Abstract
Fragile X is the most common genetic cause of intellectual disability and autism. Previous studies have showed that partial inhibition of metabotropic glutamate receptor signaling is sufficient to correct behavioral phenotypes in a mouse model of Fragile X, including audiogenic seizures, open field hyperactivity, and social behavior. These phenotypes model well the epilepsy (15%), hyperactivity (20%) and autism (30%) that are co-morbid with Fragile X in human patients (Schneider et al., 2009). Identifying reliable and robust mouse phenotypes to model cognitive impairments is critical considering the 90% co-morbidity of Fragile X and intellectual disability. Recent work characterized a five-choice visuospatial discrimination assay testing cognitive flexibility, in which Fragile X model mice show impairments associated with decreases in synaptic proteins in prefrontal cortex (Krueger et al., 2011). In this study, we sought to determine whether instrumental extinction, another process requiring prefrontal cortex, is altered in Fragile X model mice, and whether downregulation of metabotropic glutamate receptor signaling pathways is sufficient to correct both visuospatial discrimination and extinction phenotypes. We report that instrumental extinction is consistently exaggerated in Fragile X model mice. However, neither the extinction phenotype nor the visuospatial discrimination phenotype is corrected by approaches targeting metabotropic glutamate receptor signaling. This work describes a novel behavioral extinction assay to model impaired cognition in mouse models of neurodevelopmental disorders, provides evidence that extinction is exaggerated in the Fragile X mouse model, and suggests possible limitations of metabotropic glutamate receptor-based pharmacotherapy.
Keywords: Fragile X, intellectual disability, metabotropic glutamate receptor 5, lovastatin, instrumental extinction
Introduction
Fragile X syndrome (FX), the leading inherited cause of intellectual disability and autism, is caused by transcriptional silencing of the FMR1 gene and loss of the gene product, Fragile X mental retardation protein (FMRP) (Verkerk et al., 1991). The Fmr1 knockout mouse (Consortium, 1994) lacks FMRP, a translational repressor (Laggerbauer et al., 2001, Li et al., 2001), resulting in increased protein synthesis (Qin et al., 2005), and has well-characterized behavioral deficits consistent with presentation of FX in humans. Specifically, FX has high comorbidity with epilepsy (~15%), hyperactivity (~20%), and autism (~30-60%) (Schneider et al., 2009). Consistent with these clinical findings, Fmr1 KO mice show increased seizure susceptibility (Yan et al., 2004), open-field activity (Consortium, 1994, Mineur et al., 2002), and social deficits (Oddi et al., 2013). Cognitive impairments in Fmr1 KO mice have been comparatively understudied, considering the ~90% comorbidity between FX and intellectual disability (Schneider et al., 2009). Fmr1 KO mice show learning deficits in multiple contexts, such as spatial and procedural learning, inhibitory avoidance, and sustained attention (D’hooge et al., 1997, Dolen et al., 2007, Koekkoek et al., 2005, Kramvis et al., 2013, Michalon et al., 2012, Moon et al., 2006, Vinueza Veloz et al., 2012). However, apart from conflicting reports of attentional dysfunction (Kramvis et al., 2013, Krueger et al., 2011, Moon et al., 2006), the majority of learning tests in Fmr1 KO mice are not sufficient to model cognitive dysfunction mediated by prefrontal cortex.
Fmr1 KO mice show impairments in a visuospatial discrimination task which are consistent with deficits in cognitive flexibility, and this impairment is linked to altered synaptic protein levels in prefrontal cortex (PFC) (Krueger et al., 2011). Extinction of operant behaviors is also an active cognitive process (Bouton, 2002) and relies on top-down control of PFC, independent of the nature of the reinforcer (Cleva et al., 2010, Millan et al., 2011, Rebec & Sun, 2005). Substantial evidence suggests metabotropic glutamate receptor 5 (mGlu5) is involved in regulating PFC-mediated operant extinction (Chesworth et al., 2013, Cleva et al., 2011, Gass & Olive, 2009, Kufahl et al., 2012), and extinction of hippocampally-encoded inhibitory avoidance is altered in Fmr1 KO mice (Dolen et al., 2007). As mGlu5-mediated processes are involved in FX pathogenesis (Bear et al., 2004), we were thus motivated to study instrumental extinction in Fmr1 KO mice.
Enhanced synaptic protein synthesis in FX is likely to be pathogenic, and pharmacological approaches to downregulate protein synthesis are currently being evaluated in clinical trials based on their success in Fmr1 KO mice (Krueger & Bear, 2011). mGlu5 signaling is positively coupled to translation through an extracellular signal-regulated kinase (ERK1/2) signaling pathway (Osterweil et al., 2010), and modest downregulation of mGlu5 or ERK1/2 signaling is sufficient to normalize protein synthesis and behavior in Fmr1 KO mice (Dolen et al., 2007, Michalon et al., 2013, Michalon et al., 2012, Osterweil et al., 2013, Yan et al., 2005). We sought to determine whether downregulation of these signaling pathways could also correct altered visuospatial discrimination and instrumental extinction in Fmr1 KO mice.
Materials and Methods
Subjects
Fmr1 KO mice (Consortium, 1994) and Grm5+/− mice (Lu et al., 1997) were obtained from Jackson Labs (stock #3558 and #3025, respectively). Both strains were backcrossed onto a C57BL/6J background for at least 6 generations at MIT and were subsequently maintained on a congenic C57BL/6J background by regular additional backcrosses. Mice were group housed with littermates and maintained on a 12:12 h light:dark cycle. Experimental cohorts consisted of male littermates that were aged 2-4 months at the time of experiments. Cohorts were obtained from WT male x Fmr1 heterozygous female breeders (for experiments involving only Fmr1 WT and Fmr1 KO groups as well as for experiments involving Fmr1 WT/vehicle, Fmr1 WT/lova, Fmr1 KO/vehicle and Fmr1 KO/lova groups) or Grm5 heterozygous males × Fmr1 heterozygous female breeders (for experiments involving Fmr1 WT/Grm5+/+, Fmr1 WT/Grm5+/−, Fmr1 KO/Grm5+/+ and Fmr1 KO/Grm5+/− groups). The number of subjects (n) varies by experiment. For the visuospatial discrimination experiments indicated in Figure 1b-c, the number of subjects by group was: Fmr1 WT/Grm5+/+: n=42, Fmr1 WT/Grm5+/−: n=34, Fmr1 KO/Grm5+/+: n=45, Fmr1 KO/Grm5+/−: n=32. For the experiments indicated in Figure 1d-e, the number of subjects by group was: Fmr1 WT/vehicle: n=33, Fmr1 WT/lova: n=23, Fmr1 KO/vehicle: n=34, Fmr1 KO/lova: n=33. For the instrumental extinction experiments indicated in Figure 2, the number of subjects by group was: Fmr1 WT: n=16, Fmr1 KO: n=16. For the instrumental extinction experiments indicated in Figure 3a-b, e-f, the number of subjects by group was: Fmr1 WT/Grm5+/+: n=14, Fmr1 WT/Grm5+/−: n=15, Fmr1 KO/Grm5+/+: n=15, Fmr1 KO/Grm5+/−: n=14. For the experiments indicated in Figure 3c-d, g-h, the number of subjects by group was: Fmr1 WT/vehicle: n=12, Fmr1 WT/lova: n=10, Fmr1 KO/vehicle: n=11, Fmr1 KO/lova: n=9. All experiments were performed blind to genotype using littermate controls. All experiments were conducted during the light phase. All procedures were approved by the MIT Committee on Animal Care.
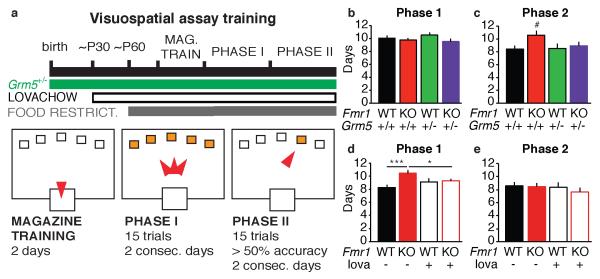
Figure 1.
Visuospatial discrimination in Fmr1 KO mice is not significantly impacted by partial reduction of mGluR5 signaling. a) Schematic showing timing of treatment strategies relative to food restriction and two phases of training on a five-choice visuospatial discrimination task. b) Phase 1 acquisition is normal in Fmr1/Grm5 cross. c) There is a trend towards delayed acquisition of phase 2 as a function of Fmr1 genotype (p=.06, n=32-45). There is not a statistically significant interaction between Fmr1 and Grm5 genotypes (WT/Grm5+/+: 8.4 +/− 0.5 days to acquisition, KO/Grm5+/+: 10.6 +/− 0.7 days, KO/Grm5+/−: 8.9 +/− 0.7 days). d) In cohorts treated chronically with lovachow or similarly formulated vehicle chow, Fmr1 KO mice show impaired phase 1 acquisition (WT/vehicle: 8.3 +/− 0.4 days, KO/vehicle: 10.4 +/− 0.5 days, ***p<.001), which is not observed following lovastatin treatment (KO/lova: 9.3 +/− 0.3 days, *p<.05). e) Lovachow/vehicle cohorts show no effect of genotype or drug treatment on phase 2 acquisition.
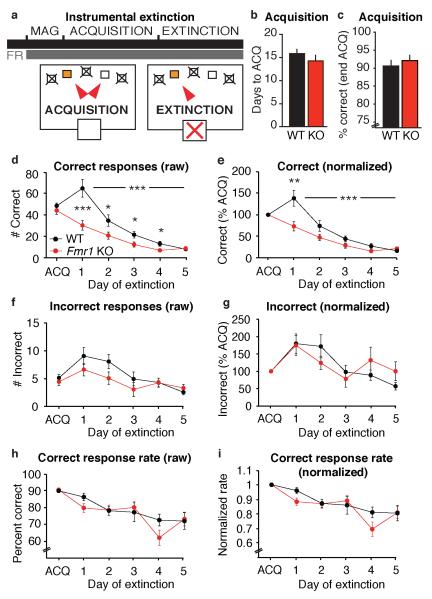
Figure 2.
Instrumental extinction is exaggerated in Fmr1 KO mice. a) Schematic shows a test with one acquisition phase, followed by five days of extinction. b) Days to acquisition and c) performance during acquisition are normal in Fmr1 KO mice. d) Extinction of correct responding is significantly exaggerated in Fmr1 KO mice (***p=.001, repeated measures two-way ANOVA), and post-hoc tests show significant exaggeration at days 1-4 (*p<.05). e) Normalization reveals significantly exaggerated extinction in Fmr1 KO mice (***p=.001), and post-hoc tests show a significant exaggeration at day 1 (**p<.01). There is no effect of Fmr1 genotype on incorrect responses (f-g) or on the rate of correct responses (h-i).
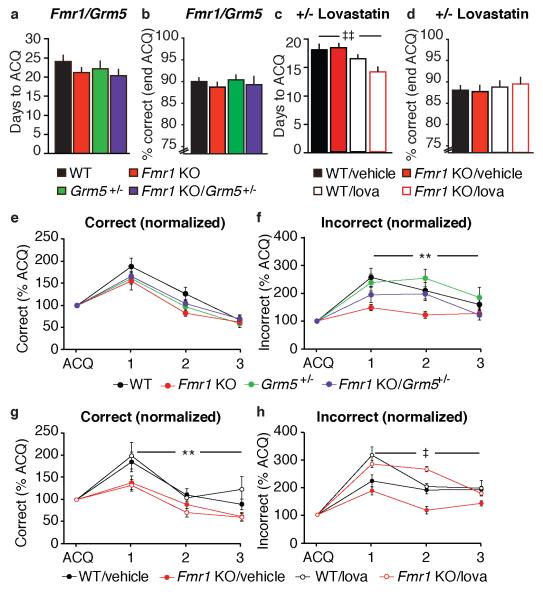
Figure 3.
Inhibition of mGluR5 and ERK signaling pathways is insufficient to correct exaggerated instrumental extinction in Fmr1 KO mice. a) Days to acquisition and b) performance during acquisition are normal in Fmr1 KO mice in Fmr1/Grm5 cross groups. c) In lovastatin/vehicle-treated groups, days to acquisition is normal in Fmr1 KO mice, but there is a significant improvement in performance with lovastatin treatment, independent of genotype (‡‡p<.01). d) In lovastatin/vehicle treated-groups, performance during acquisition is normal in Fmr1 KO mice. e) In Fmr1/Grm5 cross cohorts, there is no significant effect of Fmr1 genotype on extinction, and no Fmr1/Grm5 interaction. f) Effect of Fmr1 genotype on incorrect responding (**p<.01). g) Extinction of an instrumental response is significant exaggerated in Fmr1 KO mice independent of lovachow treatment (**p<.01). h) Effect of lovachow treatment on incorrect responding (‡p<.05). There is no effect of Fmr1 genotype and no genotype × drug interaction.
Behavioral apparatus
All experiments were performed using operant conditioning chambers (Med Associates) which had five nose-poke apertures on one wall of the chamber and a pellet dispenser and magazine for delivering food rewards (20 mg dustless precision pellets, BioServ) on the opposite wall. A house light over the food magazine permanently illuminated the chamber. Stimulus lights inside the nosepoke apertures could be controlled individually to provide visual cues during acquisition and extinction, as noted.
Food restriction
In all experiments, daily food restriction began after P60. During food restriction, the body weight of subjects was maintained at 85-90% free-feeding weight by providing unrestricted access to food for 1.5-2 hours per day immediately following daily experimental testing. Mice did not have access to food for the remainder of the day. The duration and time of feeding were kept consistent within each cohort to avoid motivation becoming a confounding variable. In some cases (where noted), experiments were conducted only 5 or 6 days per week. In these cases, ad libitum access to food was allowed on non-experimental days.
Acquisition of a five-choice visuospatial discrimination
Our five-choice visuospatial discrimination task was based on Krueger et al., PNAS, 2011. All training sessions lasted 15 minutes. Magazine training: Subjects were placed in the behavioral apparatus and each entry into the food magazine was reinforced by one food pellet on a fixed-time 10-s schedule. Subjects received two consecutive days of magazine training. Phase 1: During phase 1 of acquisition, all five nosepoke apertures were illuminated and active. To receive a reward, the subject had to respond in any illuminated aperture (“correct” response) and then return to the magazine for reward. Rewards were delivered on a fixed FR1 schedule. Illumination of apertures temporarily ceased during reward delivery until the reward was retrieved. On the first two days of phase 1, all mice were “primed” to respond in the apertures by placing a food pellet in each of the five apertures before the beginning of the session. In addition, any mouse recording less than five correct responses for two days in a row would be primed on the following day. To reach criterion and advance to the next training phase, mice were required to complete 15 trials within the 15 minute session on two consecutive days. Phase 2: During phase 2, only one aperture was illuminated at random on each trial. To receive a reward, the subject had to respond in the illuminated aperture (“correct” response) and then return to the magazine. “Incorrect” responses were defined as any response into a non-illuminated aperture and had no programmed consequence. To reach criterion and complete the task, mice had to complete 15 or more trials and achieve a 50% or greater success rate (defined as correct responses / [correct responses + incorrect responses]) on two consecutive days. Mice were excluded if they failed to reach criterion on phase 2 within 20 days. Experiments were conducted 6-7 days per week. All procedures were consistent with Krueger et al., PNAS, 2011.
Acquisition and extinction of an instrumental response
Three of the five nosepoke apertures (the outer two apertures and the center aperture) were closed using metal plugs (Med Associates), leaving only two open. Magazine training: Two days of magazine training occurred as described above. Acquisition: One of the two apertures was illuminated, and food rewards were delivered via the magazine following a correct response. Illumination of apertures temporarily ceased during reward delivery. The same aperture was illuminated on each trial within a session, and between sessions over days. Half of subjects had the left aperture illuminated and half had the right aperture illuminated. To encourage responding, rewards were delivered on a fixed FR1 schedule for the first 10 rewards, then on a variable VR2 schedule (randomly requiring either 1, 2, or 3 responses) for the following rewards. To reach criterion and advance to extinction training, mice were required to complete 15 trials with a greater than 75% success rate on five consecutive days. Extinction: For 3-5 consecutive days following acquisition, extinction sessions were conducted in which no rewards were delivered following a response in either aperture. Groups: Fmr1 KO/WT cohorts were trained 7 days per week. Fmr1 KO/Grm5+/− and Fmr1 KO ± lovastatin cohorts were trained 5 days per week. Extinction training was always conducted on three consecutive days following at least two consecutive days of acquisition. Therefore subjects in Fmr1 KO/Grm5+/− and Fmr1 KO ± lovastatin cohorts were trained to criterion for a range of 5-9 days to enable three consecutive extinction days following the final two acquisition days.
Lovastatin treatment
Lovastatin or vehicle was formulated in the food at a dose of 10 mg/kg/day, as published (Osterweil et al., 2013, Yamada et al., 2000). “Lovachow” was administered for one month prior to the beginning of experiments, from P30-P60. During experiments, lovachow was used for the 1.5-2 hour unrestricted feeding. Lovastatin or vehicle were not present in reward pellets.
Statistical analysis
Where noted, extinction data (plotted as correct responses or incorrect responses) was normalized within animal to the last five days of acquisition averaged together. Two-way ANOVA was used to analyze five-choice and two-choice acquisition data where a 2×2 experimental design was used (Fmr1 genotype × Grm5 genotype and Fmr1 genotype × lovastatin treatment). Student’s t-test was used to compare two-choice acquisition where two experimental groups were used (Fmr1 KO and WT). To analyze extinction data, either a two-factor or three-factor repeated measures ANOVA was used. The repeated measure was days of extinction, one factor was Fmr1 genotype, and where applicable, the other factor was either Grm5 genotype or lovastatin treatment. Student’s t-test or two-way ANOVA was used to compare raw baseline responding between groups. Post-hoc Bonferroni tests were used. Outliers greater than 2 standard deviations from the mean were excluded. N represents number of animals and error bars are ± SEM.
Results
Impaired visuospatial discrimination in Fmr1 KO mice is not significantly reversed by partial reduction of mGluR5 signaling
Fmr1 KO mice show deficits in a visuospatial discrimination task which are consistent with impairments in cognitive flexibility (Krueger et al., 2011). In this assay, mice are placed in an operant conditioning chamber with five nose-poke apertures and a food magazine (Figure 1a). Mice are trained on a visuospatial discrimination in two phases. In phase 1, all nosepoke apertures are illuminated and a response in any illuminated aperture results in reward delivery. In phase 2, only one aperture is randomly illuminated, and only a response in this aperture results in food reward. A previous study indicated that acquisition of phase 2, but not phase 1, is delayed in Fmr1 KO mice, indicating that these mice may be impaired in the ability to flexibly respond to changing reward contingencies (Krueger et al., 2011). Based on previous success in improving other Fmr1 KO phenotypes, we asked whether two interventions could correct this cognitive phenotype: (1) genetic cross with Grm5+/− mice (heterozygous for deletion of mGluR5) and (2) chronic administration of lovastatin for four weeks prior to testing, which has been shown to modestly reduce ERK1/2 signaling. We quantified the number of training days required to reach criterion for each phase (15 correct trials on two consecutive days for phase 1 and 15 correct trials on two consecutive days with over 50% correct for phase 2, see Methods).
Genetic cross of Fmr1+/− females and Grm5+/− males produced male offspring of four experimental genotypes: Fmr1 WT/Grm5+/+, Fmr1 KO/Grm5+/+, Fmr1 WT/Grm5+/−, and Fmr1 KO/Grm5+/−. There was no effect of Fmr1 genotype or Grm5 genotype on phase 1 acquisition in this group (Figure 1b; n = 32-45; two-way ANOVA: main effect of Fmr1 genotype, F1,133 = 1.634, p = .203; main effect of Gmr5 genotype, F1,133 < 1; Fmr1 × Grm5 interaction, F1,133 < 1). We saw a trend suggesting an effect of Fmr1 genotype on phase 2 acquisition, but this did not reach statistical significance (Figure 1c; two-way ANOVA: main effect of Fmr1 genotype, F1,149 = 3.591, p = .060). There was no significant interaction between Fmr1 and Grm5 genotype (F1,149 = 1.678, p = .197).
Lovastatin, an indirect downregulator of ERK1/2 signaling, has been shown to correct audiogenic seizures as well as altered synaptic plasticity and excitability in Fmr1 KO mice (Osterweil et al., 2013). Chronic lovastatin dosing in food (“lovachow”) also corrects seizures in Fmr1 KO mice, but this approach has not yet been tested on cognitive deficits. Animals were tested in the visuospatial discrimination task following four weeks of lovachow or vehicle and concurrent with active dosing (Figure 1a). Fmr1 KO mice surprisingly showed a delay in phase 1 operant acquisition not previously reported (Figure 1d; n = 24-38; two-way ANOVA: main effect of Fmr1 genotype, F1,131 = 6.889, p = .010). This delay in days to criterion was not observed following chronic lovastatin dosing (genotype × drug interaction, F1,131 = 4.990, p = .027). Post-hoc analysis revealed significant differences between WT/vehicle and KO/vehicle groups (p<.001), as well as KO/vehicle and KO/lovachow groups (p<.05). There was no concurrent delay in phase 2 acquisition in Fmr1 KO mice and no effect of lovastatin (Figure 1e; two-way ANOVA: main effect of genotype, F1,119 < 1; main effect of drug treatment, F1,119 < 1; genotype × treatment interaction, F1,119 < 1).
Instrumental extinction is exaggerated in Fmr1 KO mice
While there was no statistically significant effect of the Grm5+/− genotype in the visuospatial discrimination task, we observed an interesting trend that might indicate an amelioration of cognitive function by mGluR5 reduction. To further investigate this possibility, we decided to expand our analysis of phenotypes related to PFC function in the Fmr1 KO mice. Due to previous reports of altered extinction of a fear response (Dolen et al., 2007), we tested whether extinction of an appetitive operant response is also altered in Fmr1 KO mice (Figure 2a; n = 16 per group). In this experiment, mice were first trained on a two-choice instrumental response, in which one aperture was illuminated and active, while the other was not illuminated and inactive. Acquisition of this task was normal in Fmr1 KO mice, as measured by days to criterion (Figure 2b; Student’s t-test, p = .657). In addition, absolute performance during the final five days of acquisition was similar between WT and Fmr1 KO mice (Figure 2c; Student’s t-test, p = .906, Figure 2d; Student’s t-test on “ACQ”, p=.456). Subsequently, mice received extinction training, during which no reward was delivered following a response in the illuminated or non-illuminated aperture. Surprisingly, we found that Fmr1 KO mice displayed exaggerated extinction learning (Figure 2d; repeated measures two-way ANOVA, main effect of genotype, F1,30 = 12.679, p = .001; genotype × day interaction, F4,120 = 8.080, p = .001; post-hoc tests: D1, p = .001, D2, p = .034, D3, p = .023, D4, p = .020, D5, p = .625). We normalized correct responses during extinction by animal to the last five days of acquisition to account for individual differences in basal response rate and found similar results (Figure 2e; repeated measures two-way ANOVA, main effect of genotype, F1,30 = 7.906, p = .009; genotype/day interaction, F4,120 = 5.982, p = .004; post-hoc tests: D1, p = .005, D2, p = .071, D3, p = .093, D4, p = .053, D5, p = .188). The number of incorrect responses also decreased over days independent of genotype (Figures 2f-g, repeated measures two-way ANOVAs, main effect of day, F4,120 = 9.822, p<.001 for raw data; F4,120 = 5.513, p<.001 for normalized data). There was no significant effect of genotype (F1,30 = 1.815, p = .188 raw and F1,30 < 1 normalized) and no interaction between genotype and day (F4,120 = 1.551, p = .204 raw and F4,120 = 1.277, p = .150 normalized). However, since we observed a trend towards a decrease in incorrect responses, we calculated the percentage of correct responses (defined as number of correct responses / (number of correct + incorrect responses)*100) to determine the effect of changes in total responses. There was no significant effect of genotype on percentage of correct responses (Figure 2h-i, repeated measures two-way ANOVAs, F1,30 < 1 raw and F1,30 < 1 normalized) or interaction between genotype and test day (F4,120= 1.563, p=.211 raw; F4,120= 1.489, p=.230 normalized), indicating that Fmr1 KO mice responded less equally at both apertures.
Partial inhibition of mGluR5 signaling fails to improve exaggerated instrumental extinction in Fmr1 KO mice
We next tested whether 50% genetic reduction of mGluR5 or chronic lovastatin would correct exaggerated instrumental extinction in Fmr1 KO mice. There was no effect of Fmr1 genotype on acquisition in either the Fmr1/Grm5 cross (Figure 3a; two-way ANOVA, main effect of Fmr1 genotype, F1,53 = 3.248, p = .077 and Figure 3b; two-way ANOVA, main effect of Fmr1 genotype, F1,53 < 1) or the Fmr1 ± lovastatin groups (Figure 3c; two-way ANOVA, main effect of Fmr1 genotype, F1,37 = 1.029, p = .317 and Figure 3d; two-way ANOVA, main effect of Fmr1 genotype, F1,37 < 1). Chronic lovastatin treatment did improve acquisition, independent of genotype (Figure 3c; two-way ANOVA, main effect of drug, F1,37 = 10.065, p = .003, but no genotype × drug interaction, F1,37 = 2.298, p = .138).
During extinction training, Fmr1/Grm5 cross cohorts (n = 14-15) showed a significant reduction in responding at the correct aperture independent of genotype (Figure 3e; repeated measures three-way ANOVA: main effect of day, F2,100 = 108.429, p<.001), but there was no main effect of either Fmr1 or Grm5 genotype (F1,50 < 1), no Fmr1/Grm5 interaction (F1,10 = 3.119, p = .084), and no Fmr1/Grm5/day interaction (F2,100 < 1). Interestingly, there was a significant effect of Fmr1 genotype on responding at the incorrect aperture (Figure 3f; repeated measures three-way ANOVA, main effect of Fmr1 genotype, F1,50 = 7.440, p = .009), but no correction by Grm5 cross (Fmr1 × Grm5 interaction, F1,50 < 1, Fmr1 × Grm5 × day interaction, F1,50 < 1). There was no difference in basal response rates during acquisition between genotypes (two-way ANOVA: main effect of Fmr1 genotype, F1,57 < 1; main effect of Grm5 genotype, F1,57 < 1; Fmr1 × Grm5 interaction, F1,57 < 1).
During extinction training, lovastatin or vehicle-treated cohorts (n = 9-12), showed a significant reduction in responding at the correct aperture independent of drug treatment, and this was significantly exaggerated in Fmr1 KO mice (Figure 3g; repeated measures three-way ANOVA, main effect of Fmr1 genotype, F1,37 = 10.175, p = .003). Chronic lovastatin treatment does not correct exaggerated extinction (main effect of drug, F1,37 < 1; genotype × drug interaction, F1,37 < 1; no significant drug × day or genotype × drug × day interactions). No significant effect of Fmr1 genotype on responding at the incorrect aperture was observed (Figure 3d; three-way repeated measures ANOVA, main effect of Fmr1 genotype, F1,37 < 1). However, lovastatin did alter responding at the incorrect aperture, independent of genotype (F1,37 = 5.885, p = .020). There was no significant genotype × treatment, genotype × day, treatment × day, or genotype × treatment × day interaction. There was no difference in basal response rates during acquisition between genotypes (two-way ANOVA: main effect of Fmr1 genotype, F1,38 = 3.484, p = .070; main effect of Gmr5 genotype, F1,38 < 1; Fmr1 × Grm5 interaction, F1,38 < 1).
Discussion
Development of reliable and robust assays to model high-level cognitive deficits in FX and other disorders could provide a valuable system in which to test the efficacy of pharmacological treatments in mice. Here, we describe a novel form of instrumental extinction learning that is altered in Fmr1 KO mice in parallel with visuospatial discrimination (Krueger et al., 2011).
Fmr1 KO mice display exaggerated instrumental extinction (Figure 2), while at the same time showing impaired visuospatial discrimination. A key element of impaired visuospatial discrimination in Fmr1 KO mice is that it is accompanied by perseverative behavior and a lack of cognitive flexibility (Kramvis et al., 2013, Krueger et al., 2011). Cognitive inflexibility - the inability to modify behavior with changing rules - has been reported in many human neuropsychiatric disorders and mouse models and involves dopaminergic signaling in both PFC and striatum (Klanker et al., 2013). Extinction of a learned instrumental behavior is different from switching rules (i.e. between acquisition phase 1 and phase 2), and thus it is not entirely surprising that extinction is exaggerated while visuospatial discrimination is impaired.
Exaggerated extinction of inhibitory avoidance, a learning paradigm whose acquisition is hippocampally encoded, has been previously reported in Fmr1 KO mice (Dolen et al., 2007). In PFC, extinction of operant drug-seeking behavior is regulated by mGlu5. Specifically, positive allosteric modulation of mGlu5 results in exaggerated extinction for multiple reinforcers and mGlu5 KO mice have delayed extinction (Chesworth et al., 2013, Cleva et al., 2011, Gass & Olive, 2009, Kufahl et al., 2012). Assuming that food rewards and drug rewards have similar saliency and involve similar circuits, our results would be consistent with enhanced mGlu5 signaling. In Fmr1 KO mice, levels of mGlu5 protein are normal. However, in the absence of the translational repressor FMRP, protein synthesis downstream of mGlu5 is enhanced in Fmr1 KO mice. ERK1/2 signaling, linking mGlu5 to protein synthesis, is also upregulated in the cortex of Fmr1 KO mice (Michalon et al., 2012). It is therefore a reasonable hypothesis that upregulation of pathways downstream of mGlu5 may account for exaggerated instrumental extinction in Fmr1 KO mice. It has previously been shown that a reduction in mGlu5 signaling can correct a wide range of behavioral phenotypes in the Fmr1 KO mice, including cognitive phenotypes (De Vrij et al., 2008, Dolen et al., 2007, Levenga et al., 2011, Michalon et al., 2012, Vinueza Veloz et al., 2012, Yan et al., 2005). In the current study, neither the 50% reduction in mGluR5 expression nor chronic treatment with lovastatin was sufficient to correct altered extinction in Fmr1 KO mice (Figure 3). However, a trend towards improvement in visuospatial discrimination was seen in the Fmr1 KO/Grm5+/− cross (Figure 1c). It is possible that additional improvements could be observed by adjusting dosages. Our choices here were guided by success in previous studies using other fragile X phenotypes (Dolen et al., 2007, Osterweil et al., 2013).
One aim of our study was to assess whether instrumental extinction and visuospatial discrimination are robust assays of PFC-mediated cognitive dysfunction in Fmr1 KO mice that can be reliably used to test potential therapeutic agents. In this context it is important to note that the impairment observed in the visuospatial discrimination assay appears to be sensitive to environmental conditions, since the Fmr1 KO phenotype observed in the Grm5 cross experiment (Figure 1b,c) is different from that observed in the lovastatin experiment (Figure 1d,e). In the Grm5 cross experiment, we saw a trend towards delayed phase 2 acquisition in Fmr1 KO mice (p=.06) as reported previously (Krueger et al., 2011). Had we not tested the Grm5 cross concurrently, the difference between Fmr1 KO and wild-type mice would have been statistically significant. However, in the lovastatin experiment, we saw no phase 2 phenotype but did see a surprising phase 1 delay which has not been previously reported. Since mice in the latter cohorts were raised on a different dietary formulation (BioServ, for both vehicle and lovachow), it is possible that this formulation altered either metabolism or the saliency of the reward (a different type of food pellet), and therefore motivation, in these cohorts. It has also been shown that dietary conditions may influence learning and memory performance in mice (Yu et al., 2010). Our data indicate that the instrumental extinction phenotype may be less sensitive to these influences and hence more suitable for the study of pharmacological interventions.
It is interesting that an improvement in operant acquisition was observed following lovastatin treatment, which was independent of Fmr1 genotype (Figure 3). These results represent unexpected effects of lovastatin on operant acquisition and deserve further study.
Ultimately, developing robust assays for altered cognition is worthwhile in Fmr1 KO mice since cognitive impairments are at the core of FX. Different mechanisms are likely to underlie separate cognitive tasks such as the visuospatial discrimination and extinction of instrumental responses, and it is therefore not surprising that treatment strategies vary in their effectiveness depending on the cognitive test. This is an important point to consider in designing clinical trials where properly defined endpoints are crucial. It is imperative to understand which cognitive behaviors may be most amenable to treatment by which drugs. The ability to measure multiple Fmr1 KO phenotypes under similar conditions could be useful for therapeutic drug screens, the results of which may provide insights into the proper endpoints to be considered for clinical trials in FX and other neurodevelopmental disorders associated with intellectual disability.
Acknowledgements
This research was supported by grants from the Fraxa Research Foundation, Autism Science Foundation, National Institute of Mental Health training grant 2T32MH074249, and National Institute of Child Health and Development grant 5RO1HD046903. We thank Dawna Bagherian for her contributions in testing performance on the visuospatial discrimination assay. Mark Bear holds patents on the use of mGluR-based pharmacotherapy for the treatment of fragile X syndrome.
References
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Lawrence AJ. The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine-seeking in mice. PLoS ONE. 2013;8:e68371. doi: 10.1371/journal.pone.0068371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Gass JT, Widholm JJ, Olive MF. Glutamatergic targets for enhancing extinction learning in drug addiction. Current neuropharmacology. 2010;8:394–408. doi: 10.2174/157015910793358169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 2011;125:10–19. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium. T.D.-B.F.X. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, Kooy RF, Oostra BA, Willems PJ, De Deyn PP. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76:367–376. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- de Vrij FMS, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiology of Disease. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of Fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kramvis I, Mansvelder HD, Loos M, Meredith R. Hyperactivity, perseveration and increased responding during attentional rule acquisition in the Fragile X mouse model. Frontiers in behavioral neuroscience. 2013;7:172. doi: 10.3389/fnbeh.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward Fulfilling the Promise of Molecular Medicine in Fragile X Syndrome. Annu. Rev. Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2587–2592. doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Hood LE, Nemirovsky NE, Barabas P, Halstengard C, Villa A, Moore E, Watterson LR, Olive MF. Positive Allosteric Modulation of mGluR5 Accelerates Extinction Learning but Not Relearning Following Methamphetamine Self-Administration. Frontiers in pharmacology. 2012;3:194. doi: 10.3389/fphar.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Human Molecular Genetics. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Levenga J, Hayashi S, de Vrij FMS, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RAM, Pop AS, GomezMancilla B, Nelson DL, Willemsen R, Gasparini F, Ben AO. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiology of Disease. 2011;42:311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A, Bruns A, Risterucci C, Honer M, Ballard TM, Ozmen L, Jaeschke G, Wettstein JG, von Kienlin M, Kunnecke B, Lindemann L. Chronic Metabotropic Glutamate Receptor 5 Inhibition Corrects Local Alterations of Brain Activity and Improves Cognitive Performance in Fragile X Mice. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of theFmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA, Crnic LS, Strupp BJ. Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behavioral Neuroscience. 2006;120:1367–1379. doi: 10.1037/0735-7044.120.6.1367. [DOI] [PubMed] [Google Scholar]
- Oddi D, Crusio WE, D’Amato FR, Pietropaolo S. Monogenic mouse models of social dysfunction: implications for autism. Behav Brain Res. 2013;251:75–84. doi: 10.1016/j.bbr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Chuang S-C, Chubykin AA, Sidorov M, Bianchi R, Wong RKS, Bear MF. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77:243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 Leads to Excessive Protein Synthesis in the Hippocampus of a Mouse Model of Fragile X Syndrome. J. Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. Journal of the experimental analysis of behavior. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, Hessl D. Fragile X syndrome -- from genes to cognition. Dev Disabil Res Rev. 2009;15:333–342. doi: 10.1002/ddrr.80. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vinueza Veloz MF, Buijsen RA, Willemsen R, Cupido A, Bosman LW, Koekkoek SK, Potters JW, Oostra BA, De Zeeuw CI. The effect of an mGluR5 inhibitor on procedural memory and avoidance discrimination impairments in Fmr1 KO mice. Genes Brain Behav. 2012;11:325–331. doi: 10.1111/j.1601-183X.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Shinnoh N, Taniwaki T, Ohyagi Y, Asahara H, Horiuchi, Kira J. Lovastatin does not correct the accumulation of very long-chain fatty acids in tissues of adrenoleukodystrophy protein-deficient mice. Journal of inherited metabolic disease. 2000;23:607–614. doi: 10.1023/a:1005634130286. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr Fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yu H, Bi Y, Ma W, He L, Yuan L, Feng J, Xiao R. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2010;28:271–276. doi: 10.1016/j.ijdevneu.2009.12.001. [DOI] [PubMed] [Google Scholar]