Abstract
Willow species have been suggested for use in the remediation of contaminated soils due to their high biomass production, fast growth, and high accumulation of heavy metals. The tolerance and accumulation of metals may vary among willow species and varieties, and the assessment of this variability is vital for selecting willow species/varieties for phytoremediation applications. Here, we examined the variations in lead (Pb) tolerance and accumulation of three cultivated varieties of Salix integra (Weishanhu, Yizhibi and Dahongtou), a shrub willow native to northeastern China, using hydroponic culture in a greenhouse. In general, the tolerance and accumulation of Pb varied among the three willow varieties depending on the Pb concentration. All three varieties had a high tolerance index (TI) and EC50 value (the effective concentration of Pb in the nutrient solution that caused a 50% inhibition on biomass production), but a low translocation factor (TF), indicating that Pb sequestration is mainly restricted in the roots of S. integra. Among the three varieties, Dahogntou was more sensitive to the increased Pb concentration than the other two varieties, with the lowest EC50 and TI for root and above-ground tissues. In this respect, Weishanhu and Yizhibi were more suitable for phytostabilization of Pb-contaminated soils. However, our findings also indicated the importance of considering the toxicity symptoms when selecting willow varieties for the use of phytoremediation, since we also found that the three varieties revealed various toxicity symptoms of leaf wilting, chlorosis and inhibition of shoot and root growth under the higher Pb concentrations. Such symptoms could be considered as a supplementary index in screening tests.
Introduction
Heavy metal contamination in water, air, or soil is a major environmental concern worldwide [1]–[3]. Excessive levels of heavy metals can be introduced into the environment by mining, smelting, electroplating, use of pesticides or fertilizers, industrial discharge, etc. [1], [4]–[7]. In China, twenty million hectares of agricultural land have been polluted with heavy metals, and it has been reported that each year over 12 million tons of grain are contaminated by toxic metals [8]. Heavy metals cannot be degraded but can be stabilized or extracted by plants, in a process known as “phytoremediation” [1], [4], [9], [10]. The phytoremediation of heavy metals is affected by several factors, such as the species of plant, solubility in soil solution, and the translocation of the heavy metals from the soil to the harvestable plant parts, etc. [11], [12]. Of these factors, the plant species plays a crucial role, and therefore, selecting appropriate plant species is important for the successful application of plant-based remediation techniques.
Lead (Pb) is one of the most toxic metals, and its concentration in agricultural soil has rapidly increased due to various anthropogenic inputs [13]. Lead is not a bio-essential element, but is easily absorbed by and accumulated in plants [14], [15]. Many studies have shown that the patterns of Pb uptake, transport and accumulation in plants are strongly governed by plant factors, and that Pb accumulation and distribution vary largely among different plant organs [3], [16]. Most studies have shown that, for the majority of plant species, Pb uptake is restricted to roots, with only a small portion being translocated to the shoots [12], [17]–[19]. Whereas hyperaccumulation is an exception, for instance, Thlaspi rotundifolium, a cadmium hyperaccumulator, is capable of accumulating up to 8,200 mg kg−1 Pb in the shoots [10]. Despite the abilities of these plants to accumulating high amounts of Pb in above-ground tissues, their application in contaminated soil remediation is often limited because of their slow growth rate and low biomass yield [20]. In contrast, other species e.g., Brassica juncea (cv.426308) have been shown to accumulate high Pb concentrations in shoots (34,500 mg kg−1) and in addition have high biomass which allows high metal removals [12]. However, their growth performance and metal-accumulating abilities can also be limited by low pH and high Pb concentration (>1, 500 mg kg−1) in the soil [21].
Plant species are selected for use in the remediation of heavy metal contamination based on factors, such as high biomass production, ability to take up and accumulate metals, deep root systems, high growth rate, and ease of planting and maintenance [1], [22]–[24]. Trees, especially those fast-growing woody species, with their large biomass and deeper, more integrated root systems, have provided a unique means for deep phytoremediation of soil or water during recent decades [11], [25], [26]. In recent years, willow species, which could be grown intensively for use in energy production, have been suggested for use in the remediation of metal contaminated soils. A number of Salix species have been studied for their ability to tolerate and accumulate heavy metals [27]–[30]. Ali et al. [9] demonstrated that Salix acmophylla can accumulate considerable amounts of Cu, Ni and Pb in different plant parts and exhibits high tolerance to these metals. Tlustos et al. [30] reported that S. smithiana Willd. is able to accumulate 456 mg kg−1 and 26.6 mg kg−1 Pb in the roots and trunk, respectively, but no more than 10 mg kg−1 Pb was observed in the twigs and leaves. Most of the studies confirmed that Salix species are able to accumulate a high concentration of Pb in the root systems and have the potential for phytostablization of Pb, and that the uptake patterns and Pb accumulation in Salix vary among species and varieties [30], [31]. However, the critical concentration causing Pb-induced phytotoxicity or growth inhibition in different Salix species or varieties is still unknown. Hydroponic methods are effective in the rapid screening for heavy metal tolerance and accumulation in plants, and have been widely used in evaluating the phytoremediation potential of willow species in recent years [18], [29].
The aim of our study was to detect the Pb accumulation potential, especially the critical toxicity thresholds based on EC50 estimates in three cultivated varieties of Salix integra using hydroponic methods. The results are important for effective selection of willow species for phytoremediation application.
Salix integra, a shrub willow native to northeastern China, Japan, Korea, and Primorsky Krai in the far southeast of Russia, has been identified as a Cd-accumulating plant [32], [33]. Cultivation and planting of S. integra for shoot and biomass production have a long history in China, and many cultivated varieties are grown in northeastern China. Yizhibi, Dahongtou and Weishanhu are three cultivated varieties of S. integra with high biomass production and easy cultivation [34]–[36]. In previous studies, we have confirmed that S. integra was able to tolerate and accumulate high concentrations of Cd and Zn in hydroponic culture, and we measured their differences in Cd uptake and accumulation, as well as the tolerance indices, in the S. integra varieties [35]–[37].
In the present study, we hypothesized that the three varieties of S. integra could take up and accumulate Pb in their roots and above-ground parts, and that Pb accumulation would vary among the varieties according to the Pb contamination level. To test this hypothesis, hydroponic culture experiments were conducted to compare the Pb tolerance and accumulation among the three varieties of S. integra and to determine the critical level of Pb for their normal growth. The results are expected to improve our understanding of metal accumulation patterns in Salix species and provide guidance for future application of S. integra in the phytoremediation of Pb contaminated soils and/or water.
Materials and Methods
Experimental site and willow preparation
The experiment was conducted in a greenhouse in Fuyang, Hangzhou, Zhejiang Province, P. R. China (30°03′N, 119°57′E) in May, 2011. The greenhouse temperature was maintained between 20–25°C, with a natural photoperiod. The S. integra varieties Weishanhu, Yizhibi and Dahongtou were obtained from native habitats in Shandong Province. No specific permits were required to extract samples from this site, which is not privately-owned, and this study did not involve protected species. Cuttings (8–10 cm) from 1-year-old stems in nursery beds at the Institute of Subtropical Forestry were selected for uniformity based on the diameter (Φ 0.4–0.5 cm) and the number of buds (4–6 buds per cutting). Cuttings were rooted in tap water for 4 wk in hydroponic pots (50 cm×35 cm×15 cm, length × width × height) and then transferred to 15 L aerated Knop's solution [38] (6.1 mM Ca (NO3)2, 2.5 mM KNO3, 1.6 mM KCl, 1.8 mM KH2PO4, 2.1 mM MgSO4 and 3.8 µM FeCl3) in each pot, maintaining a constant pH of 5.5 using 1 M HCl or 1 M NaOH.
To avoid possible Pb precipitation caused by the presence of phosphorus (P) in the nutrient solution, the P concentration was kept at a maximum of 0.04 µM according to Zhivotovsky et al. [18].
Experimental design
After 2 wk of plant growth in the aerated solution, Pb treatments with the final concentration of 47 µM (T1), 123 µM (T2), 178 µM (T3), and 196 µM Pb (T4) were applied as Pb(NO3)2 for 14 days, with normal nutrient solution as the control. Each treatment was replicated three times, with each replicate consisting of one pot containing six plants for each variety. These pots were arranged in a completey randomized block design.
The metal and nutrient solutions were replaced every 2 d to ensure consistency. Metal-related phytotoxicity symptoms were recorded throughout the experiment. At the end of the growth and Pb treatment period, the plant tissues were separated into roots, wood (the original cuttings), new shoots, and leaves, washed with tap water and rinsed with deionized water. The root length, surface area, volume, diameter and number of root tips of each plant were determined using root scan apparatus (Epson V700), equipped with WinRHIZO software (Regent Instruments Co.). The root tissues were then washed in 1 M HCl solution and rinsed again with deionized water. Samples were dried at 70°C for 48 h and the dry weights were recorded.
Estimation of chlorophyll content
As a non-destructive measurement, we used an Opti-Sciences CCM-200 chlorophyll-meter to estimate the chlorophyll content by recording the Chlorophyll Concentration Index (CCI).
Pb analysis in plant tissues
All plant tissue samples were ashed at 500°C in a muffle furnace, and the ash was dissolved in 10 ml of 1 M HCl solution, and diluted in double deionized water to a 50 ml volume prior to analysis. The total Pb concentration in the liquid samples was determined using inductively coupled plasma optical emission spectrometer (Varian 725-ES, Palo Alto, CA, USA). Certified reference materials (Mixed shoots of shrubs from Pb-Zn mine tailings, GBW 07602, China) were used to ensure the quality of analyses. Good agreement was obtained between our method and certified values. The total metal contents (mg plant−1) in the roots and above-ground tissues (wood, new shoots, and leaves) were calculated by multiplying the tissue dry weight by the metal concentration.
Determination of tolerance index
The tolerance index (TI) was determined to assess the ability of the willow varieties to grow in the presence of a given concentration of Pb according to the following equation [39]:
where DW is the dry weight of the roots or above-ground tissues of the willow.
Determination of translocation factor
The translocation factor (TF) indicates the efficiency of the plant to translocate the accumulated metal from its roots to the aerial parts. It is calculated as follows [40]:
where Caerial parts is the concentration of metal in the above-ground tissues and Croots is the concentration of metal in the roots.
Determination of EC50
The EC50 (effective concentration) is the concentration of the metal that causes a 50% decrease in plant biomass, as compared with the control. Critical toxicity thresholds based on EC50 estimates were determined for the roots and above-ground tissues (combined wood, new shoots, and leaves) for each variety using non-linear regressions to fit curves [41]. A multivariate model using different variances was used to compare the EC50s among willow varieties [42]:
where “g” is the measured biomass. “g1” and “g2” are the parameters to be estimated and “x” is the treatment.
Statistical methods
Data were analyzed using the statistical package Data Processing System (DPS 13.01) [43], Origin7.5 and Excel 2003 for Windows. All data were tested for homogeneity of variance and normality. Differences among treatments and cultivars were analyzed by one-way or two-way ANOVA (P<0.05) according to Fisher's LSD test.
Results
Visual symptoms
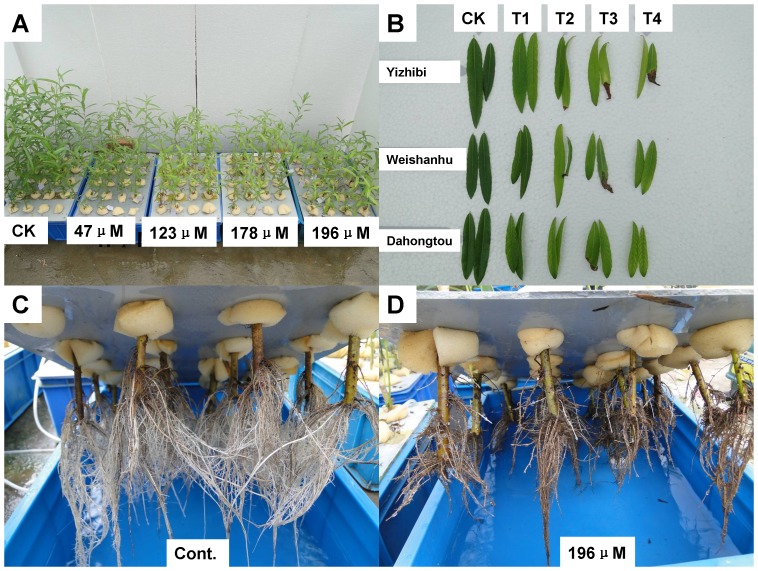
After 5 d of Pb exposure, the foliage of willow plants treated with 123,178 and 196 µM began to yellow and wilt from the leaf tip. Weishanhu treated with 123 µM Pb exhibited the earliest leaf wilting. Dahongtou exhibited more pronounced symptoms, including interveinal chlorosis of the basal leaf part, which became more severe on the day of harvest (Fig 1B). The Chlorophyll Concentration Index (CCI) at harvesting was significantly lowered (P<0.05) by Pb in all three varieties, and no significant differences were observed among the three varieties (Table 1). In addition, the willow plants had smaller leaves with higher Pb concentrations (178 and 196 µM Pb) (Figure 1B).
Figure 1. Growth development of S. integra exposed to different Pb concentrations for 14 days and metal related symptoms in roots and leaves.
(A) Growth of S. integra after exposure to 0, 47, 123, 178, and 196 µM of Pb treatments for 14 days. (B) Leaf symptoms of three varieties under different Pb treatments for 14 days. T1, T2, T3 and T4 represent 47, 123, 178, and 196 µM of Pb respectively. (C) Root systems of willows grown in the control. (D) Root systems of willows grown under 196 µM Pb treatment.
Table 1. Chlorophyll Concentration Index (CCI) of leaves of three varieties of S. integra grown in various Pb concentrations for 14 days.
| Varieties | |||
| Pb (µM) | Yizhibi | Weishanhu | Dahongtou |
| 0 | 13.05±0.93a | 13.77±0.16a | 11.79±1.14a |
| 47 | 8.50±0.53b | 8.84±0.53b | 8.40±0.08b |
| 123 | 5.58±0.23c | 6.39±1.01c | 6.20±1.47c |
| 178 | 6.13±0.78c | 6.18±0.68c | 6.13±0.72c |
| 196 | 4.78±0.13c | 5.43±0.16c | 5.27±0.13c |
Values are mean ± S.D. (n = 6), and data with different letters in the same column indicate a significant difference at P<0.05 according to Fisher's LSD test.
After 14 d of Pb treatment, the shoot growth was significantly reduced (Figure 1A) in all varieties for all Pb treatments, compared with the control, but no significant differences were observed among the 47, 123, 178, and 196 µM Pb treatments.
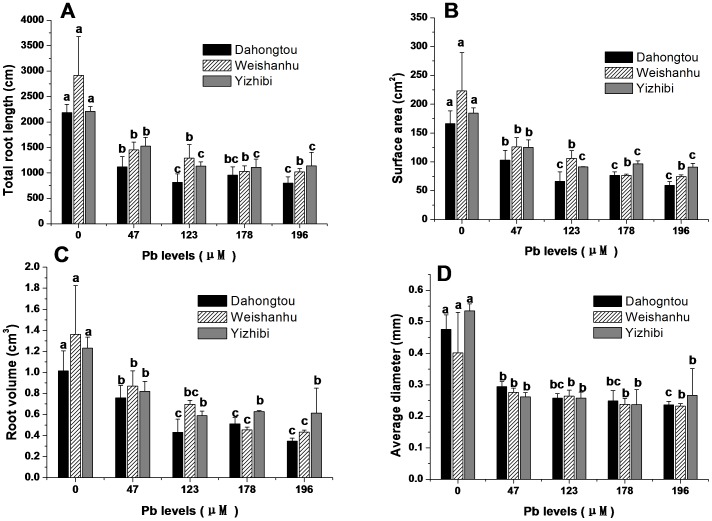
The roots exhibited different levels of blackening and their growth was stunted after exposure to Pb regardless of the Pb concentration, or willow variety (Figure 1C, D). The symptoms became more severe with increasing Pb concentrations. However, no significant differences were observed among the three varieties of S. integra. Besides blackening, the total root length and surface area of three varieties were also reduced significantly by the Pb treatments (P<0.05), but the reduction was more pronounced in Dahongtou than in Weishanhu or Yizhibi (Figure 2A, B). There was a similar reduction in root volume to that observed in the total root length and surface area in the three varieties (Figure 2C). The root diameters were significantly reduced by the Pb treatments (Figure 2D), but there were no significant differences among varieties or Pb levels.
Figure 2. Root characteristics of three S. integra varieties exposed to different Pb concentrations for 14 days.
(A) Total root length. (B) Surface area. (C) Root volume. (D) Average diameter. Data points and error bars represent means ± S.D. of three replicates (n = 3). Different letters indicate significant differences (P<0.05) across the treatments according to Fisher's LSD test.
Biomass production and tolerance Index
The dry weights of the roots and above-ground tissues (wood, new shoots, and leaves) of S. integra varieties are presented in Table 2. The largest root and above-ground tissue biomass for the Pb treatments were recorded for Weishanhu (0.12 and 1.66 g plant−1 respectively). Compared with the control, the dry weight of the above-ground tissues of three varieties reduced significantly (P<0.05) at higher Pb treatment (178 and 196 µM). While at lower Pb treatment (47 µM), no significant differences were observed in the dry weight of the above-ground tissues of Weishanhu and Yizhibi, whereas there was a significant (P<0.05) reduction in that of Dahongtou under the same Pb treatments.
Table 2. Dry weight (DW) (g) of root and aboveground tissues (combined wood, new shoots and leaves) and shoot length (cm) of three willow varieties grown in various Pb concentrations for 14 days.
| Varieties | |||
| Pb treatment (µM) | Yizhibi | Weishanhu | Dahongtou |
| DW of Aboveground tissue (g) | |||
| 0 | 1.64±0.10ab | 1.88±0.27a | 1.90±0.07a |
| 47 | 1.70±0.19a | 1.66±0.13ab | 1.50±0.15b |
| 123 | 1.38±0.12bc | 1.62±0.08abc | 1.46±0.25bc |
| 178 | 1.43±0.10cd | 1.56±0.09bc | 1.35±0.09bc |
| 196 | 1.15±0.14d | 1.36±0.09c | 1.20±0.09c |
| DW of Root (g) | |||
| 0 | 0.093±0.007a | 0.12±0.01a | 0.10±0.01a |
| 47 | 0.083±0.013a | 0.12±0.03a | 0.08±0.00a |
| 123 | 0.052±0.003b | 0.08±0.01b | 0.06±0.01b |
| 178 | 0.057±0.003b | 0.07±0.01b | 0.06±0.00b |
| 196 | 0.062±0.007b | 0.08±0.02b | 0.05±0.01b |
| Shoot length (cm) | |||
| 0 | 40.03±1.15a | 45.34±1.77a | 39.94±4.73a |
| 47 | 31.47±0.79b | 32.37±3.22b | 28.97±0.87b |
| 123 | 31.31±3.30b | 31.67±1.17b | 28.72±1.68b |
| 178 | 28.39±2.36bc | 20.56±9.85bc | 27.78±3.50b |
| 196 | 25.81±3.64c | 26.28±2.01c | 17.17±3.33c |
Values are mean ± S.D. (n = 6), and data with different letters in the same column indicate a significant difference at P<0.05 according to Fisher's LSD test.
For all varieties, there were no significant differences in the dry weight of the roots between the control and the 47 µM Pb treatment, whereas a significant decrease (P<0.05) in root dry weight was observed in all three varieties exposed to Pb concentrations of 123,178 and 196 µM, compared with the control. However, the root biomass of three varieties did not change significantly across the Pb concentraitons.
Shoot growth was significantly inhibited (P<0.05) by Pb addition in all the varieties especially at the highest Pb concentration. The longest shoot length for all the treatments, including the control, was measured in Weishanhu (45.34 cm).
The TIs observed in each variety for the different Pb concentrations are listed in Table 3. For Yizhibi and Weishanhu, the TIs decreased significantly at 196 µM Pb, whereas at 123 and 178 µM Pb, they were only slightly reduced compared to the treatment with 47 µM Pb. For Dahongtou, there were no significant differences in TI among the treatments. The TI varied among the three varieties under the different treatments. Weishanhu had the highest TI at all Pb concentrations, except at 47 µM, when the highest TI was measured for Yizhibi.
Table 3. Tolerance index (TI) in three S. integra varieties after 14 days of exposure to increasing concentrations of Pb.
| Varieties | |||
| Pb treatment (µM) | Yizhibi | Weishanhu | Dahongtou |
| 47 | 95.80±4.50a | 89.20±5.90a | 79.91±10.04a |
| 123 | 81.37±9.09ab | 85.83±9.38ab | 76.69±15.92a |
| 178 | 80.92±1.76ab | 82.72±12.89ab | 70.43±2.32a |
| 196 | 66.87±9.72b | 73.15±15.83b | 62.78±2.54a |
Values are mean ± S.D. (n = 6), and data with different letters in the same column indicate a significant difference at P<0.05 according to Fisher's LSD test.
Toxicity thresholds
The Pb toxicity thresholds of the three Salix varieties were determined for the roots and above-ground tissues (Table 4). All EC50 values were >200 µM with the highest EC50 value recorded in the roots (258.5 µM) and above-ground tissues (537.5 µM) of Weishanhu. Dahongtou had the lowest EC50 values in these tissues. For all three varieties, the EC50 values in the roots were lower than those in the above-ground tissues (P<0.05).
Table 4. Calculated EC50 toxicity thresholds, models, R and P. for roots and the aboveground tissues (combined wood, new shoots and leaves) of three willow varieties exposed to increasing levels of lead.
| S. integra varieties | Model | R | P | EC50 (µM) |
| Roots | ||||
| Yizhibi | y = 0.091727*e −0.002786x | 0.8925 | 0.0416 | 242.6 |
| Weishanhu | y = 0.123656*e −0.002779x | 0.9566 | 0.0108 | 258.5 |
| Dahogntou | y = 0.095516*e −0.003416x | 0.9897 | 0.0013 | 204.5 |
| Aboveground tissue | ||||
| Yizhibi | y = 1.7132*e −0.001531x | 0.8685 | 0.0561 | 481.3 |
| Weishanhu | y = 1.8468*e −0.001252x | 0.9132 | 0.0303 | 537.5 |
| Dahogntou | y = 1.8086*e −0.001921x | 0.9298 | 0.0221 | 335.9 |
Metal concentration in plant tissue and translocation factor
The Pb concentrations in the roots, wood, new shoots and leaves varied significantly (P<0.05) across treatments, but no significant differences were observed among the varieties, with the exception of wood. For the roots and leaves, there were significant differences (P<0.05) in Pb concentration across the varieties × treatment interactions (Table 5).
Table 5. Two-way ANOVA analysis for Pb concentration in root, new shoots, wood, and leaves.
| Variable | root | New shoots | wood | leaves | ||||
| F | P | F | P | F | P | F | P | |
| Variety | 1.04 | 0.3960 | 3.22 | 0.0942 | 5.81 | 0.0277 | 0.60 | 0.5697 |
| Treatment | 19.64 | 0.0003 | 14.52 | 0.0010 | 82.81 | 0.0000 | 7.87 | 0.0071 |
| Variety × treatment | 5.67 | 0.0003 | 1.65 | 0.1561 | 1.14 | 0.3664 | 9.65 | 0.0000 |
Lead concentrations in the control were generally below the detection limit. The highest Pb concentrations were found in the roots for all varieties and ranged from 6,799–24,597 mg kg−1 (Table 6). All varieties had the lowest Pb concentration in their roots with the 47 µM Pb treatment, ranging from 6,799–9,632 mg kg−1. There was a steady increase in the root Pb concentration in Yizhibi and Dahongtou with increasing Pb treatment concentration, and a significant increase (P<0.05) was observed in all varieties at 47 µM Pb treatment. The Pb root concentration in Weishanhu increased significantly with 123 µM Pb, whereas decreased when the Pb treatments ≥123 µM. No significant differences in the root Pb concentration of the three varieties were observed between the 178 µM and 196 µM Pb treatments.
Table 6. Average Pb concentrations (mg kg−1) in dry plant tissues of S. integra exposed to various Pb treatments for 14 days.
| Varieties | ||||
| Plant tissue | Pb µM | Yizhibi | Weishanhu | Dahongtou |
| Leaves | 0 | ND | ND | ND |
| 47 | 29.3±11.6b | 27.7±5.4a | 20.0±8.4b | |
| 123 | 44.9±7.5a | 36.9±9.4a | 89.3±19.6a | |
| 178 | 15.4±4.7c | 14.3±3.6b | 16.7±5.8bc | |
| 196 | 13.7±2.7c | 9.9±3.8b | 8.6±3.8bc | |
| New shoots | 0 | ND | ND | ND |
| 47 | 29.8±9.3a | 33.4±4.7a | 36.1±10.4a | |
| 123 | 17.2±1.7a | 14.6±2.9b | 27.4±10.5a | |
| 178 | 19.7±9.8a | 43.0±7.2a | 34.1±1.1a | |
| 196 | 25.9±8.5a | 34.1±13.1a | 49.4±23.5a | |
| Wood | 0 | ND | ND | ND |
| 47 | 244.±82.4c | 216.0±37.5c | 328.0±118.3c | |
| 123 | 548.3±147.8b | 470.3±49.7b | 574.0±114.2b | |
| 178 | 765.0±147.5a | 655.0±117.9a | 788.7±135.1ab | |
| 196 | 865.0±69.1a | 611.0±64.6a | 930.3±199.5a | |
| Root | 0 | ND | ND | ND |
| 47 | 9,632.3±2,268.6b | 6,799.7±1,918.1c | 8,318.7±1,827.6c | |
| 123 | 18,271.0±3,334.5a | 19,629.0±1,799.5a | 13,999.0±9,64.2b | |
| 178 | 18,074.7±4,816.4a | 15,340.7±3,954.3ab | 23,998.0±2,760.0a | |
| 196 | 21,471.0±1,274.9a | 14,391.7±3,738.0b | 24,597.0±1,855.1a | |
Values are mean ± S.D. (n = 6), and data with different letters in the same column indicate a significant difference at P<0.05 according to Fisher's LSD test.
The Pb concentration in the wood tissue for all the varieties and treatments ranged from 216–930 mg kg−1, with the highest Pb concentration displayed by Dahongtou with the 196 µM Pb treatment. With increasing Pb concentration, the wood tissue Pb concentration significantly increased (P<0.05) in all three varieties.
The shoot Pb concentration in all three varieties increased significantly (P<0.05) under all Pb treatments compared with the control, however, no significant differences were observed among treatments except in Weishanhu, whose shoot Pb concentration decreased significantly with the 123 µM Pb treatment. The highest Pb concentration in new shoots was observed in Dahogntou (49.4 mg kg−1) under 196 µM Pb, while the lowest was observed in Weishanhu (14.6 mg kg−1) under 123 µM Pb.
The highest Pb concentrations in the leaves for all varieties were observed with treatment of 123 µM Pb. The leaf Pb concentrations declined significantly (P<0.05) with increasing Pb treatment concentration.
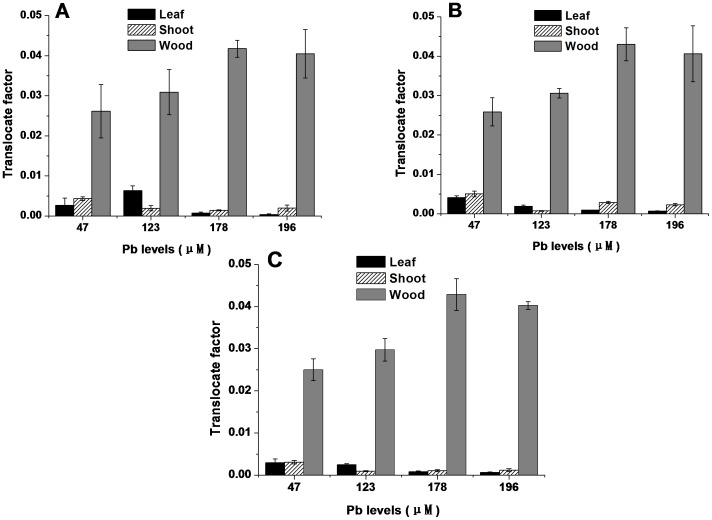
The TF can be used to evaluate the capacity of a plant to translocate heavy metals from the roots to the harvested parts. There were significant (P<0.05) differences in the TFs of three varieties among the different tissue types and among different Pb treatments. For all the varieties, the TFs of wood were much higher than those of leaf or new shoots, and increased gradually with increasing Pb treatment concentration, indicating more effective translocation in S. integra from the roots to the wood than to the leaves or new shoots (Fig 3). The TFs of leaves and new shoots were extremely low (<0.05) and decreased with increasing Pb concentration in solutions, with the exception of Dahogntou, which displayed a distinctive increase under 123 µM Pb treatment (Fig 3A).
Figure 3. Translocation factor (TF) of leaf, new shoots and wood in three varieties of S. integra for the different Pb treatment.
(A) Dahongtou. (B) Weishanhu. (C) Yizhibi.
Metal contents in above-ground tissues and roots
The Pb concent of the above-ground tissues (the combined wood, new shoots and leaves) varied significantly across the treatments and varieties × treatment interactions (P<0.05). The variety × treatment interaction effects were also significant (P<0.05) in the roots (Table 7), whereas no significant differences in the Pb concent were observed in the roots or above-ground tissues among the varieties.
Table 7. Two-way ANOVA analysis for Pb concent in root and the aboveground tissues.
| Variable | root | aboveground | ||
| F | P | F | P | |
| Variety | 0.33 | 0.7290 | 5.00 | 0.0527 |
| Treatment | 1.89 | 0.2327 | 28.47 | 0.0006 |
| Variety × treatment | 4.44 | 0.0044 | 2.92 | 0.0298 |
The highest root Pb content in Weishanhu was found in the treatment of 123 µM Pb concentration (Table 8), whereas Yizhibi and Dahongtou had the highest Pb content in their roots with the 178 or 196 µM Pb treatments.
Table 8. Lead content (mg plant−1) in root and aboveground tissue (wood, new shoots, and leaves) of three S. integra varieties exposed to different Pb concentrations for 14 days.
| Varieties | ||||
| plant tissue | Pb µM | Yizhibi | Weishanhu | Dahongtou |
| Root | 47 | 0.85±0.26c | 0.77±0.16b | 0.69±0.15c |
| 123 | 0.95±0.21bc | 1.56±0.29a | 0.83±0.20bc | |
| 178 | 1.01±0.21b | 1.11±0.22b | 1.33±0.13a | |
| 196 | 1.32±0.17a | 1.11±0.38b | 1.17±0.17ab | |
| Aboveground tissue | 47 | 0.22±0c | 0.23±0.01c | 0.33±0.06c |
| 123 | 0.50±0.09b | 0.52±0.03b | 0.63±0.06b | |
| 178 | 0.70±0.12a | 0.68±0.07a | 0.74±0.07ab | |
| 196 | 0.65±0.05ab | 0.55±0.04b | 0.88±0.13a | |
Values are mean ± S.D. (n = 6), and data with different letters within the same column indicate a significant difference at P<0.05 according to Fisher's LSD test.
The highest Pb contents in the above-ground tissues for both Yizhibi and Weishanhu were displayed under 178 µM Pb treatment, whereas the highest Pb content for Dahongtou was exhibited under 196 µM Pb treatment. All varieties had the lowest Pb content in their roots and aboveground tissues with the 47 µM Pb treatment.
Discussion
Excess Pb causes a number of toxicity symptoms in plants, and the non-specific symptoms include stunted growth, chlorosis and inhibition of root growth [44]. In our study, at Pb concentrations ≥123 µM, willows exhibited stunted growth, leaf dehydration and chlorosis, and severely reduced root biomass. Similar Pb toxicity symptoms were observed in previous studies [45]–[48]. According to Sharma and Dubey [44], these toxicity symptoms are some of the physiological responses to metal treatments exhibited by plants, because the presence of Pb in the cell, even in small amounts, can potentially cause a wide range of adverse effects on physiological processes, such as inhibition of enzymatic activities, disturbed mineral nutrition, water imbalance, altered hormonal status, and altered membrane permeability.
After exposure to different Pb concentrations, there were significant variations in the dry weights of the three varieties of S. integra. In general, the root biomass was significantly lower than the above-ground biomass among the varieties and treatments. The root dry weights of the three varieties did not change significantly at the lowest Pb concentration of 47 µM, while the above-ground biomass decreased significantly at the same concentration. This suggests that the shoot growth of S. integra was more inhibited than the root's at lower Pb concentrations. In contrast, in a previous study [18], the roots of S. lucida, S. serissima, S. sachalinensis L., S. miyabeana L., and S. nigra clones showed a significant reduction in biomass at a similar concentration of Pb (48 µM). This indicates that there is inter-specific variability in sensitivity to environmental stress such as Pb increasing concentrations in soil solution. The roots are in direct contact with Pb, and provide the primary route for metal ion penetration [49]. Plant roots rapidly respond to absorbed Pb, through a reduction in root growth and changes in branching pattern [44]. In our study, the development of root morphology of the three varieties were significantly inhibited by all Pb treatments, which is consistent with the behavior of other species, e.g., Picea abies [50] and Zea mays [51]. Studies have shown that the inhibition of root growth under Pb stress is a result of Pb-induced inhibition of cell division in root tips [52]. Weirzbicka [53] reported that the roots of onion (Allium cepa) exhibited a reduction in root growth, mitotic irregularities and chromosome stickiness when exposed to different concentrations of Pb nitrate. Yang et al. [54] also observed a disturbance in alignment of microtubules in Oryza sativa in the presence of Pb, and damage to microtubules is now considered one of the key components of Pb-induced damage in plants [44], [52].
Lead has also been reported to decrease the chlorophyll content by imparing the uptake of essential elements such as Mg and Fe by plants [55], or by increasing chlorophyllase activity in Pb-treated plants [56]. In our study, we also observed severe chlorosis in the leaves of the three varieties of S. integra and a significant decrease in the chlorophyll concentration index, however, further study is required to investigate the reasons for these damages in leaves with Pb treatment.
Assessing metal tolerance is of paramount importance when selecting plants for utilization in phytoremediation [40]. To characterize metal tolerance in plants, one of the most common parameters used is the tolerance index (TI) [57]. Willows have shown significant variations in tolerance across species and clones. In previous studies, significant variations in metal tolerance were found among willow species and clones exposed to cadmium, copper, or arsenic [29], [38], [58], [59]. S. integra was also confirmed to have a high capacity for cadmium and zinc uptake [32], [33], [35], but no information about the Pb tolerance and accumulation was reported. There are many cultivated varieties of S. integra in China, and the three varieties evaluated in this study were chosen because of their different tolerances to Cd treatment [35]–[37]. Although TI varies with increasing Pb concentration, all three varieties of S. integra tested can be defined as highly tolerant (TI >60) to Pb according to the scheme proposed by Lux et al. [60], with Weishanhu being the most tolerant variety in our study.
The EC50 value is another important parameter that describes the tolerance of plant species in multiple concentration tests [57]. In our study, we observed that the EC50 values of the roots were lower than those of the above-ground parts of the plants, suggesting that the roots of S. integra were more sensitive to Pb treatment. A similar behavior was observed in S. lucida, S. serissima, S. sachalinensis L., S. miyabeana L., and S. nigra clones [18]. However, more interesting are the extremely high EC50 values for the roots and aboveground tissues of the three varieties which we observed in S. integra. Previously, EC50 values for willows in the presence of Pb in hydroponic culture were recorded as 6–32 µM [18], while we measured values >200 µM. High tolerance to metals is a key factor for a plant to successfully colonize metallic soil. Based on the observed EC50 values, Weishanhu was found to be the most tolerant of the three varieties, and could be a suitable candidate for Pb phytostablization in contaminated areas.
A number of studies have shown that Pb accumulates preferentially in roots [19], [61], and our results are consistent with this observation. Sharma and Dubey [44] reported that Pb is confined in roots probably because it binds to ion exchangeable sites on the cell wall, with further extracellular precipitation as Pb carbonates. It has also been reported that Pb has a strong ability to bind to the carboxyl groups of galacturonic and glucuronic acids in the cell wall, which limits the apoplastic transport of this metal [62]. In addition, the Casparian strip of the endodermis plays an important role in restricting Pb transport across the endodermis into other tissues [63]. In addition to the physical barrier that causes poor translocation of Pb from the roots to new shoots, some researchers reported that a short-term exposure to a heavy metal could also result in poor translocation of Pb [64]. Zhivotovsky [18] conducted a short-term hydroponical test to show that Salix may need more time to adapt to a specific environment to improve the transport of Pb to above-ground plant tissues. In our study, we also used a short-term screening test, and found Pb accumulation patterns were similar to those of previous studies. However, it is interesting that we observed higher concentrations of Pb in the wood cuttings than in the new shoots and leaves, which showed that Pb absorbed by the roots was transported to the woody parts more readily than to the new shoots and leaves. We also observed that Dahogntou exposed to 123 µM Pb was most efficient in translocating Pb into the leaves. In addition, the highest Pb root concentration we recorded (24,597 mg kg−1) was higher than those that have been reported in the literature for Salix (4,164–23,023 mg kg−1) grown in either soil or solution [18], [65]. Our results indicate that the roots of S. integra can both tolerate and accumulate high Pb concentrations; however, the tolerance mechanism in the root system is still unknown. Further research is required to evaluate whether this tolerance to Pb in roots is acquired by blocking the entrance of Pb into the root cells, or by some metabolic mechanisms that detoxify Pb toxicity.
Conclusions
Willows have potential for phytoremediation due to their high biomass productivity, high transpiration rate, and species-specific heavy metal uptake [28], [66], [67]. S. integra is a fast-growing shrub willow, and in China, it is generally cultivated by short-rotation plantation, with the new shoots harvested for use in weaving. Yizhibi, Dahogntou and Weishanhu are three cultivated varieties of S. integra, that display excellent in biomass production and environmental adaptation [34], [35]. We demonstrated that S. integra can tolerate, transport and accumulate various levels of Pb when exposed to different Pb concentrations, and there is no significant difference in the tolerance to Pb among the varieties. For effective phytoextration, we are interested in plants that exhibit not only high tolerance to heavy metals, but also high accumulation of metals in tissues with high biomass production. We observed that Dahongtou had higher Pb contents in the above-ground tissues and the highest TF in leaves under 123 µM Pb. These results bring new insight into the selection of candidates for Pb phytoextraction. However, the results are based on a short-term study, the potential effectiveness of S. integra Dahongtou in phytoextraction should be tested in the long-term and open-field studies. In summary, all the varieties, especially Weishanhu and Yizhibi, have high TI and EC50, demonstrating their high tolerance and lower sensitivity to Pb and, thus, their suitability for phytostabilization of Pb.
Acknowledgments
We sincerely thank the Academic Editor and anonymous reviewers for their valuable comments on improving the paper. We acknowledge Dr. Zhenli He from University of Florida, and Xiaojiao Han from Research Institute of Subtropical Forestry for their suggestions and correction of English writing.
Funding Statement
This work was financially supported by Ministry of Science and Technology of China (#2010DFB33960 and #2012AA100602), the National Natural Science Foundation of China (#31400526), and the Fundamental Research Funds from the Central Universities (# 2014FZA6000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—Concepts and applications. Chemosphere 91: 869–881. [DOI] [PubMed] [Google Scholar]
- 2. Kachenko AG, Singh B, Bhatia NP (2007) Heavy metal tolerance in common fern species. Australian journal of botany 55: 63–73. [Google Scholar]
- 3. Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment 368: 456–464. [DOI] [PubMed] [Google Scholar]
- 4. Chehregani A, Malayeri B (2007) Removal of heavy metals by native accumulator plants. International Journal of Agriculture and Biology 9: 462–465. [Google Scholar]
- 5. Fulekar M, Singh A, Bhaduri AM (2009) Genetic engineering strategies for enhancing phytoremediation of heavy metals. African Journal of Biotechnology 8: 529–535. [Google Scholar]
- 6. Mehmood T, Chaudhry M, Tufail M, Irfan N (2009) Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchemical Journal 91: 94–99. [Google Scholar]
- 7. Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 2011: 1–21. [Google Scholar]
- 8. Cheng S (2003) Heavy metal pollution in China: origin, pattern and control. Environmental Science and Pollution Research 10: 192–198. [DOI] [PubMed] [Google Scholar]
- 9. Ali M, Vajpayee P, Tripathi R, Rai U, Singh S, et al. (2003) Phytoremediation of lead, nickel, and copper by Salix acmophylla Boiss: role of antioxidant enzymes and antioxidant substances. Bulletin of environmental contamination and toxicology 70: 0462–0469. [DOI] [PubMed] [Google Scholar]
- 10.Baker A, Reeves R, McGrath S (1991) In situ decontamination of heavy metal polluted soils using crops of metal-accumulating plants-a feasibility study. In situ bioreclamation Boston, Butterworth-Heinemann: 600–605.
- 11.Komives T, Gullner G (2006) Dendroremediation: the use of trees in cleaning up polluted soils. Phytoremediation Rhizoremediation: Springer. pp. 23–31.
- 12. Kumar PN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environmental Science & Technology 29: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Wai OW, Li Y, Coles BJ, Ramsey MH, et al. (2000) Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Applied Geochemistry 15: 567–581. [Google Scholar]
- 14.Freitas EV, Nascimento CW, Souza A, Silva FB (2013) Citric acid-assisted phytoextraction of lead: A field experiment. Chemosphere: 213–217. [DOI] [PubMed]
- 15. Islam E, Yang X, Li T, Liu D, Jin X, et al. (2007) Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi . Journal of hazardous materials 147: 806–816. [DOI] [PubMed] [Google Scholar]
- 16. Yang Q, Shu W, Qiu J, Wang H, Lan C (2004) Lead in paddy soils and rice plants and its potential health risk around Lechang Lead/Zinc Mine, Guangdong, China. Environment International 30: 883–889. [DOI] [PubMed] [Google Scholar]
- 17. Grejtovský A, Markušová K, Nováková L (2008) Lead uptake by Matricaria chamomilla. L Plant, Soil and Environment 54: 47–54. [Google Scholar]
- 18. Zhivotovsky OP, Kuzovkina JA, Schulthess CP, Morris T, Pettinelli D, et al. (2010) hydroponic screening of willows (Salix L.) for lead tolerance and accumulation. International journal of phytoremediation 13: 75–94. [DOI] [PubMed] [Google Scholar]
- 19. Wierzbicka M (1999) Comparison of lead tolerance in Allium cepa with other plant species. Environmental Pollution 104: 41–52. [Google Scholar]
- 20. Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to Brassica species: implications for phytoremediation. Journal of Environmental Quality 26: 776–781. [Google Scholar]
- 21. Kuzovkina YA, Zhivotovsky OP, Schulthess CP, Pettinelli D (2010) Plant selection for a pilot phytoremediation study at a former skeet range. Remediation Journal 20: 93–105. [Google Scholar]
- 22. Ghosh M, Singh S (2005) A review on phytoremediation of heavy metals and utilization of it's by products. Asian J Energy Environ 6: 1–18. [Google Scholar]
- 23. Marques AP, Rangel AO, Castro PM (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Critical Reviews in Environmental Science and Technology 39: 622–654. [Google Scholar]
- 24. Suresh B, Ravishankar G (2004) Phytoremediation-A novel and promising approach for environmental clean-up. Critical reviews in biotechnology 24: 97–124. [DOI] [PubMed] [Google Scholar]
- 25. Kuzovkina YA, Volk TA (2009) The characterization of willow (Salix L.) varieties for use in ecological engineering applications: Co-ordination of structure, function and autecology. Ecological Engineering 35: 1178–1189. [Google Scholar]
- 26.Rockwood D, Naidu C, Carter D, Rahmani M, Spriggs T, et al.. (2004) Short-rotation woody crops and phytoremediation: Opportunities for agroforestry? New Vistas in Agroforestry: Springer. pp. 51–63.
- 27. Mleczek M, Łukaszewski M, Kaczmarek Z, Rissmann I, Golinski P (2009) Efficiency of selected heavy metals accumulation by Salix viminalis roots. Environmental and Experimental Botany 65: 48–53. [Google Scholar]
- 28. Pulford I, Watson C (2003) Phytoremediation of heavy metal-contaminated land by trees—a review. Environment international 29: 529–540. [DOI] [PubMed] [Google Scholar]
- 29. Purdy JJ, Smart LB (2008) Hydroponic screening of shrub willow (Salix spp.) for arsenic tolerance and uptake. International Journal of Phytoremediation 10: 515–528. [DOI] [PubMed] [Google Scholar]
- 30. Tlustoš P, Száková Ji, Vysloužilová M, Pavlíková D, Weger J, et al. (2007) Variation in the uptake of arsenic, cadmium, lead, and zinc by different species of willows Salix spp. grown in contaminated soils. Central European Journal of Biology 2: 254–275. [Google Scholar]
- 31. Zimmer D, Kruse J, Baum C, Borca C, Laue M, et al. (2011) Spatial distribution of arsenic and heavy metals in willow roots from a contaminated floodplain soil measured by X-ray fluorescence spectroscopy. Science of the Total Environment 409: 4094–4100. [DOI] [PubMed] [Google Scholar]
- 32. Harada E, Hokura A, Takada S, Baba K, Terada Y, et al. (2010) Characterization of cadmium accumulation in willow as a woody metal accumulator using synchrotron radiation-based X-ray microanalyses. Plant Cell Physiol 51: 848–853. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Chen G-C, Zhang J, Shi X, Wang R (2011) Uptake of cadmium from hydroponic solutions by willows (Salix spp.) seedlings. African Journal of Biotechnology 10: 16209–16218. [Google Scholar]
- 34. Tian Y LWW, Fang S Z (2012) Effects of harvest rotation on the growth, wicker production and quality of willow shrub.pdf. Chinese Journal of Nanjing Forestry University (Natural Science Edition) 36: 86–90. [Google Scholar]
- 35. Yang WD, Chen YT (2008) Differences in Uptake and Tolerance to Cadmium in Varieties of Salix integra [J]. Forest Research 6: 857–861. [Google Scholar]
- 36. Yang WD, Chen YT (2009) Tolerance of different varieties of Salix integra to high zinc stress. Zhongguo Shengtai Nongye Xuebao/Chinese Journal of Eco-Agriculture 17: 1182–1186. [Google Scholar]
- 37. Wang SF, Shi X, Sun HJ, Chen YT, Yang XE (2013) Metal uptake and root morphological changes for two varieties of Salix integra under cadmium stress. Acta Ecologica Sinica 33: 6065–6073. [Google Scholar]
- 38. Magdziak Z, Kozlowska M, Kaczmarek Z, Mleczek M, Chadzinikolau T, et al. (2011) Influence of Ca/Mg ratio on phytoextraction properties of Salix viminalis. II. Secretion of low molecular weight organic acids to the rhizosphere. Ecotoxicology and environmental safety 74: 33–40. [DOI] [PubMed] [Google Scholar]
- 39. Wilkins D (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytologist 80: 623–633. [Google Scholar]
- 40. Zacchini M, Pietrini F, Mugnozza GS, Iori V, Pietrosanti L, et al. (2009) Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water, Air, and Soil Pollution 197: 23–34. [Google Scholar]
- 41.Neter J, Wasserman W, Kutner MH (1996) Applied linear statistical models: Irwin Chicago.
- 42. Schabenberger O, Tharp BE, Kells JJ, Penner D (1999) Statistical tests for hormesis and effective dosages in herbicide dose response. Agronomy Journal 91: 713–721. [Google Scholar]
- 43. Su S, Cai F, Si A, Zhang S, Tautz J, et al. (2008) East learns from West: Asiatic honeybees can understand dance language of European honeybees. PLoS One 3: e2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma P, Dubey RS (2005) Lead toxicity in plants. Brazilian Journal of Plant Physiology 17: 35–52. [Google Scholar]
- 45. HO W, Ang LH, LEE DK (2008) Assessment of Pb uptake, translocation and immobilization in kenaf Hibiscus cannabinus L.) for phytoremediation of sand tailings. Journal of Environmental Sciences 20: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 46. Liu W, Zhou Q, Zhang Y, Wei S (2010) Lead accumulation in different Chinese cabbage cultivars and screening for pollution-safe cultivars. Journal of environmental management 91: 781–788. [DOI] [PubMed] [Google Scholar]
- 47. Rezvani M, Zaefarian F (2011) Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Australian Journal of Agricultural Engineering 2: 114–119. [Google Scholar]
- 48. Verma S, Dubey R (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science 164: 645–655. [Google Scholar]
- 49. Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A (2002) Accumulation and detoxification of lead ions in legumes. Phytochemistry 60: 153–162. [DOI] [PubMed] [Google Scholar]
- 50. Godbold D, Kettner C (1991) Lead Influences Root Growth and Mineral Nutrition of Picea abies Seedlings. Journal of plant physiology 139: 95–99. [Google Scholar]
- 51. Obroucheva N, Bystrova E, Ivanov V, Antipova O, Seregin I (1998) Root growth responses to lead in young maize seedlings. Plant and soil 200: 55–61. [Google Scholar]
- 52. Eun SO, Shik Youn H, Lee Y (2000) Lead disturbs microtubule organization in the root meristem of Zea mays. Physiologia Plantarum 110: 357–365. [Google Scholar]
- 53. Wierzbicka M (1994) Resumption of mitotic activity in Allium cepa L. root tips during treatment with lead salts. Environmental and experimental botany 34: 173–180. [Google Scholar]
- 54. Yang Y-Y, Jung J-Y, Song W-Y, Suh H-S, Lee Y (2000) Identification of rice varieties with high tolerance or sensitivity to lead and characterization of the mechanism of tolerance. Plant Physiology 124: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burzynski M (1987) Influence of lead and cadmium on the absorption and distribution of potassium, calcium, magnesium and iron in cucumber seedlings. Acta Physiologiae Plantarum 9: 229–238. [Google Scholar]
- 56. Drazkiewicz M (1994) Chlorophyllase: occurrence, functions, mechanism of action, effects of external and internal factors (Review). Photosynthetica 30: 321–331. [Google Scholar]
- 57.Köhl K, Lösch R (1999) Experimental characterization of heavy metal tolerance in plants. Heavy metal stress in plants: Springer. pp. 371–389.
- 58. Kuzovkina YA, Knee M, Quigley MF (2004) Cadmium and copper uptake and translocation in five willow (Salix L.) species. International Journal of Phytoremediation 6: 269–287. [DOI] [PubMed] [Google Scholar]
- 59. Punshon T, Dickinson N (1999) Heavy metal resistance and accumulation characteristics in willows. International Journal of Phytoremediation 1: 361–385. [Google Scholar]
- 60. Lux A, Šottníková A, Opatrná J, Greger M (2004) Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum 120: 537–545. [DOI] [PubMed] [Google Scholar]
- 61. Geebelen W, Vangronsveld J, Adriano DC, Van Poucke LC, Clijsters H (2002) Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris. Physiologia Plantarum 115: 377–384. [DOI] [PubMed] [Google Scholar]
- 62. Rudakova E, Karakis K, Sidorshina T (1988) Role of plant cell membranes in uptake and accumulation of metal ions. Fiziologiia i biokhimiia kul'turnykh rastenii = Physiology and biochemistry of cultivated plants 20: 3–12. [Google Scholar]
- 63. Seregin I, Ivanov V (1997) Histochemical investigation of cadmium and lead distribution in plants. Russian Journal of Plant Physiology 44: 791–796. [Google Scholar]
- 64. Dos Santos Utmazian MN, Wieshammer G, Vega R, Wenzel WW (2007) Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environmental Pollution 148: 155–165. [DOI] [PubMed] [Google Scholar]
- 65. Borišev M, Pajević S, Nikolić N, Pilipović A, Krstić B, et al. (2009) Phytoextraction of Cd, Ni, and Pb using four willow clones (Salix spp.). Polish Journal of Environmental Studies 18: 553–561. [Google Scholar]
- 66. Evangelou MW, Deram A, Gogos A, Studer B, Schulin R (2012) Assessment of suitability of tree species for the production of biomass on trace element contaminated soils. Journal of hazardous materials 209: 233–239. [DOI] [PubMed] [Google Scholar]
- 67. Jensen JK, Holm PE, Nejrup J, Larsen MB, Borggaard OK (2009) The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environmental Pollution 157: 931–937. [DOI] [PubMed] [Google Scholar]