Abstract
Reproductive biotechnologies such as in vitro fertilization (IVF) and somatic cell nuclear transfer (SCNT) enable improved reproductive efficiency of animals. However, the birth rate of in vitro-derived embryos still lags behind that of their in vivo counterparts. Thus, it is critical to develop an accurate evaluation and prediction system of embryo competence, both for commercial purposes and for scientific research. Previous works have demonstrated that in vitro culture systems induce alterations in the relative abundance (RA) of diverse transcripts and thus compromise embryo quality. The aim of this work was to analyze the RA of a set of genes involved in cellular stress (heat shock protein 70-kDa, HSP70), endoplasmic reticulum (ER) stress (immunoglobulin heavy chain binding protein, Bip; proteasome subunit β5, PSMB5) and apoptosis (BCL-2 associated X protein, Bax; cysteine aspartate protease-3, Caspase-3) in bovine blastocysts produced by IVF or SCNT and compare it with that of their in vivo counterparts. Poly (A) + mRNA was isolated from three pools of 10 blastocysts per treatment and analyzed by real-time RT-PCR. The RA of three of the stress indicators analyzed (Bax, PSMB5 and Bip) was significantly increased in SCNT embryos as compared with that of in vivo-derived blastocysts. No significant differences were found in the RA of HSP70 and Caspase-3 gene transcripts. This study could potentially complement morphological analyses in the development of an effective and accurate technique for the diagnosis of embryo quality, ultimately aiding to improve the efficiency of assisted reproductive techniques (ART).
Introduction
Recent studies regarding the outcome of in vitro fertilization (IVF) and somatic cell nuclear transfer (SCNT) have demonstrated that embryonic developmental competence can be severely compromised without apparent correlation with morphological changes. Good quality embryos classified according to morphological criteria may have different developmental capacities, with only a certain percentage of these embryos being capable of establishing pregnancy after transfer into recipients [1]. In addition, developmental competence of in vitro-produced embryos following transfer is significantly lower than that of those produced in vivo [2]. In conclusion, qualitative or morphological evaluation alone is not sufficient in providing a precise and efficient estimation of embryo quality and embryonic developmental potential [3].
In modern cattle production, there is vast information demonstrating that micromanipulation and embryo culture conditions can have a dramatic effect on gene expression patterns, which can be disadvantageous to the quality of the resulting blastocyst [4]–[14].
Several studies have proven the broad applicability of real-time reverse transcription polymerase chain reaction (qRT-PCR) for differential gene expression studies. Researchers have revealed differences in the relative abundance (RA) of genes involved in the development and metabolism of in vivo and in vitro embryos [15], [16]. However, to our knowledge, no studies have exclusively compared the expression profile of genes specifically related to stress and apoptosis in bovine blastocysts, which are important parameters to consider in the assessment of embryo quality.
The analysis of transcripts from genes essential in early embryonic development provides a tool for the assessment of embryo quality and optimization of in vitro culture conditions and production protocols. Studies on in vitro-produced and cultured blastocysts exhibit alterations related to intercellular contact, compaction, differentiation, cell stress, and apoptosis. In addition, although mRNA expression patterns are highly conserved, there are crucial differences in the RA of transcripts of genes involved in blastocyst development, which may partly explain the differences in the quality of such embryos [13], [14], [17], [18].
The ability to identify precise changes in the expression profile of genes involved in both the response to cellular stress and the early stages of apoptosis would benefit in vitro embryo production by enabling, for example, the analysis of the physiological status of oocytes and embryos produced by different maturation and in vitro culture systems. The objective of the present study was to analyze the RA of a set of genes involved in cellular stress (heat shock protein 70-kDa, HSP70), endoplasmic reticulum (ER) stress (immunoglobulin heavy chain binding protein, Bip; proteasome subunit β5, PSMB5) and apoptosis (BCL-2 associated X protein, Bax; cysteine aspartate protease-3, Caspase-3) in bovine blastocysts produced by IVF or SCNT and compare it with that of their in vivo counterparts.
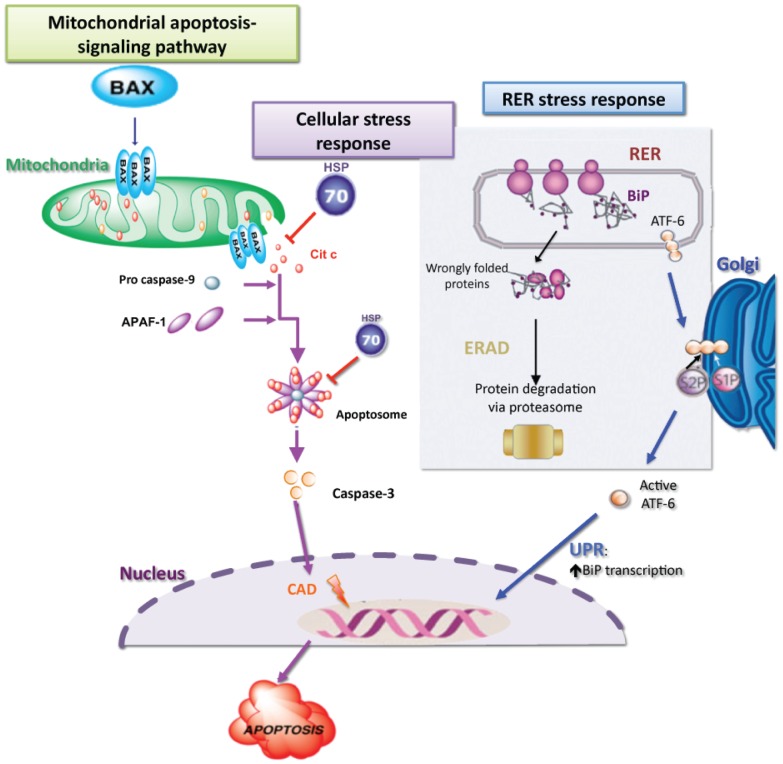
Heat shock protein 70-kDa, HSP70, is a prominent cytoprotective factor that confers tolerance as a consequence of cellular stress or transfection. HSP70 expression inhibits the induction of apoptosis, thus conferring protection to damaged cells [19]–[21]. Therefore, monitoring the expression pattern of this gene contributes to understanding the physiological state of a cell or organism. Immunoglobulin heavy chain binding protein, Bip, is a chaperone member of the HSP family located in the lumen of the rough ER [22], and PSMB5 eliminates aberrant or misfolded proteins as a result of stress within the ER. Conditions inducing apoptosis [23]–[26] as well as gene expression analysis of apoptosis associated genes [27]–[28] are well studied in bovine preimplantation embryos. Cysteine aspartate protease-3, Caspase-3, is responsible for the activation of caspase-activated DNase (CADs) for DNA fragmentation [1] and Bax is a pro-apoptotic gene member of BCL-2 family genes. Both of these genes are involved in early stages of apoptosis, which can occur prior to any visible changes in morphology (Figure 1).
Figure 1. Signaling pathways active in cellular stress conditions.
Since classical methods for gene transcript detection require large amounts of initial RNA, they are not suitable for application in oocytes and embryos [3], [29]. However, qRT-PCR is a highly sensitive technique that allows determining the RA of a target transcript as well as simultaneously amplifying an endogenous gene to be used as control.
The aim of the present work was to compare the RA of selected transcripts involved in cellular stress, ER stress, and apoptosis by qRT-PCR. This assay may be used to complement morphological analyses, ultimately providing a quantitative technique to assess the developmental potential and quality of bovine embryos produced by application of assisted reproductive technologies.
Materials and Methods
Chemicals, reagents and culture media for in vitro embryo production
All chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise indicated.
Ethics statement
The protocol for this study (reference number 02/2011) was approved by the Committee on the Ethics of Animal Experiments of the Universidad Nacional de San Martin. Protocol development was approved as recommended by the National Institutes of Health (NIH) Guide for the Care and Use of Animals.
Collection and in vitro maturation of bovine oocytes
Bovine ovaries from VISOM S.A slaughterhouse (Los Polvorines, Buenos Aires) were transported to the laboratory within 3 h at 25°C in sterile saline solution supplemented with 0.1 mg/mL streptomycin. For aspiration, follicles 2–10 mm in diameter were punctured using 19-G needles connected to a vacuum pump. Follicular fluid containing cumulus-oocyte complexes (COCs) were collected in sterile 50-mL centrifuge tubes (Corning, New York, USA). Only COCs with homogeneous cytoplasm and at least three layers of unexpanded cumulus cells were selected for in vitro maturation (IVM) [30]. Forty to fifty COCs were cultured in 400 µl of maturation medium and incubated at 38.5°C in 5% CO2 in air with maximum humidity for 24 h prior to their use. The maturation medium consisted of M199 supplemented with 100 µM cysteamine, 0.2 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL gentamicin, 25.1 mM NaHCO3, 0.1 IU recombinant human follicle-stimulating hormone (FSH) (Puregón, Organon, Ca, USA) and 10% fetal calf serum (FCS) (Gibco, New Zealand).
In vitro fertilization (IVF) procedure
Frozen–thawed semen from Brangus bulls with proven performance in IVF was used. All samples (500-µl straws) from each bull belonged to the same commercial batch. Thawing was performed by immersing straws for 30 s in a 37°C water bath.
Matured COCs were transferred to new four-well plates containing 400 µl of in vitro fertilization-synthetic oviductal fluid (IVF-SOF) medium, containing 107.7 mM NaCl, 7.1 mM KCl, 12 mM KH2PO4, 5 mM NaHCO3, penicillin 25 UI, 73 mM sodium pyruvate, 3% BSA, 2 mM fructose, 18 mM CaCl22H2O, and 54 mM sodium lactate, supplemented with 50 µg/mL heparin. Motile sperm were separated using the Percoll gradient (90%/60%/30%) method. The thawed semen was centrifuged at 600 g for 20 min. Spermatozoa were washed twice using Hepes buffered-TALP, H-TALP (114 mM NaCl, 3.1 mM KCl, 0.4 mM NaH2PO4.H2O, 10 mM sodium lactate (60%), 25 mM NaHCO3, 1.4 mM caffeine, 2 mM CaCl2.2H2O, 0.5 mM MgCl2.6H2O and 10 mM HEPES) and centrifuged at 200 g for 5 min. Spermatozoa were resuspended in IVF-SOF medium. Finally, sperm cells were added to reach a final concentration of 2×106 cells/mL and co-incubated with COCs for 24 h in a humidified atmosphere of 5% CO2 in air at 38.5°C.
In vitro culture
Presumptive zygotes were denuded 24 h after fertilization by gently vortexing for 3 min. After removal of all of the adhering cumulus cells, presumptive zygotes were placed into four-well plates in groups of 50 in 400-µl drops of synthetic oviductal fluid (SOFaa) under mineral oil. Culture medium SOFaa consisted of 5 mM NaHCO3, 107.7 mM NaCl, 7.1 mM KCl, 1.2 mM KH2PO4, 1.5 mM MgSO4, 7.3 mM sodium pyruvate, 0.2 mM L-glutamine, 1.8 mM sodium citrate, 1.8 mM CaCl22H2O, 5.4 mM sodium lactate, 1X essential aminoacids, 1X non-essential amino acids, 2.8 mM de myoinositol, 5 mg/mL BSA. Embryos were cultured for 7 days at 38.5°C in 5% CO2, 5% O2, 90% N2 with maximum humidity (Sanyo MCO 175M, Japan). Only morphologically normal embryos were included in the study.
Somatic cell nuclear transfer (SCNT)
Primary bovine fetal fibroblasts (BFF) were established from a day 25 postconception fetus by disaggregation of whole body without head and viscera and cultured in DMEM (Gibco) supplemented with 10% fetal FCS at 38.5°C in 5% CO2 and humidified air. All procedures were performed as previously described [31], with minor modifications. After 18 h of maturation, loosely associated cumulus cells were removed by vortexing for 1–2 min in Hepes-buffered SOF (H-SOF) (SOFaa supplemented with 20 mM HEPES) containing 1 mg/mL hyaluronidase. Denuded oocytes were washed and returned to maturation medium. Enucleation process was initiated within 30 min of oocyte denudation under an inverted microscope (Nikon Eclipse TE-300, Nikon, Japan) and micromanipulators (NT 88 V3, Nikon Narishige, Japan).
Oocytes were incubated for 5–10 min in H-SOF supplemented with 1 mg/mL Hoechst 33342 at 38°C, subsequently placed into manipulation drops (H-SOF supplemented with 1% FCS covered with mineral oil) and enucleated after a brief exposure to UV light (Nikon Filter Set 01; Nikon, Tokyo, Japan) to determine the location of DNA.
Passage 3 donor BFF cells were collected from the culture plates by trypsinization using 0.05% trypsin-EDTA, washed twice, and finally resuspended in H-SOF. Cells were picked up with the transfer needle and slipped into the perivitelline space of enucleated oocytes. Cell-cytoplast couplets were fused immediately after cell transfer using a 0.5-mm gap fusion chamber (BTX, San Diego, CA, USA) overlaid with sorbitol fusion medium (0.25 M sorbitol, 0.5 mM magnesium acetate, 0.1% BSA) with 25 µsec of 2.4 kV/cm pulse (BTX Electrocell Manipulator 630, Harvard Apparatus, MA, USA). The post-fusion culture medium consisted of H-SOF supplemented with 5% FCS and 5 mg/mL cytochalasin B. After 1 h, any non-fused couplets underwent a second fusion procedure as described above. Twenty minutes after the second fusion procedure, fused couplets were activated by the exposure of the structures to 5 µM ionomycin in H-SOF medium for 4 min, then rinsed three times in H-SOF and allocated to a 4-h culture in 2 mM 6-DMAP at 38.5°C in 5% CO2 in air with maximum humidity. After this treatment, presumptive embryos were rinsed in H-SOF and cultured as described previously.
In vivo production of embryos
Braford and Brangus cows from the experimental herds of the Instituto de Investigaciones Biotecnológicas (Buenos Aires, Argentina) were superovulated using an intravaginal device with 1 g medroxyprogesterone (Syntex, Argentina). On the same day (day 0), cows were administered with an IM dose of 30 IU of progesterone (Rio de Janeiro, Argentina) and 27 mg of estradiol benzoate (estradiol-17β) (Rio de Janeiro, Argentina). On day 4, cows received an ovarian stimulation protocol twice daily for four days (AM-PM, every 12 h), with decreasing concentrations of (FSH) (Folltropin, Bioniche, Canada) as follows: 40 mg on day 4, 30 mg on day 5, 20 mg on day 6, and 10 mg on day 7. On day 7, the intravaginal device was removed and cows were administered with 500 mg of coprostenol (a synthetic analog of prostaglandin F2a) (Syntex) and ovulation was induced with GnRH (Gonasyn, Syntex). On day 9, double inseminations were performed, separated by an interval of 8 h. The embryos were recovered on day 16 of the protocol (7 days after artificial insemination) using a uterine three-way lavage Foley catheter and 1 L of wash medium phosphate buffered saline (PBS), supplemented with 50 µg/mL kanamycin. At the end of embryo recovery, donors received 2 mL of coprostenol to avoid pregnancies of unrecovered embryos.
Real-time reverse transcription polymerase chain reaction (qRT-PCR)
Groups of 10 embryos from the three sources (in vivo, IVF, SCNT) were homogenized in TRIzol reagent. To increase total RNA concentration, 3.5 µg of yeast tRNA was added as a carrier (Invitrogen, Life Technologies, Carlsbad, CA, USA) using a tissue homogenizer. Total RNA was prepared from TRIzol homogenates according to the manufacturer's instructions. Briefly, Poly(A)+ RNA was purified from total RNA using the PolyA Tract mRNA Isolation System (Promega, Madison, WI, USA) with the only modification being a 10-fold reduction in recommended reagent amounts for all steps [32]. Messenger RNA (mRNA) was transcribed using Superscript II enzyme (Invitrogen, Life Technologies) following the manufacturer's instructions. After reverse transcription, all the samples were diluted accordingly (1/2, 1/10) and used as template. The RT-qPCR reactions were carried out in an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Primer sequences were designed using Primer Express Software v3.0 (Applied Biosystems Foster City, CA, USA). We selected the primer sets that amplified the sequences as close as possible to the 3′ end of the target genes (Table 1). Amplicons were 60–100 bp long. The reactions were carried out with the SYBRGREEN PCR Master Mix (Invitrogen) [32]. To verify that the SYBR Green dye detected only one PCR product, all the reactions were subjected to the heat dissociation protocol after the final cycle of the PCR [33]. All the samples were tested against Glyceraldehyde3-phosphate dehydrogenase (GAPDH) as a reference gene for data normalization. Each qRT-PCR quantitation experiment was performed in triplicate for three independently generated cDNA templates. Group means were analyzed for statistical significance using a t-test with the software Analyse-it for Microsoft Excel (Analyse-it Software, Ltd. England, UK).
Table 1. List of primers used for qRT-PCR for the specific selected genes.
| Gene identification | Primer sequences (forward and reverse, 5→3) | Accession Number | Product length (bp) |
| BAX (BCL-2 associated X protein) | F: GCGCATCGGAGATGAATTG | NM_173894.1 | 59 |
| R: CCACAGCTGCGATCATCCT | |||
| Caspase-3 (cysteine aspartate protease) | F: TACTTGGGAAGGTGTGAGAAAACTAA | NM_001077840.1 | 71 |
| R: AACCCGTCTCCCTTTATATTGCT | |||
| HSP70 (heat shock protein 70 - kDa) | F: CCATCTTTTGTCAGTTTCTTTTTGTAGTA | NM_203322.2 | 75 |
| R: GGAAGTAAACAGAAACGGGTGAA | |||
| BIP (immunoglobulin heavy chain binding protein) | F: GATTGAAGTCACCTTTGAGATAGATGTG | NM_001075148.1 | 85 |
| R: GATCTTATTTTTGTTGCCTGTACCTTT | |||
| PSMB5 (proteasome subunit β5) | F: GCTTCTGGGAGAGGCTGTTG | NM_001037612.1 | 69 |
| R: CGGAGATGCGTTCCTTGTTT | |||
| GAPDH (Glyceraldehyde3-phosphate dehydrogenase) | F: GGTTGTCTCCTGCGACTTCAA | NM_001034034.1 | 64 |
| R: AATGCCAGCCCCAGCAT |
Statistical analysis
Statistical analyses were conducted using InfoStat Software for Windows (Universidad Nacional de Córdoba, Argentina). Treatment effects were assessed by one-way or two-way ANOVA followed by Tukey multiple-comparison post-hoc test to identify individual differences between means. When the data exhibited a skewed distribution, square-root transformations of the basic variables were performed before the statistical analysis. The experimental groups were always analyzed in parallel. The number of biological (n) material was established as previously described [13]. All values are presented as means with their corresponding SEM. Statistical significance was set at P≤0.05.
Results
In vitro embryo production
A total of 37 embryos were produced by IVF, with a global blastocyst production efficiency of 22.6%, whereas 30 embryos that were generated by SCNT, exhibited an overall efficiency of 18% (Table 2). Finally, 30 in vivo-derived embryos were used as control (Table 3).
Table 2. Global efficiency of assisted reproductive technologies on cleavage and blastocyst rates.
| Reproductive technique | Total Oocytes | Total 2-cell embryos on day 2 (%) | Total Blastocysts/presumptive zygotes on day 8 (%) |
| IVF | 196 | 164 (83.7) 160 (59.7) | 37 (22.6) 30 (18.8) |
| SCNT | 268 |
a Data are expressed as mean ± standard error of the mean (SEM). B Three replicates were performed.
Table 3. In vivo embryo production.
| Cow | Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Total |
| No. of blastocysts | 8 | 6 | 7 | 4 | 5 | 30 |
Embryos produced through assisted reproductive technologies and in vivo-derived embryos were compared using differential staining. No statistical differences were found between the different sources of blastocysts with regard to cell numbers (data not shown).
Gene expression analysis
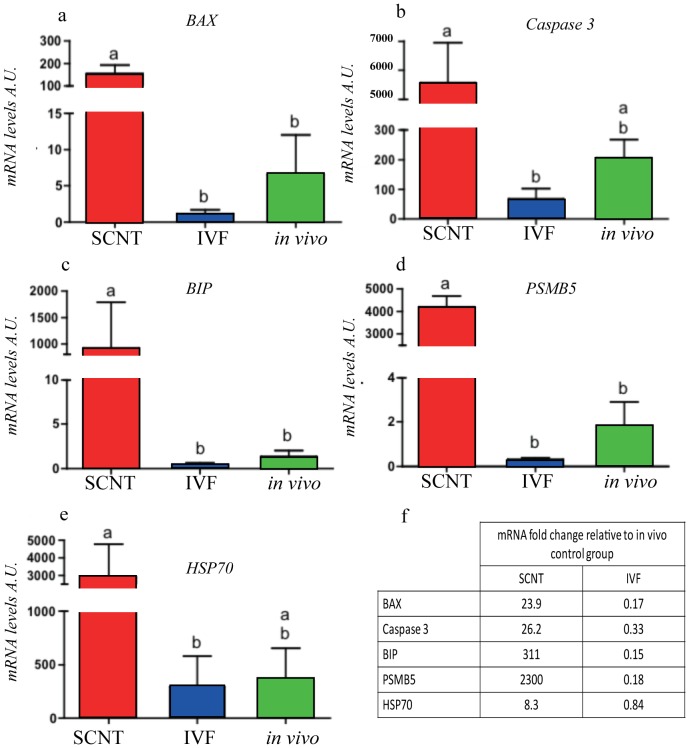
A series of real-time PCR assays was established to detect mRNA expression levels of Bax, Caspase-3, Bip, PSMB5, and HSP70. The RA of transcripts from SCNT- and IVF-derived embryos were compared with that of in vivo-produced control embryos. The expression levels of the pro-apoptotic Bax gene were significantly higher (p = 0.0001) in SCNT-derived embryos (23.9 fold higher) that the in vivo blastocyst group (Figure 1a). On the other hand, for IVF and in vivo control embryos, we found no statistical differences in Bax expression (Figure 2a). Similar mRNA expression profiles were observed in blastocysts from the three sources for the Bip and PSMB5 genes (Figure 2c, d). The RA of transcripts for PSMB5 was significantly increased (p<0.0001) in SCNT blastocysts compared with the in vivo produced group. In parallel, as illustrated in Figure 2c, the expression levels for Bip were 311 fold higher in SCNT embryos than in the control group (p = 0.0209). Although the standard deviation between the groups of blastocysts produced by SCNT was considerable, embryos produced either by IVF or in vivo generally showed significantly (p<0.05) lower RA of transcripts for Bax, Bip and PSMB5 than embryos produced by SCNT.
Figure 2. Quantification of mRNA levels for (a) Bax (b) Caspase-3 (c) Bip (d) PSMB5 proteasome subunit β5 and (e) HSP70 by real-time RT-PCR in bovine blastocysts produced either in vitro (IVF or SCNT) or in vivo.
(f) Table of mRNA expression levels presented as fold change relative to control embryos. Normalized transcription levels are shown as mean ± standard error of the mean (SEM). Different superscripts indicate statistical differences between treatment groups (P<0.05). Data were obtained from three replicates of independent groups of 10 embryos each.
Interestingly, while higher gene expression was observed for the selected genes in SCNT embryos compare to the in vivo derived control embryos, the IVF embryos had a tendency for lower levels of expression of these genes compare to control. No statistical differences were found between the assisted reproductive technique IVF and in vivo control embryos with regard to the Caspase-3 and HSP70 gene expression patterns (Figure 2b,e). However, the RA of mRNA for these two genes in SCNT-derived embryos was higher than that of the IVF group, 26.2 vs 0.33 and 8.3 vs 0.84 for Caspase-3 and HSP70, respectively, as compared to the control group.
Our results led us to focus on the genes selected as possible markers for embryonic stress. As shown in Figure 2, the SCNT technique significantly enhanced the expression levels of the genes selected.
Discussion
It has been shown that the use of several assisted reproductive technologies such as IVF and SCNT can affect embryo quality in terms of gene expression and epigenetic marks in oocytes and embryos as compared with in vivo-produced embryos [34], [35]. Large offspring syndrome (LOS) is a molecular phenomenon characterized by difficult parturition due to increased birth weight of the calves, fetal and neonatal losses, or an unhealthy status, occasionally reported in in vitro-produced embryos [30]. As indicators of embryo quality and viability, we analyzed a group of key genes involved in the response to generalized cellular stress, ER stress, and regulation and activation of programmed cell death. We compared the mRNA expression profiles for Bax, Caspase-3, Bip, PSMB5, and HSP70 in bovine embryos that were produced by different assisted reproductive technologies.
In recent years, temporal expression profiles of various gene transcripts have been studied at different stages of embryonic development [17], [35], [36], [37]. The genes analyzed in these previous studies are involved in the pre- and post-implantation processes of embryo development, as compaction and cavitation (E-cadherin, Connexin 41 and 43), metabolism (Glut 5, Glut 1, IGF II, G6PDH), DNA methylation (DNMT), oxidative stress (SOX, SOD and MnSOD), apoptosis (Bax, caspase-7 and p53), trophoblast integrity (INFτ) and signs of growth factors (IGF and IGFR). To ensure non-maternal origin, we analyzed the RA in embryos that were at the blastocyst stage, where the levels of mRNA and transcriptional regulator factors are under the control of embryonic genome. Our results show that the RA of three of the stress indicators studied was significantly increased in SCNT embryos as compared with that of in vivo-derived blastocysts for Bax, PSMB5 and Bip.
Previous reports have shown a correlation between the effect of the culture system on the incidence of apoptosis and the level of the pro-apoptotic gene, Bax, in the quality and clinical normality of in vitro-produced embryos [32]. The RA of Bax is significantly higher when SOF medium is used for cultures of in vitro-derived bovine blastocysts compared with the in vivo-produced counterparts. Blastocysts co-cultured in the presence of somatic cells also show up-regulated Bax levels [2], [13], [38]. Moreover, it is noteworthy that this gene is also overexpressed in in vitro bovine blastocysts cultured in SOF medium supplemented with serum [39].
Furthermore, it has been suggested that the interaction of the expression of Bax and HSP70 may occur under stress conditions, with suppression of Bax activation in cells with high HSP70 levels [40]. In the present study, we found no significant difference in the abundance of transcripts for HSP70 and Caspase-3. However, conflicting results have been reported and the potential use of mRNA expression of BCL-2, Bax, Caspase-3 and Caspase-7 as markers for apoptosis in bovine blastocysts has not been clarified in single embryo detection [41]. Previous reports have demonstrated that bovine blastocysts produced in vitro with low amounts of activated group II caspase activity have increased potential for development to the hatched blastocyst stage [1]. These reports also suggest that determination of caspase activity may be a useful tool for the selection of embryos that are to be transferred into recipients [1].
The increased expression of PSMB5 and Bip found in the present study is perhaps not surprising, suggesting that SCNT-derived embryos were under conditions of ER stress. When ER stress conditions persist, initiation of apoptotic processes is promoted. Furthermore, the significantly higher transcript levels of Bax could imply initial activation of apoptotic mechanisms, but the low levels of Caspase-3 reveals that the apoptotic caspase cascade signaling system was not yet activated. This could be explained because caspases are secreted as inactive procaspases, which are only active after further modification. Biologically active caspase-3 and -7 are executioner caspases only activated during the process of apoptosis by active precursor caspases-8 and -9. Taking this into account, mRNA levels of caspases can only reflect the amount of procaspases, but not of the level of biologically active caspases. Very little is known on the exact timing of the successive steps in the apoptotic pathways of early embryos. An apoptotic response is thought to depend on the balance between cell survival and cell death inducers [42]. Here, the hatching rate based on the total number of blastocysts was similar in embryos resulting from mature oocytes that were either fertilized or enucleated to perform SCNT.
With regard to IVF-derived embryos, no significant differences were detected in the RA of the genes investigated as compared with that of in vivo-produced blastocysts. Besides the fact that higher gene expression was observed for the selected genes in SCNT embryos compare to the in vivo derived control embryos, the IVF embryos had a tendency for lower levels of expression. This could be explained considering that genes expressed in the IVF embryos may be specifically affected by in vitro oocyte maturation and fertilization. Control embryos are produced from oocytes matured and fertilized in vivo. Previous studies compared in vivo matured, fertilized, developed (AI) and in vivo matured, fertilized but in vitro cultured (IVD) embryos to solely in vitro produced IVF embryos, they discovered that genes involved in RNA processing and binding were differentially expressed, that is, down-regulated in the IVF embryos [43]. Moreover, gene expression differences between the IVF and SCNT embryos may be partially attributed to developmental competence variability (compared with in vivo embryos), as well as the maternally inherited genetic variability among IVF embryos.
Differential mRNA expression in bovine blastocysts obtained by different reproductive techniques produces blastocysts of divergent quality. Even though the embryos were morphologically comparable, classified as Grade 1 (excellent quality), the mRNA expression pattern was dissimilar. The RA differences found may explain the lower developmental capacity of SCNT embryos following transfer, which adversely affects their ability to establish pregnancy and produce a live calf.
Conclusions
The present work analyzes the RA of a set of genes involved in cellular stress (HSP70), ER stress (Bip, PSMB5), and apoptosis (Bax, Caspase-3) in bovine blastocysts produced by IVF or SCNT as compared with that of their in vivo counterparts. The results presented in this paper propose a new set of stress-indicator genes that provide a widespread idea of the quality of pre-implantation bovine embryos. The apoptosis incidence in embryos is influenced mainly by the conditions and characteristics of maturation of the oocyte from which they are originated [1].
In this study, the oocyte in vitro maturation, as well as the embryo in vitro culture processes for the IVF and SCNT embryo generation followed exactly the same procedures. This allowed alleviating the potential biases in the comparison of gene expression patterns due to IVC conditions. The high incidence of pregnancy loss and neonatal death after SCNT has been hypothesized to result from incomplete nuclear reprogramming. Several studies have compared gene expression in somatic donor cells and the embryos resulting from them [44], [45]. In the present study, we observed higher gene expression for the selected genes in SCNT embryos compare to the IVF, and the in vivo derived control embryos. We cannot rule out the possibility of incomplete nuclear reprogramming in our donor cells. In a previous study, a comparisson of global gene expression profiles of individual bovine SCNT blastocysts with their somatic donor cells and fertilized control embryos suggested that the commonly observed low developmental efficiency of NT embryos may not be largely due to nuclear reprogramming during early embryo development (reprogramming of the somatic donor cell genome from a differentiated to a totipotent status, i.e., gene dedifferentiation) but may be potentially caused by abnormal gene reprogramming during postimplantation fetal/placental development [45]. Overall, our study could potentially complement morphological qualitative analysis by helping to develop an efficient, effective, and accurate technique to diagnose embryo quality, ultimately aiding to improve the efficiency of artificial reproductive techniques.
Acknowledgments
The authors are grateful to Dr. Pascal Mermillod at Physiologie de la Reproduction et des Comportements INRA-CNRS-Université de Tours-Haras Nationaux, France for carefully reading our work and for his helpful comments. We acknowledge the help provided by Carolina Herrera.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
Funding support by grant of Universidad Nacional de San Martín, number 002/2011. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jousan FD, De Castro LA, Brad AM, Roth Z, Hansen PJ (2008) Relationship between group II caspase activity of bovine preimplantation embryos and capacity for hatching. J. Reprod Dev vol 54: No.3. [DOI] [PubMed] [Google Scholar]
- 2. Lonergan P, Rizos D, Gutiérrez-Adán A, Moreira PM, Pintado B, et al. (2003) Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 69: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 3. Wrenzycki C, Herrmann D, Lucas-Hahn A, Lemme E, Korsawe K, et al. (2004) Gene expression patterns in in vitro-produced and somatic nuclear transfer-derived preimplantation bovine embryos: relationship to the large offspring syndrome? Anim Reprod Sci 82–83: 593–603. [DOI] [PubMed] [Google Scholar]
- 4. Eckert J, Niemann H (1998) mRNA expression of leukaemia inhibitory factor (Lif) and its receptor subunits glycoprotein 130 and Lif-receptor-beta in bovine embryos derived in vitro or in vivo. Mol Hum Reprod 4: 957–965. [DOI] [PubMed] [Google Scholar]
- 5. Wrenzycki C, Herrmann D, Carnwath JW, Niemann H (1999) Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol Reprod Dev 53: 8–18. [DOI] [PubMed] [Google Scholar]
- 6. Wrenzycki C, Herrmann D, Korsawe K, Hadeler K-G, Niemann H (2000) Relative abundance of specific mRNAs in bovine embryos produced in vivo or in vitro employing two different culture systems. Theriogenology 53: 415. [Google Scholar]
- 7. Wrenzycki C, Herrmann D, Keskintepe L, Martins A Jr, Sirisathien S, et al. (2001) Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Hum Reprod 16: 893–901. [DOI] [PubMed] [Google Scholar]
- 8. Doherty AS, Mann MRW, Tremblay KD, Bartolomei MS, Schultz RM (2000) Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 62: 1526–1535. [DOI] [PubMed] [Google Scholar]
- 9. Lee KF, Chow JFC, Xu JS, Chan STH, Ip SM, et al. (2001) A comparative study of gene expression in murine embryos developed in vivo, cultured in vitro, and cocultured with human oviductal cells using messenger ribonucleic acid differential display. Biol Reprod 64: 910–917. [DOI] [PubMed] [Google Scholar]
- 10. Lequarre AS, Feugang JM, Malhomme O, Donnay I, Massip A, et al. (2001) Expression of Cu/Zn and Mn superoxide dismutases during bovine embryo development: influence of in vitro culture. Mol Reprod Dev 58: 45–53. [DOI] [PubMed] [Google Scholar]
- 11. Minami N, Sasaki K, Aizawa A, Miyamoto M, Imai H (2001) Analysis of gene expression in mouse 2-cell embryos using fluorescein differential display: comparison of culture environments. Biol Reprod 64: 30–35. [DOI] [PubMed] [Google Scholar]
- 12. Rief S, Sinowatz F, Stojkovic M, Einspanier R, Wolf E, et al. (2002) Effects of a novel co-culture system on development, metabolism and gene expression of bovine embryos produced in vitro. Reproduction 124: 543–56. [PubMed] [Google Scholar]
- 13. Rizos D, Lonergan P, Boland MP, Arroyo-Garcia R, Pintado B, et al. (2002) Analysis of differential mRNA expression between bovine blastocysts produced in different culture systems: Implications for blastocyst quality. Biol Reprod 66: 589–595. [DOI] [PubMed] [Google Scholar]
- 14. Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, de la Fuente J, Boland MP, et al. (2003) Bovine embryo culture in the presence or absence of serum: Implications for blastocyst development, cryotolerance and messenger RNA expression. Biol Rep 68: 236–243. [DOI] [PubMed] [Google Scholar]
- 15. Niemann H, Wrenzycki C (2000) Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology 53: 21–34. [DOI] [PubMed] [Google Scholar]
- 16. Arroyo-Garcia R, Oter M, Martinez-Zapater JM, Pintado B, de la Fuente J, et al. (2001) RT-AFLP-based analysis of specific genetic expression of in vivo or in vitro produced bovine blastocysts. Theriogenology 55: 409. [Google Scholar]
- 17. Gutiérrez-Adán A, Rizos D, Fair T, Moreira PN, Pintado B, et al. (2004) Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol Reprod Dev 68: 441–448. [DOI] [PubMed] [Google Scholar]
- 18. Lonergan P, Rizos D, Gutiérrez-Adán A, Fair T, Boland MP (2003) Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod Dom Anim 38: 259–267. [DOI] [PubMed] [Google Scholar]
- 19. Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27: 437–496. [DOI] [PubMed] [Google Scholar]
- 20. Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, et al. (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3: 839–843. [DOI] [PubMed] [Google Scholar]
- 21. Shinichi T, Reed JC, Homma S (2003) Heat-shock proteins as regulators of apoptosis. Oncogene 22: 9041–9047. [DOI] [PubMed] [Google Scholar]
- 22.Gething MJ (1999) Role and regulation of the ER chaperone BiP. Seminars in Cell and Developmental Biology vol 10. [DOI] [PubMed]
- 23. Paula-Lopes FF, Hansen PJ (2002) Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol. Reprod 66: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 24. Paula-Lopes FF, Hansen PJ (2002) Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem Biophys Res Commun 295: 37–42. [DOI] [PubMed] [Google Scholar]
- 25. Krininger CE, Stephens SH, Hansen PJ (2002) Developmental changes in inhibitory effects of arsenic and heat shock on growth of pre-implantation bovine embryos. Mol Reprod Dev 63: 335–340. [DOI] [PubMed] [Google Scholar]
- 26. Feugang JM, de Roover R, Moens A, Léonard S, Dessy F, et al. (2004) Addition of b-mercaptoethanol or Trolox at the morula/blastocyst stage improves the quality of bovine blastocysts and prevents induction of apoptosis and degeneration by prooxidant agents. Theriogenology 61: 71–90. [DOI] [PubMed] [Google Scholar]
- 27. Fear JM, Hansen PJ (2011) Developmental changes in expression of genes involved in regulation of apoptosis in the bovine preimplantation embryo. Biol. Reproduction 84: 43–51. [DOI] [PubMed] [Google Scholar]
- 28. Melka MG, Rings F, Hölker M, Tholen E, Havlicek V, et al. (2010) Expression of apoptosis regulatory genes and incidence of apoptosis in different morphological quality groups of in vitro-produced bovine pre-implantation embryos. Reprod Dom Anim 45: 915–921. [DOI] [PubMed] [Google Scholar]
- 29. Robert C, Hue I, McGraw S, Gagne D, Sirard MA (2002) Quantification of cyclin B1 and p34(cdc2) in bovine cumulus-oocyte complexes and expression mapping of genes involved in the cell cycle by complementary DNA macroarrays. Biol Reprod 67: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 30. Mucci N, Aller J, Kaiser GG, Hozbor F, Cabodevila J, et al. (2006) Effect of estrous cow serum during bovine embryo culture on blastocyst development and cryotolerance after slow freezing or vitrification. Theriogenology 65 (8): 1551–62. [DOI] [PubMed] [Google Scholar]
- 31. Baldassarre H, Wang B, Pierson J, Neveu N, Sneek L, et al. (2004) Prepubertal propagation of transgenic cloned goats by laparoscopic ovum pick-up and in vitro embryo production. Cloning Stem Cells 6 (1): 25–9. [DOI] [PubMed] [Google Scholar]
- 32. Brocco MA, Pollevick GD, Frasch AC (2003) Differential regulation of polysialyltranferases during hippocampus development. Implications for neuronal survival. J. Neurosci Res vol 74: 744–753. [DOI] [PubMed] [Google Scholar]
- 33. Ririe KM, Rasmussen RP, Wittwer CT (1997) Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Analytical Biochem 245: 154–160. [DOI] [PubMed] [Google Scholar]
- 34. Niemann H, Wrenzycki C, Lucas-Hahn A, Brambrink T, Kues W, et al. (2002) Gene expression patterns in bovine in vitro-produced and nuclear transfer-derived embryos and their implications for early development. Cloning Stem Cells 4 (1): 29–38. [DOI] [PubMed] [Google Scholar]
- 35. Wrenzycki C, Herrmann D, Carnwath JW, Niemann H (2007) Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology 68 S: S77–S83. [DOI] [PubMed] [Google Scholar]
- 36. Lazzari G, Wrenzycki C, Herrmann D, Duchi R, Kruip T, et al. (2002) Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol Reprod 67: 767–75. [DOI] [PubMed] [Google Scholar]
- 37. Lonergan P, Gutiérrez-Adán A, Rizos D, Pintado B, de la Fuente JD, et al. (2003) Relative messenger RNA abundance in bovine oocytes collected at the LH surge and matured in vitro or their counterparts matured in vivo. Theriogenology 59: 493. [Google Scholar]
- 38. Lonergan P (2005) Predicting embryo quality: mRNA expression and the preimplantation embryo. Reprod Biomed Online 11: 340–8. [DOI] [PubMed] [Google Scholar]
- 39. Yang MY, Rajamahendran R (1999) Involvement of apoptosis in bovine blastocysts produced in vitro. Theriogenology 51 336: Abstract. [Google Scholar]
- 40. Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD (2005) Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 280: 38729–3. [DOI] [PubMed] [Google Scholar]
- 41. Vandaele L, Goossens K, Peelman L, Van Soom A (2008) mRNA expression of Bcl-2, Bax, caspase-3 and -7 cannot be used as a marker for apoptosis in bovine blastocysts. Anim Reprod Sci 168–173 [DOI] [PubMed] [Google Scholar]
- 42. Badr H, Bongioni G, Abdoon ASS, Kandil O, Puglisi R (2007) Gene expression in the in vitro-produced preimplantation bovine embryos. Zygote 15: 355–367. [DOI] [PubMed] [Google Scholar]
- 43. Smith SL, Everts RE, Sung LY, Du F, Page RL, et al. (2009) Gene expression profiling of single bovine embryos uncovers significant effects of in vitro maturation, fertilization and culture. Mol Reprod Dev 76(1): 38–47. [DOI] [PubMed] [Google Scholar]
- 44. Beyhan Z, Forsberg EJ, Eilertsen KJ, Kent-First M, First NL (2007) Gene expression in bovine nuclear transfer embryos in relation to donor cell efficiency in producing live offspring. Mol Reprod Dev 74 (1): 18–27. [DOI] [PubMed] [Google Scholar]
- 45. Smith SL, Everts RE, Tian XC, Du F, Sung LY, et al. (2005) Global gene expression profiles reveal significant nuclear reprogramming by the blastocyst stage after cloning. Proc Natl Acad Sci U S A 102(49): 17582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.