Abstract
Purpose
Glioblastoma (GBM) is the most common form of malignant glioma in adults. Although protected by both the blood brain- and blood tumor-barriers, T cells actively infiltrate GBM. Previous work has shown that IDO, CTLA-4 and PD-L1 are dominant molecular participants in the suppression of GBM immunity. This includes IDO-mediated regulatory T cell (Treg; CD4+CD25+FoxP3+) accumulation, the interaction of T cell-expressed, CTLA-4, with dendritic cell-expressed, CD80, as well as the interaction of tumor- and/or macrophage-expressed, PD-L1, with T cell-expressed, PD-1. The individual inhibition of each pathway has been shown to increase survival in the context of experimental GBM. However, the impact of simultaneously targeting all three pathways in blood tumor-barriers, GBMs are actively infiltrated by T cells.
Experimental Design and Results
In this report, we demonstrate that, when dually-challenged, IDO-deficient tumors provide a selectively competitive survival advantage against IDO-competent tumors. Next, we provide novel observations regarding tryptophan catabolic enzyme expression, before showing that the therapeutic inhibition of IDO, CTLA-4 and PD-L1 in a mouse model of well-established glioma maximally decreases tumor-infiltrating Tregs, coincident with a significant increase in T cell-mediated long-term survival. In fact, 100% of mice bearing intracranial tumors were long-term survivors following triple combination therapy. The expression and/or frequency of T cell-expressed CD44, CTLA-4, PD-1 and IFN-γ depended on timing after immunotherapeutic administration.
Conclusions
Collectively, these data provide strong pre-clinical evidence that combinatorially-targeting immunosuppression in malignant glioma is a strategy that has high potential value for future clinical trials in patients with GBM.
Keywords: immunosuppression, tryptophan, glioblastoma, metabolism, immunotherapy
Introduction
Glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults, with >50,000 patients currently living with the disease (1, 2). Although patients with GBM may undergo aggressive surgical resection, irradiation and chemotherapy, the overall survival remains at only 14.6 months post-diagnosis (3). GBM has been genomically stratified into four major subgroups: 1) neural, 2) pro-neural, 3) classical and 4) mesenchymal (4). Different subtypes arise due to distinct pro-oncogenic events, possess novel response rates to therapy and show diverse patterns of T cell infiltration (5). Given the heterogeneity of GBM, it has been a challenge to find therapeutically-targetable genes that are expressed by all four genomic subtypes.
Indoleamine 2,3 dioxygenase 1 (IDO) is a tryptophan catabolic enzyme not normally expressed in the CNS parenchyma at appreciable levels (6), but is induced in 90% of GBM (7). Moreover, higher IDO expression is more often observed in GBM when compared with low-grade glioma (8), suggesting that the degree of transformation may play a role in IDO expression levels. Moreover, GBM patients with higher IDO expression levels exhibit a shorter overall survival. Recent work from our laboratory has identified that IDO expression by brain tumor cells, rather than other cells capable of expressing IDO, is critically-important in tumor-induced suppression of the anti-tumor response (9). This was an unexpected finding given that in peripheral tumor models, IDO expressed by dendritic cells appears to play a critical role in suppressing the anti-tumor immune response (10-12).
Immunosuppression in glioblastoma is a characteristic hallmark associated with the recruitment of myeloid-derived suppressor cells (13, 14), increased interleukin-10 and transforming growth factor-beta (15-17), as well as the accumulation of regulatory T cells (Treg; CD4+CD25+FoxP3+) (18, 19). The latter component is a potently immunosuppressive subset that in GBM is characterized by the constitutively high level of CTLA-4, GITR and is predominantly represented as being thymus-derived (20). Based on previous studies in peripheral tumor models demonstrating the impact of IDO on Treg activation (21), expansion (22) and/or recruitment (23), we recently asked if there was a dominant cell type that required IDO to regulate Treg levels in brain tumors (9). Our original hypothesis assumed that dendritic cell (DC)-expressed IDO would be required, based on previous work showing their role in Treg modulation (10, 21, 24). However, upon more careful examination, while IDO-expressing DCs control Treg expansion, this mechanism depends on the conversion of CD4+CD25− non-Treg into CD4+CD25+(FoxP3+) Treg (25-27). Given that glioma-resident Treg are primarily thymus-derived (20), this likely explains why IDO−/−DC failed to impact Treg levels in brain tumors.
Here, we first extend our previous observations by determining the immunodominance of IDO-competent and IDO-deficient malignant glioma, ultimately revealing that timing of tumor implantation plays a significant role in tumor rejection. Next, we determined the therapeutic impact of inhibiting IDO, alone, or when combined with the current standard-of-care therapy, Temozolomide (TMZ; Temodar), unexpectedly revealing that the enzymatic inhibition of IDO does not play a crucial role in significantly increasing overall survival from malignant glioma. Importantly, we tested the individual and combined impact of inhibiting CTLA-4, PD-L1, and IDO in models of established glioma, demonstrating a robust decrease in tumor-resident Treg concurrent with increased survival when all three targets were inhibited simultaneously. However, when we tested the impact of a similar strategy in an aggressive intracranial melanoma model, only a modest effect on survival was observed. Collectively, these data significantly enhance our understanding of therapeutically-targetable immunosuppressive pathways active in brain tumors.
Methods
Mice and cell lines
C57BL/6 (wild-type; Cat# 000664), IDO−/− (Cat# 005897), Rag1−/− (Cat# 002216) and OT-II (Cat# 004194) mice were obtained from Jackson Laboratories (Bar Harbor, ME), maintained in the University of Chicago Carlson Barrier Facility and intracranially-injected between the ages of 6 and 8 weeks. All mouse strains used in the included studies were on the C57BL/6 background and were provided autoclaved food pellets and water ad libitum. All surgical procedures were completed in accordance with National Institutes of Health guidelines on the care and use of laboratory animals for research purposes. All studies performed on mice were approved by the IACUC of the University of Chicago. Mice were euthanized by cervical dislocation. GL261 and B16-F10 cells were obtained from the NCI Frederick National Tumor Repository Lab (no authentication of the cell lines was conducted) and cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum as well as streptomycin (100 mg/ml) and penicillin (100 U/ml) at 37°C in a humidified atmosphere of 95 % air/5 % CO2. All cell culture products were purchased from Gibco Invitrogen (Carlsbad, CA).
Mouse orthotopic intracranial-injection model
A detailed description can be found in the Supplemental Materials and Methods section.
Reagents and treatments
A detailed description can be found in the Supplemental Materials and Methods section.
Western blotting
A detailed description can be found in the Supplemental Materials and Methods section.
Flow cytometry and T cell stimulation
A detailed description can be found in the Supplemental Materials and Methods section.
Statistics
Data were analyzed using Prism 4.0 software (GraphPad Software). Experiments were repeated at least two times each. Data are represented as the mean ± SEM for all figure panels in which error bars are shown. The P values represent ANOVA for groups of 3 or more, whereas 2-tailed unpaired Student t tests were used for paired groups. A P value of less than 0.05 was considered statistically significant.
Results
The role of IDO and antigen specificity in glioma immunity
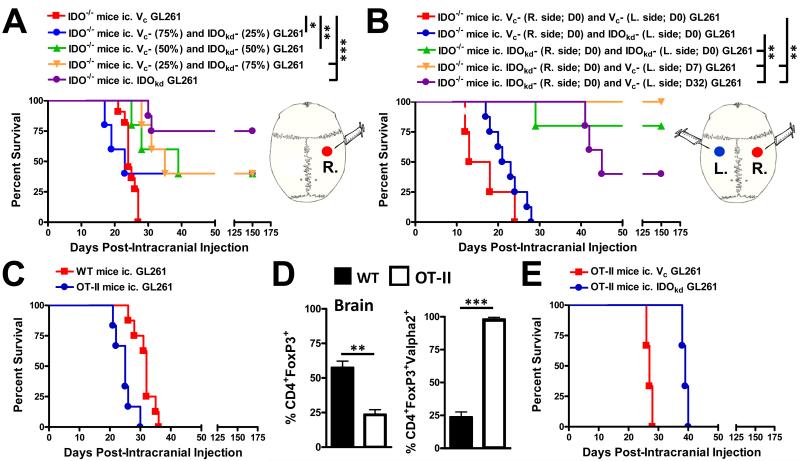
The genetic ablation of IDO in glioma cells results in the spontaneous rejection of brain tumors mediated by T cells (9). Previous work demonstrating that the majority of patient GBM specimens are >50% positive for IDO (7) suggests that this tryptophan catabolic enzyme tonically maintains suppression of the anti-tumor response. To determine the minimum number of IDO-deficient cells in a brain tumor required to induce tumor rejection, we mixed IDO-competent and IDO-deficient GL261 cells at various ratios and tested the effects on survival in IDO1-deficient (IDO−/−) mice. As shown in Figure 1A, 100% of glioma-bearing mice with IDO-competent (Vc) tumor cells died with a median overall survival of 24 days. In contrast, glioma-bearing mice with tumors mixed with IDO-competent and -deficient (IDOkd) tumor cells at 3:1, 1:1 or 1:3, resulted in 40% of mice surviving for up to 150 days (P<0.05, P<0.01, P<0.001, respectively). However, even with the survival advantage conveyed by the different ratios of IDO-deficient glioma cells, it was still overall lower when compared to the group of mice intracranially-injected with IDO-deficient cells, alone, which resulted in 75% of mice surviving for up to 150 days post-ic. (P<0.001).
Figure 1.
The rejection of IDO-competent and -deficient brain tumors is context-dependent. (A) Survival analysis of indoleamine 2,3 dioxygenase knockout (IDO−/−) mice intracranially-injected (ic.) with a total of 4×105 GL261 cells transduced with -scrambled shRNA (vector control, Vc), -shRNA specific to IDO (IDO knockdown, IDOkd), or mixed (Vc + IDOkd) at different ratios of cells. *P < 0.05; **P < 0.01; ***P < 0.001. (n = 5 - 11/group) (B) Survival analysis of IDO−/− mice ic. Injected with 4×105 Vc or IDOkd GL261 cells in the right (R. side) and left (L. side) cerebral hemispheres, simultaneously [Day 0 (D0)], or 4×105 IDOkd GL261 were ic. injected in the right hemisphere (D0), followed by an ic. injection of 4×105 Vc GL261 cells at 7- or 32- days post-ic. (D7 or D32, respectively) (n = 4 - 8/group). **P < 0.01. (C) Survival analysis of wild-type (WT) or OT-II (CD4+ T cells specific to chicken ovalbumin 323-339 I-Ab) mice ic. injected with 4×105 unmodified GL261 (n = 5 - 7/group). (D) The frequency of CD4+FoxP3+ regulatory T cells (Treg; left) and frequency of Treg bearing the Vα2 receptor isolated from brain tumors derived from unmodified GL261 cells analyzed at 3 weeks post-ic. Treg were initially gated on CD3 and CD4. Bar graphs are shown as mean ± SEM (n = 4 - 5 mice/group). (E) Survival analysis of WT or OT-II mice ic. injected with 4×105 Vc or IDOkd GL261 cells (n = 3 mice/group). For survival experiments, mice were analyzed for up to 150 days and results reflect the data from two independent experiments
To determine the nature and strength of the anti-tumor response induced by IDO-deficient glioma cells, we established IDO-competent and/or IDO-deficient tumor cells in both cerebral hemispheres of IDO−/− mouse brain to better understand IDO-dependent glioma-induced immunodominance. As shown in Figure 1B, when mice were simultaneously injected IDO-competent cells on both sides of the mouse brain, 100% of mice died with a median survival of 15.5 days post-ic. Interestingly, when mice were simultaneously injected IDO-competent and -deficient cells in opposite cerebral hemispheres, 100% of mice died with a median survival of 22 days. This was in contrast to the survival benefit imparted when IDO-deficient glioma cells were intracranially-injected into both cerebral hemispheres, resulting in 80% of mice surviving up to 150 days post-ic. (P<0.001). When taking into consideration the results from Figure 1A, these data collectively suggest that the microenvironment within IDO-competent gliomas is sufficient to induce a coordinated immunosuppressive response that overcomes the anti-tumor response elicited by completely IDO-deficient satellite tumors in the brain. We next tested if the prior establishment of IDO-deficient tumors would be sufficient for rejecting IDO-competent tumors. In mice already bearing IDO-deficient tumors, when IDO-competent glioma cells were implanted early (ie. 7 days post-ic.), 100% of mice survived, whereas a later re-challenge with IDO-competent glioma cells (ie. 32 dp-ic.) led to only 40% of mice surviving (P<0.001, respectively). These data highlight the contextual nature of the anti-tumor immune response mediated by IDO-deficient glioma cells and suggest that timing of re-challenge plays a critical role with regard to overcoming tumor-induced immunosuppression and consequent effects on survival.
Given the potent effects of glioma cell-specific IDO-deficiency on the induction of productive tumor immunity, we next wondered if this effect required antigen specificity or whether the non-specific presence of CD4+ T cells was sufficient for increasing overall survival. As expected, Figure 1C shows that neither wild-type (WT) nor OT-II (antigen restricted CD4+ T cells to chicken ovalbumin) mice mounted a long-term survival effect when intracranially-injected with normal (IDO-competent) glioma cells. Notably, both WT and OT-II mice showed a significant accumulation of Treg in the brain, although OT-II mice had relatively decreased levels (P<0.01). When OT-II mice were intracranially-injected with IDO-competent or -deficient glioma cells, neither group could mount a long-term (ie. up to 150 days post-ic.) survival response. These data suggest that antigen-specific CD4+ T cells are required for mediating the anti-tumor response in the context of IDO-deficient brain tumors.
IDO expression and combinatorial targeting with temozolomide in glioma
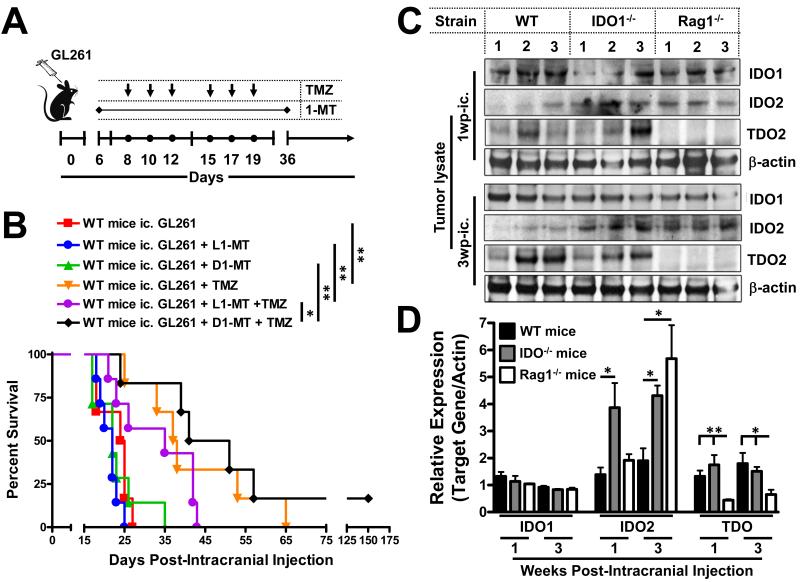
Previous work from peripheral tumor models has shown that inhibiting IDO with the well-characterized molecular agent, 1-methyltryptophan (1-MT), requires the addition of a chemotherapeutic agent to provide a significant antitumor response (10). To address this, we hypothesized that 1-MT would synergize with temozolomide (TMZ), the current standard-of-care chemotherapeutic agent for patients with glioblastoma, to achieve a synergistic antitumor-mediated survival benefit. As shown in Figure 2A,B, neither L1-MT nor D1-MT in glioma-bearing mice significantly impacted overall survival, when compared to control untreated mice with a median survival of 24.5 days post-ic. In contrast, the treatment of mice with TMZ, alone, led to an increased median survival of 37.5 days post-ic. (P<0.01). To our surprise, neither the addition of L1-MT nor D1-MT increased survival further, vs. treatment with TMZ, alone. However, there was a small but significant survival advantage when D1-MT was coupled with TMZ, compared to L1-MT with TMZ, reflected by the median overall survival of 46- vs. 35-dp-ic (P<0.05).
Figure 2.
Impact of IDO inhibition and tryptophan catabolic enzyme expression in brain tumors. (A) Timeline illustrating when oral (through the drinking water) 1-MT (2mg/mL) and i.p.-injected TMZ was administered to mice after intracranial injection (ic.) of 4×105 GL261 cells. (B) Survival analysis represents mice that received the L- or D-stereoisomer of 1-MT, TMZ, or a combination of each 1-MT stereoisomer with TMZ (n = 6/group). *P < 0.05; **P < 0.01. (C) Representative Western blots and (D) relative quantitative analysis for IDO1, IDO2, TDO and chicken β-actin in GL261 cell-based glioma lysates isolated at 1 and 3 weeks post- ic. in WT, IDO−/− and Rag1−/− mice (n = 3/group). *P < 0.05; **P < 0.01.
Given the surprisingly low level of efficacy upon treatment with 1-MT and TMZ, we hypothesized that additional tryptophan catabolic pathways are present in the context of glioma, given the recent evidence that IDO2 and TDO also play a role in tumor immunity (28, 29). We quantified the expression of IDO1, IDO2 and TDO from normal (IDO-competent) GL261 cell-based tumor lysates at 1- and 3-weeks post-ic. from WT, IDO−/− and Rag1−/− (lack functional T and B cells) mice in Figure 2 C,D. Not surprisingly, when compared to WT mice, IDO expression was unchanged in both IDO−/− and Rag1−/− mice, concordant with data showing that tumors require IDO to suppress tumor immunity, as well as inferring that the tumor is a primary source of IDO expression. Interestingly, IDO2 was expressed in WT mice bearing glioma and that expression was enhanced by peripheral IDO-deficiency (P<0.05), as well as the temporal-sensitivity to the absence of T cells (P<0.05). Equally, if not more intriguing was the expression of TDO in WT and IDO−/− mice that was potently decreased by the absence of functional T cells (P<0.05). As far as we know, this is the first reported observation that the immune system plays a role in regulating TDO expression in tumors. Collectively, these data suggest that due to the presence of IDO2 and TDO in glioma, therapeutic intervention against all three mammalian tryptophan catabolic enzymes may require simultaneous blockade to significantly impact brain tumor immunity.
A durable survival benefit after CTLA-4/PD-L1/IDO blockade in glioma
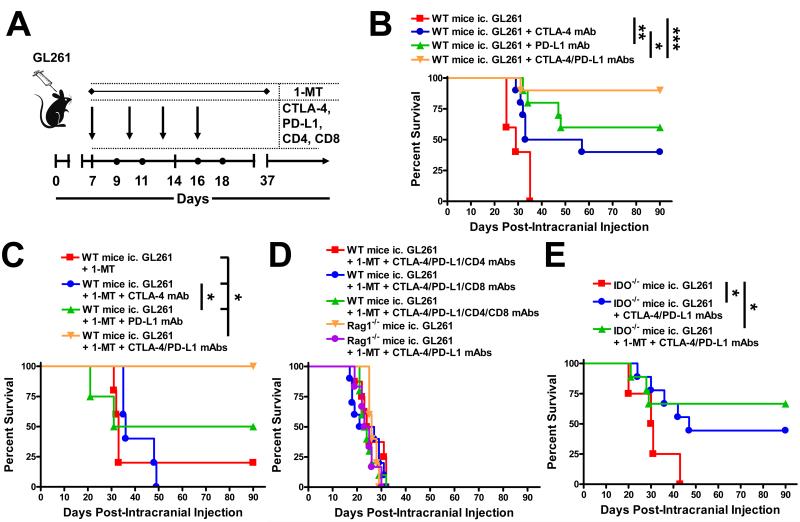
As an alternative to the combination of 1-MT with chemotherapy, we decided to pursue an approach that utilized 1-MT with CTLA-4 and PD-L1 blockade, instead, given the recent clinical success for end-stage melanoma patients administered with CTLA-4 and PD-1 mAbs (30). As shown in Figure 3A,B, 100% of untreated mice died with a median survival of 29 days post-ic. In contrast, 40%, 60% and 90% of mice treated with CTLA-4 mAb, PD-L1 mAb and co-administered CTLA-4 and PD-L1 mAbs, respectively, were still alive at 90 days post-ic., demonstrating an extraordinary survival benefit in glioma. To determine the impact of 1-MT on this approach, we analyzed mice bearing glioma and administered 1-MT, alone, or in combination with CTLA-4 mAb, PD-L1 mAb or co-administered with both CTLA-4 and PD-L1 mAbs (Figure 3C). Excitingly, 100% of mice treated with the triple therapy showed durable survival, when compared to only 20% of mice treated with 1-MT, alone (P<0.05). To test whether this triple immunotherapy required T cells to mediate the survival effect, we co-administered CTLA-4 mAb, PD-L1 mAb and 1-MT with CD4- and/or CD8-depleting mAb(s), which resulted in complete abrogation of any survival benefit (Figure 3D). Similarly, when we treated Rag1−/− mice with the triple therapy, a similar lack of survival benefit was observed, confirming that T cells are required for this approach to be effective. Finally, we tested this effect in IDO−/− mice, given the recent data showing a synergistic benefit of CTLA-4 or PD-1/PD-L1 blockade in B16-F10 cell-based peripheral tumors (31). As shown in Figure 3E, peripheral IDO deficiency negated the maximal amount of survival benefit, as seen in WT mice, with only 67% of mice that received the triple therapy surviving to 90 days post-ic. Collectively, these data demonstrate that the triple immunotherapy is highly effective at increasing survival in glioma-bearing mice and suggests a complex mechanism that requires peripheral cell expression of IDO to maximize therapeutic benefits.
Figure 3.
Early blockade of CTLA-4, PD-L1 and IDO leads to prolonged T cell-mediated survival against brain tumors. (A) Timeline demonstrating the intracranial injection of 4×105 GL261 cells followed by the i.p. injection of CTLA-4, PD-L1, CD4 and/or CD8 mAbs at days 7, 10, 13 and 16 post-ic. and the oral availability of D1-MT on days 7 through 37 post-ic. (B) Survival analysis of WT mice intracranially (ic.) with 4×105 GL261 cells and treated with CTLA-4 mAb (clone 9H10), PD-L1 mAb (clone 10F.9G2) or both CTLA-4 and PD-L1 mAbs (n = 10/group). *P < 0.05; **P < 0.01; ***P < 0.001. (C) Survival analysis of WT mice ic. injected with 4×105 GL261 cells and treated with D1-MT, D1-MT and CTLA-4 mAb, D1-MT and PDL1 mAb or D1-MT, CTLA-4 and PD-L1 mAbs (n = 5/group). *P < 0.05. (D) Survival analysis of WT (n = 10/group) or Rag1−/− (n = 5 - 6/group) mice ic. injected with 4×105 GL261 cells, with or without treatment with D1-MT, CTLA-4 and PD-L1 mAbs, with or without depletion for CD4+- and/or CD8+-T cells. (E) Survival analysis of IDO−/− mice ic. injected with 4×105 GL261 cells, alone (n = 4/group), or treated with D1-MT, CTLA-4 mAb and/or PD-L1 mAb (n = 9/group). *P < 0.05.
Triple therapy against CTLA-4, PD-L1 and IDO decreases Treg in glioma
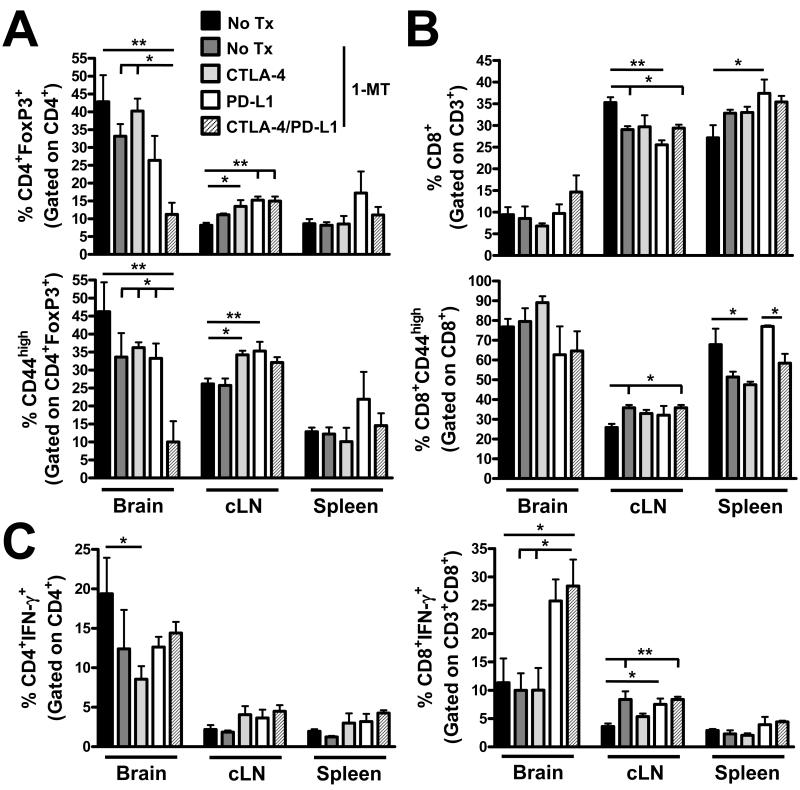
Regulatory T cells (Treg; CD4+CD25+FoxP3+) are potently immunosuppressive T cells that infiltrate human GBM (19), suppress the cytotoxic effector arm (32) and promote pathogenesis in experimental brain tumor models (18, 20, 33). Therefore, depleting them directly or utilizing immunotherapy to neutralize their presence is a major on-going goal for improving standard-of-care treatment for GBM patients. As shown in Figure 4A, brain-resident Treg levels were decreased by treatment with triple CTLA-4, PD-L1, IDO blockade, but not by 1-MT alone, nor by combinations of 1-MT with CTLA-4 or PD-L1 mAbs, vs. untreated control mice (P<0.01). Interestingly, this effect was also seen for the frequency of Treg expressing high levels of CD44 (P<0.01), a marker of antigen experience. In contrast, the triple therapy neither affected the frequency of brain-resident cytolytic CD8+ T cells (Tc), nor did it affect the level of antigen-experienced Tc (Figure 4B). Moreover, while the triple therapy did not affect the levels of IFN-γ expression in brain-infiltrating CD4+ T cells, when compared to control, it was associated with higher IFN-γ levels in Tc cells (P<0.05). Taken together, triple blockade of CTLA-4, PD-L1 and IDO in glioma-bearing mice decreases antigen-experienced Treg levels, while coincidently increasing armed cytolytic T cells.
Figure 4.
Early [1 week post-intracranial injection (wp-ic.)] therapeutic blockade against CTLA-4, PD-L1 and IDO decreases Treg levels and increases IFN-γ+ Tc in brain tumors. Wild-type (WT) mice ic. injected with 4×105 GL261 cells and left untreated or administered D1-MT, CTLA-4 mAb (clone 9H10) and/or PD-L1 mAbs (clone 10F.9G2). The frequency of (A) CD4+FoxP3+ and CD4+FoxP3+CD44+ Treg, (B) CD8+ and CD8+CD44+ Tc or (C) CD4+IFN-γ+ and CD8+IFN-γ+ effector-Tonv and -Tc, respectively, isolated from the brain, bilateral deep and superficial cervical draining lymph nodes (cLN) and the spleen of glioma-bearing mice at 3 wpic. left untreated, or administered D1-MT, CTLA-4 and/or PD-L1 (n = 5/group). All T cell populations were initially identified by the expression of CD3. *P < 0.05; **P < 0.01.
Given the dramatic effects of simultaneous CTLA-4, PD-L1 and IDO blockade on brain-resident Treg and Tc in glioma-bearing mice, we hypothesized that, in addition to the effect on overall T cell frequency, there would also be an effect on the expression of immunomodulatory targets and/or receptors. Both CTLA-4 and PD-1 have previously been shown to be potentially high value targets in experimental mouse models of malignant glioma (34, 35). To understand their expression on T cells in the context of a responsive and productive anti-tumor response, we analyzed Treg, Tconv and Tc for these molecules at 3 wp-ic. to determine whether the expression changes commensurately. As shown in Supplementary Figure 1A, while the triple immunotherapy did not change the expression of PD-1 on either Treg or Tc, PD-1 mean fluorescence intensity (MFI) on Tconv increased from 369 ± 49 in untreated glioma-bearing mice to 790 ± 181 in mice that received triple immunotherapy (P<0.01). In contrast, the triple immunotherapy decreased CTLA-4 expression on Treg from 1527 ± 176 in untreated mice to 715 ± 222 in mice receiving triple therapy, while it increased on Tconv from 434 ± 72 to 1076 ± 272 with triple therapy (P<0.001, respectively) (Supplementary Figure 1B). Collectively, these data suggest that the Tconv subset is particularly sensitive to the effects of CTLA-4/PD-L1/IDO blockade and that as Treg levels decrease due to the effects of triple immunotherapy, the CD4+ T cell expression for PD-1 and CTLA-4 changes commensurately.
CTLA-4/PD-L1/IDO blockade targets Treg and enhances survival from established glioma
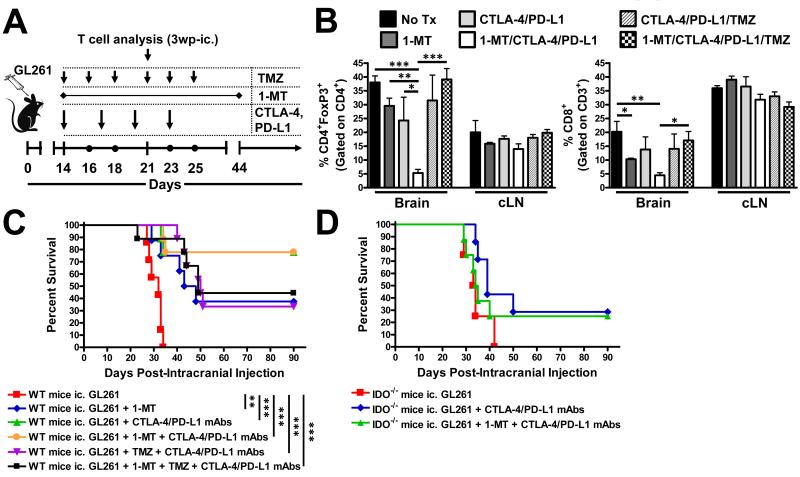
Since our previous observation in smaller, less-established brain tumors suggested the triple therapy primarily decreased Treg cells, we wondered whether this effect would be maintained in larger, more well-established glioma; a time point that co-incides with brain tumors that are ≥ 2 mm in diameter (9). As shown in Figure 5A,B, there was a dramatic decrease in Treg levels from 38 ± 2% in untreated mice to 5.3 ± 1% in mice treated with triple therapy (P<0.001), which was reversed with the addition of TMZ back to 39 ± 4% (P<0.001). Importantly, the triple therapy decreased Treg levels, when compared to dual treatment with CTLA-4/PD-L1 mAbs (P<0.05), suggesting that all three targets require therapeutic modulation to significantly affect this T cell subset in glioma. Interestingly, triple therapy also decreased brain-resident Tc levels, when compared to untreated mice, suggesting a possible overall reduction in inflammation-mediated by this approach. Importantly, this effect did not translate into differences in the level of CD44high-, CTLA-4+-, PD-1+-, Aiolos+CD39+- Treg and Tc cells, although it did have an effect on the level of BTLA expressed by tumor-infiltrating Tc (Supplementary Figure 2 A-G). As shown in Figure 5C, untreated WT mice had a median overall survival of 32 days post-ic. with 100% mortality. In contrast, 1-MT alone, increased the median survival to 45.5 days post-ic. with 38% of mice alive at 90 days post-ic. (P<0.01). Incredibly, both co-administration of CTLA-4/PD-L1 mAbs or triple therapeutic CTLA-4/PD-L1/IDO blockade resulted in durable survival for 78% of glioma-bearing mice (P<0.001). Not surprisingly, the concurrent addition of TMZ to this therapeutic regimen decreased maximal survival, suggesting that active chemotherapy in the context of productive anti-tumor immunity is an undesirable approach. Interestingly, when the dual and triple therapies were utilized in IDO−/− mice bearing glioma, maximal therapeutic efficacy was unattainable (Figure 5D), reinforcing our previous observation that peripheral IDO is required for effective immunotherapy in the context of CNS tumors. Ultimately, these data show exciting pre-clinical results demonstrating the efficacy of either dual or triple immunotherapy utilizing CTLA-4, PD-L1 and/or IDO blockade.
Figure 5.
Late [2 weeks post-intracranial injection (wp-ic.)] blockade of CTLA-4, PD-L1 and IDO blockade decreases Treg levels and increases survival against brain tumors. (A) Timeline demonstrating the intracranial injection of 4×105 GL261 cells followed by the i.p. injection of CTLA-4 and PD-L1 mAbs at days 14, 17, 20 and 23 post-ic., the oral availability of D1-MT on days 14 through 44 post-ic. and i.p. administration of TMZ on days 14, 16, 18, as well as 21, 23 and 25 post-ic. (B) The frequency of CD4+FoxP3+ Treg and CD8+ Tc isolated from the brain, bilateral deep and superficial cervical draining lymph nodes (cLN) and the spleen of tumor-bearing mice at 3 wp-ic. (n = 5/group). (C) Survival analysis of WT mice ic. injected with 4×105 GL261 cells, alone (n = 7/group), or treated with (D)1-MT, CTLA-4 mAb (clone 9H10), PD-L1 mAb (clone 10F.9G2) and/or TMZ (n = 9 - 10/group). (D) Survival analysis of IDO−/− mice ic. injected with 4×105 GL261 cells, alone (n = 4/group), or treated with D1-MT, CTLA-4 mAb (clone 9H10), PD-L1 mAb (clone 10F.9G2) and/or TMZ (n = 7 - 8/group). *P < 0.05; **P < 0.01; ***P < 0.001.
Intracranial melanoma is poorly responsive to CTLA-4/PD-L1/IDO blockade
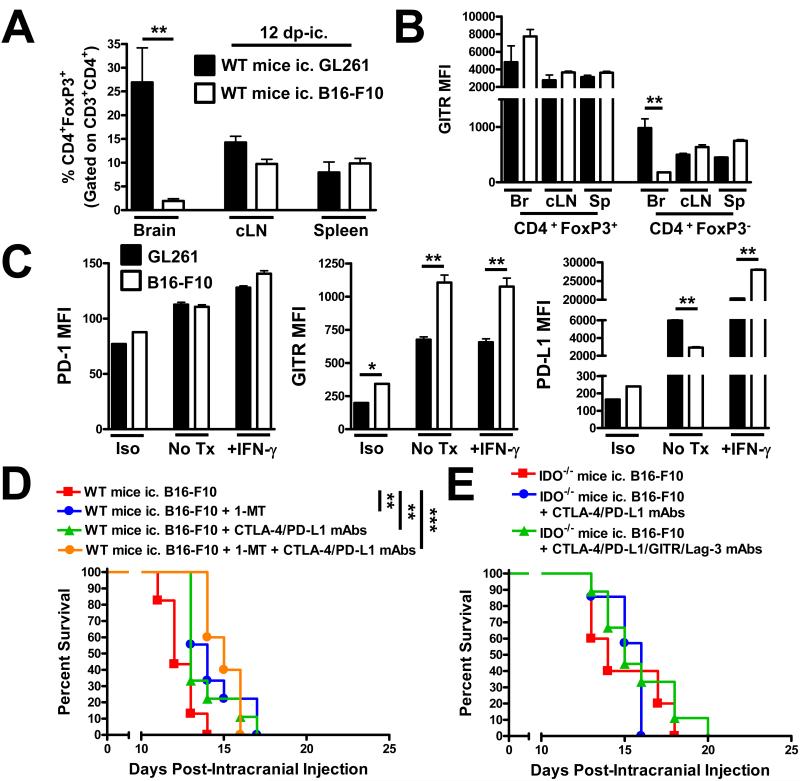
GL261 cell-based brain tumors robustly recruit Treg, which has been shown to be one of the ways that CNS malignancies subvert the anti-tumor response. However, we were curious as to whether this was a tumor intrinsic phenomenon, or whether any malignant intracranial tumor ultimately causes Treg accumulation. As shown in Figure 6A, WT mice analyzed at 12 days post-ic. Injection of GL261 tumor possess 27 ± 7% Treg in the brain, when compared to B16-F10 tumors that recruit only 2 ± 0.3% Treg (P<0.01). This is not a reflection of a difference in overall Treg phenotype, since Tregs isolated from both types of intracranial tumors exhibit the same level of GITR, a prototypical receptor that is highly expressed by Treg and known to regulate their function in tumors (Figure 6B) (36). When the GL261 and B16-F10 cell lines were analyzed, in vitro, for PD-1, GITR and PD-L1, quantifiable differences in GITR and PD-L1 were observed after stimulation with IFN-γ (Figure 6C). However, whether these differences affect Treg accumulation has yet to be established. To determine the effects of our triple immunotherapeutic approach against intracranial melanoma, we began immunotherapy at 3 days post-ic. in WT mice given the known aggressiveness of B16-F10 cells (Figure 6D). To our surprise, 1-MT alone (P<0.01), CTLA-4/PD-L1 blockade (P<0. 01), as well as combining all three reagents (P<0.001) increased overall survival. However, the overall benefit was limited to days, rather than months, as we had observed in GL261 cell-based brain tumors. Moreover, no difference in overall survival was found when a similar approach was used in IDO−/− mice (Figure 6E), nor when that approach was further combined with GITR and Lag-3 mAbs. These data suggest that the efficacy of inhibiting CTLA-4, PD-L1 and IDO in brain tumors will depend on context and based on the data we have presented here, will be more effective in tumors reliant on Treg accumulation, rather than aggressive tumors known to migrate and evade the immune response by alternative mechanisms.
Figure 6.
Intracranial melanoma is infiltrated by decreased Treg levels and is not amenable to durable survival with CTLA-4/PD-L1/IDO blockade. (A) Wild-type mice (WT) intracranially-injected (ic.) with 4×105 GL261- or 2 - 5×103 B16-F10-cells were analyzed for Treg levels in the brain, bilateral deep and superficial cervical draining lymph nodes (cLN) and the spleen of tumor-bearing mice at 12 days post-intracranial injection (dp-ic.) (n = 4 - 6/group). (B) Mean fluorescence intensity (MFI) of GITR expression on Treg and Tconv analyzed at 12 dp-ic. as described in (A). (C) MFI for surface expression of PD-1, GITR and PD-L1 on GL261 (black bars) and B16-F10 (white bars) after 24 h in culture and stained with isotype mAb (Iso), untreated (No Tx) or treated with 10ng/mL IFN-γ (+IFN-γ). (D) Wild-type mice were intracranially-injected with 2 - 5×103 B16-F10 cells followed by the i.p. injection of CTLA-4 and PD-L1 mAbs at days 3, 7, 10 and 13 (when possible) post-ic., as well as the oral availability of D1-MT on days 3 through the end of the survival experiment. (E) Wild-type mice were intracranially-injected with 2 - 5 × 103 B16-F10 cells followed by the i.p. injection of CTLA-4 and PD-L1 mAbs at days 3, 7, 10 and 13 (when possible) post-ic., as well as the oral availability of D1-MT on days 3 through the end of the survival experiment. Survival analysis of WT mice ic. injected with 4×105 GL261 cells, alone (n = 7/group), or treated with D1-MT, CTLA-4 mAb (clone 9H10), PD-L1 mAb (clone 10F.9G2) and/or TMZ (n = 9 - 10/group). Bar graphs are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Current standard-of-care treatment for patients initially diagnosed with glioblastoma multiforme (GBM), includes surgical resection, radiation and chemotherapy with Temodar (temozolomide). However, this aggressive regimen can leave undesirable side-effects, with an average overall survival advantage of only 14.6 month post-diagnosis. These grim potential outcomes have served as rationale to develop alternative approaches for treating patients with high-grade primary brain tumors of which immunotherapy is a leading candidate for producing effective, durable and long-lasting patient benefits (32, 37, 38). While these highly promising studies warrant further investigation, it is helpful to also understand the redundant and compensatory immunosuppressive pathways that undermine the efficacy of immunotherapy, as well as general anti-tumor immunity (39, 40). Among these pathways, IDO plays a central role in regulating immunosuppression, since the genetic ablation of IDO, specifically in glioma cells, leads to the spontaneous and rapid rejection of brain tumors (9). Other high value targets that have been demonstrated to regulate immunosuppression in glioma include CTLA-4 (34), PD-L1 (41), PD-1 (35). However, to the best of our knowledge, no previous study simultaneously targeted these regulatory hubs in the context of malignant glioma.
Through the mixture of IDO-competent and -deficient glioma cells, we began our investigation by asking the simple question: which proportion of IDO-deficient cells induces an anti-tumor immune response that results in a durable survival advantage. The data indicate that mice with brain tumors composed of >50% IDO-competent glioma cells mice had a shorter overall survival, when compared to those tumors with a composition of ≥50% IDO-deficient glioma cells. This observation is concordant with previous immunocytochemical analysis in human glioblastoma (GBM), demonstrating that the majority of GBM cases are >50% positive for IDO in tumor cells (7). Interestingly, when IDO-competent and -deficient brain tumors were independently established in contralateral cerebral hemispheres, the IDO-competent tumors were capable of abrogating any survival effect normally attributable to IDO-deficient tumors. This was not simply due to the burden of tumor cells intracranially-injected into the mouse brain, since when IDO-deficient glioma cells were dually-injected, the majority of mice survived and lived for up to 150 days post-ic. Rather, this may reflect the difference in the programming and/or recruitment of stromal cells that IDO-deficient glioma cells recruit to induce anti-tumor immunity. Importantly, the factors recruited by IDO-deficient glioma cells during the priming phase leading to anti-tumor immunity are sufficient to cause rejection of IDO-competent tumor cells, with a diluted level of effectiveness if challenged after priming has already occurred. Finally, given our previous data suggesting that CD4+ T cells are required to reject IDO-deficient tumors based on the lack of long-term durable survival in CD4−/− mice (9), we determined the relevance of antigen specificity in this survival mechanism. As expected, OT-II mice bearing CD4+ T cells universally antigen-restricted to chicken ovalbumin, a non-glioma expressed protein, were incapable of rejecting both IDO-competent and -deficient brain tumors. Collectively, these data suggest a critical role for IDO in suppressing the anti-tumor response that depends on the proportion of IDO-expressing cells, factors differentially-induced/recruited depending on IDO-competency, as well as the requirement of TCR specificity to tumor and/or stromal antigens.
Given the dominant role of IDO in suppressing immune-mediated glioma rejection, we next asked whether inhibiting the IDO pathway via 1-MT would be sufficient to recapitulate our observations with genetic silencing. Based on previous studies demonstrating that, 1-MT alone, does not lead to tumor rejection but that, 1-MT in combination with chemotherapy leads to productive tumor immunity (10), we chose to study the treatment of glioma with 1-MT in combination with TMZ. We found that both levorotary (L) and dextrorotary (D) stereoisomers of 1-MT were ineffective at increasing overall survival from glioma burden when administered at 2 mg/mL in the drinking water. However, when both D1-MT and L1-MT were combined at 5 mg/mL they showed a significant anti-tumor effect, possibly reflecting a higher dosing requirement due to poor blood brain barrier permeability. Interestingly, when either L1-MT or D1-MT were co-administered with TMZ under the dosing regimen used in this study, neither combinatorial regimen increased survival when compared to mice treated with TMZ alone. This suggests that, in contrast to peripheral tumor models, the synergism between IDO inhibition and glioma-induced death may differ in sensitivity towards this therapeutic regimen. Alternatively, the lack of synergistic anti-tumor effect could be due to repeated and frequent dosing with TMZ, which may abrogate the establishment of a productive immune response. Another possible explanation includes compensation due to kynurenine production by IDO2 and/or TDO. In support of this hypothesis, we found that all three mammalian tryptophan catabolic enzymes, IDO1, IDO2 and TDO, were expressed in brain tumors. Recent evidence suggests that both IDO2 and TDO contribute to the regulation of immunity (42) and/or progression of glioma (43).
Given that 1-MT and TMZ did not produce beneficial results when compared to TMZ, alone, we hypothesized that coupling IDO inhibition with the regulation of other powerful immunomodulatory mediators would produce a synergistic survival response in glioma-bearing mice. Data from peripheral tumor models previously demonstrated that combining CTLA-4 and PD-1 and/or PD-L1 inhibition is an attractive method for reducing immunosuppression and/or reactivating productive anti-tumor RESPONSE (44-46). Based on this literature, we wondered if this approach would also yield benefits in the context of aggressive neoplasms within the central nervous system (CNS), a site normally considered to be relatively immune privileged. Much to our surprise, the simultaneous therapeutic inhibition of CTLA-4 and PD-L1 at 1 week following glioma cell implantation led to a remarkably high survival rate of 90% in glioma-bearing mice. Notably, this survival was durable over a 90-day period of observation. The addition of 1-MT to CTLA-4 and PD-L1 blockade was also associated with a high survival rate of 100% in glioma-bearing mice. Notably, the survival benefit induced by triple immunotherapy was completed abrogated when the immune system was depleted for CD4+ and/or CD8+ T cells. This implies that there is a coordinated mechanism of action between both T cell subsets to carry out effective glioma immunity. However, the temporal requirement after therapeutic initiation for each T cell subtype, which cells they must interact with in the tumor microenvironment and whether those interactions mediate direct or indirect antitumor effects, have yet to be determined.
One unexpected finding from our study was that CTLA-4, PD-L1 ± IDO blockade in mice deficient for peripheral IDO (ie. non-glioma derived) showed decreased overall survival, when compared to WT mice. This is a somewhat paradoxical observation given the recent finding that host-derived IDO plays a critical role in anti-tumor immunity when coupled with either CTLA-4 or PD-1 blockade (31). However, it is important to keep in mind that what is often observed in the CNS, regardless of the presence or absence of neoplasm, does not recapitulate an identical response, peripherally. This is likely due, in part, to the presence of the blood brain barrier, lack of a developed lymphatic system, the normally low expression of MHC class II, as well as the different stromal cells within the CNS parenchyma.
We found that, regardless of early or late blockade for CTLA-4, PD-L1 and IDO, Treg levels were overall decreased. Interestingly, the late administration of immunotherapy also induced a decrease in brain tumor-infiltrating Tc. This latter observation has potentially important implications related to the overall inflammatory state IN the brain. Theoretically, any immunotherapy will generate some degree of inflammation associated with the production of pro-inflammatory cytokines and cell death associated with tumor rejection. Thus, a therapy that is effective for killing tumor cells, but recruits fewer leukocytes to the CNS, is highly desirable. This is particularly notable since the common way to decrease inflammation in the CNS for GBM patients is by administering Decadron, a glucocorticoid that will likely marginalize an active T cell-mediated response. Thus, while overall survival is similar between dual and triple therapeutic approaches, the additional advantage of decreased inflammatory cell infiltration warrants further investigation.
Our investigation found that CTLA-4/PD-L1 mAb ± 1-MT treatment in the context of intracranially-injected GL261 (glioma) cell-based brain tumors resulted in a highly effective and durable survival advantage, while the intracranially-injected B16-F10 (melanoma) tumor model, showed a dramatically reduced level of efficacy. Other notable differences included a significantly decreased level of Treg infiltration in B16-F10 cell-based tumors with higher levels of IFN-γ-inducible GITR and B7-H1 expression. Interestingly, there was no difference in survival when B16-F10 cells were intracranially-injected in IDO−/− mice, again highlighting the contextual difference between eradicating peripheral tumors as previously shown (31) with the data we have included here. Given the capability that both GL261 and B16-F10 cells express IDO and consequently modulate anti-tumor immunity (9, 47), it will be interesting to determine whether both types of tumors express similar levels of IDO, IDO2 and TDO.
In summary, we have extended our previous observations delineating the impact of IDO in brain tumors, demonstrated that all three mammalian tryptophan catabolic enzymes are present and have found a potent method to induce the rejection of primary tumors by virtue of CTLA-4 and PD-L1 blockade. By coupling this therapeutic regimen with 1-MT, we were able to reduce Treg and Tc levels in established brain tumors with similar levels of overall survival and durable efficacy. Since clinical-grade analogs are available for all three agents that we tested, this strategy has high therapeutic value for patients with GBM. Whether this approach will be equally effective for those patients that are initially diagnosed versus those who present with recurrent tumors has yet to be explored and is difficult to model experimentally. Also, our data suggests that concomitant administration of CTLA-4/PD-L1/IDO blockade and TMZ is not advantageous. Accordingly, we plan to determine whether staggering the treatments avoids therapeutic abrogation in the future. Ultimately, this work serves a proof-of-concept that this type of approach works and is relevant for treating patients with incurable malignant glioma.
Supplementary Material
Translational Relevance.
The use of agents that reverse immunosuppression in tumors is an approach that is gaining clinical traction. Excitingly, Wolchok et al. (2013) recently demonstrated that the combination of immune checkpoint blockade inhibitors (via CTLA-4 and PD-1 mAbs) resulted in an objective response in end-stage melanoma patients. However, whether a similar type of approach will be effective in patients with intracranial tumors has remained an elusive question. Here, we first extended our previous findings by investigating the role(s) of IDO1 in brain tumors, followed by the presentation of pre-clinical findings demonstrating a highly effective therapeutic strategy that simultaneously targets immune checkpoints and tryptophan catabolism in brain tumors. Given the current interest for exploring clinical trials utilizing CTLA-4, PD-(L)1 and/or IDO blockade in brain tumor patients, these results serve as an important proof-of-concept supporting the future pursuit of this strategy in patients with incurable glioblastoma.
Acknowledgements
We thank Dr. Mario R. Mautino, PhD, for making significant intellectual contributions towards this project and manuscript.
Funding: This work was supported by an American Brain Tumor Association Discovery Grant (D.A.W.), as well as NIH grants NIH F32 NS073366 (D.A.W.), NIH K99 NS082381 (D.A.W.), R01 CA138587 (M.S.L.), NIH R01 CA122930 (M.S.L.), NIH U01 NS069997 (M.S.L.).
Footnotes
Conflict of Interest Disclosure: The authors declare that no competing interests exist.
References
- 1.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-oncology. 2010;12:520–7. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CBTRUS . CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. Central Brain Tumor Registry of the United States; Hinsdale, IL: 2011. [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clinical cancer research. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. Journal of Immunology. 2009;182:3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Medicine. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72:1031–8. doi: 10.1227/NEU.0b013e31828cf945. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl tryptophan correlates with antitumor responses. Cancer research. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of Clinical Investigation. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. Journal of Molecular Medicine. 2008;86:145–60. doi: 10.1007/s00109-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-Oncology. 2010;12:351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-Oncology. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Wu A, Fan Y, Wang Y. Increased transforming growth factor-beta2 in epidermal growth factor receptor variant III-positive glioblastoma. Journal of Clinical Neuroscience. 2011;18:821–6. doi: 10.1016/j.jocn.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146:317–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Nitta T, Hishii M, Sato K, Okumura K. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Research. 1994;649:122–8. doi: 10.1016/0006-8993(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 18.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. Journal of Neurosurgery. 2006;105:430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 19.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro-Oncology. 2011;13:1308–23. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of Clinical Investigation. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–63. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prendergast GC, Metz R, Muller AJ. IDO recruits Tregs in melanoma. Cell Cycle. 2009;8:1818–9. doi: 10.4161/cc.8.12.8887. [DOI] [PubMed] [Google Scholar]
- 24.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. Journal of Immunology. 2009;183:2475–83. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. Journal of Immunology. 2008;181:5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proceedings of the National Academy of Sciences. 2010;107:2159–64. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–11. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microscopy Research and Technique. 2001;52:401–10. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. Journal of the American College of Surgeons. 2009;208:781–7. doi: 10.1016/j.jamcollsurg.2008.12.018. discussion 7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of Experimental Medicine. 2013;210:1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS One. 2012;7:e31046. doi: 10.1371/journal.pone.0031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clinical Cancer Research. 2006;12:4294–305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 34.Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clinical cancer research. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 35.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International Journal of Radiation Oncology*Biology*Physics. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-Oncology. 2014;16:274–9. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunology, Immunotherapy. 2012;61:2033–44. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert opinion on emerging drugs. 2012;17:181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, Wen PY, et al. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Review of Vaccines. 2013;12:597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature Medicine. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 42.Metz R, Smith C, Duhadaway JB, Chandler P, Baban B, Merlo LM, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. International Immunology. 2014 doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 44.Binder DC, Schreiber H. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors--Letter. Cancer Research. 2014;74:632. doi: 10.1158/0008-5472.CAN-13-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Research. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng X, Koropatnick J, Li M, Zhang X, Ling F, Ren X, et al. Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. Journal of Immunology. 2006;177:5639–46. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.