Abstract
Background
Surgeons frequently obtain intraoperative cultures at the time of revision total joint arthroplasty. The use of broth or liquid medium before applying the sample to the agar medium may be associated with contamination and false-positive cultures; however, the degree to which this is the case is not known.
Questions/purposes
We (1) calculated the performance characteristics of broth-only cultures (sensitivity, specificity, positive predictive value, and negative predictive value) and (2) characterized the organisms identified in broth to determine whether a specific organism showed increased proclivity for true-positive periprosthetic joint infection (PJI).
Methods
A single-institution retrospective chart review was performed on 257 revision total joint arthroplasties from 2009 through 2010. One hundred ninety (74%) had cultures for review. All culture results, as well as treatment, if any, were documented and patients were followed for a minimum of 1 year for evidence of PJI. Cultures were measured as either positive from the broth only or broth negative. The true diagnosis of infection was determined by the Musculoskeletal Infection Society criteria during the preoperative workup or postoperatively at 1 year for purposes of calculating the performance characteristics of the broth-only culture.
Results
The sensitivity, specificity, positive predictive value, and negative predictive value were 19%, 88%, 13%, and 92%, respectively. The most common organism identified was coagulase-negative Staphylococcus (16 of 24 cases, 67%). Coagulase-negative Staphylococcus was present in all three true-positive cases; however, it was also found in 13 of the false-positive cases.
Conclusions
The broth-only positive cultures showed poor sensitivity and positive predictive value but good specificity and negative predictive value. The good specificity indicates that it can help to rule in the presence of PJI; however, the poor sensitivity makes broth-only culture an unreliable screening test. We recommend that broth-only culture results be carefully scrutinized and decisions on the diagnosis and treatment of infection should be based specifically on the Musculoskeletal Infection Society criteria.
Level of Evidence
Level IV, diagnostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Surgeons should consider the possibility of infection before performing a revision total joint arthroplasty (TJA) of the hip or knee [2, 3, 6, 21]. When there is diagnostic ambiguity, intraoperative cultures often are performed, and these cultures may influence the postoperative care of these patients, sometimes resulting in more surgery or the use of extended courses of parenteral antibiotics. Besides the expense, inconvenience, and possible morbidity to patients, this course of treatment can lead to the development of antibiotic-resistant organisms [10, 16].
False-positive culture results may lead to unnecessary or dangerous interventions. When an individual culture is analyzed, the result commonly is reported as negative, positive, or positive from the broth only (or liquid medium). The definition of periprosthetic joint infection (PJI) as described by the Musculoskeletal Infection Society (MSIS) relies in part on culture results. The MSIS criteria do not distinguish between positive cultures obtained on solid media (which may be less likely to be contaminated) from cultures that are positive in the broth only (which may be more likely to be contaminated) [24]. The use of enrichment broth as an adjunct to direct plating has been recommended to improve the recovery of clinically relevant isolates [4, 5, 11, 14, 20, 22]. However, high rates of contamination have been reported with this technique and its usefulness has been questioned [7, 8, 12, 13, 17, 18, 23]. Additionally, it is not known whether certain organisms identified in broth cultures pose a more virulent threat and should be treated more aggressively than other organisms that are identified.
Since the culture results are critically important in shaping the course of treatment, we sought to determine the reliability of cultures from the broth only after revision TJA and how to address the organisms found exclusively in the broth medium. Specifically, we (1) calculated the performance characteristics of broth-only cultures (sensitivity, specificity, positive predictive value, and negative predictive value) and (2) characterized the organisms identified in broth to determine whether a specific organism showed increased proclivity for true-positive PJI. Overall, the performance characteristics and organism profile of broth cultures will give insight into the reliability of broth-only culture results in diagnosing and treating PJI.
Patients and Methods
The study was performed at a 319-bed, high-volume joint arthroplasty, community hospital in the suburbs of Philadelphia, PA, USA. We completed a retrospective chart review of 257 consecutive revision TJAs from January 1, 2009, through December 31, 2010. Revision procedures that sought specifically to treat PJI that had already been diagnosed (ie, irrigation and débridement and/or resection arthroplasty) were not included. Intraoperative cultures were not available (ie, not obtained) at the time of surgery during 67 TJAs that were believed to have a low index of suspicion for infection, including cases where the preoperative diagnosis was dislocation/instability (30), periprosthetic fracture (18), polyethylene wear (eight), component loosening (six), and mechanical complication (five). These TJAs were therefore excluded, leaving 190 revisions (133 TKA revisions and 57 THA revisions) for review. The preoperative diagnoses of the included revisions were component loosening (69), mechanical complication (52), painful implant (49), polyethylene wear (13), dislocation/instability (four), and periprosthetic fracture (three). The mean time from index surgery to revision was 3.5 years (range, 0.4–11.4 years).
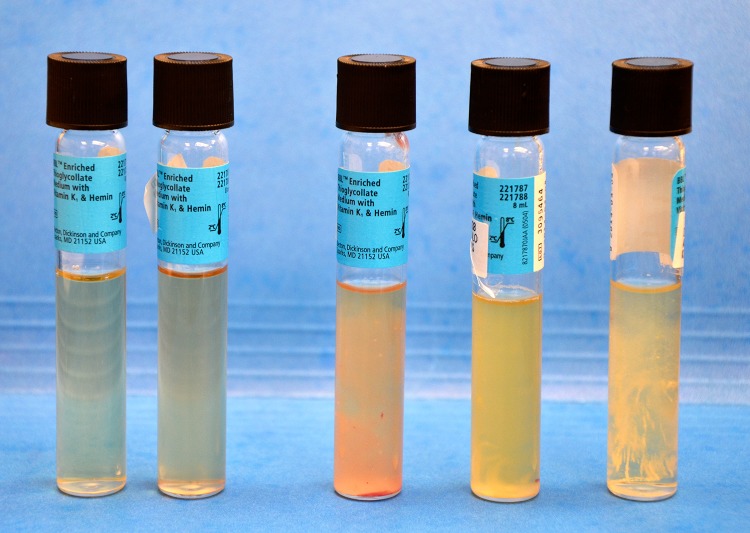
At the time of revision surgery, three or more specimens were sent for culture. The specimens included tissue and/or fluid sent to the microbiology laboratory in a sterile container. In the microbiology laboratory, the specimens were prepared under a laminar airflow hood by adding 1 to 2 mL thioglycollate liquid medium followed by mechanically grinding down of the tissue. A drop of the resultant specimen was used for direct plating (ie, smeared on each of three agar plates containing a blood, chocolate, or anaerobic medium). Additionally, after grinding the tissue, two to three drops of the specimen were placed into the original test tube containing the thioglycollate liquid medium (broth). All specimens were then incubated and checked for isolates daily for a total of 5 days. If the broth appeared to be turbid at any point in time, it was then plated and incubated for 5 days (Fig. 1).
Fig. 1.
Five test tube thioglycollate specimens with increasing turbidity from left to right are shown. If significant turbidity is seen, as in the three specimens on the right, the specimen is smeared onto agar plates and incubated.
All culture results, as well as treatment, if any, were recorded and patients were followed for a minimum of 1 year (mean 2.2 years; range, 1.2–3.2 years) for evidence of PJI. Culture results were reported from the microbiology department as positive (from direct plating), positive broth only, or negative. For the purposes of statistical analysis, these culture results were categorized into two groups based on the broth results: positive from the broth only or broth negative (ie, negative culture or a specimen that was positive from direct plating, but negative from the broth). We reviewed data from the preoperative workup including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fluid aspiration results, and other studies, if available. Postoperatively, charts were reviewed to see whether patients underwent further workup for infection and/or were treated operatively or nonoperatively for PJI.
The sensitivity, specificity, and positive and negative predictive values were determined for the broth-only cultures. True PJI results were defined as patients who met the criteria as described by the MSIS. Patients were considered to have true PJI if two cultures were positive or a sinus tract was present. PJI also was considered true if four of the following six were present: elevated CRP and ESR, elevated synovial white blood cell count, elevated synovial neutrophil percentage, positive histologic analysis, intraarticular purulence, or a single positive culture [24]. Positive cultures from the broth were not counted as part of the MSIS criteria for determining infection (ie, two positive broth cultures were not considered a true PJI unless other factors met the MSIS criteria). True PJI negative results were defined as patients who were free from infection 1 year after surgery and did not match the criteria for MSIS-positive PJI.
The binomial distribution was used to estimate 95% CIs for sensitivity, specificity, and positive and negative predictive values. The defined groups were test positive (broth-only positive), test negative (broth negative), disease positive (MSIS positive), and disease negative (MSIS negative). Fisher’s exact test for 2 x 2 contingency tables was used to determine whether the probability of being a true positive was statistically associated with the presence of a specifically identified organism. These analyses were performed using R statistical software (Version 3.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Broth cultures were found to be highly specific and had a high negative predictive value; however, sensitivity and positive predictive value were very poor. The culture results from the 190 TJA revisions revealed 24 (13%) broth-positive and 166 (87%) broth-negative cases. Of the 24 cases that were positive from the broth, 17 (71%) were positive from one, five (21%) from two, and two (8%) from three of the specimens taken at the time of surgery. Sixteen of the 190 (8%) were considered disease positive for PJI based on the MSIS criteria. Three of the broth-only cases met the MSIS criteria for PJI and were considered to be true positives. Two of these cases had only one of three positive broth specimens and one of the cases had three of three positive broth specimens. Twenty-one broth-positive cases did not meet the criteria and were considered false positives. One hundred fifty-three of the broth-negative cases were considered true negatives and 13 met the MSIS criteria for PJI and were considered false negatives. The sensitivity, specificity, positive predictive value, and negative predictive value (with 95% CIs) for the broth-only culture test were 19% (4%–46%), 88% (82%–93%), 13% (3%–32%), and 92% (87%–96%), respectively (Table 1).
Table 1.
Calculation of sensitivity, specificity, positive predictive value, and negative predictive value of broth-only culture results after total joint arthroplasty revision
| Broth | MSIS | Predictive value | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 3 (true positive) | 21 (false positive) | 13% positive predictive value |
| Negative | 13 (false negative) | 153 (true negative) | 92% negative predictive value |
| Sensitivity/specificity | 19% sensitivity | 88% specificity | |
MSIS = Musculoskeletal Infection Society.
Multiple offending organisms were identified in the broth cultures. Two-thirds of the isolates from the broth contained coagulase-negative Staphylococcus (CNS). The remaining isolates consisted of Enterococcus faecalis, Escherichia coli, Enterobacter agglomerans, Bacillus, β-hemolytic Group B Streptococcus, methicillin-sensitive Staphylococcus aureus, Proteus, and Bacteroides. In six of the 24 cases, multiple organisms were isolated (Table 2). CNS was identified in all three of the true-positive cases; however, this was not significant as it was found in 13 of the false-positive cases as well (p = 0.526). Proteus and Bacteroides were found in addition to CNS in one of the true-positive cases. Interestingly, one of the patients with true-positive results required an irrigation and débridement of the hip for a draining wound 2 weeks postoperatively and the isolate from the irrigation and débridement (E coli) was different from the broth-only isolate (CNS), suggesting the broth culture may have actually been falsely positive.
Table 2.
List of organisms isolated from the broth only
| Number of isolates | Organism |
|---|---|
| 30 | Total |
| 16 | Coagulase-negative Staphylococcus |
| 3 | Enterococcus faecalis |
| 2 | Escherichia coli |
| 2 | Enterobacter agglomerans |
| 2 | Bacillus species |
| 2 | Group B Streptococcus |
| 1 | Methicillin-sensitive Staphylococcus aureus |
| 1 | Proteus mirabilis |
| 1 | Bacteroides fragilis |
Six of the 24 specimens grew multiple organisms.
Discussion
Results of intraoperative cultures impact the diagnosis and treatment of PJI [1–3, 6, 13, 19, 24]. However, interpretation of “positive from the broth-only cultures” has been questioned, as well as the validity of broth-only cultures as a tool for diagnosis and treatment of PJI [7, 8, 12, 13, 17, 18, 23]. This is important because unnecessary treatment with antibiotics due to a false-positive test can result in significant emotional and financial hardship on patients, morbidity, and development of antibiotic resistance. However, not treating an infected patient due to a false-negative result can have even more devastating consequences. We therefore determined the performance characteristics of broth cultures and characterized the organisms uniquely identified in broth-only cultures.
The main limitations of our paper are its retrospective nature, the number of patients not included because no cultures were obtained, possibility of cross-contamination, short duration of followup, and a lack of a clear treatment algorithm. As with all retrospective studies, there exists the potential for selection bias and this study did not include 67 patients who underwent revision surgery during this period but did not have cultures sent at the time of surgery. Since they were believed to have a low index of suspicion for PJI, it is possible that excluding their results may have actually improved the broth-only results (ie, if included, perhaps more false-positive results would have been seen). We were able to obtain records on all 190 patients who did have culture results. Patients were followed for a minimum of 1.2 years after revision TJA. It is possible that this is an insufficient followup duration to detect indolent infections: what we considered contaminants at 1 year could perhaps reveal themselves to be clinical infections at 2 years. Sterile techniques practiced by the surgeon and staff are crucial to prevent cross-contamination in the specimens retrieved, such as using new sterile instruments for each retrieval. Due to the retrospective design of this study, we are unable to ensure that each staff member took all necessary precautions to ensure sterility. One of the driving forces to study the issue of broth-only cultures was the fact that we had no consensus on the treatment of these patients. Being unsure of the appropriate course of action, many treatments were prescribed from intravenous or oral antibiotics to no antibiotics at all. Some of these treatments may, in fact, have eradicated or suppressed some infections sufficiently that they were not diagnosed using the MSIS criteria; this limitation would tend to decrease the apparent utility of a positive broth-only culture in this study, and this limitation needs to be considered very seriously.
The first goal of our study was to determine the diagnostic utility of broth-only culture results. The broth-only positive cultures showed poor sensitivity and positive predictive value (19% and 13%, respectively) but good specificity and negative predictive value (88% and 92%, respectively). These results indicate that broth-only positive cultures may be helpful in ruling in PJI; however, the high number of false positives led to a very poor positive predictive value. The lack of the broth to pick up 13 disease-positive patients led to a very poor sensitivity. Many studies that recommended utilizing these enrichment broths as an adjunct to direct plating were older and did not specifically analyze joint fluid specimens [4, 5, 11, 14, 20, 22]. The origins of evaluating media for isolating organisms goes back to the early 1900s, but recommendation for use of broth specifically for backup began in the early 1970s [15]. Senneville et al. [22] reported that 26 of 144 (18.1%) known infected joint specimens would have been missed without the addition of the broth medium. These older studies have been questioned extensively [7, 8, 12, 13, 17, 18, 23]. Meredith et al. [17] found a nearly 100% false-positive rate of broth-only cultures from cerebrospinal fluid when the offending organisms were CNS. Morris et al. [18] noted that 73% of isolates from the broth were contaminants and that only 3% of 376 isolates from various sources were thought to be clinically relevant. Derby et al. [7] found that only two of 317 patients (0.6%) had their treatment altered on the basis of positive broth-only results.
Our second goal was to describe the bacteriology of the broth-only cultures. Though we identified CNS as the most common offending organism (found in 2/3 of the cases), it was not predictive of being a true isolate from the surgical case. It was present in each of the three true-positive cases but also in 13 of the false-positive cases. Both Proteus and Bacteroides were isolated from the broth of one of the true-positive patients. Due to the small number of true-positive results, we were unable to identify an organism that would be clearly worrisome for a true infection when identified. Interestingly, there were no cases of methicillin-resistant S aureus and therefore we cannot comment on the virulence of this resistant organism if it was identified in the broth cultures. Of note, E faecalis, E coli, E agglomerans, Bacillus, β-hemolytic Group B Streptococcus, and methicillin-sensitive S aureus were all found to be false positives. Perhaps a larger study with a larger sample size could improve the power to determine whether there is truly no difference. Further study on this topic is necessary. Ideally, a prospective study would be performed with a defined algorithm of treatment (or no treatment) for patients with positive cultures from the broth only.
Based on our findings, we recommend that broth-only culture results be carefully scrutinized and decisions on the diagnosis and treatment of infection should be based specifically on the MSIS criteria [24]. Similar studies questioned the usefulness of Gram stains, which show similar sensitivities and specificities [9, 19, 25]. The good specificity of broth-only cultures shows they can help to rule in the presence of PJI; however, the poor sensitivity makes it an unreliable screening test.
Acknowledgments
The authors thank the Main Line Health Microbiology Department (Philadelphia, PA, USA) for analysis and reporting of the broth-only culture data.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 3.Bernard L, Lubbeke A, Stern R, Bru JP, Feron JM, Peyramond D, Denormandie P, Arvieux C, Chirouze C, Perronne C, Hoffmeyer P. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review. Scan J Infect Dis. 2004;36:410–416. doi: 10.1080/00365540410015240. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright CP, Stock F, Gill VJ. Improved enrichment broth for cultivation of fastidious organisms. J Clin Microbiol. 1994;32:1825–1826. doi: 10.1128/jcm.32.7.1825-1826.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton H. Body fluid specimens, synovial fluid specimens. In: Dalton HP, Nottebart HC Jr, editors. Interpretative Medical Microbiology. New York, NY: Churchill Livingstone; 1986. pp. 875–900. [Google Scholar]
- 6.Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:760–770. [DOI] [PubMed]
- 7.Derby P, Davies R, Oliver S. The value of including broth cultures as part of a routine culture protocol. J Clin Microbiol. 1997;35:1101–1102. doi: 10.1128/jcm.35.5.1101-1102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz FR, Koontz FP, Found EM, Marsh JL. The importance of positive bacterial cultures of specimens obtained during clean orthopaedic operations. J Bone Joint Surg Am. 1991;73:1200–1207. [PubMed] [Google Scholar]
- 9.Ghanem E, Ketonis C, Restrepo C, Joshi A, Barrack R, Parvizi J. Periprosthetic infection: where do we stand with regard to Gram stain? Acta Orthop. 2009;80:37–40. doi: 10.1080/17453670902804943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbath S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on the surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916–2921. doi: 10.1161/01.CIR.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg HD, Baron EJ, D’Amato RF, Johnson RC, Murray PR, Rogers FG, von Graevenitz A. Recommendations for the isolation of bacteria from clinical specimens. In: Balows A, Hausler WJ Jr, Herrmann KL, Isenberg HD, Shadomy HJ, editors. Manual of Clinical Microbiology. 5. Washington, DC: American Society for Microbiology; 1991. pp. 216–221. [Google Scholar]
- 12.Levine BR, Evans BG. Use of blood culture vial specimens in intraoperative detection of infection. Clin Orthop Relat Res. 2001;382:222–231. doi: 10.1097/00003086-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Marin M, Esteban J, Meseguer MA, Sanchez-Somolinos M. Microbiological diagnosis of bone-joint infections. Enferm Infecc Microbiol Clin. 2010;28:534–540. doi: 10.1016/j.eimc.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Marsik FJ. Central nervous system specimens. In: Dalton HP, Nottebart HC Jr, editors. Interpretive Medical Microbiology. New York, NY: Churchill Livingstone; 1986. p. 196. [Google Scholar]
- 15.McMinn MT, Crawford JJ. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970;19:207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meehan J, Jamali AA, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:2480–2490. doi: 10.2106/JBJS.H.01219. [DOI] [PubMed] [Google Scholar]
- 17.Meredith FT, Phillips HK, Reller LB. Clinical utility of broth cultures of cerebrospinal fluid from patients at risk for shunt infections. J Clin Microbiol. 1997;35:3109–3111. doi: 10.1128/jcm.35.12.3109-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris AJ, Wilson SJ, Marx CE, Wilson ML, Mirrett S, Reller LB. Clinical impact of bacteria and fungi recovered only from broth cultures. J Clin Microbiol. 1995;33:161–165. doi: 10.1128/jcm.33.1.161-165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oethinger M, Warner DK, Schindler SA, Kobayashi H, Bauer TW. Diagnosing periprosthetic infection: false positive intraoperative Gram stains. Clin Orthop Relat Res. 2011;469:954–960. doi: 10.1007/s11999-010-1589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhold CE, Nickolai DJ, Piccinini TE, Byford BA, York MK, Brooks GF. Evaluation of broth media for routine culture of cerebrospinal and joint fluid specimens. Am J Clin Pathol. 1988;89:671–674. doi: 10.1093/ajcp/89.5.671. [DOI] [PubMed] [Google Scholar]
- 21.Ritter MA, Stringer EA. Intraoperative wound cultures: their value and long-term effect on the patient. Clin Orthop Relat Res. 1981;155:180–185. [PubMed] [Google Scholar]
- 22.Senneville E, Savage C, Nallet I, Yazdanpanah Y, Giraud F, Migaud H, Dubreuil L, Courcol R, Mouton Y. Improved aero-anaerobe recovery from infected prosthetic joint samples taken from 72 patients and collected intraoperatively in Rosenow’s broth. Acta Orthop. 2006;77:120–124. doi: 10.1080/17453670610045795. [DOI] [PubMed] [Google Scholar]
- 23.Tsukayama PT, Estrada R, Gusheo RB. Infection after total hip arthroplasty. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Workgroup Convened by the Musculoskeletal Infection Society New definition for periprosthetic joint infection. J Arthroplasty. 2011;26:1136–1138. doi: 10.1016/j.arth.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Zwiel MG, Stroh DA, Johnson AJ, Marker DR, Mont MA. Gram stains have limited application in the diagnosis of infected total knee arthroplasty. Int J Infect Dis. 2011;15:e702–e705. doi: 10.1016/j.ijid.2011.05.015. [DOI] [PubMed] [Google Scholar]