Abstract
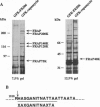
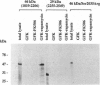
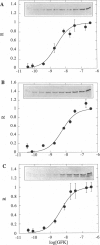
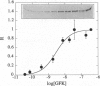
Complexed with its intracellular receptor, FKBP12, the natural product rapamycin inhibits G1 progression of the cell cycle in a variety of mammalian cell lines and in the yeast Saccharomyces cerevisae. Previously, a mammalian protein that directly associates with FKBP12-rapamycin has been identified and its encoding gene has been cloned from both human (designated FRAP) [Brown, E.J., Albers, M.W., Shin, T.B., Ichikawa, K., Keith, C.T., Lane, W.S. & Schreiber, S.L. (1994) Nature (London) 369, 756-758] and rat (designated RAFT) [Sabatini, D.M., Erdjument-Bromage, H., Lui, M., Tempst, P. & Snyder, S.H. (1994) Cell 78, 35-43]. The full-length FRAP is a 289-kDa protein containing a putative phosphatidylinositol kinase domain. Using an in vitro transcription/translation assay method coupled with proteolysis studies, we have identified an 11-kDa FKBP12-rapamycin-binding domain within FRAP. This minimal binding domain lies N-terminal to the kinase domain and spans residues 2025-2114. In addition, we have carried out mutagenesis studies to investigate the role of Ser2035, a potential phosphorylation site for protein kinase C within this domain. We now show that the FRAP Ser2035-->Ala mutant displays similar binding affinity when compared with the wild-type protein, whereas all other mutations at this site, including mimics of phosphoserine, abolish binding, presumably due to either unfavorable steric interactions or induced conformational changes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers M. W., Brown E. J., Tanaka A., Williams R. T., Hall F. L., Schreiber S. L. An FKBP-rapamycin-sensitive, cyclin-dependent kinase activity that correlates with the FKBP-rapamycin-induced G1 arrest point in MG-63 cells. Ann N Y Acad Sci. 1993 Nov 30;696:54–62. doi: 10.1111/j.1749-6632.1993.tb17142.x. [DOI] [PubMed] [Google Scholar]
- Albers M. W., Williams R. T., Brown E. J., Tanaka A., Hall F. L., Schreiber S. L. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993 Oct 25;268(30):22825–22829. [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Buechler Y. J., Taylor S. S. Mutations in the autoinhibitor site of the regulatory subunit of cAMP-dependent protein kinase I. Replacement of Ala-97 and Ser-99 interferes with reassociation with the catalytic subunit. J Biol Chem. 1991 Feb 25;266(6):3491–3497. [PubMed] [Google Scholar]
- Cafferkey R., Young P. R., McLaughlin M. M., Bergsma D. J., Koltin Y., Sathe G. M., Faucette L., Eng W. K., Johnson R. K., Livi G. P. Dominant missense mutations in a novel yeast protein related to mammalian phosphatidylinositol 3-kinase and VPS34 abrogate rapamycin cytotoxicity. Mol Cell Biol. 1993 Oct;13(10):6012–6023. doi: 10.1128/mcb.13.10.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo V., Crews C. M., Vik T. A., Bierer B. E. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M. I., Katz H., Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Francavilla A., Carr B. I., Starzl T. E., Azzarone A., Carrieri G., Zeng Q. H. Effects of rapamycin on cultured hepatocyte proliferation and gene expression. Hepatology. 1992 May;15(5):871–877. doi: 10.1002/hep.1840150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Helliwell S. B., Wagner P., Kunz J., Deuter-Reinhard M., Henriquez R., Hall M. N. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol Biol Cell. 1994 Jan;5(1):105–118. doi: 10.1091/mbc.5.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992 Jul 2;358(6381):70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Morice W. G., Brunn G. J., Wiederrecht G., Siekierka J. J., Abraham R. T. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J Biol Chem. 1993 Feb 15;268(5):3734–3738. [PubMed] [Google Scholar]
- Morice W. G., Wiederrecht G., Brunn G. J., Siekierka J. J., Abraham R. T. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993 Oct 25;268(30):22737–22745. [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Stan R., McLaughlin M. M., Cafferkey R., Johnson R. K., Rosenberg M., Livi G. P. Interaction between FKBP12-rapamycin and TOR involves a conserved serine residue. J Biol Chem. 1994 Dec 23;269(51):32027–32030. [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Brizuela L., Elliston K., Sigal N. H., Siekierka J. J. FKB1 encodes a nonessential FK 506-binding protein in Saccharomyces cerevisiae and contains regions suggesting homology to the cyclophilins. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1029–1033. doi: 10.1073/pnas.88.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]