYP51 gene isolated from clinical strains of C. albicans [86] (although not all of these mutations confer an azole-resistant phenotype). In addition, the CYP51 genes from both azole-resistant and azole-susceptible clinical C. albicans strains may contain several amino acid substitutions [87]. Most of these substitutions cluster into three ‘hotspots’ [87] located within amino acid residues 105-165, 266-287 and 405-488. Not all amino acid substitutions contribute equally to azole resistance. K143R, S405F, G464S, R467K and I471T have been recovered only from azole-resistant strains, whereas E266D and V488I have been recovered from both azole-resistant and azole-susceptible strains, suggesting that these two latter mutations are probably not responsible for conferring azole resistance [86].
A number of the C. albicans CYP51 point mutations have previously been expressed in Escherichia coli, purified and biochemically characterised. These include Y132H [88], F145L [88, 89], I471T [90] and S279F [91]. In addition, several mutations were expressed in Saccharomyces cerevisiae, and the isolated membrane fractions (G464S [92] and R467K [93]) and purified enzyme (T315A) were used to study azole resistance [94].
A. fumigatus
Aspergillus infections are a problem in both immunocompetent and immunocompromised individuals. Approximately 2 to 10 % of immunosuppressed patients suffer from acute IA annually [95], whilst three million immunocompetent patients worldwide are estimated to have CPA. Aspergillus is also responsible for an estimated four million cases of ABPA. Both CPA and ABPA require long-term use of azoles for management of the conditions [96]. Azole resistance is therefore a concern for these patients and also amongst cystic fibrosis patients [97–99]. The mortality rate amongst patients with multiazole-resistant invasive aspergillosis is 88 % [100]. Increasing azole resistance amongst A. fumigatus strains will result in increased morbidity and mortality amongst aspergillosis patients as well as placing an extra resource burden (financial and manpower) on hospitals with an increasing reliance on amphotericin B and caspofungin to clear fungal infections which needs to be administered and monitored in a clinical setting. Since 1998, azole resistance in A. fumigatus clinical isolates has been increasing around the world. The global ARTEMIS surveillance programme found that 5.8 % of A. fumigatus clinical isolates showed elevated minimum inhibitory concentrations (MICs) to one or more triazoles [101], whilst the SCARE programme in Europe found that 3.4 % of A. fumigatus clinical isolates were azole-resistant [100]. In the Netherlands, ~10 % of all clinical isolates are now itraconazole-resistant [100] compared to Manchester (UK) where 23 and 31 % of isolates were azole-resistant in 2008 and 2009 [102].
Aspergillus spp. contain two CYP51 isoenzymes, CYP51A and CYP51B. Studies with A. fumigatus strains containing gene knock-outs of CYP51A and CYP51B have demonstrated that both isoenzymes can fulfil the role of sterol 14α-demethylase in A. fumigatus in vivo, resulting in no phenotypic difference [103–105]. A conditional mutant study indicated that in the absence of both CYP51A and CYP51B, growth was abolished but not when performed individually [106]. The main azole resistance mechanism in A. fumigatus is the development of point mutations in CYP51A, and this form was found to have less affinity for azole drugs than CYP51B suggesting a molecular basis for this based on changes in the protein responsible for activity at higher concentrations of drug [107]. Other azole resistance mechanisms such as overexpression of CYP51 isoforms A [96, 108, 109] and B [110], point mutations in CYP51B [111, 112] and the induction and overexpression of the drug transporters atrF, AfuMDR3, AfuMDR4 and Cdr1B [109, 113–115] have been described. Other novel mechanisms that confer azole resistance in A. fumigatus strains such as the P88L mutation in transcription factor hapE [116] are still being discovered.
Recently, Lockhart et al. [101] found that 60 % of clinical A. fumigatus isolates contained triazole resistance mutations within CYP51A. The five most frequent CYP51A mutations or ‘hotspots’ reported in A. fumigatus are G54, L98, G138, M220 and G448 [96]. These five mutations have been confirmed to confer azole resistance in A. fumigatus through gene replacement experiments (though L98H also required a modified promoter sequence) [108, 111, 117–121]. Nearly 40 different CYP51A point mutations in clinical A. fumigatus isolates have been reported, although not all mutations confer an azole resistance phenotype [122, 123] and can occur on their own or in combination with other mutations. The CYP51A point mutations G54, G138, M220 and G448 are thought to have arisen during triazole therapy of patients in the clinic, whilst TR34/L98H and TR46/Y121F/T289A (which both contain tandem repeats in the promoter region of the gene) are thought to have arisen in the environment in the Netherlands as a response to the use of agricultural triazole fungicides on crops [118, 124–126]. Interestingly, even at the time of the first report of itraconazole resistance in A. fumigatus (1997) [127], the possibility of an environmental origin of the resistance was recognised [128].
C. neoformans
Cryptococcosis is a particularly problematic systemic fungal infection in HIV/AIDS immunocompromised patients from sub-Saharan Africa and is caused by the opportunistic basidiomycete yeast pathogen C. neoformans [14] leading to infections of the lungs and brain. Meningoencephalitis is the most lethal manifestation of cryptococcosis with a life expectancy of less than a month if untreated [129]. Pathogenic Cryptococcus species cause disease in almost one million people annually with over 620,000 deaths, and a third of all HIV/AIDS deaths are attributable to Cryptococcus species infection [14]. Current treatment options are limited to only a few drugs, namely initial induction therapy with a combination of amphotericin B and flucytosine followed by a maintenance regime of fluconazole [129]. Even after administering the recommended treatment, 3-month mortality rates of 10 to 20 % are common [130, 131]. In addition, adopting such treatment is costly (with amphotericin B requiring intravenous administration), especially for developing countries where mortality rates can approach 100 % [132, 133]. Most Cryptococcus infections of humans and nearly all cryptococcal infections of HIV/AIDS patients are caused by C. neoformans var. grubii, the most prevalent being the H99 strain, although Cryptococcus gattii (found in trees and primarily located in the tropics and sub-tropics) infection is increasing in prevalence amongst immunocompetent individuals, especially in NW America and Africa [134].
Azole resistance associated with enhanced resistance in the CYP51 activity in C. neoformans was first observed in isolates from AIDS patients in 1995 [135]. Resistance, especially towards fluconazole, is becoming an increasing problem amongst Cryptococcus species in the clinic due to prolonged maintenance treatment regimens [136]. Three main mechanisms for increased azole resistance in C. neoformans have been identified. Firstly, point mutations in CYP51 have been identified that confer resistance to azole antifungals. The CYP51 G468S mutation [137] confers resistance to fluconazole, whilst the Y145F mutation (equivalent to Y132F in C. albicans and several other species) [138] confers resistance to fluconazole and voriconazole but increases susceptibility to itraconazole and posaconazole. Secondly, C. neoformans overcomes stress induced by exposure to azoles, especially fluconazole, by duplication of chromosome 1 (selective disomy) which contains two important genes CYP51, the azole target enzyme, and AFR1, the major transporter of azoles in C. neoformans, promoting increased expression levels of these two proteins [139]. Thirdly, general genome plasticity of C. neoformans strains post-infection [140] presents the challenge of a constantly moving target in terms of effective drug therapies in combating cryptococcosis. Presently, there are no published studies comparing purified mutant C. neoformans CYP51 proteins against wild-type CYP51 protein in terms of substrate and azole binding affinities and inhibition of CYP51 catalysis by azole antifungal agents. Again, such biochemical characterisations of mutant versus wild-type enzymes would provide useful insights into the azole resistance mechanism of this fungal pathogen.
Other species of fungi have shown some resistance to azole antifungal drugs. The prolonged treatment regimens often required to treat dermatological infections have led to the emergence of azole-resistant T. rubrum and M. globosa strains, especially against fluconazole [141–143], although no CYP51 point mutations have so far been identified in azole-resistant isolates with the mechanism(s) of azole resistance remaining unclear [144]. However, fluconazole resistance in Histoplasma capsulatum in AIDS patients has been correlated with a single mutation (Y136F) in CYP51 [145].
CYP51 mediated azole resistance in agriculture
Azole fungicides are used to treat several economically important arable crops to minimise crop damage caused by plant fungal pathogens. These pathogens include Oculimacula yallundae and Tapesia yallundae which cause eyespot disease in wheat and barley, Blumeria graminis (powdery mildew in wheat and barley), Puccinia triticina (brown rust in wheat), Mycosphaerella fijiensis (cause of black sigatoka in bananas and plantain), Erysiphe necator (powdery mildew in grapes), Rhynchosporium secalis (leaf scald in cereals), Cercospora beticola (leaf spot in sugar beet), Venturia inaequalis (apple scab), Penicillium digitatum (mold on citrus fruit), Zymoseptoria tritici (formerly called Mycosphaerella graminicola and Septoria tritici and the cause of septoria leaf blotch in wheat) and Fusarium sp. (headblight).
There are presently no reports of mutations in the CYP51 of O. yallundae, T. yallundae, P. digitatum, R. secalis, C. beticola, V. inaequalis or Fusarium spp. that correlated with azole resistance. However, CYP51 mutations have been documented in B. graminis (Y136F and K147Q), E. necator (Y136F) [146, 147], P. triticina (Y134F) [148], M. fijiensis (Y136F, A313G, Y461D, Y463D, Y463H and Y463N) [149] and Z. tritici (over 30 CYP51 mutations) [150]. In addition, constitutive overexpression of CYP51 contributes to azole resistance in field isolates of Z. tritici [151], P. digitatum [152, 153], P. triticina [148] and V. inaequalis [154, 155]. Azole resistance in P. digitatum [156] and B. cinerea [157] has also been found to be mediated by the overexpression of efflux transporters. Resistance to azoles in R. secalis has been found to be mediated by selection of strains possessing both CYP51A and CYP51B, where previously the majority of isolates possessed a single CYP51B [158].
Mutations in the sole CYP51 enzyme present in Z. tritici (formerly called M. graminicola) are well documented [58, 150, 159–164]. Azoles have been used extensively since the early 1980s to combat septoria wheat blotch which has given rise to a large number of CYP51 mutations, with up to eight coding changes in one haplotype, reducing the effectiveness of some compounds. CYP51 alterations are the most common form of azole resistance in Z. tritici. Mutations at 14 residues have been reported, which appear in different combinations resulting in over 30 CYP51 variants [150]. However, some mutations are lethal in isolation and may only exist in combination with other mutations enabling CYP51 function to be maintained. Mutations are often found in combinations with different CYP51 variants leading to resistance to different azole compounds [159, 162–164]. Studies of mutations, including heterologous systems and protein modelling, have provided insight into the development of resistance to different azoles [160, 161] and the differential nature of resistance [159]. The impact of various combinations of mutations in Z. tritici is complex and has been comprehensively reviewed in relation to the current status of variants in the field by Cools and Fraaije [150].
Several of the Z. tritici CYP51 mutations are equivalent to those seen in other fungal species. Mutations at the equivalent residue to Y137 are the most widely reported azole resistance mutations found in both clinical and agricultural isolates including E. necator, B. graminis, P. triticina and M. fijiensis [78, 138, 145, 146, 148, 149, 165–167]. The mutation Y137F was prevalent in the 1990s and later in 2000 in combination with S542T and confers resistance to triadimenol. However, triadimenol is no longer used and Y137F and Y137F-S542T (double mutation) are now rarely seen in the population as newer azoles have been selected for other mutations. Alterations at residues 459-461 in Z. tritici confer resistance to many azoles but are also required to accommodate some other mutations (e.g. I381V, V136A) which are lethal on their own [160]. Mutations in this region are also found in combination in C. albicans [82, 86, 167], M. fijiensis [149] and A. fumigatus [59]. The mutation D134G is another mutation found in combination, which has also been identified in the structurally equivalent region in C. albicans (G129A) where it has also only been observed in combination with other mutations [78].
The use of many different azoles to combat Z. tritici has led to a shift in variants found in the population adapting to the azole challenge. Of particular concern at present are isolates carrying V136A, I381V and D134G which have reduced sensitivity to prochloraz, epoxiconazole and prothioconazole [150]. Another recently emerged mutation, S524T, decreases sensitivity to all azoles, but when combined with L50S, D134G, V136A and Y461S, it is less sensitive to prothioconazole, the most recently introduced fungicide [58]. The majority of Z. tritici isolates from the field now contain mutations in CYP51 and demonstrate an extreme change due to selective pressure from azole use. This extensive development of mutations in Z. tritici CYP51 compared to the present status of clinical fungal strains may represent an indicator of the mutations that may eventually occur in clinical species given similar selection pressures.
Assessing azole susceptibility of CYP51
DNA sequencing of strains is used to identify point mutations within CYP51 genes. Correlated with MIC data, they indicate those mutations that may confer resistance. Further genetic analysis, including mutating genes in host organisms and heterologous expression of mutated and wild-type CYP51s, can be used to functionally assess the effect such mutations have on azole resistance [105, 108, 111, 117–121, 160]. However, biochemical analysis allows an assessment of the impact CYP51 susceptibility alone has on resistance.
Substrate and azole ligand binding studies using UV–visible spectroscopy under oxidative conditions and determination of the azole concentration required for 50 % inhibition of CYP51 catalysis (IC50 value) using purified recombinant CYP51 enzymes are valuable tools in assessing the susceptibility of CYP51 enzymes to particular azole compounds and the impact of point mutations on that susceptibility. These methods are usually performed using truncated (soluble) CYP51 enzymes since eukaryotic CYP51 enzymes generally express poorly in E. coli without modification of the N-terminal membrane anchor region. Due to the difficulty in expressing full-length eukaryotic CYP51 proteins, no systematic study has been performed to assess the impact of the N-terminal membrane anchor region on eukaryotic CYP51 catalytic efficiency, azole susceptibilities, ligand binding affinities and structural/spatial conformation. However, we have studied the difference between truncated and full-length forms of Homo sapiens CYP51. The removal of the N-terminal membrane anchor of H. sapiens CYP51 increased the turnover number with lanosterol by 3.7-fold compared to the full-length enzyme [168], suggesting that the N-terminus impedes optimal catalysis in CYP51 in vitro reconstitution assays. The N-terminal region did not affect the azole or sterol ligand binding properties as both the full-length and truncated H. sapiens CYP51 bound pharmaceutical azole antifungal agents and lanosterol with similar affinities [168]. Further studies are required to fully elucidate the effects of the removal of the N-terminal membrane anchor on the catalytic properties of CYP51 enzymes both in free solution and in lipid bilayers/membranes. However, comparison of wild-type CYP51s against those that contain point mutations makes it possible to biochemically determine whether a compound is likely to be effective against strains harbouring point mutations in CYP51 and whether azoles are likely to be effective against a species. Furthermore, these studies in conjunction with in silico protein modelling enable a better understanding of azole–CYP51 interactions including which structural elements of azole antifungals confer increased selectivity for a specific CYP51 enzyme and will assist in the design of the next generation of azole antifungals both for agriculture and the clinic.
Ligand binding studies
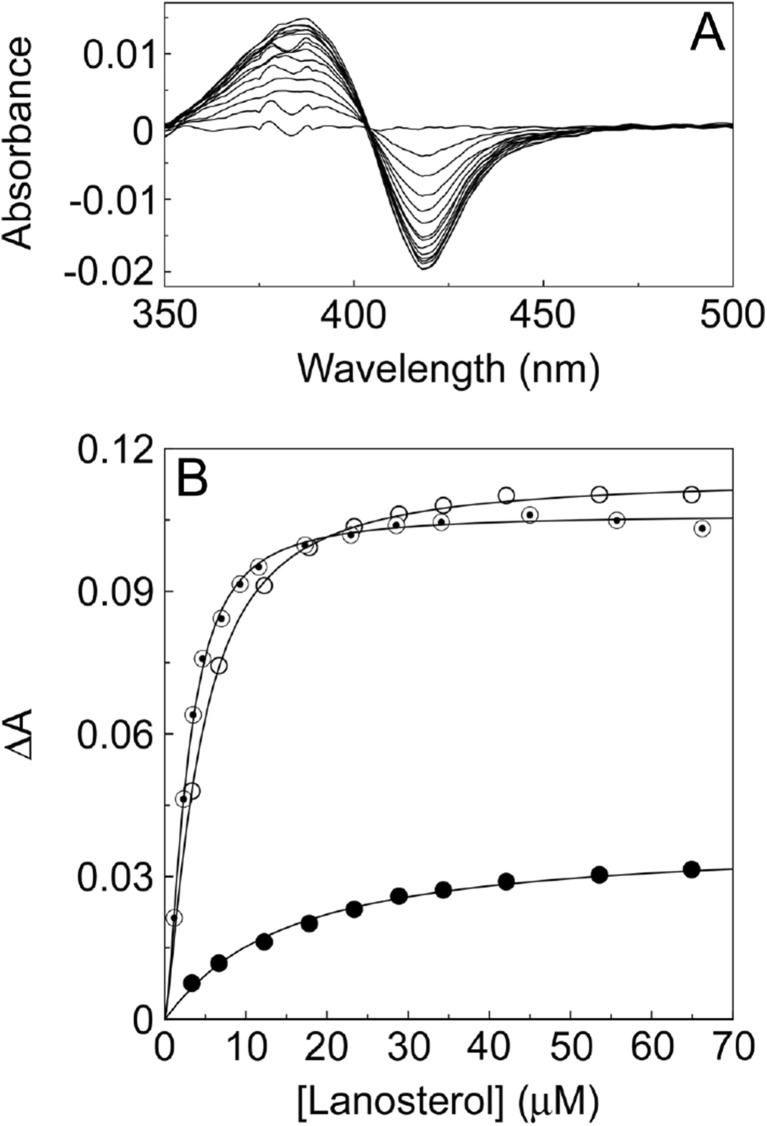
Progressive titration of CYP51 enzymes with their sterol substrates (lanosterol for mammals and yeasts, lanosterol/eburicol for higher fungi and obtusifoliol for plants) give type I difference spectra (Fig. 3a) with an absorbance maximum at 380–390 nm and an absorbance minimum at 415–420 nm. Type I binding spectra are formed when the substrate (or other molecules) displaces the water molecule coordinated as the sixth ligand to the low-spin hexa-coordinated heme prosthetic group, causing the heme to adopt the high-spin penta-coordinated conformation [169]. These spectra are useful for investigating substrate affinity with the CYP51 protein by determining the dissociation constant for the enzyme–substrate complex (Ks) (Fig. 3b), often an indicator of catalytic efficiency, and will quickly identify whether a CYP51 point mutation alters the binding affinity for substrate. The Ks values for wild-type, S279F and I471T C. albicans CYP51 enzymes were 13.5, 7.1 and 4.8 μM, respectively [90, 91]. Therefore, the S279F and I471T mutant proteins had higher apparent affinities for lanosterol than the wild-type enzyme suggesting increased catalytic efficiency was one way these two point mutations conferred a reduction in azole susceptibility. The type I difference spectrum only measures the low- to high-spin state conversion, and studies have shown that the CYP–substrate complexes can exist as an equilibrium of high- and low-spin states [170].
Fig. 3.
Lanosterol binding studies with 5 μM wild-type, S279F and I471T C. albicans CYP51 proteins. The type I difference spectrum obtained for the wild-type protein is shown a along with the lanosterol saturation plots b for wild-type (black circle), S279F (white circle) and I471T (◉) C. albicans CYP51. The Michaelis–Menten equation was used to fit the data and derive K s values
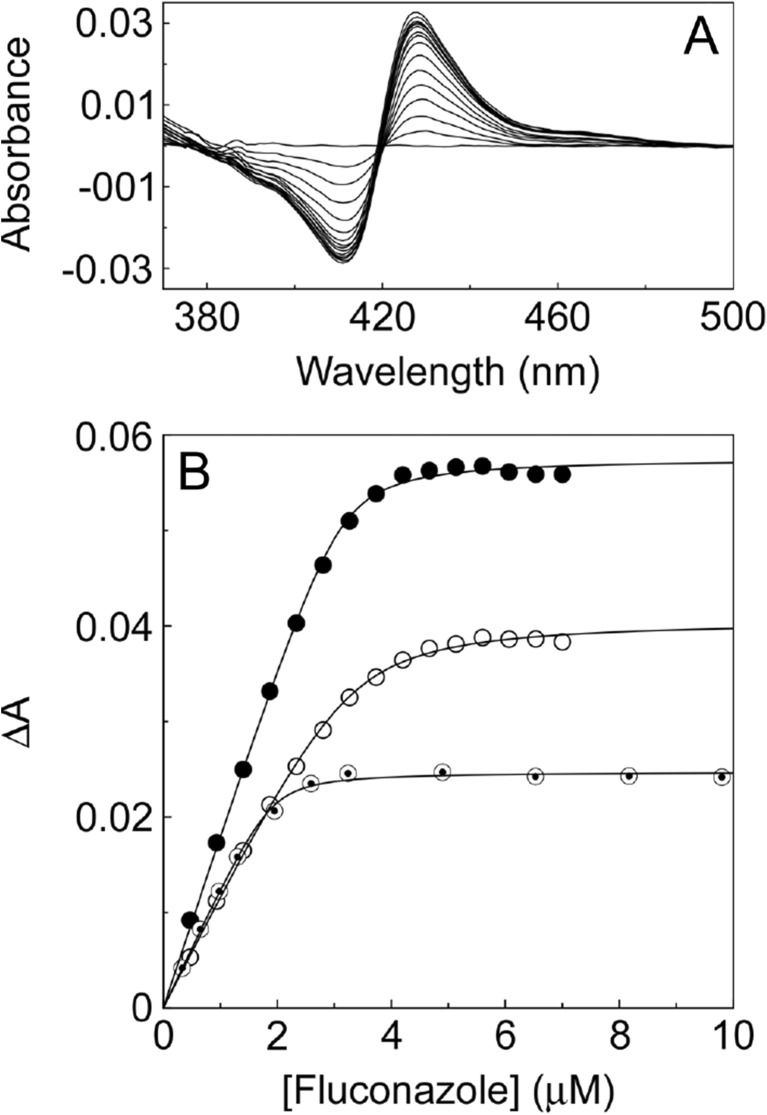
Progressive titration of CYP51 proteins with azole antifungal agents gives type II difference spectra (Fig. 4a) with an absorbance maximum at 425–435 nm and an absorbance minimum at 390–410 nm [169]. Type II difference spectra arise due to the triazole ring N-4 nitrogen or the imidazole ring N-3 nitrogen coordinating as the sixth ligand with the heme iron [171]. Fluconazole saturation curves (Fig. 4b) were best fitted using a rearrangement of the Morrison equation [172], indicating that the observed binding was ‘tight’. Tight binding is observed when the dissociation constant for a ligand (Kd) is similar to or lower than the concentration of the enzyme present [173]. The fluconazole Kd values for wild-type, S279F and I471T C. albicans CYP51 enzymes were 56 ± 4, 241 ± 18 and 84 ± 17 nM [90, 91]. This suggests the S279F point mutation confers reduced fluconazole susceptibility through weakening fluconazole binding, whereas the I471T mutation binds fluconazole with similar affinity to the wild-type CYP51 enzyme. Azole ligand binding experiments are useful to screen new azole fungicides for potential effectiveness against the target CYP51 and that they should ideally exhibit reduced affinity towards the host CYP51 homologue. Candidate azole fungicides that bind weakly to the target CYP51 are unlikely to be effective inhibitors in situ unless the compound is a pro-fungicide which breaks down over time to release the active component such as prothioconazole [174, 175].
Fig. 4.
Fluconazole binding studies with wild-type, S279F and I471T C. albicans CYP51 proteins. The type II difference spectrum obtained for the wild-type protein is shown a along with the fluconazole saturation plots b for wild-type (black circle), S279F (white circle) and I471T (◉) C. albicans CYP51. A rearrangement of the Morrison equation [172] was used to fit the data and determine K d values
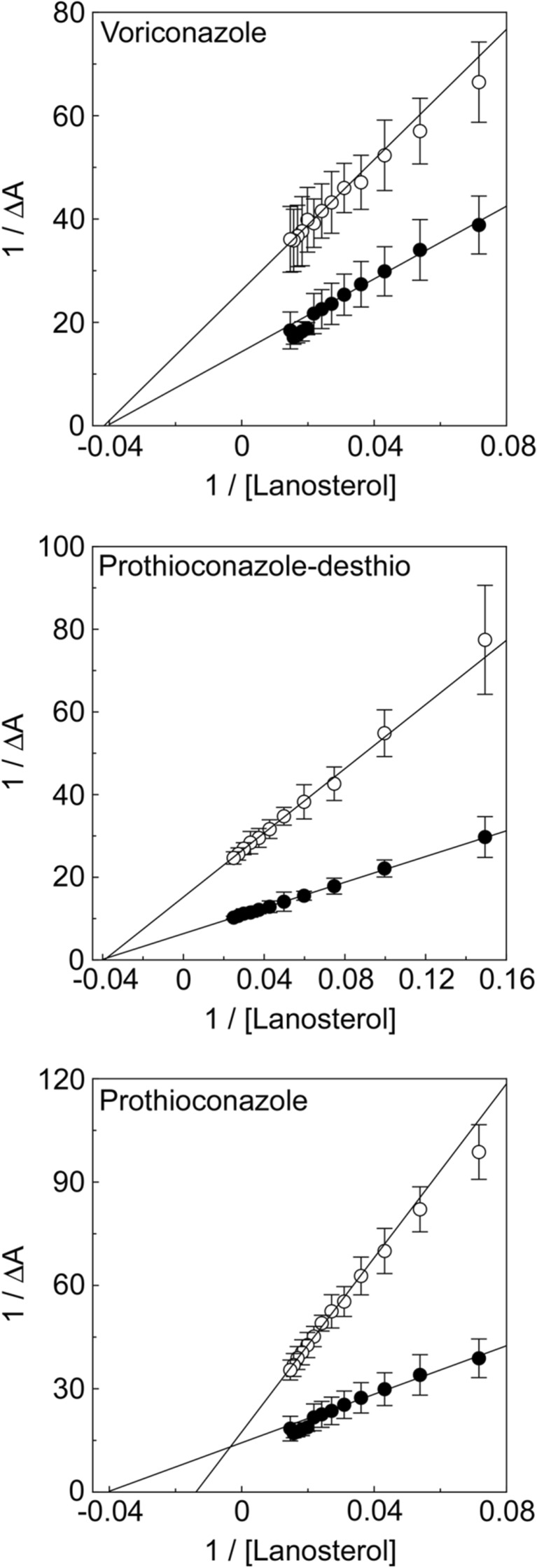
Type I substrate binding studies can also be used to gain insight into how a CYP51 inhibitor disrupts/alters substrate binding. This is achieved by progressively titrating the CYP51 enzyme with substrate in the presence of fixed concentrations of inhibitor, such as voriconazole, prothioconazole and prothioconazole-desthio. A secondary Lineweaver-Burk plot of 1/ΔA against 1/[substrate] can elucidate the type of inhibition being observed towards lanosterol binding (Fig. 5). Voriconazole and prothioconazole-desthio (Fig. 5a, b) clearly show non-competitive inhibition (converging lines that meet at 1/ΔA = 0) as would be expected for an azole antifungal agent. In contrast, prothioconazole inhibition appeared to be competitive (converging lines at 1/[lanosterol] = 0) [175] suggesting competition with lanosterol for the same binding site. The primary and strongest interaction between an azole antifungal and a CYP51 enzyme is through the direct coordination of the triazole N-4 nitrogen atom as the sixth ligand of the heme ferric ion. CYP51 substrates do not bind directly through coordination with the heme ferric ion but instead bind through numerous interactions with the amino acid side chain residues that line the substrate access channel and binding pocket in CYP51. As the azole and substrate binding sites are different, non-competitive inhibition would be expected to occur unless the primary binding mechanism of the azole antifungal was no longer through direct coordination with the heme ferric ion but was through interactions of the azole side chain groups with the amino acid residues lining the substrate access channel. In such circumstances, the antifungal agent would be potentially competing for the same sites as lanosterol, which appeared to be the case for the pro-fungicide prothioconazole. Type I substrate binding studies can also be utilised to study the interactions of non-azole CYP51 inhibitors, especially if the inhibitor binds within the CYP51 substrate binding pocket.
Fig. 5.
Inhibition of lanosterol binding to C. albicans CYP51 by azole fungicides. Type I difference spectra were measured during the progressive titration of 10 μM CYP51 with lanosterol in the absence and presence of azole fungicides. Lineweaver-Burk plots were constructed from the type I binding spectra obtained in order to compare lanosterol binding in the absence (black circle) and presence (white circle) of 4 μM voriconazole, 4 μM prothioconazole-desthio and 100 μM prothioconazole. One representative example of each experiment is shown, although all experiments were performed in triplicate
CYP51 catalysis studies
CYP51 catalysis studies using the CYP51 reconstitution assay system developed by Lepesheva et al. [176] allow detailed inhibition studies to be performed. The reconstitution assay utilises recombinant CYP51 protein and a partner cytochrome P450 reductase in suspension with substrate (lanosterol) in an artificial lipid bilayer of dilauryl phosphatidylcholine (DLPC) along with a reduced nicotinamide adenine dinucleotide phosphate (NADPH) regeneration system. If the recombinant CYP51 protein has low catalytic activity after purification, up to 50 μl of the membrane fraction (isolated from E. coli of the expression clone) can be used instead by omitting the DLPC from the assay. The reaction is initiated with NADPH, shaken at the desired temperature for a predetermined time before termination and extraction with ethyl acetate. Dried reaction metabolites are then derivatization with N,O-bis(trimethylsilyl)trifluoroacetamide and tetramethylsilane prior to analysis by gas chromatography–mass spectrometry [175]. Azole IC50 determinations can easily be performed by the inclusion of 2.5 μl of ×200 stock azole solutions in dimethylformamide, followed by preincubation for 10 min prior to commencement of the reaction with NADPH [168].
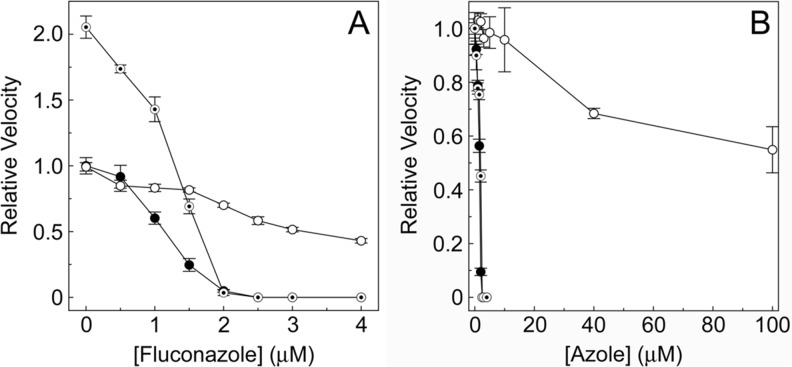
The resultant IC50 plot of relative velocity against azole concentration (Fig. 6a) quickly identifies azole drug candidates that strongly inhibit the target CYP51 enzyme and is the enzymological equivalent of the whole organism MIC50 determinations often performed to determine the effectiveness of an azole fungicide. The IC50 values for fluconazole with wild-type, S279F and I471T C. albicans CYP51 (Fig. 6a) were 1.39, 3.28 and 1.28 μM, respectively, confirming the S279F point mutation had reduced the fluconazole susceptibility of the CYP51 enzyme, whilst the I471T point mutation had not affected the observed fluconazole susceptibility; however, I471T had more than 2-fold higher catalytic turnover than the wild-type and S279F enzymes. Even though the Ks value obtained for S279F was half that of the wild-type enzyme, this did not result in an increased enzyme reaction velocity for S279F, confirming that the binding affinity for substrate is not always the rate limiting step. In contrast, I471T had nearly a 3-fold lower Ks value than the wild-type enzyme, and this did result in more than 2-fold increase in reaction velocity [90, 91].
Fig. 6.
Azole IC50 determinations. a The CYP51 activities of 2.5 μM wild-type (black circle), S279F (white circle) and I471T (◉) C. albicans CYP51 were determined at fluconazole concentrations ranging from 0 to 4 μM. A relative velocity of 1.0 corresponds to the velocity observed with the wild-type enzyme in the absence of fluconazole (0.062 nmol min−1). b The CYP51 activities of 2.5 μM wild-type C. albicans CYP51 were determined at voriconazole (black circle) and prothioconazole-desthio (◉) concentrations ranged from 0 to 4 μM and prothioconazole (white circle) concentrations ranged from 0 to 100 μM. Relative velocities of 1.0 equate to actual velocities of 0.080, 0.098 and 0.087 nmol min−1 for the IC50 determinations with voriconazole, prothioconazole and prothioconazole-desthio, respectively. Mean values from three replicates are shown along with associated standard error bars
The IC50 values for voriconazole, prothioconazole-desthio and prothioconazole with wild-type C. albicans CYP51 (Fig. 6b) were 1.6, 1.9 and ~120 μM, respectively. As expected, voriconazole and prothioconazole-desthio strongly inhibited C. albicans CYP51 activity with IC50 values approximately half the CYP51 concentration indicative of tight binding inhibitors. Prothioconazole, however, weakly inhibited C. albicans CYP51 with 55 % residual activity remaining in the presence of 100 μM prothioconazole. IC50 determinations can also be performed using non-azole CYP51 inhibitors broadening the range of inhibitors that can be screened and studied. For a more comprehensive picture of azole inhibition of the target CYP51 enzyme, the IC50 determinations can be performed at different CYP51 concentrations and different substrate concentrations (resources permitting). However, performing sterol substrate Km determinations is complicated by the discontinuous nature of the CYP51 assay system making determination of initial reaction velocities difficult.
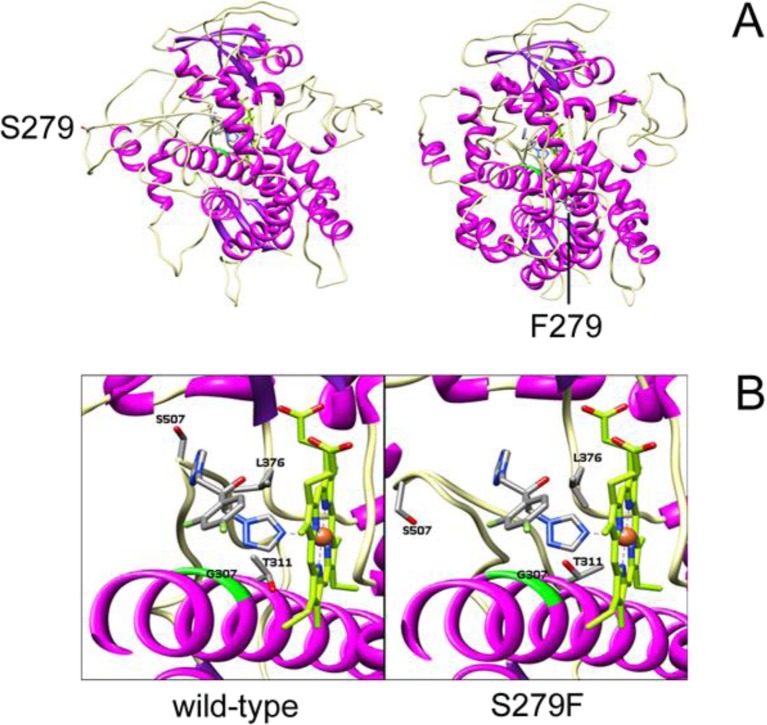
Structural modelling of the wild-type C. albicans CYP51 protein indicated S279 was located outside the protein, on an external β turn. With the S279F substitution, F279 became incorporated within a short section of α helix preceding the long I helix, resulting in a large movement of position (Fig. 7a). During fluconazole docking, the oxygen atom of S507 interacts with the second triazole ring of fluconazole in the wild-type C. albicans CYP51 protein, assisting in orientating fluconazole so that a more favourable binding conformation to heme is achieved (Fig. 7b). In contrast, for the S279F point mutation, this S507-fluconazole interaction is absent, providing an explanation for the higher Kd value observed with fluconazole [91].
Fig. 7.
Structural modelling of wild-type and S279F C. albicans CYP51 proteins with and without fluconazole docked. a Wild-type protein, with S279 located peripherally on an external β turn. The S279F mutant protein also shown has F279 incorporated within a short section of α helix preceding the long I helix. b The wild-type protein shows fluconazole in the centre (coloured by element) and the haem group to the right, and residues predicted to be within the range of contact of fluconazole are labelled. The S279F mutant protein is also shown with the S279F substitution leading to conformational changes that result in S507 being removed from interaction with fluconazole but still bordering the haem cavity
Therefore, studying the biochemical properties of CYP51 enzymes is important to validate the in silico models and that the two techniques can then be used together to further understand the mechanisms of resistance exhibited by CYP51 point mutations, develop appropriate strategies to combat the growing problem of azole resistance in fungal pathogens and screen for new antifungals.
Specificity of azole fungicides for the target CYP51 enzyme
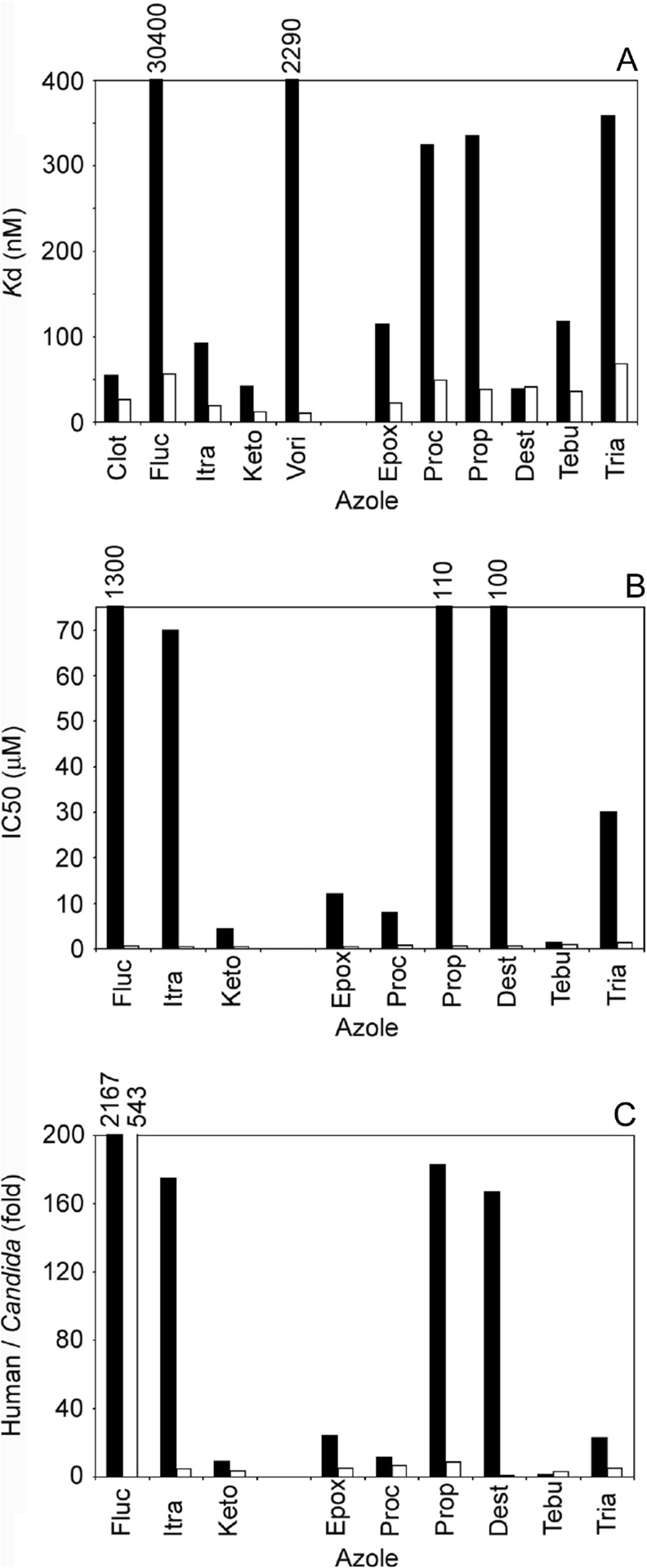
The ideal new clinical azole antifungal drug/fungicide should have a far greater affinity for the target fungal CYP51 enzyme than the host CYP51 analogue to avoid disruption of the host’s sterol biosynthetic pathway. It must also prove to be effective against fungal CYP51 point mutations that confer resistance against previous azole antifungal compounds. In addition, the azole drug/fungicide should bind poorly to other cytochrome P450 enzymes in the host to avoid the disruption of important biosynthetic and regulatory pathways, such as the endocrine system and liver drug/xenobiotic metabolism in mammals. Designing such an ideal azole drug/fungicide is extremely challenging and will inevitably lead to compromises being made. As a consequence, it is important that any new azole compound is fully characterised in terms of selectivity for its drug target over the host homologue and that interactions with other CYPs are investigated. In a recent publication, the selectivity of five therapeutic azole antifungal drugs and seven agricultural azole fungicides for C. albicans CYP51 over the human homologue [168] demonstrated that azole binding and IC50 studies can be used as a measure of selectivity for an azole compound. Comparison of the Kd values (Fig. 8a) obtained from type II binding spectra indicated that the selectivity of the azole compounds for C. albicans CYP51 over the human homologue varied considerably from 0.95-fold for prothioconazole-desthio to 543-fold for fluconazole. Likewise, comparison of the IC50 values (Fig. 8b) also showed variable selectivity from 1.3-fold with tebuconazole to ~1,300-fold with fluconazole for C. albicans CYP51 over the human homologue. Fold selectivity based on IC50 values was significantly greater for itraconazole (175-fold), propiconazole (183-fold) and prothioconazole-desthio (167-fold) than observed with Kd values (4.8-, 8.8- and 0.95-fold) (Fig. 8c). This was primarily due to the high IC50 values obtained with human CYP51 for these three azoles even though the Kd values obtained were relatively low. The differences observed between Kd determinations and IC50 values may in part be due to the CYP51 enzyme adopting a slightly different structural conformation when inserted into a lipid bilayer along with substrate, cytochrome P450 reductase redox partner and NADPH. Therefore, azole ligand binding studies are useful in excluding new compounds that bind poorly to the target CYP51 enzyme, but IC50 studies are required to confirm that high initial binding affinities are translated into effective inhibition of the target CYP51 enzyme. Conventional MIC studies can then be performed using the target organism to confirm biological effectiveness against clinical and agricultural isolates. Fluconazole had the highest selectivity for the fungal CYP51 enzyme over the human homologue by a significant margin with some of the agricultural fungicides, such as tebuconazole, having the poorest selectivity [168]. Similar studies could be performed using CYP51 enzymes from fungal plant pathogens and the host CYP51 (CYP71) homologues to determine selectivity of the agricultural fungicides.
Fig. 8.
Comparison of azole binding parameters for C. albicans and human CYP51 enzymes. a K d values were determined for azoles binding to human (black bar) and C. albicans (white bar) CYP51 proteins from ligand saturation curves using a rearrangement of the Morrison equation [172]. b IC50 values for azole antifungal drugs and fungicides. IC50 values were determined for azoles binding to human (black bar) and C. albicans (white bar) CYP51 proteins using the CYP51 reconstitution assay system. c Fold selectivity for the fungal drug target over the human homologue was calculated by dividing the IC50 values (black bar) and K d values (white bar) for human CYP51 by those obtained for C. albicans CYP51
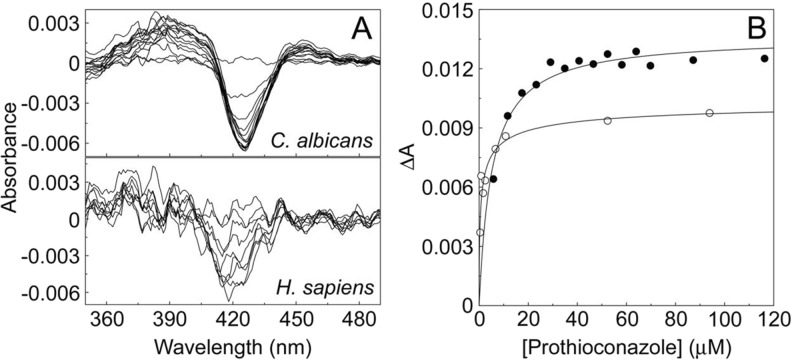
Prothioconazole is a special case and does not behave like the other azoles when interacting with C. albicans and human CYP51 proteins. Ligand binding experiments (Fig. 9) gave weak type I binding spectra typical of substrate binding and not the typical type II interaction observed when azole antifungals coordinate through the triazole N-4 or imidazole N-3 nitrogen atom with the haem ferric ion. In addition, prothioconazole did not appear to inhibit human CYP51 and only very weakly inhibited C. albicans CYP51. This alternate behaviour is attributed to the ‘bulky’ sulphur atom covalently attached adjacent to the triazole N-4 nitrogen atom prevented direct coordination to the haem ferric ion. Further work on prothioconazole has shown that prothioconazole is readily converted to the desthio form which accounts for the antifungal effect [175].
Fig. 9.
Prothioconazole binding with C. albicans and human CYP51 proteins. a Weak type I difference spectra were obtained using 5 μM solutions of the CYP51 proteins during progressive titration with prothioconazole. b Ligand saturation curves were constructed from the change in absorbance against prothioconazole concentration using the Michaelis–Menten equation
In silico structural modelling of CYP51 enzymes
In silico structural modelling is increasingly being used to investigate CYP51-mediated azole resistance [119, 161, 177–179]. These investigations have primarily focussed on differences in azole ligand docking between the wild-type enzyme and the point mutations found in the CYP51 enzymes present in azole-resistant strains from the clinic or field. Ideally, azole ligand docking studies need to be performed using the template of the species CYP51 of interest. However, this is not possible, as crystallising membrane-bound CYP51 proteins of eukaryotes with the N-terminal membrane anchor intact is difficult. Presently, six CYP51 enzyme structures have been resolved by X-ray crystallography. They are the eukaryote CYP51 enzymes from Trypanosoma brucei [180], Trypanosoma cruzi [181], Leishmania infantum [182], H. sapiens [183] and S. cerevisiae [184] and the prokaryote CYP51 enzyme from Mycobacterium tuberculosis [185]. All of the eukaryotic CYP51 enzymes, with the exception of S. cerevisiae, were crystallised in truncated form (N-terminal membrane anchor removed). To date, the only fungal, membrane-bound, non-truncated CYP51 to be crystallised and the structure resolved is that of S. cerevisiae CYP51 [184]. This is an important development which may increase the reliability of models in the future with regards to fungal specific regions of CYP51.
As more CYP51 crystal structures are published, the more refined these models have become. Most of the CYP51 point mutations observed in azole-resistant clinical C. albicans isolates were not predicted by models to interact directly or through hydrophobic interactions with the main azole antifungal drugs, since they are based on homology models to a single bacterial enzyme [177–179]. Recently, modelling techniques based on using multiple cytochrome P450 proteins as templates have been used to describe how point mutations in C. albicans and Z. tritici CYP51 might exert their effect in conferring azole resistance [58, 91, 161]. For example, the model constructed for C. albicans S279F revealed a large change in conformation compared to the wild type which precludes an important interaction with fluconazole, accounting for the resistant phenotype [91]. These scaffolds have proved particularly useful in understanding the importance of a region associated with azole resistance mutations in Z. tritici [161], C. albicans [82, 86, 167], A. fumigatus [59] and M. fijiensis [149]. Models of Z. tritici CYP51 [58, 125] predicted this fungal-specific region (Y459–Y461) to be integral to the active site and directly impact azole binding, which was consistent with in vivo findings whilst past models had predicted this region to be on the surface of the protein [186]. Models of mutations in this region directly impact azole binding and are consistent with in vivo findings. These multiple scaffold models are also useful in predicting the impact of individual and combinations of mutations [58, 91, 161]. Interactions with docked ligands and substrates can be predicted, explaining the differential azole sensitivities seen in different variants. Models of Z. tritici CYP51 have also shown that successive mutations have led to an enlarged active site pocket reducing the interaction of azoles with surrounding amino acids and therefore reducing sensitivity to azoles [58, 161]. In silico models may therefore be a tool to aid the management of fungicides in agriculture in response to existing and developing resistance. These models can also be used without prior biological knowledge of a specific mutation. They can be used to predict how new compounds will bind and how existing compounds will bind to mutants for which there is no in vitro data. This is the case with azole-resistant mutants of Z. tritici CYP51 and A. fumigatus CYP51A, which have not yet been expressed and purified, precluding biochemical comparison of the azole and substrate binding properties, CYP51 catalytic efficiency and azole inhibitory properties (IC50 values) of these proteins. In the case of Z. tritici, there is still a requirement for a functional cytochrome P450 reductase partner in order to be able to obtain catalytic efficiency and IC50 data. In the absence of the availability of such biochemical analyses, in silico structural models are of particular importance.
Problems encountered with current azole antifungals
Since the haem prosthetic group is not unique to CYP51 but is shared by all cytochrome P450 (CYPs), and all CYP51s are similar, containing structurally conserved regions [187], there may be significant cross-reaction and toxicity problems with azoles. Firstly, azole compounds may inhibit human CYP51, resulting in a reduction in the synthesis of cholesterol and affect the endocrine system [188]. Secondly, CYPs are the most abundant form of drug-metabolising enzymes in humans; therefore, there is the potential for drug–drug interactions due to the inhibition of other human CYPs [189] which may cause adverse effects. There is increasing concern in Europe over the effects of agricultural azoles in mammals [190, 191] and the hepatotoxicity of these fungicides [192]. Hepatotoxicity may be caused by induction of the expression of liver cytochrome P450 enzymes (CYP1, CYP2 and CYP3 families), which in turn increases the abundance of reactive oxygen species in liver cells. This may result in lipid peroxidation and DNA damage [192]. In addition, azoles may also inhibit liver P450 enzymes, interfering in the phase I metabolism of xenobiotics [192–195]. Alterations in cytochrome P450 in humans associated with cholesterol, steroid, vitamin D and eicosanoid synthesis are known to cause diseases [196]. Therefore, interference in these pathways may cause adverse effects. Azoles can disrupt the endocrine system by inhibiting several highly substrate-selective cytochrome P450 enzymes (CYP11A1, CYP11B2, CYP17A1, CYP19A1 and CYP21A2) involved in mammalian steroid hormone biosynthesis. This can lead to impaired reproduction, alterations in sexual differentiation, impaired growth and development and the formation of hormone-dependent cancers [38, 39, 190, 191, 197]. In addition, there is growing evidence that inhibition of CYP46A1 by azoles impairs 24S-hydroxycholesterol synthesis in the brain and can result in visual disturbances [198]. Recent analysis has shown that the azole affinity for CYP46A1 and fungal CYP51 enzymes is similar [199].
Many yeasts and fungi that are causative agents of clinical infections, such as Candida species and Aspergillus species, are also present in the general environment and are exposed to the selective pressure of agricultural azoles in the field. There are now concerns that azole resistance in clinical infections has emerged through the use of agricultural azoles [200]. Aspergillus is a relatively weak phytopathogen, though it is abundant in the environment and therefore exposed to azoles through treatment of other agriculturally important species. Recently, point mutations in A. fumigatus CYP51A that confer resistance to azole antifungal agents were attributed to resistance acquired outside the clinic, in the general environment [125, 200–202]. Unlike resistance acquired as a result of clinical azole treatment, wild-type strains were not recovered from patients infected with either of the two resistant strains CYP51A TR34/L98H and TR46/Y121F/T289A [125], isolated from some azole-naïve patients.
Resistance acquired in the environment is a growing concern. It is likely that more azole-resistant Aspergillus sp. strains that have evolved in the environment will appear in clinical isolates. It is possible that other opportunistic pathogens, such as Fusarium spp. may also emerge as resistant strains in the clinic. Fusarium species cause many types of infection in humans, ranging from superficial infections (keratitis and onychomycosis) to systemic infections in immunocompromised patients [203]. Around 50 % of cases are caused by Fusarium solani, around 20 % by Fusarium oxysporum and around 10 % each by Fusarium verticilliodis and Fusarium moniliforme [203]. As yet, azole resistance mediated by CYP51 mutations has not been observed either in the field or the clinic. However, Fan et al. have generated Y123H mutations in CYP51B and a mutant overexpressing CYP51A in F. verticillioides in the laboratory by subjecting the strain to successive selection with azole [204]. Both strains are prochloraz-resistant and exhibit some cross-resistance to triazoles. This indicates the potential for azole resistance to arise in Fusarium spp. and may apply to other opportunistic pathogens, which are also exposed to agricultural azole treatments in the environment.
In addition to agricultural use, many fungicides are used both by industry and domestically to prevent fungal growth and decay. Difenoconazole, tebuconazole and propiconazole are used to treat lawns. Tebuconazole present in ‘Copper azole type B’ [25] and propiconazole are used to treat wood. Propiconazole is also licenced for use in adhesives, paints, leather, paper and textiles [26] and is the active compound in antimold paints. Also, copper corrosion inhibitors added to aqueous coolant systems also contain azoles (tolyltriazole and benzotriazole) [27]. Therefore, environmentally prevalent fungi will be exposed to these azoles [57]. In addition, azole compounds are used in toiletries and shampoos (climbazole and ketoconazole), and excreted medicines (clotrimazole) will also increase the environmental exposure of fungi to azoles [205].
Summary
CYP51 is an effective drug target that has enabled the introduction of a large number of new antifungal compounds to be developed for use against it over the past four decades. Compounds with other modes of action have also been introduced to target fungal diseases, such as echinocandins and strobilurins; however, resistance to these quickly developed [206–208] and compounds targeting CYP51 are still an essential tool in tackling fungal diseases. There is a necessity to develop new antifungal compounds with increased selectivity for the fungal CYP51 enzyme over the human homologue and other human CYPs and which will also minimise the risk of cross-resistance. Currently, several new azole and non-azole CYP51 inhibitors are being developed for the next generation of antifungal drugs [176, 209–214]. It may also be possible to design new compounds which are pro-fungicides in a manner similar to that of prothioconazole [174, 175].
Understanding which agricultural azoles may drive resistance to clinical azoles (especially in those species such as Aspergillus which are already intrinsically resistant to many clinical azoles) is of the upmost importance. It is therefore necessary to be able to determine differences between agriculturally and clinically important strains and their susceptibility to azoles. Traditionally, antifungals used in agriculture may be less specific for fungal CYP51 and may cause cross-resistance problems with human CYPs (as demonstrated with human CYP51 [168]). Azole compounds which are not closely related structurally to medical azoles are best used in agriculture. In addition to binding and inhibition of human CYPs, azole compounds may also induce some human CYPs. Therefore, the potential for drug–drug interactions and toxicity needs to be addressed.
All these factors must be considered when designing new antifungals, and the assessment of CYP51 susceptibility, through the biochemical techniques described above, is central to the design and assessment of such compounds. However, the increasing concern over cross-resistance emanating from environmental use of azoles may need to be addressed through altering agricultural antifungal treatment regimens. Interestingly, triazole fungicides were used on crops nearly two decades before triazole antifungal drugs were routinely used in the clinic to treat systemic infections of humans [215]; therefore, the recent emergence of cross-resistance may be due to a change in use of agricultural antifungals or a change towards azoles which interact with fungal CYP51 enzymes more akin to clinical azoles. Alternative antifungal strategies including mixes of azoles with non-azoles and a rotation in the use of antifungals with different modes of action may aid to stem the increase in cross-resistance.
The continued discovery of inhibitors of CYP51 in fungi as drugs and fungicides shows the success of this approach and the difficulty of discovering other targets to control fungal infection. Management of resistance or tolerance is possible, although even in azole sensitive fungi cure rates in the clinic are not ideal. Molecular modelling may allow the cycling of fungicide use as implied by the emerging pattern of resistance on exposure to CYP51 inhibitors in agriculture, and something similar could arise in the clinic as resistant strains to current therapeutics become sensitive to earlier drugs. As more CYP51 structures from pathogenic fungi are solved, in silico structural modelling will continually improve enabling modelling of azole docking to drug-resistant CYP51 mutants and use as a predictive tool to decide on therapy strategies such as whether cycling of azole drugs would prove beneficial in the clinic. Future work will require the investigation of the enzymology to dissect the roles of mutations in reducing azole affinity, changing enzyme turnover rates and compensating for the role of other mutations on activity and strain fitness. Previous fluconazole IC50 studies with C. albicans CYP51 have shown that some point mutations (F145L) cause only a modest increase in the IC50 value but facilitate low residual CYP51 activity at high azole concentrations [88] compared to the wild-type CYP51 that exhibits no such residual activity. This ‘leaky’ phenotype deserves further investigation to elucidate the molecular mechanism of resistance involved. Long range as well as close proximity amino acid sequence changes relative to the active site can be examined for conferring these effects. Better inhibitors with reduced toxicity that are resistance breakers and less stable compounds that break down in the environment are needed for the longer term.
Acknowledgments
This work was in part supported by the European Regional Development Fund/Welsh Government funded BEACON research programme.
We are grateful to the Engineering and Physical Sciences Research Council National Mass Spectrometry Service Centre at Swansea University for assistance in GC/MS analyses.
Contributor Information
Diane E. Kelly, Phone: +44 (0)1792 292207, Email: d.kelly@swansea.ac.uk
Steven L. Kelly, Phone: +44 (0)1792 292207, Email: s.l.kelly@swansea.ac.uk
References
- 1.Shelton BK. Opportunistic fungal infections in the critically ill. Crit Care Nurs Clin N Am. 2000;12(3):323–340. [PubMed] [Google Scholar]
- 2.GAFFI (2014) Global action fund for fungal infection; http://www.gaffi.org/
- 3.WHO (2014) http://www.who.int/mediacentre/factsheets/fs094/en/
- 4.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 5.Matee MI, Scheutz F, Moshy J. Occurrence of oral lesions in relation to clinical and immunological status among HIV-infected adult Tanzanians. Oral Dis. 2000;6(2):106–111. doi: 10.1111/j.1601-0825.2000.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith E, Orholm M. Trends and patterns of opportunistic diseases in Danish AIDS patients 1980-1990. Scand J Infect Dis. 1990;22(6):665–672. doi: 10.3109/00365549009027119. [DOI] [PubMed] [Google Scholar]
- 7.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 8.Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care. 2010;16(5):445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 10.Guinea J, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol Infect. 2010;16(7):870–877. doi: 10.1111/j.1469-0691.2009.03015.x. [DOI] [PubMed] [Google Scholar]
- 11.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51(4):361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 12.Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864–872. doi: 10.2471/BLT.11.089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37(4):865–872. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 14.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 15.Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin N Am. 2003;17(1):41–57. doi: 10.1016/s0891-5520(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 16.WHO (2013) http://www.who.int/mediacentre/factsheets/fs282/en/
- 17.Ashbee HR, Scheynius A. Malassezia. In: Ashbee HR, Bignell EM, editors. Pathogenic yeasts. The yeast handbook. Berlin Heidelberg: Springer; 2010. [Google Scholar]
- 18.Gupta AK, et al. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51(5):785–798. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Batra R, et al. Malassezia Baillon, emerging clinical yeasts. FEMS Yeast Res. 2005;5(12):1101–1113. doi: 10.1016/j.femsyr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Lakshmipathy DT, Kannabiran K. Review on dermatomycosis: pathogenesis and treatment. Nat Sci. 2010;2(7):726–731. [Google Scholar]
- 21.Jackson CJ, et al. Strain identification of Trichophyton rubrum by specific amplification of subrepeat elements in the ribosomal DNA nontranscribed spacer. J Clin Microbiol. 2000;38(12):4527–4534. doi: 10.1128/jcm.38.12.4527-4534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowyer P, Denning DW. Environmental fungicides and triazole resistance in Aspergillus. Pest Manag Sci. 2014;70(2):173–178. doi: 10.1002/ps.3567. [DOI] [PubMed] [Google Scholar]
- 23.ECPA, European Crop Protection Association (2012) The assessment of the economic importance of azoles in european agriculture: wheat case study. NOMISMA, Bologna, Italy. available at; http://www.ecpa.eu/article/agriculture-today/assessment-economic-importance-azoles-european-agriculture-wheat-case-stud
- 24.Bhanderi B. Antifungal drug resistance—concerns for veterinarians. Vet World. 2009;2(5):204–207. [Google Scholar]
- 25.US forest service. Types of wood preservative. Available at; http://www.fs.fed.us/td/pubs/pdfpubs/pdf06772809/pdf06772809dpi72pt03.pdf
- 26.EPA, Environmental Protection Agency. Registration eligibility decision; Propiconazole. available from http://www.epa.gov/oppsrrd1/REDs/propiconazole_red.pdf2006
- 27.GE, GE Water and Process Technologies (2011) A product of ecomagination. Halogen resistant azole capability profile (cp101.pdf.) available at https://knowledgecentral.gewater.com/
- 28.Shyadehi AZ, et al. The mechanism of the acyl-carbon bond cleavage reaction catalyzed by recombinant sterol 14 alpha-demethylase of Candida albicans (other names are: lanosterol 14 alpha-demethylase, P-45014DM, and CYP51) J Biol Chem. 1996;271(21):12445–12450. doi: 10.1074/jbc.271.21.12445. [DOI] [PubMed] [Google Scholar]
- 29.Kelly SL, et al. The biodiversity of microbial cytochromes P450. Adv Microb Physiol. 2003;47:131–186. doi: 10.1016/s0065-2911(03)47003-3. [DOI] [PubMed] [Google Scholar]
- 30.Marichal P, et al. Accumulation of 3-ketosteroids induced by itraconazole in azole-resistant clinical Candida albicans isolates. Antimicrob Agents Chemother. 1999;43(11):2663–2670. doi: 10.1128/aac.43.11.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martel CM, et al. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54(11):4527–4533. doi: 10.1128/AAC.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanden Bossche H, et al. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother. 1993;37(10):2101–2105. doi: 10.1128/aac.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fromtling RA. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev. 1988;1(2):187–217. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell PE. A century of fungicide evolution. J Agric Sci. 2005;143(01):11–25. [Google Scholar]
- 35.Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. Part I. J Am Acad Dermatol. 1994;30(5 Pt 1):677–698. [PubMed] [Google Scholar]
- 36.Heeres J, et al. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979;22(8):1003–1005. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JH, et al. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86(3):503–513. [PubMed] [Google Scholar]
- 38.Pont A, et al. Ketoconazole blocks testosterone synthesis. Arch Intern Med. 1982;142(12):2137–2140. [PubMed] [Google Scholar]
- 39.Pont A, et al. Ketoconazole blocks adrenal steroid synthesis. Ann Intern Med. 1982;97(3):370–372. doi: 10.7326/0003-4819-97-3-370. [DOI] [PubMed] [Google Scholar]
- 40.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36(5):630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 41.Maertens JA. History of the development of azole derivatives. Clin Microbiol Infect. 2004;10(s1):1–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 42.Gothard P, Rogers TR. Voriconazole for serious fungal infections. Int J Clin Pract. 2004;58(1):74–80. doi: 10.1111/j.1368-5031.2004.0099.x. [DOI] [PubMed] [Google Scholar]
- 43.Scott LJ, Simpson D. Voriconazole : a review of its use in the management of invasive fungal infections. Drugs. 2007;67(2):269–298. doi: 10.2165/00003495-200767020-00009. [DOI] [PubMed] [Google Scholar]
- 44.Denning DW, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–571. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 45.Bartroli J, et al. New azole antifungals. 2. Synthesis and antifungal activity of heterocyclecarboxamide derivatives of 3-amino-2-aryl-1-azolyl-2-butanol. J Med Chem. 1998;41(11):1855–1868. doi: 10.1021/jm970726e. [DOI] [PubMed] [Google Scholar]
- 46.Bartroli J, et al. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J Med Chem. 1998;41(11):1869–1882. doi: 10.1021/jm9707277. [DOI] [PubMed] [Google Scholar]
- 47.Pasqualotto AC, Thiele KO, Goldani LZ. Novel triazole antifungal drugs: focus on isavuconazole, ravuconazole and albaconazole. Curr Opin Investig Drugs. 2010;11(2):165–174. [PubMed] [Google Scholar]
- 48.Fung-Tomc JC, et al. In vitro activity of a new oral triazole, BMS-207147 (ER-30346) Antimicrob Agents Chemother. 1998;42(2):313–318. doi: 10.1128/aac.42.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minassian B, et al. In vitro activity of ravuconazole against Zygomycetes, Scedosporium and Fusarium isolates. Clin Microbiol Infect. 2003;9(12):1250–1252. doi: 10.1111/j.1469-0691.2003.00755.x. [DOI] [PubMed] [Google Scholar]
- 50.Odds FC. Drug evaluation: BAL-8557—a novel broad-spectrum triazole antifungal. Curr Opin Investig Drugs. 2006;7(8):766–772. [PubMed] [Google Scholar]
- 51.Warn PA, Sharp A, Denning DW. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J Antimicrob Chemother. 2006;57(1):135–138. doi: 10.1093/jac/dki399. [DOI] [PubMed] [Google Scholar]
- 52.Odds F, et al. In vitro and in vivo activities of the novel azole antifungal agent r126638. Antimicrob Agents Chemother. 2004;48(2):388–391. doi: 10.1128/AAC.48.2.388-391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanden Bossche H, et al. The novel azole R126638 is a selective inhibitor of ergosterol synthesis in Candida albicans, Trichophyton spp., and Microsporum canis. Antimicrob Agents Chemother. 2004;48(9):3272–3278. doi: 10.1128/AAC.48.9.3272-3278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meerpoel L, et al. Synthesis and in vitro and in vivo structure-activity relationships of novel antifungal triazoles for dermatology. J Med Chem. 2005;48(6):2184–2193. doi: 10.1021/jm0494772. [DOI] [PubMed] [Google Scholar]
- 55.Geria AN, Scheinfeld NS. Pramiconazole, a triazole compound for the treatment of fungal infections. IDrugs. 2008;11(9):661–670. [PubMed] [Google Scholar]
- 56.McDougal P (2006) Agriservice report
- 57.ECDPC, European Centre for Disease Prevention and Control (2013) Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. Stockholm
- 58.Cools HJ, et al. Impact of recently emerged sterol 14{alpha}-demethylase (CYP51) variants of Mycosphaerella graminicola on azole fungicide sensitivity. Appl Environ Microbiol. 2011;77(11):3830–3837. doi: 10.1128/AEM.00027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard SJ, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15(7):1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56(Suppl 1):i5–i11. doi: 10.1093/jac/dki218. [DOI] [PubMed] [Google Scholar]
- 62.Sims CR, Ostrosky-Zeichner L, Rex JH. Invasive candidiasis in immunocompromised hospitalized patients. Arch Med Res. 2005;36(6):660–671. doi: 10.1016/j.arcmed.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Snelders E, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5(11):e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson PF, et al. Defective sterol C5-6 desaturation and azole resistance: a new hypothesis for the mode of action of azole antifungals. Biochem Biophys Res Commun. 1989;164(3):1170–1175. doi: 10.1016/0006-291x(89)91792-0. [DOI] [PubMed] [Google Scholar]
- 65.Eddouzi J, et al. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob Agents Chemother. 2013;57(7):3182–3193. doi: 10.1128/AAC.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly SL, et al. Mode of action and resistance to azole antifungals associated with the formation of 14 alpha-methylergosta-8,24(28)-dien-3 beta,6 alpha-diol. Biochem Biophys Res Commun. 1995;207(3):910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- 67.Rinaldi MG. Biology and pathogenicity of Candida species, pathogenesis, diagnosis and treatment. In: Bodey GP, editor. Candidiasis, pathogenesis, diagnosis and treatment. New York: Raven; 1993. pp. 1–20. [Google Scholar]
- 68.Marriott DJ, et al. Determinants of mortality in non-neutropenic ICU patients with candidaemia. Crit Care. 2009;13(4):R115. doi: 10.1186/cc7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magill SS, et al. The epidemiology of Candida colonization and invasive candidiasis in a surgical intensive care unit where fluconazole prophylaxis is utilized: follow-up to a randomized clinical trial. Ann Surg. 2009;249(4):657–665. doi: 10.1097/SLA.0b013e31819ed914. [DOI] [PubMed] [Google Scholar]
- 70.Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horn DL, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 72.Neofytos D, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 73.Pfaller MA, et al. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J Clin Microbiol. 2002;40(3):852–856. doi: 10.1128/JCM.40.3.852-856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2(2):73–85. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 75.Tobudic S, Kratzer C, Presterl E. Azole-resistant Candida spp.—emerging pathogens? Mycoses. 2012;55:24–32. [Google Scholar]
- 76.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11(2):382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Löffler J, et al. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol Lett. 1997;151(2):263–268. doi: 10.1111/j.1574-6968.1997.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 78.Sanglard D, et al. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42(2):241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanglard D, et al. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39(11):2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venkateswarlu K, et al. Resistance to fluconazole in Candida albicans from AIDS patients correlated with reduced intracellular accumulation of drug. FEMS Microbiol Lett. 1995;131(3):337–341. doi: 10.1111/j.1574-6968.1995.tb07797.x. [DOI] [PubMed] [Google Scholar]
- 81.Cernicka J, Subik J. Resistance mechanisms in fluconazole-resistant Candida albicans isolates from vaginal candidiasis. Int J Antimicrob Agents. 2006;27(5):403–408. doi: 10.1016/j.ijantimicag.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Chau AS, et al. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob Agents Chemother. 2004;48(6):2124–2131. doi: 10.1128/AAC.48.6.2124-2131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coste A, et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell. 2007;6(10):1889–1904. doi: 10.1128/EC.00151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franz R, et al. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42(12):3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldman GH, et al. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis. 2004;50(1):25–32. doi: 10.1016/j.diagmicrobio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Morio F, et al. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn Microbiol Infect Dis. 2010;66(4):373–384. doi: 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Marichal P, et al. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology. 1999;145(Pt 10):2701–2713. doi: 10.1099/00221287-145-10-2701. [DOI] [PubMed] [Google Scholar]
- 88.Kudo M, et al. Effects of Y132H and F145L substitutions on the activity, azole resistance and spectral properties of Candida albicans sterol 14-demethylase P450 (CYP51): a live example showing the selection of altered P450 through interaction with environmental compounds. J Biochem. 2005;137(5):625–632. doi: 10.1093/jb/mvi073. [DOI] [PubMed] [Google Scholar]
- 89.Bellamine A, Lepesheva GI, Waterman MR. Fluconazole binding and sterol demethylation in three CYP51 isoforms indicate differences in active site topology. J Lipid Res. 2004;45(11):2000–2007. doi: 10.1194/jlr.M400239-JLR200. [DOI] [PubMed] [Google Scholar]
- 90.Warrilow AG, et al. Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51) Antimicrob Agents Chemother. 2010;54(10):4235–4245. doi: 10.1128/AAC.00587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warrilow AG, et al. S279 point mutations in Candida albicans sterol 14-alpha demethylase (CYP51) reduce in vitro inhibition by fluconazole. Antimicrob Agents Chemother. 2012;56(4):2099–2107. doi: 10.1128/AAC.05389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelly SL, et al. The G464S amino acid substitution in Candida albicans sterol 14alpha-demethylase causes fluconazole resistance in the clinic through reduced affinity. Biochem Biophys Res Commun. 1999;262(1):174–179. doi: 10.1006/bbrc.1999.1136. [DOI] [PubMed] [Google Scholar]
- 93.Lamb DC, et al. The R467K amino acid substitution in Candida albicans sterol 14alpha-demethylase causes drug resistance through reduced affinity. Antimicrob Agents Chemother. 2000;44(1):63–67. doi: 10.1128/aac.44.1.63-67.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamb DC, et al. The mutation T315A in Candida albicans sterol 14alpha-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J Biol Chem. 1997;272(9):5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 95.Verweij PE, Denning DW. The challenge of invasive aspergillosis: increasing numbers in diverse patient groups. Int J Infect Dis. 1997;2(2):61–63. [Google Scholar]
- 96.Denning DW, Perlin DS. Azole resistance in Aspergillus: a growing public health menace. Future Microbiol. 2011;6(11):1229–1232. doi: 10.2217/fmb.11.118. [DOI] [PubMed] [Google Scholar]
- 97.Terpstra P, et al. Filamentous fungi in the Netherlands among CF patients. Mycoses. 2011;54(Suppl 2):152–152. [Google Scholar]
- 98.Mortensen KL, et al. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol. 2011;49(6):2243–2251. doi: 10.1128/JCM.00213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burgel PR, et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob Agents Chemother. 2012;56(2):869–874. doi: 10.1128/AAC.05077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Linden JW, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis. 2011;17(10):1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lockhart SR, et al. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. 2011;55(9):4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bueid A, et al. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65(10):2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 103.Mellado E, et al. Targeted gene disruption of the 14-alpha sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob Agents Chemother. 2005;49(6):2536–2538. doi: 10.1128/AAC.49.6.2536-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia-Effron G, et al. Differences in interactions between azole drugs related to modifications in the 14-alpha sterol demethylase gene (cyp51A) of Aspergillus fumigatus. Antimicrob Agents Chemother. 2005;49(5):2119–2121. doi: 10.1128/AAC.49.5.2119-2121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martel CM, et al. Complementation of a Saccharomyces cerevisiae ERG11/CYP51 (sterol 14alpha-demethylase) doxycycline-regulated mutant and screening of the azole sensitivity of Aspergillus fumigatus isoenzymes CYP51A and CYP51B. Antimicrob Agents Chemother. 2010;54(11):4920–4923. doi: 10.1128/AAC.00349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu W, et al. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 2007;3(3):e24. doi: 10.1371/journal.ppat.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warrilow AG, et al. Expression, purification, and characterization of Aspergillus fumigatus sterol 14-alpha demethylase (CYP51) isoenzymes A and B. Antimicrob Agents Chemother. 2010;54(10):4225–4234. doi: 10.1128/AAC.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Albarrag AM, et al. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob Agents Chemother. 2011;55(11):5113–5121. doi: 10.1128/AAC.00517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slaven JW, et al. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet Biol. 2002;36(3):199–206. doi: 10.1016/s1087-1845(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 110.Buied A, et al. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J Antimicrob Chemother. 2013;68(3):512–514. doi: 10.1093/jac/dks451. [DOI] [PubMed] [Google Scholar]
- 111.da Silva Ferreira ME, et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob Agents Chemother. 2004;48(11):4405–4413. doi: 10.1128/AAC.48.11.4405-4413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diaz-Guerra TM, et al. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2003;47(3):1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nascimento AM, et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother. 2003;47(5):1719–1726. doi: 10.1128/AAC.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowyer P, et al. Identification of novel genes conferring altered azole susceptibility in Aspergillus fumigatus. FEMS Microbiol Lett. 2012;332(1):10–19. doi: 10.1111/j.1574-6968.2012.02575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fraczek MG, et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2013;68(7):1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 116.Camps SM, et al. Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One. 2012;7(11):e50034. doi: 10.1371/journal.pone.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mann PA, et al. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14alpha-demethylase. Antimicrob Agents Chemother. 2003;47(2):577–581. doi: 10.1128/AAC.47.2.577-581.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mellado E, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51(6):1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Snelders E, et al. The structure-function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: the mechanism of L98H azole resistance. Fungal Genet Biol. 2011;48(11):1062–1070. doi: 10.1016/j.fgb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 120.Mellado E, et al. Substitutions at methionine 220 in the 14alpha-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother. 2004;48(7):2747–2750. doi: 10.1128/AAC.48.7.2747-2750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krishnan Natesan S, et al. In vitro-in vivo correlation of voriconazole resistance due to G448S mutation (cyp51A gene) in Aspergillus fumigatus. Diagn Microbiol Infect Dis. 2012;74(3):272–277. doi: 10.1016/j.diagmicrobio.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 122.Alanio A, et al. Azole preexposure affects the Aspergillus fumigatus population in patients. Antimicrob Agents Chemother. 2012;56(9):4948–4950. doi: 10.1128/AAC.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Escribano P, et al. Aspergillus fumigatus strains with mutations in the cyp51A gene do not always show phenotypic resistance to itraconazole, voriconazole, or posaconazole. Antimicrob Agents Chemother. 2011;55(5):2460–2462. doi: 10.1128/AAC.01358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chowdhary A, et al. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013;9(10):e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van der Linden JW, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57(4):513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 126.Camps SM, et al. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol. 2012;50(8):2674–2680. doi: 10.1128/JCM.00335-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Denning DW, et al. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41(6):1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Venkateswarlu K (1996) Azole antifungal drugs mode of action and resistance, p140. Sheffield University
- 129.Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 130.Lortholary O, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20(17):2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 131.Dromer F, et al. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4(2):e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mwaba P, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77(914):769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.French N, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16(7):1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 134.Byrnes EJ, 3rd, et al. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect. 2011;13(11):895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lamb DC, et al. Resistant P45051A1 activity in azole antifungal tolerant Cryptococcus neoformans from AIDS patients. FEBS Lett. 1995;368(2):326–330. doi: 10.1016/0014-5793(95)00684-2. [DOI] [PubMed] [Google Scholar]
- 136.Perfect JR, Cox GM. Drug resistance in Cryptococcus neoformans. Drug Resist Updat. 1999;2(4):259–269. doi: 10.1054/drup.1999.0090. [DOI] [PubMed] [Google Scholar]
- 137.Rodero L, et al. G484S amino acid substitution in lanosterol 14-alpha demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob Agents Chemother. 2003;47(11):3653–3656. doi: 10.1128/AAC.47.11.3653-3656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sionov E, et al. Identification of a Cryptococcus neoformans cytochrome P450 lanosterol 14alpha-demethylase (Erg11) residue critical for differential susceptibility between fluconazole/voriconazole and itraconazole/posaconazole. Antimicrob Agents Chemother. 2012;56(3):1162–1169. doi: 10.1128/AAC.05502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sionov E, et al. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6(4):e1000848. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu G, et al. Variation in chromosome copy number influences the virulence of Cryptococcus neoformans and occurs in isolates from AIDS patients. BMC Genomics. 2011;12:526. doi: 10.1186/1471-2164-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balkis MM, et al. Mechanisms of fungal resistance: an overview. Drugs. 2002;62(7):1025–1040. doi: 10.2165/00003495-200262070-00004. [DOI] [PubMed] [Google Scholar]
- 142.Santos DA, Hamdan JS. In vitro activities of four antifungal drugs against Trichophyton rubrum isolates exhibiting resistance to fluconazole. Mycoses. 2007;50(4):286–289. doi: 10.1111/j.1439-0507.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 143.Kim D, et al. Functional expression and characterization of CYP51 from dandruff-causing Malassezia globosa. FEMS Yeast Res. 2011;11(1):80–87. doi: 10.1111/j.1567-1364.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- 144.Hryncewicz-Gwozdz A, et al. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia. 2013;176(1–2):49–55. doi: 10.1007/s11046-013-9655-y. [DOI] [PubMed] [Google Scholar]
- 145.Wheat LJ, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother. 2006;57(6):1235–1239. doi: 10.1093/jac/dkl133. [DOI] [PubMed] [Google Scholar]
- 146.Delye C, Laigret F, Corio-Costet MF. A mutation in the 14 alpha-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl Environ Microbiol. 1997;63(8):2966–2970. doi: 10.1128/aem.63.8.2966-2970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gadoury DM, et al. Grapevine powdery mildew (Erysiphe necator): a fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol Plant Pathol. 2012;13(1):1–16. doi: 10.1111/j.1364-3703.2011.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stammler G, et al. Role of the Y134F mutation in cyp51 and overexpression of cyp51 in the sensitivity response of Puccinia triticina to epoxiconazole. Crop Prot. 2009;28(10):891–897. [Google Scholar]
- 149.Cañas-Gutiérrez GP, et al. Analysis of the CYP51 gene and encoded protein in propiconazole-resistant isolates of Mycosphaerella fijiensis. Pest Manag Sci. 2009;65(8):892–899. doi: 10.1002/ps.1770. [DOI] [PubMed] [Google Scholar]
- 150.Cools HJ, Fraaije BA. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag Sci. 2012;69(2):150–155. doi: 10.1002/ps.3348. [DOI] [PubMed] [Google Scholar]
- 151.Cools HJ, et al. Overexpression of the sterol 14alpha-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Manag Sci. 2012;68(7):1034–1040. doi: 10.1002/ps.3263. [DOI] [PubMed] [Google Scholar]
- 152.Ghosoph JM, et al. Imazalil resistance linked to a unique insertion sequence in the PdCYP51 promoter region of Penicillium digitatum. Postharvest Biol Technol. 2007;44(1):9–18. [Google Scholar]
- 153.Sun X, et al. PdMLE1, a specific and active transposon acts as a promoter and confers Penicillium digitatum with DMI resistance. Environ Microbiol Rep. 2013;5(1):135–142. doi: 10.1111/1758-2229.12012. [DOI] [PubMed] [Google Scholar]
- 154.Schnabel G, Jones AL. The 14alpha-demethylasse(CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology. 2001;91(1):102–110. doi: 10.1094/PHYTO.2001.91.1.102. [DOI] [PubMed] [Google Scholar]
- 155.Pfeufer EE, Ngugi HK. Orchard factors associated with resistance and cross resistance to sterol demethylation inhibitor fungicides in populations of Venturia inaequalis from Pennsylvania. Phytopathology. 2012;102(3):272–282. doi: 10.1094/PHYTO-04-11-0117. [DOI] [PubMed] [Google Scholar]
- 156.Nakaune R, et al. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl Environ Microbiol. 1998;64(10):3983–3988. doi: 10.1128/aem.64.10.3983-3988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kretschmer M, et al. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009;5(12):e1000696. doi: 10.1371/journal.ppat.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hawkins NJ et al (2014) Paralog re-emergence: a novel, historically contingent mechanism in the evolution of antimicrobial resistance. Mol Biol Evol [DOI] [PMC free article] [PubMed]
- 159.Fraaije BA, et al. A novel substitution I381V in the sterol 14alpha-demethylase (CYP51) of Mycosphaerella graminicola is differentially selected by azole fungicides. Mol Plant Pathol. 2007;8(3):245–254. doi: 10.1111/j.1364-3703.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 160.Cools HJ, et al. Heterologous expression of mutated eburicol 14{alpha}-demethylase (CYP51) proteins of Mycosphaerella graminicola demonstrates effects on azole fungicide sensitivity and intrinsic protein function. Appl Environ Microbiol. 2010;76:2866–2872. doi: 10.1128/AEM.02158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mullins JG, et al. Molecular modelling of the emergence of azole resistance in Mycosphaerella graminicola. PLoS One. 2011;6(6):e20973. doi: 10.1371/journal.pone.0020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leroux P, et al. Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag Sci. 2007;63(7):688–698. doi: 10.1002/ps.1390. [DOI] [PubMed] [Google Scholar]
- 163.Leroux P, Walker AS. Multiple mechanisms account for resistance to sterol 14alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag Sci. 2010;67(1):44–59. doi: 10.1002/ps.2028. [DOI] [PubMed] [Google Scholar]
- 164.Stammler G, et al. Frequency of different CYP51-haplotypes of Mycosphaerella graminicola and their impact on epoxiconazole-sensitivity and -field efficacy. Crop Prot. 2008;27(11):1448–1456. [Google Scholar]
- 165.Delye C, Bousset L, Corio-Costet MF. PCR cloning and detection of point mutations in the eburicol 14alpha-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr Genet. 1998;34(5):399–403. doi: 10.1007/s002940050413. [DOI] [PubMed] [Google Scholar]
- 166.Wyand RA, Brown JK. Sequence variation in the CYP51 gene of Blumeria graminis associated with resistance to sterol demethylase inhibiting fungicides. Fungal Genet Biol. 2005;42(8):726–735. doi: 10.1016/j.fgb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 167.Perea S, et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001;45(10):2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Warrilow AG, et al. Azole affinity of sterol 14alpha-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob Agents Chemother. 2013;57(3):1352–1360. doi: 10.1128/AAC.02067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jefcoate CR (1978) Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy pp258-279. In Fleischer S, Packer L (ed) Biomembranes Part C, Methods in Enzymology. Vol. 52. Elsevier inc. USA [DOI] [PubMed]
- 170.Lange R, Bonfils C, Debey P. The low-spin/high-spin transition equilibrium of camphor-bound cytochrome P-450. Effects of medium and temperature on equilibrium data. Eur J Biochem. 1977;79(2):623–628. doi: 10.1111/j.1432-1033.1977.tb11847.x. [DOI] [PubMed] [Google Scholar]
- 171.Jefcoate CR, Gaylor JL, Calabrese RL. Ligand interactions with cytochrome P450. I. Binding of primary amines. Biochemistry. 1969;8:3455–3463. doi: 10.1021/bi00836a049. [DOI] [PubMed] [Google Scholar]
- 172.Lutz JD, et al. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem Pharmacol. 2009;77(2):258–268. doi: 10.1016/j.bcp.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Copeland RA. Evaluation of enzyme inhibitors in drug discovery: a guide for medicinal chemists an pharmacologists. New York: Wiley-Interscience; 2005. pp. 178–213. [PubMed] [Google Scholar]
- 174.Parker JE, et al. Mechanism of binding of prothioconazole to Mycosphaerella graminicola CYP51 differs from that of other azole antifungals. Appl Environ Microbiol. 2011;77(4):1460–1465. doi: 10.1128/AEM.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parker JE, et al. Prothioconazole and prothioconazole-desthio activities against Candida albicans sterol 14-alpha-demethylase. Appl Environ Microbiol. 2013;79(5):1639–1645. doi: 10.1128/AEM.03246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lepesheva GI, et al. Sterol 14alpha-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chem Biol. 2007;14(11):1283–1293. doi: 10.1016/j.chembiol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rupp B, et al. Molecular design of two sterol 14α-demethylase homology models and their interactions with the azole antifungals ketoconazole and bifonazole. J Comput Aided Mol Des. 2005;19(3):149–163. doi: 10.1007/s10822-005-3692-7. [DOI] [PubMed] [Google Scholar]
- 178.Ji H, et al. A three-dimensional model of lanosterol 14alpha-demethylase of Candida albicans and its interaction with azole antifungals. J Med Chem. 2000;43(13):2493–2505. doi: 10.1021/jm990589g. [DOI] [PubMed] [Google Scholar]
- 179.Xiao L, et al. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14{alpha}-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob Agents Chemother. 2004;48(2):568–574. doi: 10.1128/AAC.48.2.568-574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lepesheva GI, et al. Crystal structures of Trypanosoma brucei sterol 14alpha-demethylase and implications for selective treatment of human infections. J Biol Chem. 2010;285(3):1773–1780. doi: 10.1074/jbc.M109.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lepesheva GI, et al. Structural insights into inhibition of sterol 14alpha-demethylase in the human pathogen Trypanosoma cruzi. J Biol Chem. 2010;285(33):25582–25590. doi: 10.1074/jbc.M110.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hargrove TY, et al. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14alpha-demethylase (CYP51) from Leishmania infantum. J Biol Chem. 2011;286(30):26838–26848. doi: 10.1074/jbc.M111.237099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Strushkevich N, Usanov SA, Park HW. Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol. 2010;397(4):1067–1078. doi: 10.1016/j.jmb.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 184.Monk BC, et al. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc Natl Acad Sci U S A. 2014;111(10):3865–3870. doi: 10.1073/pnas.1324245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Podust LM, Poulos TL, Waterman MR. Crystal structure of cytochrome P450 14alpha -sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci U S A. 2001;98(6):3068–3073. doi: 10.1073/pnas.061562898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alvarez-Rueda N, et al. Amino acid substitutions at the major insertion loop of Candida albicans sterol 14alpha-demethylase are involved in fluconazole resistance. PLoS One. 2011;6(6):e21239. doi: 10.1371/journal.pone.0021239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lepesheva GI, Waterman MR. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta. 2007;1770(3):467–477. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zarn JA, Bruschweiler BJ, Schlatter JR. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ Health Perspect. 2003;111(3):255–261. doi: 10.1289/ehp.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Masubuchi Y, Horie T. Toxicological significance of mechanism-based inactivation of cytochrome p450 enzymes by drugs. Crit Rev Toxicol. 2007;37(5):389–412. doi: 10.1080/10408440701215233. [DOI] [PubMed] [Google Scholar]
- 190.Taxvig C, et al. Endocrine-disrupting properties in vivo of widely used azole fungicides. Int J Androl. 2008;31(2):170–177. doi: 10.1111/j.1365-2605.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 191.Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94(1):3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- 192.Korashy HM, et al. Induction of cytochrome P450 1A1 by ketoconazole and itraconazole but not fluconazole in murine and human hepatoma cell lines. Toxicol Sci. 2007;97(1):32–43. doi: 10.1093/toxsci/kfm012. [DOI] [PubMed] [Google Scholar]
- 193.Nesnow S, Padgett WT, Moore T. Propiconazole induces alterations in the hepatic metabolome of mice: relevance to propiconazole-induced hepatocarcinogenesis. Toxicol Sci. 2011;120(2):297–309. doi: 10.1093/toxsci/kfr012. [DOI] [PubMed] [Google Scholar]
- 194.Babin M, et al. Cytochrome P4501A induction caused by the imidazole derivative Prochloraz in a rainbow trout cell line. Toxicol In Vitro. 2005;19(7):899–902. doi: 10.1016/j.tiv.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 195.Hasselberg L, et al. Interactions between xenoestrogens and ketoconazole on hepatic CYP1A and CYP3A, in juvenile Atlantic cod (Gadus morhua) Comp Hepatol. 2005;4(1):2. doi: 10.1186/1476-5926-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pikuleva IA, Waterman MR. Cytochromes p450: roles in diseases. J Biol Chem. 2013;288(24):17091–17098. doi: 10.1074/jbc.R112.431916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thompson DF, Carter JR. Drug-induced gynecomastia. Pharmacotherapy. 1993;13(1):37–45. [PubMed] [Google Scholar]
- 198.Shafaati M, et al. The antifungal drug voriconazole is an efficient inhibitor of brain cholesterol 24S-hydroxylase in vitro and in vivo. J Lipid Res. 2010;51(2):318–323. doi: 10.1194/jlr.M900174-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mast N, et al. Antifungal azoles: structural insights into undesired tight binding to cholesterol-metabolizing CYP46A1. Mol Pharmacol. 2013;84(1):86–94. doi: 10.1124/mol.113.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Verweij PE, et al. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9(12):789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 201.Snelders E, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One. 2012;7(3):e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Snelders E, et al. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol. 2009;75(12):4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20(4):695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fan J, et al. The Y123H substitution perturbs FvCYP51B function and confers prochloraz resistance in laboratory mutants of Fusarium verticillioides. Plant Pathol. 2013;198(3):821–835. [Google Scholar]
- 205.Dallet M, OSPAR Commission (2013) Background document on clotrimazole (2013 update). London
- 206.Ishii H, et al. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology. 2001;91(12):1166–1171. doi: 10.1094/PHYTO.2001.91.12.1166. [DOI] [PubMed] [Google Scholar]
- 207.Walker LA, Gow NAR, Munro CA. Fungal echinocandin resistance. Fungal Genet Biol. 2010;47(2):117–126. doi: 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Torriani SF, et al. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag Sci. 2009;65(2):155–162. doi: 10.1002/ps.1662. [DOI] [PubMed] [Google Scholar]
- 209.Podust LM, et al. Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography. Antimicrob Agents Chemother. 2007;51(11):3915–3923. doi: 10.1128/AAC.00311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Doyle PS, et al. A nonazole CYP51 inhibitor cures Chagas’ disease in a mouse model of acute infection. Antimicrob Agents Chemother. 2010;54(6):2480–2488. doi: 10.1128/AAC.00281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ekins S, et al. Three-dimensional quantitative structure-activity relationship analysis of human CYP51 inhibitors. Drug Metab Dispos. 2007;35(3):493–500. doi: 10.1124/dmd.106.013888. [DOI] [PubMed] [Google Scholar]
- 212.Konkle ME, et al. Indomethacin amides as a novel molecular scaffold for targeting Trypanosoma cruzi sterol 14alpha-demethylase. J Med Chem. 2009;52(9):2846–2853. doi: 10.1021/jm801643b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Korosec T, et al. Novel cholesterol biosynthesis inhibitors targeting human lanosterol 14alpha-demethylase (CYP51) Bioorg Med Chem. 2008;16(1):209–221. doi: 10.1016/j.bmc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 214.Podust LM, et al. Interaction of Mycobacterium tuberculosis CYP130 with heterocyclic arylamines. J Biol Chem. 2009;284(37):25211–25219. doi: 10.1074/jbc.M109.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Morton V, Staub T (2008) A short history of fungicides. Online APSnet Features