Abstract
Background
Osteochondral allografting is an option for successful treatment of large articular cartilage defects. Use of osteochondral allografting is limited by graft availability, often because of loss of chondrocyte viability during storage.
Questions/purposes
The purpose of this study was to compare osteochondral allografts implanted in canine knees after 28 days or 60 days of storage for (1) initial (1 week) safety and feasibility; (2) integrity and positioning with time (12 weeks and 6 months); and (3) gross, cell viability, histologic, biochemical, and biomechanical characteristics at an endpoint of 6 months.
Methods
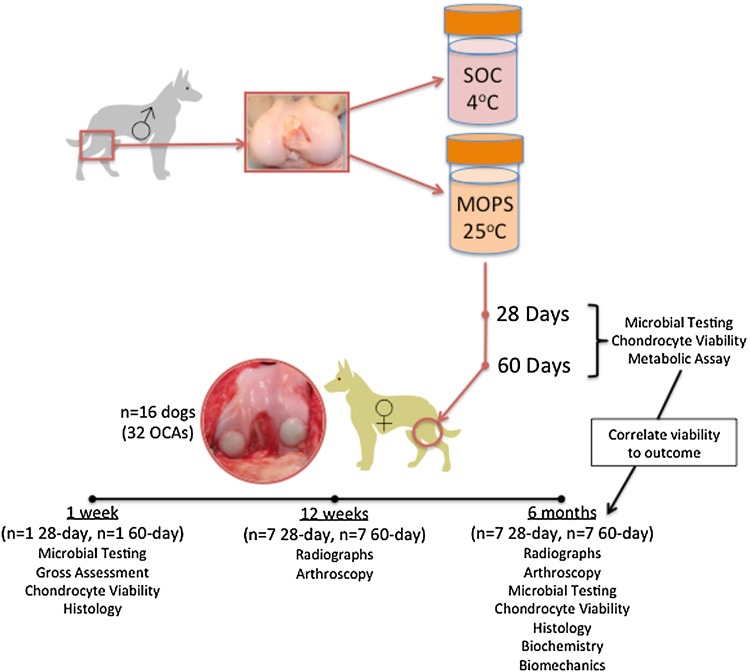
With Institutional Animal Care and Use Committee approval, adult dogs (n = 16) were implanted with 8-mm cylindrical osteochondral allografts in the lateral and medial femoral condyles of one knee. Osteochondral allografts preserved for 28 or 60 days using either the current tissue bank standard-of-care (SOC) or a novel system (The Missouri Osteochondral Allograft Preservation System, or MOPS) were used, creating four treatment groups: SOC 28-day, MOPS 28-day, SOC 60-day, and MOPS 60-day. Bacteriologic analysis of tissue culture and media were performed. Dogs were assessed by radiographs and arthroscopy at interim times and by gross, cell viability, histology, biochemistry, and biomechanical testing at the 6-month endpoint.
Results
With the numbers available, there was no difference in infection frequency during storage (5% for SOC and 3% for MOPS; p = 0.5). No infected graft was implanted and no infections occurred in vivo. MOPS grafts had greater chondrocyte viability at Day 60 (90% versus 53%; p = 0.002). For 60-day storage, MOPS grafts were as good as or better than SOC grafts with respect to all outcome measures assessed 6 months after implantation.
Conclusions
Donor chondrocyte viability is important for osteochondral allograft success. MOPS allows preservation of chondrocyte viability for up to 60 days at sufficient levels to result in successful outcomes in a canine model of large femoral condylar articular defects.
Clinical Relevance
These findings provide a promising development in osteochondral allograft technology that can benefit the quantity of grafts available for use and the quality of grafts being implanted.
Introduction
Articular cartilage defects resulting from traumatic injuries, osteochondrosis, or arthritis are commonly encountered in patients. Osteochondral allografting is a unique treatment option; it is a biologic technique that can functionally restore even very large articular cartilage defects with viable hyaline cartilage and subchondral bone. This method has been in use clinically for more than 30 years, primarily in the knee [21]. Numerous studies have reported that osteochondral allografts are associated with 10-year survivorship between 71% and 85% and up to 74% at 15 years [9, 13, 14, 16, 17, 19, 20, 31, 37]. Overall, outcomes after osteochondral allograft treatment have been good to excellent, even in the athletic population in which 88% of patients returned to sport, including 79% returning to their preinjury level of sport [24, 31].
Although osteochondral allografting has proven clinical safety and efficacy, its use is limited by availability and logistical issues involving graft procurement, disease testing, and storage before implantation. These issues depend on the relatively short time for which sufficient chondrocyte viability in the grafts can be maintained using current tissue storage protocols. Chondrocyte viability has been reported to be critically important for maintaining the biochemical and biomechanical properties of osteochondral allografts, which correlate directly to the clinical success of the surgery [1, 19, 26, 39]. Several storage methods have been investigated to try to optimize chondrocyte viability with each showing noticeable declines in chondrocyte viability after Day 14, decreasing below acceptable levels (typically considered to be 70% viable cells) by 28 days after procurement [2, 5–8, 25, 28, 29, 33, 34, 36, 39]. Mandatory disease testing procedures require 14 days before tissues can be released from the tissue bank to the surgeon for implantation. As such, a narrow window of time (eg, 14 days) for size matching, scheduling surgery, and transporting tissues exists to allow for optimal use of donor tissues. We wished to find a way to preserve osteochondral allograft tissue in a manner that maintains chondrocyte viability at acceptable levels for a longer time than current tissue bank protocols permit. To this end, we developed The Missouri Osteochondral Allograft Preservation System (MOPS), a serum-free tissue preservation method [11] that has prolonged the time for maintenance of acceptable levels of chondrocyte viability in osteochondral tissues to more than twice as long as the current standard-of-care based on in vitro assessments [15, 32].
The purpose of this study was to validate MOPS in vivo with respect to functional outcomes of osteochondral allografts preserved using the MOPS compared with osteochondral allografts preserved using the current standard-of-care method for tissue banks. Specifically, we compared allografts implanted in canine knees after 28 days or 60 days of storage in terms of (1) initial (1 week) safety and feasibility; (2) integrity and positioning with time (12 weeks and 6 months); and (3) gross, cell viability, histologic, biochemical, and biomechanical characteristics at an endpoint of 6 months.
Materials and Methods
With approval from our institution’s Animal Care and Use Committee, the stifles (knees) of purpose-bred adult (2 to 6 years old) male mongrel dogs were aseptically harvested after humane euthanasia was performed for reasons unrelated to this study. The knees were randomly assigned to one of four groups: (1) Standard-of-care (SOC) 28, 28-day storage at 4° C in standard tissue bank media (n = 12); MOPS [35] 28, 28-day storage at room temperature (25° C) in MOPS media (n = 12); SOC 60, 60-day storage at 4° C in standard tissue bank media (n = 12); and MOPS 60, 60-day storage at room temperature in MOPS media (n = 12).
All soft tissues were removed in the operating room and the knees were fully inspected to ensure no disease was present. For samples assigned to the SOC groups, the distal femurs were placed in standard storage media and stored in a dedicated refrigerator at 4° C for 28 days or 60 days. For samples assigned to the MOPS group, the distal femurs were placed in defined media (custom closed containers) and stored in a dedicated clean room at room temperature (25° C) for 28 or 60 days. Samples of media were collected weekly for both groups for microbial testing and metabolic assay for MOPS osteochondral allografts.
For analysis of osteochondral allograft tissue metabolism, a subset of samples was tested using the resazurin (Sigma Aldrich, St Louis, MO, USA) assay. For the assay, 6 mL of resazurin (100 μg/mL) was added to each sample and incubated overnight at room temperature. After incubation, a 200-µL sample of the media was placed on a black 96-well plate, and the level of fluorescence (metabolic activity count) in the sample was read using a spectrophotometer (Synergy™ HT; BioTek, Winooski, VT, USA).
Surgery and Aftercare
Sixteen adult mongrel female dogs (2 to 5 years old, 25 to 35 kg body weight, obtained from Marshall Farms BioResources, North Rose, NY, USA; USDA #21-A-008), judged free of osteoarthritis in all joints based on physical and orthopaedic examination by two veterinary orthopaedic surgeons (JLC, SPF) and radiographs of the hips, elbows, and knees, were enrolled in the study (Fig. 1). At 28 or 60 days after graft procurement, the dogs were premedicated with xylazine, morphine, and atropine; anesthetized with propofol; and maintained on isoflurane in oxygen inhaled through endotracheal intubation. The dogs were prepared for aseptic surgery of the right knee. A lateral parapatellar approach with arthrotomy was performed in the right knee, and 8-mm cylindrical focal defects were created in the weightbearing areas of the lateral and medial femoral condyles using commercially available allograft instrumentation. These defects were filled with site-matched 8-mm diameter x 8-mm depth osteochondral allografts obtained from the adult male cadaveric distal femurs preserved by either SOC or MOPS and implanted using the calibrated press-fit method of the allograft system (Fig. 2). At each time (28 days or 60 days after graft procurement), each knee received one SOC graft and one MOPS osteochondral allograft, which were alternated between medial and lateral femoral condyles. At the time of implantation, samples of the bone and cartilage from each distal femur and from the preservation media were obtained and processed for microbial testing.
Fig. 1.
This study design flow chart shows the process used to test and analyze the SOC and MOPS groups. SOC = standard-of-care; MOPS = The Missouri Osteochondral Allograft Preservation System; OCAs = osteochondral allografts.
Fig. 2.
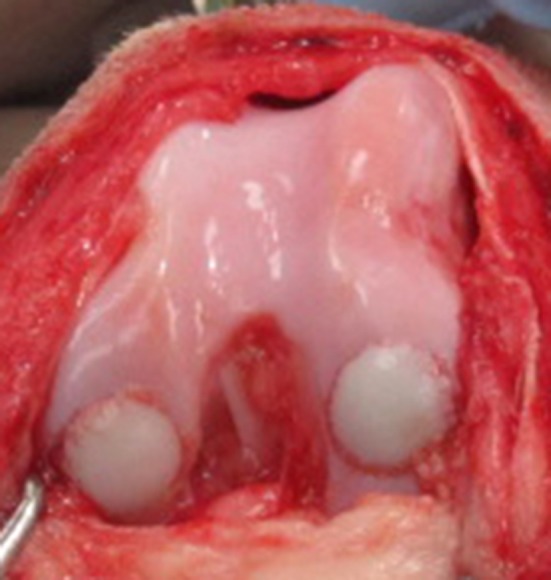
This intraoperative image shows 8-mm osteochondral allografts preserved by either SOC (left) or MOPS (right) and implanted in the medial and lateral femoral condyles of dogs using the press-fit method of a commercially available allograft system.
Soft-padded, full-limb bandages were placed on the surgically treated limb of each dog. The dogs were recovered and analgesics (morphine followed by oral analgesics) were provided postoperatively for 48 hours and then as needed based on physical parameters indicating the presence of pain. The bandages were maintained for 1 week to limit flexion and extension of the stifles during the initial healing period. The dogs were restricted to their kennels with monitored daily out-of-kennel exercise in a confined area for the entire study period.
Initial Feasibility and Safety Testing
One dog for each implantation time (n = 2 dogs; 28-day osteochondral allograft storage and 60-day osteochondral allograft storage) was humanely euthanized 1 week after surgery by an intravenous overdose of pentabarbitol/phenytoin. Synovial fluid from both knees was obtained and processed for microbial testing. The knee of each dog was examined for gross appearance of the grafts and all other tissues. Sections from each graft then were obtained and immediately prepared for determination of cell viability. Chondrocyte viability in constructs was assessed using two stains to detect live and dead cells (Molecular Probes®; Life Technologies, Grand Island, NY, USA) as per the manufacturer’s suggested protocol, where live cells are stained green with calcein-AM and dead cells stained blue with SYTOX® blue (Life Technologies). Percent chondrocyte viability for each osteochondral allograft was quantified using digital image analysis.
Each osteochondral allograft was placed in 10% neutral-buffered formalin fixative. After fixation, tissues were decalcified using 10% EDTA in phosphate-buffered saline until the endpoint of decalcification was reached as indicated by the ammonium oxalate test (ie, absence of detectable calcium in the decalcifying fluid). After decalcification, tissues were dehydrated, paraffin-embedded, and cut on a microtome in 5-µm sections for histopathologic examination of each graft. Specimens were deparaffinized, rehydrated, and stained with hematoxylin and eosin to determine cell distribution and morphologic features of the tissue, toluidine blue to assess proteoglycan distribution, and picrosirius red to determine collagen integrity. Osteochondral sections were evaluated by two board-certified veterinary pathologists (KK, CCB) who were blinded to dog number, group, and clinical findings and scored based on criteria described in the Osteoarthritis Research Society International (OARSI) histologic assessment system for dogs [11].
For microbial testing, synovial fluid was submitted in trypticase soy broth and tissue was submitted in thioglycollate enrichment broth in triplicate such that two incubation temperatures and aerobic and anaerobic conditions could be evaluated. For each sample, aerobic testing was performed at room temperature and 35° C, and anaerobic testing was performed at 35° C. All cultures were maintained for 14 days. Samples with evidence of growth were plated for bacterial, yeast, fungal, and spore-forming bacterial identification.
All dogs were implanted successfully and recovered without complications. Immediate postoperative radiographs showed appropriate implantation of all grafts with no evidence of technical errors.
Radiographic Assessments
Craniocaudal (anteroposterior), and mediolateral digital radiographic views of surgically treated knees were obtained in a standardized fashion the day before surgery, immediately postoperatively, and at 12 weeks and 6 months after surgery. The radiographs were evaluated by one board-certified veterinary radiologist (CRC) blinded to dog number, group, and clinical signs using an established subjective system [30]. Subjective findings related to allograft integrity and positioning also were described.
Arthroscopic Assessments
At 12 weeks and 6 months after surgery, arthroscopic evaluation of surgically treated knees was performed using cranio(antero)lateral and cranio(antero)medial portals. All articular surfaces of the patella, femur, and tibia were examined and subjectively assessed by one board-certified veterinary orthopaedic surgeon (JLC) with respect to graft appearance and any associated articular cartilage damage. Synovitis and meniscal disorders also were subjectively assessed.
Gross Examination
Remaining dogs (n = 14) were humanely euthanized 6 months after surgery by intravenous overdose of pentabarbitol/phenytoin. Both knees of each dog were carefully disarticulated. The articular surfaces of the patella, femur, and tibia were photographed and subjectively assessed. Each graft site was harvested individually with its surrounding cartilage and bone and divided in half to allow for cell viability and histologic assessments (½) and biochemical and biomechanical assessments (½) to be performed on every sample. Matched sections from the contralateral untreated limb were harvested and assessed in the same fashion to serve as normal (native tissue) controls.
Cell Viability
Sections from each sample were prepared for determination of cell viability. Cell viability in grafts and surrounding cartilage was assessed using the LIVE/DEAD® Assay Kit (Molecular Probes®; Life Technologies) as described previously.
Histologic Assessment
For histology, each osteochondral allograft was placed in 10% neutral-buffered formalin fixative until processed for histologic examination. After fixation, tissues were processed as previously described. Osteochondral sections were evaluated by two board-certified veterinary pathologists (KK, CCB) who were blinded to dog number, group, and clinical findings and scored for structural disorders, chondrocyte disorders, proteoglycan loss, collagen integrity, tidemark integrity, and subchondral bone plate changes based on criteria described in the OARSI histologic assessment system for dogs [12].
Biochemical Assessment
The biochemical content from articular cartilage sections of each osteochondral allograft was assessed by first measuring the sample’s wet weight, lyophilizing overnight, and then measuring dry weight. Gross water content was determined from the difference. Once dry, the samples were digested with papain overnight at 60° C. Aliquots of the digest were analyzed for glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue dye-binding assay. An additional aliquot was assessed for collagen content using a colorimetric assay to detect ortho-hydroxyproline (OHP) content. OHP content was converted to total collagen content using the conversion of 1:10 ratio of OHP:collagen. Each biochemical constituent (GAG and collagen) was normalized to the tissue dry weight.
Biomechanical Assessment
The material properties of the cartilage in the graft in the femoral condyles were determined and compared with cartilage in the site-matched region of the contralateral femoral condyles using previously reported methods [15]. In brief, biomechanical testing (3.9-mm diameter indenter and 4.0-mm diameter sample well) was performed using a computer-controlled load frame (Instron® 8821s; Instron, Norwood, MA, USA) and optical position tracking (Certus®; Northern Digital Incorporated, Waterloo, Ontario, Canada). After equilibration under a tare load (5 N), the tissue was deformed to 10% tissue strain at a ramp rate of 1 μm/second followed by dynamic loading with a sinusoidal strain (± 5%) at 1 Hz. Strain measurements were performed using optical position tracking (Certus®). These measurements yielded an instantaneous tissue modulus (EY) at the end of the stress-relaxation test and the dynamic modulus at 1 Hz (G) from the sinusoidal test.
Definition of a Successful Outcome
For the dogs in this study, a successful outcome was defined as an osteochondral allograft that was associated with graft integration, maintenance of hyaline cartilage, lack of associated cartilage disorder, and lack of fibrillation, fissuring, or fibrous tissue infiltration of the allograft based on subjective radiographic, arthroscopic, gross, and histologic assessments at 6 months after implantation.
Statistical Analyses
All statistical analyses were performed using a computer software program (Sigma Plot®, San Rafael, CA, USA). Data from each group at each time were combined and means ± SDs were determined. Pearson product moment test was used to assess strength and significance of correlations. A t-test was performed to determine differences between groups with respect to chondrocyte viability. A one-way ANOVA was performed to determine differences among groups with respect to each outcome measure of continuous data (chondrocyte viability, metabolic activity counts, GAG, collagen, biomechanical assessments) at each time. Rank sum test was performed to determine differences among groups with respect to each outcome measure of categorical data (histologic scores) at each time. Significance was set at p less than 0.05. Odds ratios were calculated to determine effect size for proportions of grafts from each group with less than 70% chondrocyte viability.
Results
Comparison of Osteochondral Allografts After 28 Days or 60 Days
The preimplantation bacterial infection rate was 5% for SOC and 3% for MOPS tissues. No infected graft was implanted and no infections occurred in vivo.
Day 28 percent chondrocyte viability ranged from 22.9% to 99% with a mean ± SD of 60.2 ± 15.6% for SOC and 82.9 ± 7.4% for MOPS (p = 0.26). Based on calculation of odds ratio, SOC-28 grafts were six times more likely than MOPS-28 grafts to have percent chondrocyte viability less than 70%. Three SOC-28 grafts and 1 MOPS-28 graft with less than 70% chondrocyte viability were implanted in long-term (6-month) recipient dogs’ knees.
Day 60 percent chondrocyte viability ranged from 24.7% to 99% with a mean ± SD of 52.7 ± 17.9% for SOC and a mean of 89.8 ± 9.1% for MOPS. Day 60 percent chondrocyte viability was greater for MOPS than for SOC grafts (p = 0.002). Eleven SOC-60 (92%) and two MOPS-60 (17%) grafts had a chondrocyte viability less than 70%. Based on calculation of odds ratio, SOC-60 grafts were 55 times more likely than MOPS-60 grafts to have percent chondrocyte viability less than 70%. Six SOC-60 grafts and one MOPS-60 graft with less than 70% chondrocyte viability were implanted in long-term (6-month) recipient dogs’ knees.
Metabolic activity counts had a strong (r = 0.95), significant (p < 0.00001), positive correlation with percent chondrocyte viability. Activity counts greater than 1000 indicated grafts maintained chondrocyte viability of 70% or greater, whereas counts less than 1000 indicated grafts maintained chondrocyte viability less than 70%.
Gross, histologic, and cell viability assessments of the two dogs assessed 1 week after implantation showed appropriate maintenance of graft integrity for all groups.
Assessment of Allograft Integrity and Positioning at 12 Weeks and 6 Months
Radiographic assessments performed at 12 weeks and 6 months after implantation showed evidence for progressive osseous integration of SOC and MOPS grafts with variable degrees of associated sclerosis. There was no radiographic evidence of failure of osseous integration for any graft. All knees showed radiographic evidence of effusion or synovial proliferation, ranging from mild to moderate. No subjective differences were noted between groups with respect to radiographic assessments (Fig. 3).
Fig. 3A–D.
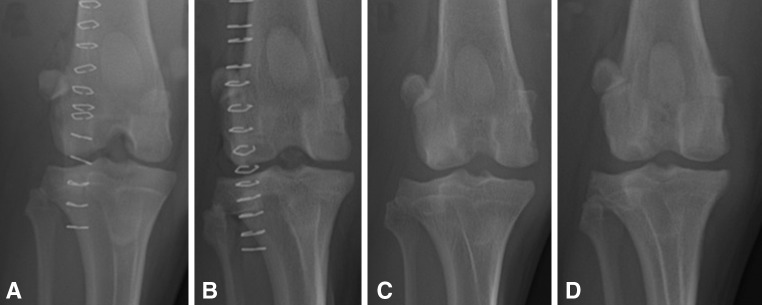
The radiographic appearances of the knees of (A) Dog 1 and (B) Dog 5 immediately after implantation of osteochondral allografts are shown. These radiographs show the appearances of the knees of (C) Dog 1 and (D) Dog 5 6 months after implantation of osteochondral allografts into the medial and lateral femoral condyles. For both of these dogs, MOPS-60 grafts were placed in the medial femoral condyles and SOC-60 grafts were placed in the lateral femoral condyles. Osseous integration of SOC and MOPS grafts is apparent based on the loss of radiolucency at the graft margins. There was no radiographic evidence for failure of osseous integration for any graft.
For 28-day preservation, MOPS and SOC grafts were similar in appearance based on arthroscopic assessments performed 12 weeks and 6 months after implantation. For 60-day preservation, MOPS grafts were subjectively superior to SOC grafts with respect to arthroscopic appearance when assessed 12 weeks and 6 months after implantation.
Assessment of Gross, Cell Viability, Histologic, Biochemical, and Biomechanical Characteristics at 6 Months
For 28-day preservation, MOPS and SOC grafts were similar in appearance based on gross assessments performed 6 months after implantation. For 60-day preservation, MOPS grafts were subjectively superior to SOC grafts with respect to gross appearance when assessed 6 months after implantation. For both preservation periods, chondrocyte viability at the time of implantation appeared to correspond well to subjective arthroscopic, gross, and histologic assessments (Fig. 4) in that grafts with viability less than 70% (1000 activity count) at the time of implantation were all considered to have failed (Table 1).
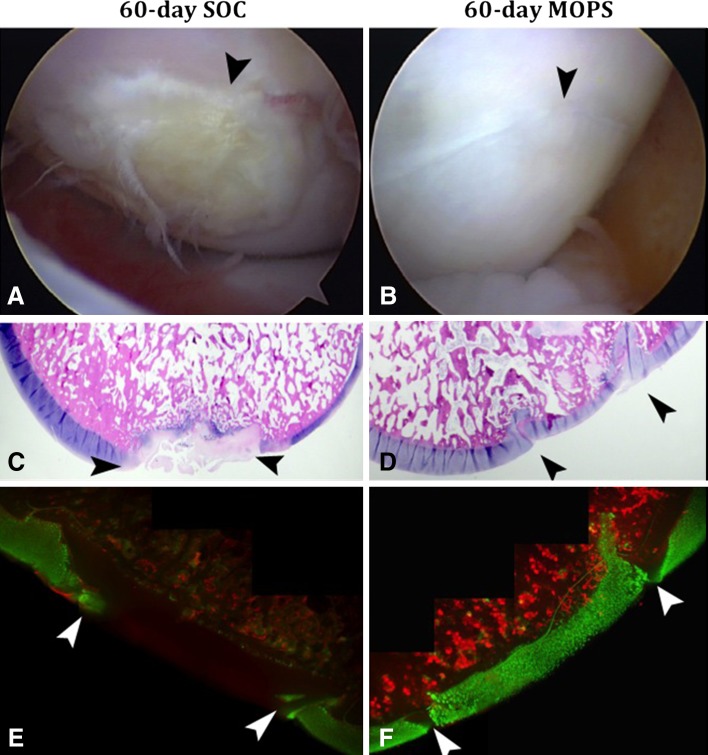
Fig. 4A–F.
Corresponding (A) SOC-60 and (B) MOP-60 arthroscopic, (C) SOC-60 and (D) MOP-60 histologic (Stain, toluidine blue; original magnification, ×2); and (E) SOC-60 and (F) MOP-60 cell viability (Stain, calcein-AM/ SYTOX® blue; original magnification, ×4) images show the appearance of osteochondral allografts stored for 60 days before implantation in the femoral condyles of dogs and assessed 6 months after surgery. The arrowheads designate the graft-host junction in each image. Osteochondral allografts stored using MOPS for 60 days before implantation were consistently better than those stored using the current SOC for tissue banks based on the subjective arthroscopic appearance of the articular cartilage surface, histologic scoring, and chondrocyte viability. SOC = standard-of-care; MOPS = The Missouri Osteochondral Allograft Preservation System.
Table 1.
Summary of chondrocyte viability, histologic scores, and outcomes
| Osteochondral allograft preservation method | Mean ± SD percent chondrocyte viability | Less than 70% chondrocyte viability | Greater than 70% chondrocyte viability implanted | Histologic score (mean ± SD) | Successful outcome (number [%]) |
|---|---|---|---|---|---|
| SOC-28 | 60.2 ± 15.6 | 33% | 4 | 13.7 ± 2.7 | 4 (57) |
| MOPS-28 | 82.9 ± 7.4 | 8% | 6 | 8.3 ± 3.8 | 6 (86) |
| SOC-60 | 52.7 ± 17.9 | 92% | 1 | 17.4 ± 2.8 | 0 (0) |
| MOPS-60 | 89.8 ± 7.4 | 17% | 6 | 10.1 ± 2.9 | 6 (86) |
Grafts with less than 70% chondrocyte viability = percentage of total donor grafts (n = 12 per group) that had less than 70% chondrocyte viability after storage; grafts with greater than 70% chondrocyte viability implanted = number of grafts with greater than 70% chondrocyte viability that were implanted in the long-term recipient dogs (n = 14); Successful outcome = number (%) of grafts in each group that were associated with graft integration, maintenance of hyaline cartilage, lack of associated cartilage disease, and lack of fibrillation, fissuring, or fibrous tissue infiltration of the allograft based on subjective radiographic, arthroscopic, gross, and histologic assessments 6 months after implantation; SOC-28 = standard-of-care 28 days; MOPS-28 = Missouri Osteochondral Allograft Preservation System 28 days; SOC-60 = standard of care 60 days; MOPS-60 = Missouri Osteochondral Allograft Preservation System 60 days. P value for 28-day mean percent chondrocyte viability between SOC and MOPS = 0.26; Day 60 chondrocyte viability between SOC and MOPS = 0.002. For 28-day and 60-day storage, OARSI histologic scores for MOPS grafts were numerically lower (less disease) compared with SOC grafts for both storage durations, however, these differences were not statistically significant (p > 0.05).
Histologic Assessment at 6 Months
For 28-day and 60-day storage, OARSI histologic scores for MOPS grafts were numerically lower (less disease) compared with SOC grafts for both storage durations, however, these differences were not statistically significant. Significantly (p < 0.05) higher (more severe disorders) OARSI scores were noted for grafts with less than 70% chondrocyte viability at the time of implantation.
Subjectively, morphologic features of hyaline cartilage and proteoglycan staining were better preserved in MOPS grafts than SOC grafts for 28-day and 60-day osteochondral allografts. Chondrocyte loss, clustering, and phenotypic changes were observed more frequently in SOC grafts than MOPS grafts, with the most severe changes noted in SOC-60 osteochondral allografts. Similarly, fibrillation, fissuring, and fibrous tissue infiltration were observed in three SOC-28 and seven SOC-60 grafts with only one MOPS-28 and one MOPS-60 osteochondral allograft showing these structural changes.
Biochemical Assessments
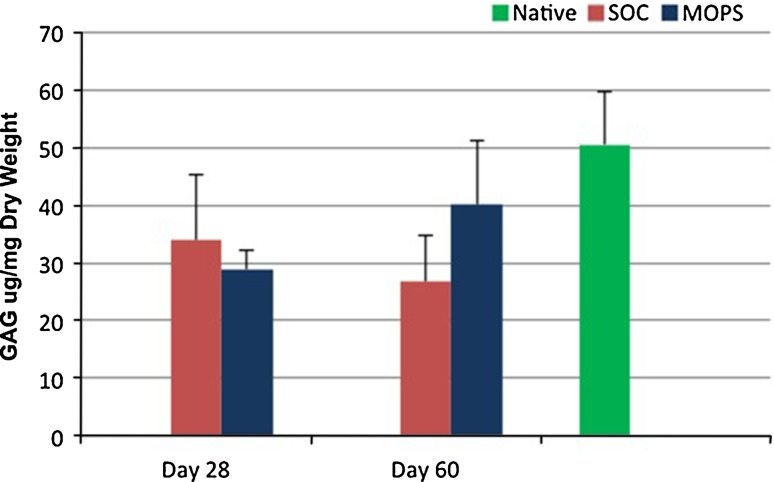
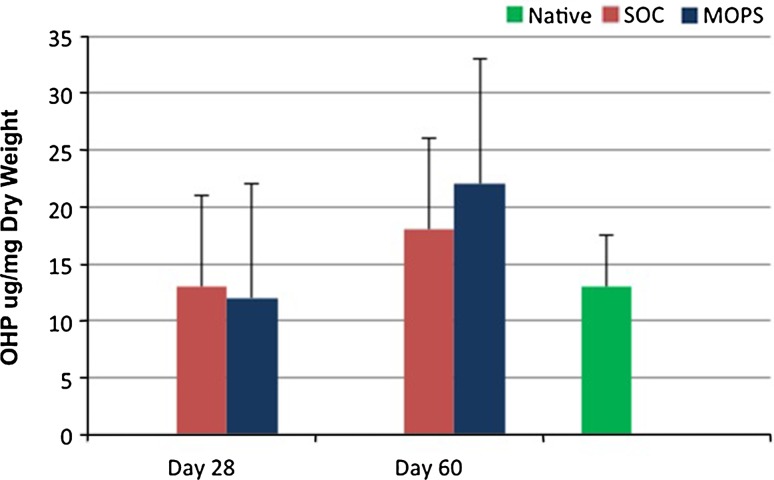
GAG content was greater (p < 0.009) in native articular cartilage compared with cartilage from SOC and MOPS 28-day grafts and SOC 60-day grafts when assessed 6 months after implantation. GAG content was not different in native articular cartilage compared with cartilage from MOPS 60-day grafts at 6 months (p = 0.34; Fig. 5). There were no differences in collagen content in native cartilage compared with cartilage from SOC and MOPS 28-day or 60-day grafts at 6 months (p = 0.28 and 0.21, respectively; Fig. 6).
Fig. 5.
Mean ± SD values for GAG content of articular cartilage from osteochondral allografts (MOPS [n = 7] and SOC [n = 7]) stored for either 28 or 60 days before implantation and assessed 6 months after implantation were compared with values for site-matched normal articular cartilage from the contralateral limbs (n = 14; Native). GAG content was significantly (p < 0.009) greater in native articular cartilage compared with cartilage from SOC and MOPS 28-day grafts and SOC 60-day grafts when assessed 6 months after implantation. GAG = glycosaminoglycan; SOC = standard-of-care; MOPS = The Missouri Osteochondral Allograft Preservation System.
Fig. 6.
Mean ± SD values for ortho-hydroxyproline content of articular cartilage from osteochondral allografts (MOPS [n = 7] and SOC [n = 7]) stored for either 28 or 60 days before implantation and assessed 6 months after implantation were compared with values for site-matched normal articular cartilage from the contralateral limbs (n = 14; Native). There were no differences in collagen content in native cartilage compared with cartilage from SOC and MOPS 28-day or 60-day grafts at 6 months (p = 0.28 and 0.21, respectively. OHP = ortho-hydroxyproline; SOC = standard-of-care; MOPS = The Missouri Osteochondral Allograft Preservation System.
Biomechanical Assessment
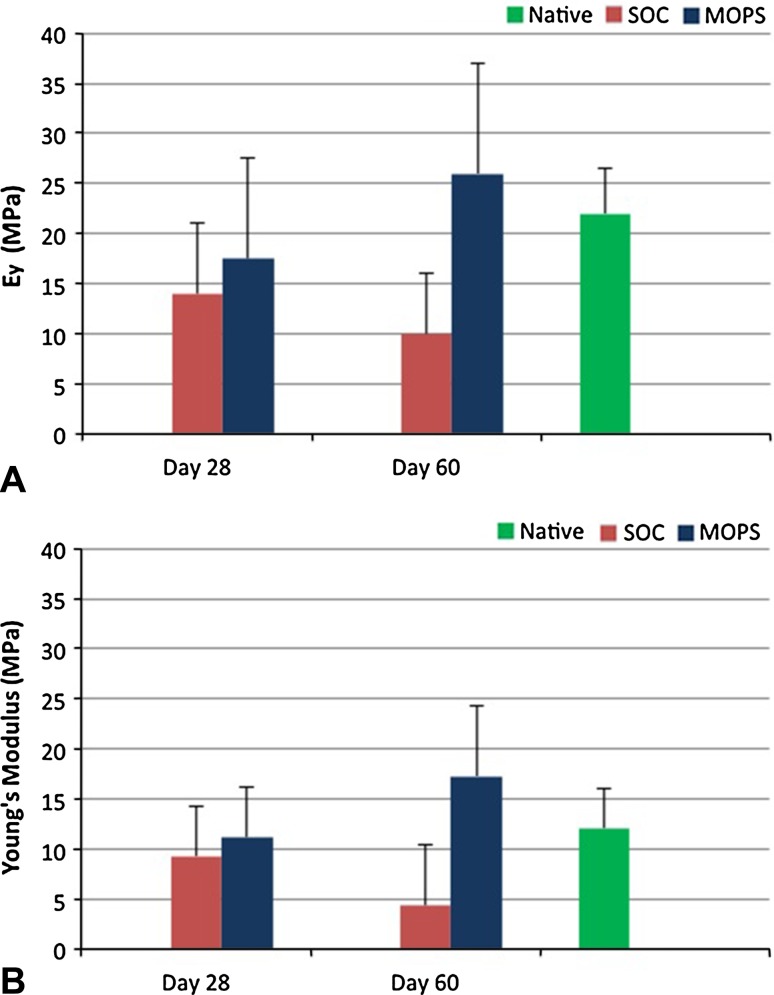
Instantaneous tissue modulus (Ey) and dynamic modulus (G) were greater (p < 0.05) in native articular cartilage and cartilage from MOPS 60-day grafts compared with SOC 60-day grafts when assessed 6 months after implantation. Instantaneous tissue modulus and dynamic modulus were not different in native articular cartilage compared with cartilage from MOPS 60-day grafts at 6 months (p = 0.12 and 0.18, respectively; Fig. 7).
Fig. 7A–B.
Mean ± SD for (A) instantaneous tissue modulus (Ey) and (B) dynamic modulus (G) of articular cartilage from osteochondral allografts (MOPS [n = 7] and SOC [n = 7]) stored for either 28 or 60 days before implantation and assessed 6 months after implantation were compared with values for site-matched normal articular cartilage from the contralateral limbs (n = 14; Native). Native and MOPS-60 were significantly higher (p < 0.05) than SOC-60. SOC = standard-of-care; MOPS = The Missouri Osteochondral Allograft Preservation System.
Discussion
While osteochondral allografting can be a successful treatment for large articular cartilage defects, use and success of allografts is limited by availability of acceptable grafts, often because of loss of chondrocyte viability during storage. We examined the feasibility of storing osteochondral allografts for up to 60 days using the MOPS system in terms of (1) whether chondrocyte viability is maintained at acceptable levels and risk for contamination is not increased over current standard-of-care storage; (2) integrity and positioning with time (12 weeks and 6 months); and (3) radiographic, arthroscopic, gross, cell viability, histologic, biochemical, and biomechanical characteristics at an endpoint of 6 months Both methods for and durations of storage evaluated in this study allowed for integration into host bone and showed lack of associated joint disorders based on longitudinal radiographic assessments. After 28 days of storage using either current tissue bank protocol (4° C) or MOPS (room temperature), osteochondral allografts implanted in the femoral condyles of dogs were associated with similarly good radiographic, arthroscopic, gross, histologic, biochemical, and biomechanical measures of graft performance performed 6 months after implantation. However, for allografts stored for 60 days before implantation, MOPS grafts performed better than SOC grafts when assessed 6 months after implantation.
The limitations of this study include the use of a canine model with a relatively small number of dogs with normal knees using a single 6-month endpoint. In addition, the clinical outcome measures used, radiographs and arthroscopy, were subjective assessments. Although these limitations can result in type II errors for results that were not significantly different between groups and affect the interpretation of the data, it is important to also consider that dogs serve as a legitimate translational model based on anatomy, joint physiology, biomechanics, and use of osteochondral allografts for successful treatment of clinical canine patients [3, 10, 18]. In addition, direct comparison of this novel storage method to the current standard of care using multiple outcome measures including histology and biomechanical testing with a study duration considered appropriate for preclinical testing by the ASTM and the US FDA lends credence to the relevance of these data for human use [4, 10, 35].
A concern with storing osteochondral allografts at temperatures greater than 4° C (eg, 25° C or 37° C) and for longer times before implantation is the potential for increased risk for graft infection. In our study, the infection rate for MOPS grafts was numerically lower than for SOC grafts with no significant difference noted between groups for up to 60 days of storage. No infected graft was implanted in dogs because all infections were detected based on microbial testing of the media before the time of implantation.
This translational animal model study verified the previously reported findings regarding the importance of donor chondrocyte viability in osteochondral allografts with respect to successful outcomes [1, 19, 22, 23, 26, 27, 38, 39]. The previously stated recommendation for a minimum of 70% chondrocyte viability in all grafts to be used clinically is supported. The data from this study suggest that donor chondrocytes contributed to maintenance of functional hyaline cartilage in the grafts based on the finding that all successful grafts had greater than 70% chondrocyte viability at the time of implantation. Grafts that failed to maintain the architecture and function of hyaline cartilage all had less than 70% chondrocyte viability at the time of implantation. Together with previous studies [1, 19, 22, 23, 26, 27, 38, 39], these data suggest that viable chondrocytes in osteochondral allografts at the time of transplantation are primarily responsible for maintenance of donor articular cartilage health long term. Therefore, optimizing chondrocyte viability in all aspects of osteochondral allografting, including procurement, processing, storage, transportation, and surgical implantation, needs to be a primary focus for research in this field. Our study addresses graft storage and provides in vivo validation for the advantages of MOPS in optimizing this critical component of the process.
The findings from the current study also validate the concern regarding use of grafts with insufficient chondrocyte viability as viability is known to rapidly decline from the completion of mandatory disease testing (Day 14) to fall below acceptable levels by Day 28 after procurement using current tissue bank protocols [2, 5–8, 25, 28, 29, 33, 34, 36, 38]. In addition, current protocols do not allow for determination of chondrocyte viability in individual grafts, leaving the judgment of graft acceptability to assumptions based on mean values from previous studies. MOPS was able to maintain sufficient (> 70%) chondrocyte viability for up to 60 days in 83% of stored allografts in this study. Previous studies [15, 32] which tested various media, temperatures, and containers for preserving allografts before implantation, suggest that it is the unique combination of these variables optimized during development of the MOPS that results in the improved graft quality noted in our study. If these findings hold true for preservation of human osteochondral tissues procured for osteochondral allografting, it would effectively triple the window for clinical use of donor tissue after disease-testing clearance for use (eg, from 14 days currently to 46 days using MOPS). If metabolic testing of storage media as described in this study can be implemented by tissue banks, the actual quality of individual osteochondral allografts can be determined before distribution to the surgeon so that only grafts with sufficient chondrocyte viability are used for patients. In this way, the quantity and quality of osteochondral allografting will be improved, which will benefit tissue banks, surgeons, the healthcare system, and, most importantly, patients receiving allografts. The medium used for MOPS is serum-free and does not contain growth factors or other additives that would require further regulatory approval before implementation. As such, tissue banks could adopt this system for preservation of osteochondral grafts intended for clinical trials.
Data derived in this study that examined the MOPS allow us to conclude that preservation of chondrocyte viability for up to 60 days at sufficient levels to result in successful outcomes in a canine model of large femoral condylar articular defects is possible. Chondrocyte viability at the time of implantation corresponded well to clinical and histologic measures of success with a relative threshold of 70% chondrocyte viability being acceptable for use. The MOPS system was better able to preserve grafts above this threshold, and allows for determination of chondrocyte viability by media assay before implantation so that graft quality can be ensured. Outcomes based on arthroscopic, gross, cell viability, histologic, biochemical, and biomechanical characteristics associated with MOPS-stored allografts were as good as or better than those using the current standard-of-care system used by tissue banks. This is a promising development in osteochondral allograft technology that potentially can benefit the quantity of grafts available for use and the quality of grafts being implanted.
Acknowledgment
We thank Samuel P. Franklin DVM, PhD, DACVS, DACVSMR (Small Animal Orthopedic Surgery, University of Georgia, College of Veterinary Medicine, Athens, GA, USA) for participation in the technical aspects of this project.
Footnotes
Several of the authors (JLC, AMS, JPS, KK, CRC, FMP, CB, CTH) received funding in the amount of USD 10,000 to USD 100,000 from Synthes (West Chester, PA, USA) and the Musculoskeletal Transplant Foundation (MTF). One or more of the authors (JLC, AMS, CTH) are patent holders of the tissue preservation system used in this study and will receive royalties for future clinical use. One of the authors (JLC) received royalties from the University of Missouri and Arthrex, Inc (Naples, FL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was done at the University of Missouri, Columbia campus.
References
- 1.Allen RT, Robertson CM, Pennock AT, Bugbee WD, Harwood FL, Wong VW, Chen AC, Sah RL, Amiel D. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33:1479–1484. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 2.Amiel D, Harwood FL, Hoover JA, Meyers M. A histological and biochemical assessment of the cartilage matrix obtained from in vitro storage of osteochondral allografts. Connect Tissue Res. 1989;23:89–99. doi: 10.3109/03008208909103906. [DOI] [PubMed] [Google Scholar]
- 3.Arnoczky SP, Cook JL, Carter T, Turner AS. Translational models for studying meniscal repair and replacement: what they can and cannot tell us. Tissue Eng Part B Rev. 2010;16:31–39. doi: 10.1089/ten.teb.2009.0428. [DOI] [PubMed] [Google Scholar]
- 4.ASTM International guideline. ASTM F2451-05 (2010) Standard Guide for In Vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage. Available at: http://www.astm.org/Standards/F2451.htm. Accessed June 16, 2014.
- 5.Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 6.Bastian JD, Egli RJ, Ganz R, Hofstetter W, Leunig M. Chondrocytes within osteochondral grafts are more resistant than osteoblasts to tissue culture at 37°C. J Invest Surg. 2011;24:28–34. doi: 10.3109/08941939.2010.523511. [DOI] [PubMed] [Google Scholar]
- 7.Black J, Shadle CA, Parsons JR, Brighton CT. Articular cartilage preservation and storage: II. Mechanical indentation testing of viable, stored articular cartilage. Arthritis Rheum. 1979;22:1102–1108. doi: 10.1002/art.1780221009. [DOI] [PubMed] [Google Scholar]
- 8.Brighton CT, Shadle CA, Jimenez SA, Irwin JT, Lane JM, Lipton M. Articular cartilage preservation and storage: I. Application of tissue culture techniques to the storage of viable articular cartilage. Arthritis Rheum. 1979;22:1093–1101. doi: 10.1002/art.1780221008. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Relat Res. 1999;360:159–168. doi: 10.1097/00003086-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Cook JL, Hung CT, Kuroki K, Stoker AM, Cook CR, Pfeiffer FM, Sherman SL, Stannard JP. Animal models for cartilage repair. Bone Joint Res. 2014;3:89–94. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook JL, Hung CT, Lima E, Stoker A, inventors. Tissue Preservation System. US patent application 2012/0177615 A1. July 12, 2012.
- 12.Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP. The OARSI histopathology initiative: recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage. 2010;18(suppl 3):S66–S79. doi: 10.1016/j.joca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 14.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469:2696–2705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrity JT, Stoker AM, Sims HJ, Cook JL. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med. 2012;40:2542–2548. doi: 10.1177/0363546512458575. [DOI] [PubMed] [Google Scholar]
- 16.Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008–1013. doi: 10.1302/0301-620X.79B6.7534. [DOI] [PubMed] [Google Scholar]
- 17.Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269–1278. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:764621. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. doi: 10.1097/01.blo.0000165845.21735.05. [DOI] [PubMed] [Google Scholar]
- 21.Gross AE, Silverstein EA, Falk J, Falk R, Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975;108:7–14. doi: 10.1097/00003086-197505000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Haudenschild DR, Hong E, Hatcher S, Jamali AA. Chondrogenic potential and homogeneity of cell populations of donor and recipient cells in a fresh osteochondral allograft: a case report. J Bone Joint Surg Am. 2012;94:e17. doi: 10.2106/JBJS.J.01969. [DOI] [PubMed] [Google Scholar]
- 23.Jamali AA, Hatcher SL, You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years: a case report. J Bone Joint Surg Am. 2007;89:166–169. doi: 10.2106/JBJS.F.00618. [DOI] [PubMed] [Google Scholar]
- 24.Krych AJ, Robertson CM, Williams RJ., 3rd Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053–1059. doi: 10.1177/0363546511435780. [DOI] [PubMed] [Google Scholar]
- 25.Linn MS, Chase DC, Healey RM, Harwood FL, Bugbee WD, Amiel D. Etanercept enhances preservation of osteochondral allograft viability. Am J Sports Med. 2011;39:1494–1499. doi: 10.1177/0363546511398645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malinin T, Temple HT, Buck BE. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am. 2006;88:762–770. doi: 10.2106/JBJS.D.02991. [DOI] [PubMed] [Google Scholar]
- 27.Pallante AL, Chen AC, Ball ST, Amiel D, Masuda K, Sah RL, Bugbee WD. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40:1814–1823. doi: 10.1177/0363546512449321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennock AT, Robertson CM, Wagner F, Harwood FL, Bugbee WD, Amiel D. Does subchondral bone affect the fate of osteochondral allografts during storage? Am J Sports Med. 2006;34:586–591. doi: 10.1177/0363546505281815. [DOI] [PubMed] [Google Scholar]
- 29.Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19:265–272. doi: 10.1055/s-0030-1248117. [DOI] [PubMed] [Google Scholar]
- 30.Roy RG, Wallace LJ, Johnston GR, Wickstrom SL. A retrospective evaluation of stifle osteoarthritis in dogs with bilateral medial patellar luxation and unilateral surgical repair. Vet Surg. 1992;21:475–479. doi: 10.1111/j.1532-950X.1992.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 31.Sherman SL, Garrity J, Bauer K, Cook JL, Stannard JP, Bugbee W. Fresh osteocondral allograft transplantation for the knee: current concepts review. J Am Acad Orthop Surg. 2014;22:121–133. doi: 10.5435/JAAOS-22-02-121. [DOI] [PubMed] [Google Scholar]
- 32.Stoker A, Garrity JT, Hung CT, Stannard JP, Cook J. Improved preservation of fresh osteochondral allografts for clinical use. J Knee Surg. 2012;25:117–125. doi: 10.1055/s-0032-1319809. [DOI] [PubMed] [Google Scholar]
- 33.Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466:1804–1809. doi: 10.1007/s11999-008-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas VJ, Jimenez SA, Brighton CT, Brown N. Sequential changes in the mechanical properties of viable articular cartilage stored in vitro. J Orthop Res. 1984;2:55–60. doi: 10.1002/jor.1100020109. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for Industry: Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed June 11, 2014.
- 36.Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831–837. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Williams RJ, III, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 38.Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, Badlani NM, Emery SC, Haghighi P, Bugbee WD. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 39.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]