Abstract
Objectives
Refugees and immigrants from developing countries settling in industrialised countries have a high prevalence of Helicobacter pylori (H. pylori). Screening these groups for H. pylori and use of eradication therapy to reduce the future burden of gastric cancer and peptic ulcer disease is not currently recommended in most countries. We investigated whether a screening and eradication approach would be cost effective in high prevalence populations.
Methods
Nine different screening and follow-up strategies for asymptomatic immigrants from high H. pylori prevalence areas were compared with the current approach of no screening. Cost effectiveness comparisons assumed population prevalence's of H. pylori of 25%, 50% or 75%. The main outcome measure was the net cost for each cancer prevented for each strategy. Total costs of each strategy and net costs including savings from reductions in ulcers and gastric cancer were also calculated.
Results
Stool antigen testing with repeat testing after treatment was the most cost effective approach relative to others, for each prevalence value. The net cost per cancer prevented with this strategy was US$111,800 (assuming 75% prevalence), $132,300 (50%) and $193,900 (25%). A test and treat strategy using stool antigen remained relatively cost effective, even when the prevalence was 25%.
Conclusions
H. pylori screening and eradication can be an effective strategy for reducing rates of gastric cancer and peptic ulcers in high prevalence populations and our data suggest that use of stool antigen testing is the most cost effective approach.
Introduction
Estimates suggest that half of the world's population is infected with H. pylori. Sero-prevalence studies in lower and middle income countries show rates of exposure above 80% [1]. H. pylori infection is usually acquired in childhood [1]. Colonisation persists for decades and is potentially lifelong, leading to chronic gastrointestinal inflammation, and stomach and duodenal ulcers.
H. pylori is also a major causative agent in the development of gastric cancer [1], and cancer occurs in 0.1–3% of those chronically infected [1]. Gastric cancer is the second most common cause of cancer death worldwide leading to 736,000 deaths annually [2], including 11000 in the United States [3]. The mean 5 year net cost of a patient with gastric cancer is over $50000 [4], and the five year survival rate is less than 20% [5]. Eradication of H. pylori has been shown to reduce progression to precancerous changes in the stomach [6], and to reduce the risk of developing gastric cancer by approximately one third [7].
Refugees and immigrants settling in western countries often have high rates of H. pylori infection (72–93%) [8], [9], as compared to the local population [8]. In Canada, overseas birth and immigration after 20 years of age were both shown to be risk factors for H. pylori infection [10]. Mexico, the largest source country for US immigration, has an H. pylori prevalence of 60% in serological surveys of 20 year olds [11]. More than 80% of African refugee children in Australia have positive stool antigen tests on arrival [9].
Approximately 12.5% (38.5 million) of the US population were born overseas, of whom 85% come from low or middle income countries [12]. Currently, neither H. pylori nor gastric cancer screening are recommended for this group if asymptomatic, with most guidelines recommending testing based on symptoms. However, it is recognised that detection based on symptoms can miss a significant burden of H. pylori infection [8] and gastric cancer [13].
A number of testing modalities exist for the detection of H. pylori. Serology is widely available and has a sensitivity of 92% (25% IQR 85–96%) and specificity of 83% (25% IQR 73–92%) depending on the test kit used [14]. Antibody levels decline slowly after eradication of H. pylori infection so a positive serology result may reflect past rather than current infection. Stool antigen testing is relatively inexpensive, monoclonal enzyme immunoassay (EIA) testing has a sensitivity of 94% (95% CI 93–95%) and specificity of 97% (95% CI 96–98%) [15]. Breath testing is a rapid, non-invasive test with a high sensitivity (95%) and specificity (98%) but is more expensive [16]. Gastroscopy with biopsy and culture remains the gold standard for H. pylori detection but is costly and more logistically challenging.
In this study we hypothesized that screening for and eradication of H. pylori in high prevalence populations would be cost-effective. Our objectives were to model the effect of various H. pylori screening strategies on the incidence of gastric cancer and ulcer disease in populations with different prevalence's of infection, and to compare the relative cost-effectiveness of each strategy (including savings accrued through prevention of gastric cancer and reduced burden of ulcer disease).
Methods
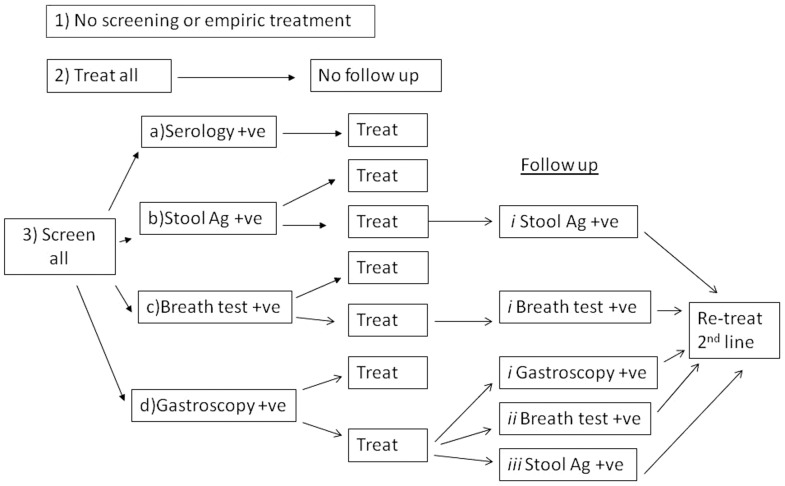
Costs of test and treat strategies were compared to no screening and to empiric treatment strategies, both of which require no testing. Nine different screening and follow-up strategies were investigated (Figure 1).The empiric treatment approach was included as a comparator and may have a role in very high prevalence populations.
Figure 1. H. pylori management strategies included in the analysis.
Costs were calculated as total costs for each strategy and benefit as the total cost of the outcome prevented. Primary benefits were the prevention of gastric cancer and the prevention of peptic ulcer disease, compared to no screening or treatment. Net costs were total costs minus savings accrued due to benefits of screening (ulcers and gastric cancers prevented).
The likelihood of a particular testing outcome was based on sensitivity and specificity values for the tests used (Table 1). Models were developed using decision analysis trees with the endpoints being total and net cost of the strategy and number of gastric cancers and ulcers prevented. Costs and treatment efficacy were based on published estimates (Table 1 & 2).
Table 1. Testing and treatment parameters used including estimated ranges around each parameter.
| Parameter | Best estimate | Lower range | Upper range | Distribution | Reference |
| Testing for H.pylori | |||||
| Breath test | |||||
| Sensitivity (%) | 95.3 | 92.2 | 97.5 | 95%CIa | [16] |
| Specificity (%) | 97.7 | 94.8 | 99.3 | 95%CI | [16] |
| Serology | |||||
| Sensitivity (%) | 92 | 85 | 96 | IQRb | [14] |
| Specificity (%) | 83 | 73 | 92 | IQR | [14] |
| Stool Antigen | |||||
| Sensitivity (%) | 94 | 93 | 95 | 95%CI | [15] |
| Specificity (%) | 97 | 96 | 98 | 95%CI | [15] |
| Gastroscopy with biopsy | |||||
| Sensitivity (%) | 95 | 90 | 99 | [36] | |
| Specificity (%) | 99 | 95 | 100 | [36] | |
| Treatment of H. pylori | |||||
| HP7 efficacy | 77% | 27% | 97% | full range | [29], [37] |
| 2nd line treatment efficacy | 90% | 85% | 95% | [38] | |
| Sequential therapy efficacy | 93% | 91% | 95% | 95%CI | [37] |
| Benefits of H.pylori eradication | |||||
| Reduction in gastric cancers | RRc 0.56 | RR 0.4 | RR 0.8 | 95%CI | [7] |
| Reduction in duodenal ulcers | RR 0.37 | RR 0.26 | RR 0.53 | 95% CI | [22] |
| Other | |||||
| Incidence of Peptic Ulcer disease | 0.19% | 0.10% | 0.19% | [39] | |
| Prevalence of Peptic Ulcer disease | 1.50% | 0.12% | 1.50% | [39] | |
| Incidence of duodenal ulcer if H. pylori +ve | 5% | 0.18% | 17% | 95% CI | [21] |
| Treatment adverse effects (all) | |||||
| Comparison | 8% | [22] | |||
| Treatment | 22% | [22] | |||
| Cancer Survival (%) | |||||
| 1 year | 41 | 39 | 42 | 95%CI | [5] |
| 2 year | 26 | 25 | 28 | 95%CI | [5] |
| 3 year | 21 | 19 | 22 | 95%CI | [5] |
| 4 year | 18 | 16 | 19 | 95%CI | [5] |
| 5 year | 16 | 14 | 17 | 95%CI | [5] |
CI = 95% Confidence Interval.
IQR = Interquartile range.
RR = Relative Risk.
Table 2. Costs of testing and treatment for H.pylori, and costs of adverse outcomes associated with H.pylori in US dollars.
| Costs (in US$) | Original figure (US$) | Converted to 2011 (US$) | Reference |
| Testing and Treatment | |||
| Physician visit | 86 | 86 | [40] |
| Serology | 29 | 30 | [41] |
| Breath test | 133 | 140 | [41] |
| Stool antigen | 21 | 22 | [41] |
| Eradication therapy | 355 | 373 | [41] |
| Gastroscopy with biopsy | 550 | 636 | [21] |
| Peptic ulcer annual cost | |||
| Peptic Ulcer Costs | 866 | 1582 | [42] |
| Gastric Cancer costs | |||
| Mean cost of gastric cancer care (5 year net in 2004) | |||
| Men | 44203 | 52712 | [4] |
| Women | 41899 | 49965 | [4] |
Each model included the cost of the physician visits, which were assumed to be only for H. pylori management and not as part of other care or screening. It was assumed tests or empiric treatment (with a proton pump inhibitor (PPI), clarithromycin and amoxicillin), would be ordered during this initial visit. Strategies with a single time-point for testing were assumed to require up to two physician visits, with the second visit being needed only for those who required treatment because of positive testing. Strategies involving retesting were assumed to require up to three physician visits. Non-medical and indirect costs were not included, in keeping with other comparable modelling papers [5]. While a small proportion of individuals remain H. pylori positive after two courses of treatment, (the second course of treatment with PPI, bismuth, tetracycline and metronidazole) and require subsequent further testing and treatment, this scenario was not included. It is recognized that certain strains of H. pylori have higher risk of progression to gastric cancer [17]. As immigrants arrive from a multitude of countries from which strain prevalence varies or is not known, a standard risk of progression to cancer was used.
Costs were calculated in US dollars for 2011. Costs from earlier years were adjusted for inflation to bring them to 2011 values (Table 2). Analyses were performed for each strategy at three prevalence values (25%, 50% and 75%) with net costs per cancer prevented calculated for each strategy (Table 3). Total costs and numbers of cancers and ulcers prevented with each strategy (expressed per 1000 patients managed) were also calculated (Table 4).
Table 3. Net cost per cancer prevented (US dollars) for each strategy at varying prevalence rates of H. pylori.
| Net cost per cancer prevented | Prevalence | ||
| Management Options | 25% | 50% | 75% |
| 1) No screening | 0 | 0 | 0 |
| 2) Treat all | 477800 | 206900 | 116600 |
| 3) Screen and Treatment | |||
| a. Serology | |||
| No follow up | 294700 | 169900 | 128300 |
| b. Stool Ag | |||
| No follow up | 219200 | 142700 | 117100 |
| i) Follow with stool Ag and retreat | 193900 | 132300 | 111800 |
| c. Breath test | |||
| No follow up | 360200 | 213800 | 165000 |
| i) Follow with breath test and retreat | 334600 | 216400 | 177000 |
| d. Gastroscopy | |||
| No follow up | 972000 | 520600 | 370200 |
| i) Follow up gastroscopy and retreat | 939900 | 577200 | 456300 |
| ii) Follow with breath test and retreat | 820200 | 460100 | 340100 |
| iii) Follow with stool Ag and retreat | 794400 | 433900 | 313700 |
Table 4. Total cost (US dollars) and number of gastric cancers and ulcers prevented for each strategy for every 1000 people managed.
| Prevalence | 25% | 25% | 25% | 50% | 50% | 50% | 75% | 75% | 75% |
| Management Options | Total cost | Cancers prevented | Ulcers prevented | Total cost | Cancers prevented | Ulcers prevented | Total cost | Cancers prevented | Ulcers prevented |
| 1) No screening | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2) Treat all | 458700 | 0.8 | 6.1 | 459000 | 1.7 | 12.1 | 459000 | 2.5 | 18.2 |
| 3) Screen and Treat | |||||||||
| a. Serology | |||||||||
| No follow up | 280500 | 0.8 | 5.6 | 366600 | 1.6 | 11.2 | 452700 | 2.3 | 16.7 |
| b. Stool Ag | |||||||||
| No follow up | 226200 | 0.8 | 5.7 | 330700 | 1.6 | 11.4 | 435100 | 2.4 | 17.1 |
| i) Follow with stool Ag and retreat | 258000 | 1.0 | 7.6 | 393200 | 2.0 | 15.2 | 528300 | 3.0 | 22.8 |
| c. Breath test | |||||||||
| c. Breath test No follow up | 343000 | 0.8 | 5.8 | 449700 | 1.6 | 11.6 | 556400 | 2.4 | 17.3 |
| i) Follow with breath test and retreat | 404800 | 1.0 | 7.6 | 569800 | 2.0 | 15.3 | 734900 | 3.0 | 22.9 |
| d. Gastroscopy | |||||||||
| No follow up | 834300 | 0.8 | 5.8 | 942200 | 1.6 | 11.5 | 1050000 | 2.4 | 17.3 |
| i) Follow up gastroscopy and retreat | 1014800 | 1.0 | 7.6 | 1296700 | 2.0 | 15.3 | 1578600 | 3.0 | 22.9 |
| ii) Follow with breath test and retreat | 894400 | 1.0 | 7.6 | 1060900 | 2.0 | 15.3 | 1227400 | 3.0 | 22.9 |
| iii) Follow with stool Ag and retreat | 865900 | 1.0 | 7.6 | 1005000 | 2.0 | 15.2 | 1144100 | 3.0 | 22.8 |
Sensitivity analysis was performed on the most cost-effective strategy in the initial analysis (stool testing with retesting of those treated). The outcome measure of interest was the net cost per cancer saved. The parameters tested were cost of managing one cancer, cost of a physician visit, cost of medication for eradication, cost of managing one peptic ulcer and lifetime risk of gastric cancer. The change in net cost per cancer saved was estimated against the proportional change in each of the five parameters. A probabilistic model was developed in which the model parameters were drawn from their full uncertainty distributions, as given in Table 1. The distributions were assumed to be normal with the mean equal to the best estimate and upper and lower range equal to the 95% area under the curve of the normal distribution. For each of 10,000 iterations, a parameter was drawn from each uncertainty distribution and results calculated; including costs, number of cancers averted, number of ulcers averted false negatives and positive results. Sensitivity to change in parameters was estimated using multivariable regression, with cost per cancer saved as the continuous outcome variable and the parameters above as the predictor variables. Linear relationships were assumed and the parameters were not transformed. Figures represent the effect on cost per cancer prevented if each parameter was increased by 1% of the original estimate used.
Results
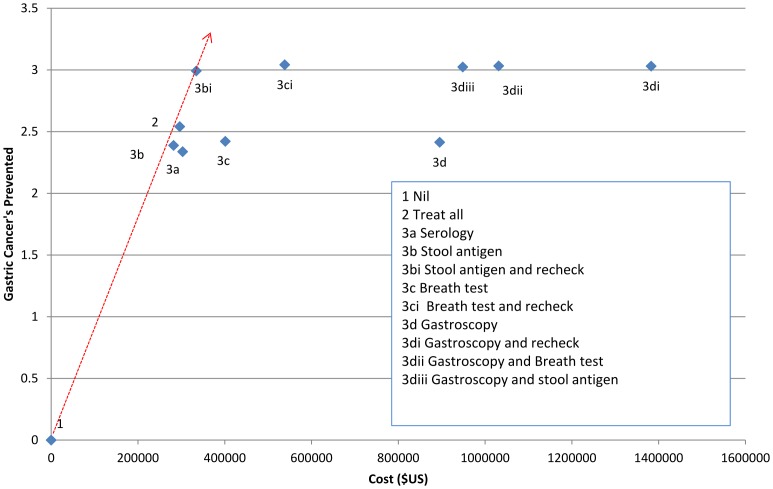
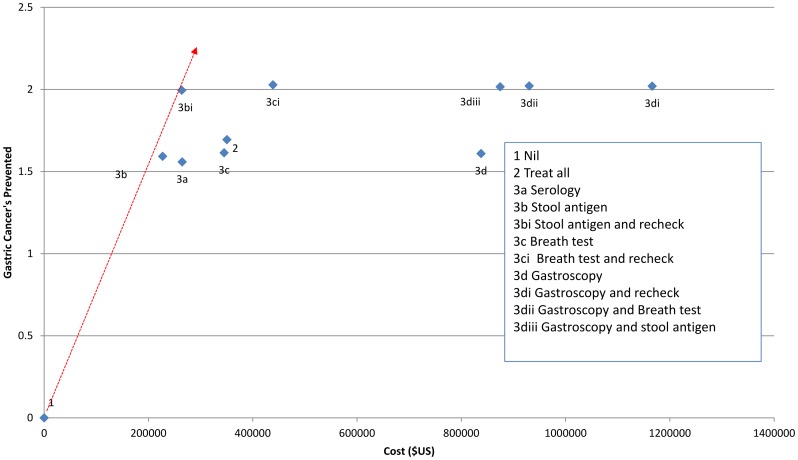
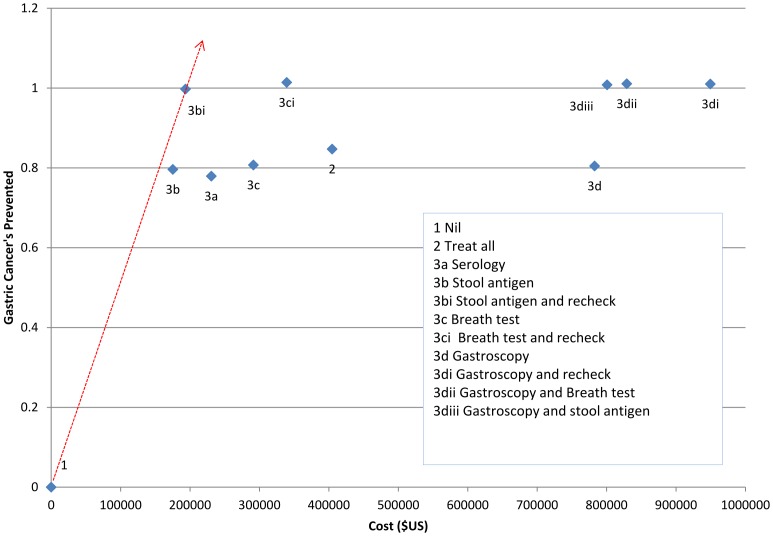
For all three prevalence rates tested, the most cost effective approach relative to others was testing with stool antigen, with treatment for those who tested positive followed by retesting and further treatment if the initial treatment failed (Figure 2, 3, 4).
Figure 2. Net cost per number of cancers prevented for different strategies for screening and treatment of H. pylori in a population with a 75% prevalence of H. pylori infection shown as an Incremental Cost Effectiveness Ratio (ICER).
Red line indicates lowest net cost per cancer prevented.
Figure 3. Net cost per number of cancers prevented for different strategies for management of H. pylori in a population with a 50% prevalence of H. pylori infection (ICER).
Red line indicates lowest net cost per cancer prevented.
Figure 4. Net cost per number of cancers prevented for different strategies for screening and treatment of H. pylori in a population with a 25% prevalence of H. pylori infection (ICER).
Red line indicates lowest net cost per cancer prevented.
When the prevalence was assumed to be 75%, the estimated net cost per cancer prevented was $111800 (strategy 3bi) (Table 3). For every 1000 people managed under this strategy we expect that 3.0 gastric cancers and 22.8 ulcers would be prevented (Table 4). At 50% prevalence the net cost per cancer prevented was estimated to be $132300, with prevention of 2.0 cancers and 15.2 ulcers per 1000 people managed. At 25% prevalence the net cost per cancer prevented was estimated to be $193900, with prevention of 1.0 cancer and 7.6 ulcers per thousand people managed (Tables 3 & 4).
Treating all individuals without screening was also a relatively cost effective strategy in a high prevalence (75%) population, with a net cost per cancer prevented of $116600 (Table 3), although the overall number of cancers (2.5/1000 treated) and ulcers (18.2/1000 treated) prevented was lower than with a strategy involving retesting and further treatment (Table 4). At a lower prevalence estimate of 25% (Table 4) the cost of treatment became a very significant burden with the treat-all strategy because most of the population would receive unnecessary treatment. At 25% prevalence the strategy with the lowest cost of testing (stool antigen testing) offered the lowest overall cost, and post treatment testing and retreatment improved the net benefit.
The use of serology was more expensive at all prevalence levels tested and breath test, although having slightly better sensitivity and specificity than stool antigen, was considerably more expensive. Any strategy that involved the use of gastroscopy had considerably higher net costs per cancer prevented (Table 3) and total costs (Table 4). The net costs were slightly lower than the total costs indicating that the cost savings from preventing gastric cancer and ulcer disease contributed only a small component of the cost/benefit of each strategy.
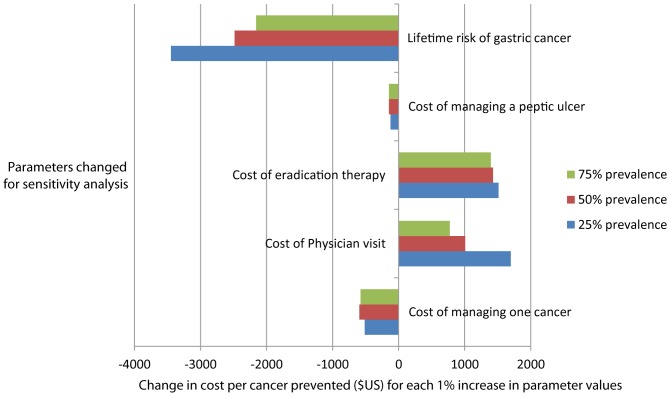
Figure 5 shows the sensitivity analysis for each of three prevalence values, using strategy 3bi, the optimal strategy. Parameters that represent the consequences of untreated H.pylori (peptic ulcer and gastric cancer) have negative values because as these costs rise, the value of eradicating H.pylori increases, the cost-effectiveness increases, and the net cost per cancer averted decreases. The parameters associated with the cost of the strategy have positive values, since as costs rise for any intervention, the cost per cancer averted rises. The cost of eradication therapy was the greatest cost associated with the strategy at high prevalence, while the risk of gastric cancer contributed significantly to the benefit of the strategy. Any increase in the estimated lifetime incidence of gastric cancer results in a significant decrease in the net cost per cancer prevented.
Figure 5. Sensitivity analysis for the most cost effective strategy (stool antigen with retesting).
Horizontal bars represent the estimated net effect on cost per cancer prevented in US Dollars with a 1% increase in the listed parameters.
Discussion
Screening and treatment of H. pylori in high-risk populations has been suggested as a means of reducing the burden of gastric cancer and peptic ulceration [8], [18] and been shown to be cost effective [19] however this is not routinely undertaken in Western countries [18]. Immigrants from developing countries represent a group with a high prevalence of H. pylori and hence a target group for screening strategies. Our modelling has shown that the costs associated with most of the available ‘test and treat’ strategies are not prohibitive. In particular, the use of a cheap and easily available stool antigen test has the potential to significantly lower the overall costs of screening, and deserves consideration in populations with high prevalence's of H. pylori. Notably the number of cancers and ulcers prevented is similar with stool antigen testing and retesting, breath test and retesting or any strategy involving gastroscopy and retesting. This indicates that the additional cost of more expensive screening strategies does not confer any significant additional benefit and reflects the similar sensitivity and specificity of these testing modalities.
Previous international modelling has shown that universal screening of 20 year olds for H. pylori is a cost effective way of reducing gastric cancer in a Chinese population [20]. Screening in lower prevalence populations has also been shown to be cost effective although the cost is significantly higher per quality adjusted life year (QALY) [5].
Our model may underestimate the benefits of screening and treatment as we did not include prevention of dyspepsia through H. pylori treatment and the associated reduced doctor visits for dyspepsia management, and potential for reduced hospital admissions [21]. The benefits of test and treat strategies may also be an underestimate as the cost of gastric cancer treatment may be considerably more than the cost estimates used in this analysis in patients over the age of 65 years [3]. The use of physician assistants or clinic nurses to order the initial testing would also lead to cost savings, and the use of more effective first line therapies could improve cost effectiveness. However, the frequency of treatment side effects, which reportedly occur in 22% of treated patients and 8% of placebo patients [22] needs to be considered and these additional side effects may increase costs.
H. pylori has been part of our gastrointestinal flora for 60000 years [23] so a recommendation for eradication should be made with caution. H. pylori prevalence rates have been falling in developed countries at the same time as allergic disease, reflux and obesity have been increasing [24]. Some randomised trial evidence demonstrates a small increase in weight after H. pylori eradication [25]. H. pylori eradication is also associated with a rise in prevalence of Barrett's Oesophagus [26], and increasing oesophageal cancer rates [27]. Concern that H. pylori eradication can lead to increased risk of gastro-oesophageal reflux disease (GERD) has not been confirmed in a large systematic review [28]. In any event this concern about oesophageal pathology is of small magnitude compared with the potential reduction in gastric cancer rates.
Antibiotic resistance to H. pylori is increasing and efficacy of standard treatment for H. pylori in many countries is now less than 80%, primarily due to clarithromycin resistance [29]. This is concerning for the implementation of a screening program. Other options that can be more effective include sequential therapy with PPI and amoxicillin for 5 days followed by PPI, clarithromycin and metronidazole for 5 days) [29], and longer courses (10–14 days) of quadruple therapy, including bismuth, tetracycline, metronidazole and a PPI [30].
Screening or empiric treatment for refugees and immigrants for infectious conditions is currently recommended for a number of pathogens. Empiric treatment for helminthic infections is cost effective and recommended in some settings [31]. Treatment costs for latent tuberculosis (TB) are over US$28000 (17,956 pounds) per episode of TB prevented [32]. TB in the United States now has a mortality less than 5% [33], compared to gastric cancer's 5 year mortality of 84% [5]. Screening and treatment for Hepatitis B virus is common in many immigrant groups, and is cost effective even at a population prevalence of less than 2% [34].
Stool sampling is currently routinely recommended for helminth detection for refugee groups arriving in many developed countries [35], and faecal antigen testing for H. pylori could be incorporated with stool testing for other pathogens, an additional important cost saving measure.
The current American College of Gastroenterology and also European guidelines do not recommend a general screen and treat strategy for H. pylori infection to reduce the risk of gastric cancer; and do not specifically address the issue of high risk populations [6], [36]. Asia Pacific guidelines, representing countries with a higher H. pylori prevalence, do recommend general screening for H. pylori in high risk populations although the strategy is not clearly defined [18].
Our data provide important evidence on which to base future recommendations.
Acknowledgments
We thank the Victorian Department of Health for funding the Refugee Health Fellow Program at the Royal Melbourne Hospital, and Christalla Hajisava for her assistance with formatting and manuscript submission.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1. McColl KEL (2010) Clinical practice. Helicobacter pylori infection. The New England Journal Of Medicine 362: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 2.Globocan (2008) Stomach Cancer Incidence, Mortality and Prevalence Worldwide in 2008.
- 3.SEER (2013) SEER Cancer Statistics Review, 1975–2010. National Cancer Institute.
- 4. Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, et al. (2008) Cost of Care for Elderly Cancer Patients in the United States. Journal of the National Cancer Institute 100: 630–641. [DOI] [PubMed] [Google Scholar]
- 5. Xie F, O'Reilly D, Ferrusi IL, Blackhouse G, Bowen JM, et al. (2009) Illustrating economic evaluation of diagnostic technologies: comparing Helicobacter pylori screening strategies in prevention of gastric cancer in Canada. J Am Coll Radiol 6: 317–323. [DOI] [PubMed] [Google Scholar]
- 6. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, et al. (2007) Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito M, Takata S, Tatsugami M, Wada Y, Imagawa S, et al. (2009) Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol 44: 365–371. [DOI] [PubMed] [Google Scholar]
- 8. De Vries AC, Van Driel HF, Richardus JH, Ouwendijk M, Van Vuuren AJ, et al. (2008) Migrant communities constitute a possible target population for primary prevention of Helicobacter pylori-related complications in low incidence countries. Scandinavian Journal Of Gastroenterology 43: 403–409. [DOI] [PubMed] [Google Scholar]
- 9. Cherian S, Forbes D, Sanfilippo F, Cook A, Burgner D (2008) The epidemiology of Helicobacter pylori infection in African refugee children resettled in Australia. The Medical Journal Of Australia 189: 438–441. [DOI] [PubMed] [Google Scholar]
- 10. Naja F, Kreiger N, Sullivan T (2007) Helicobacter pylori infection in Ontario: prevalence and risk factors. Canadian Journal Of Gastroenterology = Journal Canadien De Gastroenterologie 21: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torres J, Lopez L, Lazcano E, Camorlinga M, Flores L, et al. (2005) Trends in Helicobacter pylori infection and gastric cancer in Mexico. Cancer Epidemiology, Biomarkers & Prevention: A Publication Of The American Association For Cancer Research, Cosponsored By The American Society Of Preventive Oncology 14: 1874–1877. [DOI] [PubMed] [Google Scholar]
- 12.MPI (2010) 2010 American Community Survey and Census Data on the Foreign Born by State. In: Institute MP, editor. [Google Scholar]
- 13. Bai Y, Li ZS, Zou DW, Wu RP, Yao YZ, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut 59: 722–728. [DOI] [PubMed] [Google Scholar]
- 14. Laheij RJ, Straatman H, Jansen JB, Verbeek AL (1998) Evaluation of commercially available Helicobacter pylori serology kits: a review. J Clin Microbiol 36: 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gisbert JP, de la Morena F, Abraira V (2006) Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 101: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 16. Vaira D, Malfertheiner P, Megraud F, Axon AT (1999) Diagnosis of Helicobacter pylori infection by HpSA test. European Helicobacter pylori HpSA Study Group. Lancet 354: 1732. [DOI] [PubMed] [Google Scholar]
- 17. Yamaoka Y, Kato M, Asaka M (2008) Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med 47: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, et al. (2009) Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal Of Gastroenterology And Hepatology 24: 1587–1600. [DOI] [PubMed] [Google Scholar]
- 19. Areia M, Carvalho R, Cadime AT, Rocha Goncalves F, Dinis-Ribeiro M (2013) Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter 18: 325–337. [DOI] [PubMed] [Google Scholar]
- 20. Yeh JM, Kuntz KM, Ezzati M, Goldie SJ (2009) Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer 124: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barton PM, Moayyedi P, Talley NJ, Vakil NB, Delaney BC (2008) A second-order simulation model of the cost-effectiveness of managing dyspepsia in the United States. Med Decis Making 28: 44–55. [DOI] [PubMed] [Google Scholar]
- 22.Ford AC, Delaney B, Forman D, Moayyedi P (2011) Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Library. [DOI] [PubMed]
- 23. Linz B, Balloux Fo, Moodley Y, Manica A, Liu H, et al. (2007) An African origin for the intimate association between humans and Helicobacter pylori. Nature 445: 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blaser MJ, Chen Y, Reibman J (2008) Does Helicobacter pylori protect against asthma and allergy? Gut 57: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, et al. (2011) Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Alimentary Pharmacology & Therapeutics 33: 922–929. [DOI] [PubMed] [Google Scholar]
- 26. Sonnenberg A, Lash RH, Genta RM (2010) A National Study of Helicobactor pylori Infection in Gastric Biopsy Specimens. Gastroenterology 139: 1894–1901.e1892. [DOI] [PubMed] [Google Scholar]
- 27. Nordenstedt H, El-Serag H (2011) The influence of age, sex, and race on the incidence of esophageal cancer in the United States (1992–2006). Scandinavian Journal Of Gastroenterology 46: 597–602. [DOI] [PubMed] [Google Scholar]
- 28. Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH (2010) Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. The American Journal Of Gastroenterology 105: 1007. [DOI] [PubMed] [Google Scholar]
- 29. Graham DY, Fischbach L (2010) Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 30.Malfertheiner P, Bazzoli F, Delchier J-C, Celiñski K, Giguère M, et al. (2011) Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. The Lancet In Press, Corrected Proof. [DOI] [PubMed]
- 31. Muennig P, Pallin D, Sell RL, Chan MS (1999) The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med 340: 773–779. [DOI] [PubMed] [Google Scholar]
- 32. Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, et al. (2011) Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 11: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC (2012) Tuberculosis Fact Sheet.
- 34. Eckman MH, Kaiser TE, Sherman KE (2011) The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis 52: 1294–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC (2012) Domestic Intestinal Parasite Guidelines.
- 36. Chey WD, Wong BC (2007) American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102: 1808–1825. [DOI] [PubMed] [Google Scholar]
- 37. Jafri NS, Hornung CA, Howden CW (2008) Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med 148: 923–931. [DOI] [PubMed] [Google Scholar]
- 38. Fischbach LA, van Zanten SV, Dickason J (2004) Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Alimentary Pharmacology & Therapeutics 20: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 39. Sung JJY, Kuipers EJ, El-Serag HB (2009) Systematic review: the global incidence and prevalence of peptic ulcer disease. Alimentary Pharmacology & Therapeutics 29: 938–946. [DOI] [PubMed] [Google Scholar]
- 40.IFHP (2010) 2010 Comparative Price Report: Medical and Hospital Fees by Country. In: of IF, Plans H, editors.
- 41. Holmes KP, Fang JC, Jackson BR (2010) Cost-effectiveness of six strategies for Helicobacter pylori diagnosis and management in uninvestigated dyspepsia assuming a high resource intensity practice pattern. BMC Health Serv Res 10: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barkun A, Leontiadis G (2010) Systematic review of the symptom burden, quality of life impairment and costs associated with peptic ulcer disease. Am J Med 123: 358–366 e352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.