Abstract
Background
Although lifestyle factors such as cigarette smoking, excessive drinking, obesity, low or no exercise, and unhealthy dietary habits have each been associated with inadequate sleep, little is known about their combined effect. The aim of this study was to quantify the overall impact of lifestyle-related factors on non-restorative sleep in the general Japanese population.
Methods and Findings
A cross-sectional study of 243,767 participants (men, 39.8%) was performed using the Specific Health Check and Guidance System in Japan. A healthy lifestyle score was calculated by adding up the number of low-risk lifestyle factors for each participant. Low risk was defined as (1) not smoking, (2) body mass index<25 kg/m2, (3) moderate or less alcohol consumption, (4) regular exercise, and (5) better eating patterns. Logistic regression analysis was used to examine the relationship between the score and the prevalence of non-restorative sleep, which was determined from questionnaire responses. Among 97,062 men (mean age, 63.9 years) and 146,705 women (mean age, 63.7 years), 18,678 (19.2%) and 38,539 (26.3%) reported non-restorative sleep, respectively. The prevalence of non-restorative sleep decreased with age for both sexes. Compared to participants with a healthy lifestyle score of 5 (most healthy), those with a score of 0 (least healthy) had a higher prevalence of non-restorative sleep (odds ratio, 1.59 [95% confidence interval, 1.29–1.97] for men and 2.88 [1.74–4.76] for women), independently of hypertension, hypercholesterolemia, diabetes, and chronic kidney disease. The main limitation of the study was the cross-sectional design, which limited causal inferences for the identified associations.
Conclusions
A combination of several unhealthy lifestyle factors was associated with non-restorative sleep among the general Japanese population. Further studies are needed to establish whether general lifestyle modification improves restorative sleep.
Introduction
A combination of healthy lifestyle factors, such as abstaining from smoking, maintaining a body mass index (BMI) of less than 25 kg/m2, consuming alcohol moderately, exercising regularly, and having a healthy diet, is reportedly associated with a significantly reduced risk of developing several diseases, such as coronary heart disease [1]–[2], type 2 diabetes mellitus [3], stroke [4], sudden cardiac death [5], chronic kidney disease [6], cancer [7]–[9], and total mortality [10]. A clear linear relationship was observed in these studies between risk reduction and the number of healthy lifestyle factors, suggesting that an analysis of combined lifestyle factors may demonstrate their influence better than analyses based on single factors due to the complexity and multiple dimensions of habitual health behaviors. In addition, maintaining an overall healthy lifestyle throughout young adulthood was strongly associated with a low cardiovascular disease risk profile in middle age regardless of sex, race, or a parental history of myocardial infarction, suggesting that genetic factors may not be very important in determining a low risk profile [11]. Most of these studies were conducted in non-Japanese populations except for a few studies [6], [9]; however, a combination of healthy lifestyle factors may play a prominent role regardless of sex, race, or genetics.
Little is known about the impact of combined lifestyle factors on inadequate sleep. There is growing evidence that inadequate sleep, which includes short sleep duration and poor sleep quality, is associated with lifestyle factors that include obesity, insufficient physical exercise, and consumption of substances such as caffeine, alcohol, and nicotine [12]. Inadequate sleep may also modify eating patterns, thereby mediating or contributing to the observed relationship between sleep disturbance and obesity [13].
Inadequate sleep is associated with several chronic diseases. Epidemiological studies have shown that short sleep duration is associated with a higher risk of lifestyle-related diseases such as obesity [14]–[17], type 2 diabetes mellitus [18]–[19], hypertension [20], dyslipidemia [21], coronary heart disease [22], and chronic kidney disease [23]. Sleep quality is important in modifying the association between sleep duration and these diseases [24]–[26].
We hypothesized that a combination of unhealthy lifestyle factors is associated with inadequate sleep. Evidence of a relationship could have important clinical and public health implications. If a combination of unhealthy behaviors is associated with inadequate sleep, lifestyle interventions have the potential to reduce its occurrence. We present the results of a large cross-sectional study on the prevalence of non-restorative sleep (NRS), typically defined as subjectively feeling unrefreshed upon waking [27], and its association with a combination of lifestyle factors in the general Japanese population.
Methods
Study population and design
This cross-sectional study used baseline data from a prospective cohort study of 667,218 participants, aged 40 to 74 years, obtained from the Japanese Specific Health Check and Guidance System (SHC) created in 2008. Twenty-four of the prefectures participating in this nationwide project (Hokkaido, Miyagi, Yamagata, Fukushima, Ibaraki, Tochigi, Tokyo, Saitama, Kanagawa, Niigata, Nagano, Ishikawa, Gifu, Osaka, Okayama, Tokushima, Kochi, Fukuoka, Saga, Nagasaki, Oita, Kumamoto, Miyazaki, and Okinawa) agreed to participate in our study and were included in the present analysis. Data were sent to and verified by an independent data center, the NPO Japan Clinical Research Support Unit (Tokyo, Japan). All participants remained anonymous, and the study was conducted according to Japanese privacy protection laws and ethical guidelines for epidemiological studies published by the Ministry of Education, Science, and Culture and the Ministry of Health, Labor, and Welfare. The study protocol was approved by the ethics committee in Fukushima Medical University (No. 1485).
The SHC has been previously described [6], [28]. Briefly, it is a new healthcare strategy initiated by the Japanese Government in 2008 for the early diagnosis and intervention of metabolic syndrome. The proportion of men to women in the SHC does not necessarily reflect the national population. This is because the SHC is designed for people who have National Health Insurance, or dependents (e.g., spouse) of salaried workers who have health insurance. In this system, participants answer a self-administered questionnaire that covers medical history, smoking habits, alcohol intake, exercise habits, and eating patterns. Trained staff then measure the height, weight, blood pressure, and waist circumference of each participant, after which serum and spot urine samples are collected. BMI is calculated by dividing body weight in kilograms by the square of height in meters. Blood samples are analyzed using an automated clinical chemical analyzer within 24 h of sampling. All blood analyses are conducted at a local, rather than a central, laboratory. Although the methods used for blood analyses are not calibrated between laboratories, analyses are performed according to the Japan Society of Clinical Chemistry-recommended methods for laboratory tests, which have been widely adopted by laboratories across Japan [29]. Participants diagnosed with metabolic syndrome are obligated to receive repeated lifestyle guidance over a six-month period after an annual health examination.
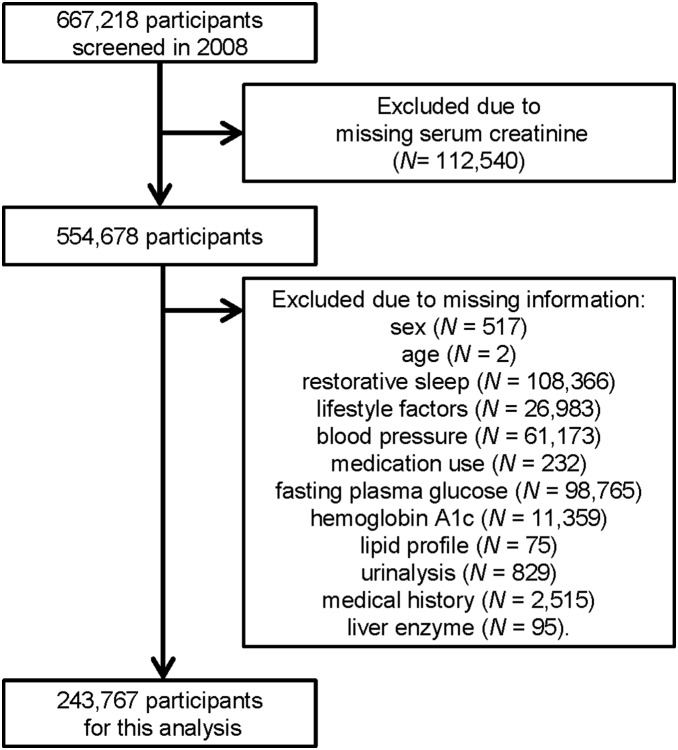
Participants from 40 to 74 years of age without missing information were included in this study. The complete selection process is presented in Figure 1.
Figure 1. Flow chart of participant selection.
Of the 667,218 SHC participants screened in 2008, we excluded anyone with missing information, resulting in a final sample size of 243,767.
Primary outcome
The primary outcome was NRS, which was assessed using this question from the self-administered questionnaire: ‘Do you feel refreshed after a night’s sleep?’ Participants answered either yes or no. NRS was considered present when the answer was ‘no’.
Lifestyle factors and covariates
For each lifestyle factor (smoking, BMI, alcohol intake, exercise habits, and eating patterns), we created a binary low-risk variable in which participants were given a score of 1 if they met the criteria for low risk or a score of 0 if otherwise, based on previous research [6]. The a priori definition of low risk was based on current literature, recommended guidelines, and realistically obtainable levels within the general population. We calculated a healthy lifestyle score by adding the total number of lifestyle factors for which each participant was at low risk. The score ranged from 0 (least healthy) to 5 (most healthy).
For smoking, we defined low risk as currently not smoking. Optimal body weight was defined as a BMI of <25 kg/m2, the standard World Health Organization cutoff for healthy weight. For alcohol, average daily consumption over 20 g was considered high risk. Alcohol consumption was assessed by the following questions: “How often do you drink alcohol (sake, shochu [distilled spirits], beer, liquor, etc.)?” to which participants responded by selecting (1) every day, (2) sometimes, or (3) rarely (can’t drink); and “How much do you drink a day, in terms of glasses of refined sake? (A glass [180 mL] of refined sake is equivalent to a medium bottle [500 mL] of beer, 80 mL of shochu (alcohol content 35 percent), a glass [double, 60 mL] of whiskey, and 2 glasses [240 mL] of wine),” to which participants responded by selecting (1) <1 drink per day, (2) 1–2 drinks per day, (3) 2–3 drinks per day, or (4) ≥3 drinks per day’. The ethanol content per drink was calculated to be equivalent to 20 g. For exercise habits, two questions were asked: ‘Are you in the habit of exercising to sweat lightly for over 30 minutes each time, two times weekly, for over a year?’ and ‘In your daily life, do you walk or do any equivalent amount of physical activity for more than one hour a day?’ Low risk patients were defined as those who answered ‘yes’ to both questions on the basis of a current Japanese guideline [30]. For eating patterns, two questions were asked: ‘Do you skip breakfast more than three times a week?’ and ‘Do you eat snacks after supper more than three times a week?’ Low risk patients were defined as those who answered ‘no’ to both questions.
Hemoglobin A1c (HbA1c) was estimated as a National Glycohemoglobin Standardization Program equivalent value using the following equation [31]: HbA1c (%) = HbA1c (Japan Diabetes Society) (%)+0.4%. Diabetes was defined in accordance with American Diabetes Association guidelines [32] as a fasting plasma glucose concentration of 126 mg/dL or higher, HbA1c of 6.5% or higher, or self-reported use of anti-hyperglycemic drugs. Hypertension was defined as using antihypertensive medications, a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or both. Hypercholesterolemia was defined as using cholesterol-lowering medications, a low-density lipoprotein (LDL) cholesterol level ≥140 mg/dL, or both. Chronic kidney disease was defined as proteinuria in urinalysis, a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2, or both [33]. Proteinuria was defined as a dipstick urinalysis score of 1+ or greater (equivalent to ≥30 mg/dL) because of poor discrimination between negative and trace positive dipstick readings [34]. Estimated GFR was calculated using the Japanese equation [35].
Statistical analysis
Data were analyzed separately by sex. First, we calculated the prevalence of NRS stratified by age categories. Age was categorized as 40–49, 50–59, 60–69, and 70–74 years. Second, we analyzed clinical and laboratory parameters stratified by the presence or absence of NRS. The chi-square test, Student’s t-test, and Mann-Whitney U test were used to assess differences among participant characteristics in relation to NRS. Spearman and Pearson correlation coefficients were calculated to evaluate the relationship among each independent variable. To evaluate the association between prevalent NRS and each variable of the healthy lifestyle score, multivariable-adjusted odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated using the category conventionally believed to be most healthy as the reference group. Data were initially adjusted for age. Next, we added age, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease to multivariate models (Model 1). Finally, we added history of stroke, heart disease, and renal failure to Model 1 (Model 2).
To assess the robustness of the main results, we conducted several subsidiary analyses. First, subgroup analyses were stratified by age categories because age is associated with NRS. Healthy lifestyle scores from 0 (least healthy) to 1 (second-least healthy) were combined into one category because there were few cases. Age was also added as a continuous variable. Second, subgroup analyses were conducted among nonusers of medications to avoid variations in results due to medications. Nonusers of medications were defined as individuals who took no medications for diabetes, hypertension, and hypercholesterolemia. Finally, the analyses were repeated after excluding participants with obesity (BMI≥25 kg/m2) to enhance the association between healthy lifestyle and NRS, as obese participants could modify their lifestyle to lose weight. Because increased BMI may be a consequence rather than a component of an unhealthy lifestyle, another healthy lifestyle score was created to incorporate all variables except BMI. This score ranged from 0 to 4 points.
P<0.05 was considered statistically significant, and all tests were two-tailed. All analyses were performed with the SPSS for Windows statistical package (Version 18.0; SPSS, Chicago, IL, USA) and Stata/MP software (Version 12.1; Stata Corp, College Station, TX, USA).
Results
Participant flow
Of the 667,218 SHC participants screened in 2008, we excluded those who did not have serum creatinine levels measured (n = 112,540) because it is not mandatory for the SHC but is included independently in some areas. We also excluded anyone with missing information (n = 310,911), resulting in a final sample size of 243,767 (Figure 1). There were no substantial differences between included and excluded participants for characteristics such as prevalence of NRS, sex, age, and healthy lifestyle score (Table S1).
Demographic characteristics of participants
Among 97,062 men and 146,705 women, 18,678 (19.2%) and 38,539 (26.3%) were identified as having NRS, respectively. Tables 1 and 2 present the associations between various clinical characteristics and NRS. Both male and female participants with NRS were younger, had higher prevalence of current smokers, higher BMI, lower prevalence of exercise habits, and higher prevalence of less healthy eating patterns. They were also more likely to have a history of heart or renal disease and less likely to have hypertension, diabetes, and chronic kidney disease. Some differences were observed between sexes. Women with NRS were less likely to have an adequate intake of daily alcohol, while this was more likely in men with NRS compared to those without NRS. In addition, the proportion of those with a history of stroke was significantly higher in women with NRS, but not in men with NRS.
Table 1. Clinical characteristics of male participants by restorative sleep achievement.
| Characteristics | Total (N = 97,062) | Restorative sleep(N = 78,384 [80.8%]) | Non-restorative sleep(N = 18,678 [19.2%]) | P value |
| Age, years | 63.9 (8.5) | 64.4 (8.2) | 61.7 (9.5) | <0.0001 |
| Healthy lifestyle score, n (%) | <0.0001 | |||
| 0 | 484 (0.5) | 360 (0.5) | 124 (0.7) | |
| 1 | 5,049 (5.2) | 3,715 (4.7) | 1,334 (7.1) | |
| 2 | 16,986 (17.5) | 13,140 (16.8) | 3,846 (20.6) | |
| 3 | 30,331 (31.3) | 24,326 (31.0) | 6,005 (32.2) | |
| 4 | 31,355 (32.3) | 25,735 (32.8) | 5,620 (30.1) | |
| 5 | 12,857 (13.3) | 11,108 (14.2) | 1,749 (9.4) | |
| Components of the healthy lifestyle score | ||||
| Current smoker, n (%) | 25,359 (26.1) | 20,214 (25.8) | 5,145 (27.5) | <0.0001 |
| Body mass index, kg/m2 | 23.6 (3.0) | 23.6 (2.9) | 23.7 (3.2) | 0.05 |
| Alcohol<20 g/day, n (%) | 67,556 (69.6) | 53,924 (68.8) | 13,632 (73.0) | <0.0001 |
| Regular exercise | ||||
| Exercise to sweat lightly, n (%) | 46,069 (47.5) | 39,055 (49.8) | 7,014 (37.6) | <0.0001 |
| Walking>1 hour/day, n (%) | 53,950 (55.6) | 45,253 (57.7) | 8,697 (46.6) | <0.0001 |
| Eating pattern | ||||
| Snacks after supper, n (%) | 12,000 (12.4) | 8,775 (11.2) | 3,225 (17.3) | <0.0001 |
| Skipping breakfast, n (%) | 11,060 (11.4) | 7,818 (10.0) | 3,242 (17.4) | <0.0001 |
| Past history, n (%) | ||||
| Stroke | 4.938 (5.1) | 4,003 (5.1) | 935 (5.0) | 0.59 |
| Heart disease | 7,964 (8.2) | 6,285 (8.0) | 1,679 (9.0) | <0.0001 |
| Renal disease | 552 (0.6) | 417 (0.5) | 135 (0.7) | 0.002 |
| Comorbidities, n (%) | ||||
| Hypertension | 49,135 (50.6) | 40,406 (51.5) | 8,729 (46.7) | <0.0001 |
| Diabetes | 14,596 (15.0) | 11,965 (15.3) | 2,631 (14.1) | <0.0001 |
| Hypercholesterolemia | 33, 258 (34.3) | 26,878 (34.3) | 6,380 (34.2) | 0.74 |
| Chronic kidney disease | 22,570 (23.3) | 18,481 (23.6) | 4,089 (21.9) | <0.0001 |
| Medication, n (%) | ||||
| Antihypertensive drugs | 30,756 (31.7) | 25,382 (32.4) | 5,374 (28.8) | <0.0001 |
| Antidiabetic medication | 6,649 (6.9) | 5,457 (7.0) | 1,192 (6.4) | 0.005 |
| Cholesterol-lowering drugs | 10,779 (11.1) | 8,856 (11.3) | 1,923 (10.3) | <0.0001 |
| Systolic pressure, mmHg | 131 (17) | 132 (17) | 130 (17) | <0.0001 |
| Diastolic pressure, mmHg | 78 (11) | 78 (11) | 78 (11) | 0.001 |
| Fasting plasma glucose, mg per 100 mL | 102 (25) | 102 (24) | 102 (26) | 0.66 |
| Hemoglobin A1c, % | 5.79 (0.79) | 5.79 (0.77) | 5.78 (0.85) | 0.02 |
| LDL cholesterol, mg per 100 mL | 120.9 (30.0) | 120.8 (29.9) | 121.1 (30.4) | 0.21 |
| Triglycerides, mg per 100 mL | 107 (77, 154) | 107 (77, 153) | 107 (76, 155) | 0.58 |
| HDL cholesterol, mg per 100 mL | 57 (15) | 57 (15) | 57 (15) | 0.96 |
| Creatinine, mg per 100 mL | 0.85 (0.23) | 0.85 (0.23) | 0.84 (0.23) | 0.03 |
| eGFR, mL min–1 per 1.73 m2 | 74.4 (16.4) | 74.2 (16.3) | 75.6 (16.9) | <0.0001 |
| Proteinuria, n (%) | 7,670 (7.9) | 6,157 (7.9) | 1,513 (8.1) | 0.26 |
Numbers in the table are means (standard deviation) for continuous variables except triglycerides (median and interquartile range) or numbers (percentages) for categorical variables.
LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
Table 2. Clinical characteristics of female participants by restorative sleep achievement.
| Characteristics | Total(N = 146,705) | Restorative sleep(N = 108,166 [73.7%]) | Non-restorativeSleep(N = 38,539 [26.3%]) | P value |
| Age, years | 63.7 (7.9) | 64.2 (7.7) | 62.6 (8.5) | <0.0001 |
| Healthy lifestyle score, n (%) | <0.0001 | |||
| 0 | 63 (0.0) | 35 (0.0) | 28 (0.1) | |
| 1 | 1,283 (0.9) | 765 (0.7) | 518 (1.3) | |
| 2 | 9,909 (6.8) | 6,502 (6.0) | 3,407 (8.8) | |
| 3 | 35,999 (24.5) | 25,208 (23.3) | 10,791 (28.0) | |
| 4 | 71,637 (48.8) | 53,100 (49.1) | 18,537 (48.1) | |
| 5 | 27,814 (19.0) | 22,556 (20.9) | 5,258 (13.6) | |
| Components of the healthy lifestyle score | ||||
| Current smoker, n (%) | 9,763 (6.7) | 6,655 (6.2) | 3,108 (8.1) | <0.0001 |
| Body mass index, kg/m2 | 22.7 (3.4) | 22.7 (3.3) | 22.6 (3.5) | <0.0001 |
| Alcohol<20 g/day, n (%) | 142,216 (96.9) | 105,038 (97.1) | 37,178 (96.5) | <0.0001 |
| Regular exercise | ||||
| Exercise to sweat lightly, n (%) | 58,932 (40.2) | 46,404 (42.9) | 12,528 (32.5) | <0.0001 |
| Walking>1 hour/day, n (%) | 76,043 (51.8) | 58,492 (54.1) | 17,551 (45.5) | <0.0001 |
| Eating pattern | ||||
| Snacks after supper, n (%) | 20,361 (13.9) | 13,630 (12.6) | 6,731 (17.5) | <0.0001 |
| Skipping breakfast, n (%) | 11,791 (8.0) | 7,429 (6.9) | 4,362 (11.3) | <0.0001 |
| Past history, n (%) | ||||
| Stroke | 3.902 (2.7) | 2,813 (2.6) | 1,089 (2.8) | 0.02 |
| Heart disease | 7,606 (5.2) | 5,271 (4.9) | 2,335 (6.1) | <0.0001 |
| Renal disease | 632 (0.4) | 436 (0.4) | 196 (0.5) | 0.007 |
| Comorbidities, n (%) | ||||
| Hypertension | 62,034 (42.3) | 46,822 (43.3) | 15,212 (39.5) | <0.0001 |
| Diabetes | 11,623 (7.9) | 8,625 (8.0) | 2,998 (7.8) | <0.0001 |
| Hypercholesterolemia | 74,371 (50.7) | 55,682 (51.5) | 18,689 (48.5) | <0.0001 |
| Chronic kidney disease | 21,762 (14.8) | 16,146 (14.9) | 5,616 (14.6) | <0.0001 |
| Medication, n (%) | ||||
| Antihypertensive drugs | 39,592 (27.0) | 29,884 (27.6) | 9,708 (25.2) | <0.0001 |
| Antidiabetic medication | 5,373 (3.7) | 3,960 (3.7) | 1,413 (3.7) | 0.96 |
| Cholesterol-lowering drugs | 28,802 (19.6) | 21,771 (20.1) | 7,031 (18.2) | <0.0001 |
| Systolic pressure, mmHg | 128 (18) | 129 (18) | 130 (18) | <0.0001 |
| Diastolic pressure, mmHg | 75 (11) | 75 (11) | 75 (11) | <0.0001 |
| Fasting plasma glucose, mg per 100 mL | 95 (18) | 95 (17) | 95 (19) | 0.03 |
| Hemoglobin A1c, % | 5.71 (0.59) | 5.72 (0.58) | 5.70 (0.60) | <0.0001 |
| LDL cholesterol, mg per 100 mL | 130.0 (30.3) | 130.2 (30.2) | 129.2 (30.8) | <0.0001 |
| Triglycerides, mg per 100 mL | 92 (68, 127) | 92 (69, 127) | 91 (67, 126) | <0.0001 |
| HDL cholesterol, mg per 100 mL | 65.7 (16.0) | 65.5 (16.0) | 66.3 (16.2) | <0.0001 |
| Creatinine, mg per 100 mL | 0.63 (0.15) | 0.63 (0.15) | 0.63 (0.16) | 0.005 |
| eGFR, mL min–1 per 1.73 m2 | 75.6 (15.9) | 75.4 (15.9) | 76.3 (16.1) | <0.0001 |
| Proteinuria, n (%) | 5,777 (3.9) | 4,169 (3.9) | 1,608 (4.2) | 0.006 |
Numbers in the table are means (standard deviation) for continuous variables except triglycerides (median and interquartile range) or numbers (percentages) for categorical variables.
LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
Associations between the healthy lifestyle score and NRS
The prevalence of NRS decreased with increasing age for both men and women (P for trend <0.0001, Figure 2), and was lower among men than women for all age groups. An inverse, dose-response relationship was observed between healthy lifestyle scores and prevalence of NRS for both male and female participants (P for trend <0.0001, Figure 3).
Figure 2. Prevalence of non-restorative sleep by sex and age.
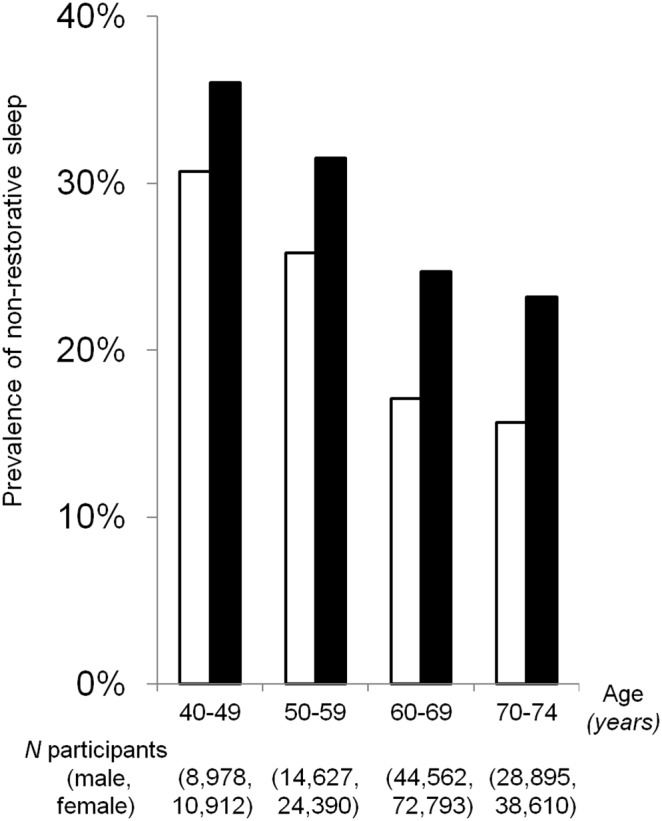
Trends were significant for both males (□; P<0.0001) and females (▪; P<0.0001).
Figure 3. Prevalence of non-restorative sleep by healthy lifestyle score.
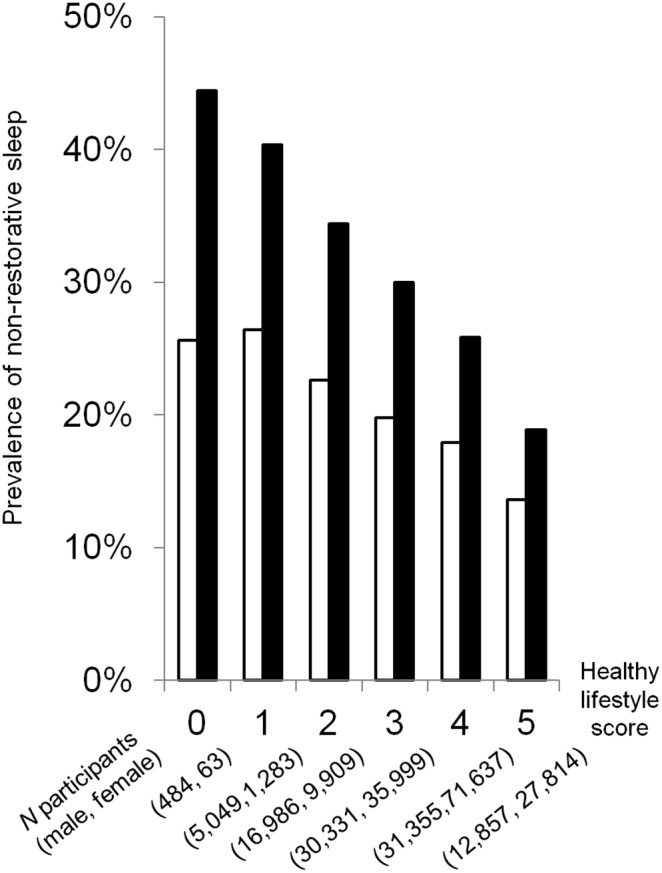
Trends were significant for both males (□; P<0.0001) and females (▪; P<0.0001).
When each variable of the healthy lifestyle score was considered individually, less healthy eating patterns and no regular exercise were associated with a higher prevalence of NRS (Table 3), but there were no apparent associations between BMI and the prevalence of NRS. Some differences were observed between men and women. Current smokers were associated with a higher prevalence of NRS in women and a lower prevalence in men. In addition, alcohol consumption was not associated with NRS in women but was associated with a lower prevalence in men.
Table 3. Multivariate analysis of the relationship between categories from the healthy lifestyle score and prevalence of non-restorative sleep (N = 243,767).
| Variable | Male (N = 97,062) | Female (N = 146,705) | ||
| Age-adjustedodds ratio(95%CI) | Multivariateodds ratioa(95%CI) | Age-adjustedodds ratio(95%CI) | Multivariateodds ratioa(95%CI) | |
| Categories | ||||
| Current smoker | ||||
| No (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.90 (0.87–0.93)**** | 0.90 (0.87–0.93)**** | 1.09 (1.04–1.14)**** | 1.09 (1.04–1.14)**** |
| Body mass index | ||||
| <25 m/kg2 (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥25 m/kg2 | 0.97 (0.93–1.00) | 0.97 (0.94–1.01) | 0.98 (0.95–1.00) | 0.99 (0.96–1.02) |
| Alcohol consumption | ||||
| <20 g/day (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥20 g/day | 0.83 (0.80–0.86)**** | 0.83 (0.80–0.86)**** | 1.03 (0.96–1.10) | 1.03 (0.96–1.10) |
| Regular exercise | ||||
| Yes (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.57 (1.51–1.62)**** | 1.57 (1.51–1.63)**** | 1.52 (1.47–1.58)**** | 1.52 (1.48–1.56)**** |
| Eating pattern | ||||
| Healthy (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| Less healthy | 1.54 (1.48–1.60)**** | 1.54 (1.48–1.60)**** | 1.44 (1.40–1.48)**** | 1.44 (1.40–1.48)**** |
| Age | ||||
| 40–49 years | 1.96 (1.85–2.08)**** | 1.95 (1.84–2.07)**** | 1.56 (1.48–1.63)**** | 1.50 (1.43–1.57)**** |
| 50–59 years | 1.63 (1.55–1.71)**** | 1.62 (1.54–1.71)**** | 1.35 (1.30–1.40)**** | 1.32 (1.27–1.37)**** |
| 60–69 years | 1.09 (1.04–1.13)**** | 1.09 (1.04–1.13)**** | 1.06 (1.03–1.09)**** | 1.05 (1.02–1.09)** |
| 70–74 years (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| Hypertension | 0.96 (0.93–1.00)* | 0.94 (0.91–0.96)**** | ||
| Diabetes mellitus | 0.99 (0.95–1.04) | 1.06 (1.02–1.11)** | ||
| Hypercholesterolemia | 0.97 (0.93–1.00) | 0.95 (0.92–0.97)**** | ||
| Chronic kidney disease | 1.04 (1.00–1.08) | 1.04 (1.00–1.08)* | ||
Adjusted for age (years), sex, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Definitions of these factors are described in the text.
*P<0.05,
**P<0.01,
***P<0.001,
****P<0.0001.
Tables 4 and 5 show that participants with a score of 0 (least healthy) had an age-adjusted OR of 1.56 (95% CI, 1.26–1.93) for men and 2.76 (95% CI, 1.68–4.56) for women, compared to those with a score of 5 (most healthy). Additional adjustments for potential consequences of an unhealthy lifestyle (i.e., hypertension, diabetes mellitus, hypercholesterolemia, and chronic kidney disease) only partially changed risk (OR for men: 1.59, 95% CI, 1.29–1.97; OR for women: 2.88, 95% CI, 1.74–4.76). This association was not changed by additional adjustments for history of stroke, heart disease, and renal failure (Model 2).
Table 4. Odds ratios for the association between the healthy lifestyle score and prevalent non-restorative sleep in men (N = 97,062).
| Healthy lifestyle score | Unadjusted | Age-adjusted | Model 1 | Model 2 |
| 0 | 2.19 (1.77–2.70)**** | 1.56 (1.26–1.93)**** | 1.59 (1.29–1.97)**** | 1.60 (1.29–1.98)**** |
| 1 | 2.28 (2.10–2.47)**** | 1.73 (1.59–1.88)**** | 1.76 (1.62–1.91)**** | 1.77 (1.63–1.92)**** |
| 2 | 1.86 (1.75–1.98)**** | 1.54 (1.44–1.64)**** | 1.56 (1.46–1.66)**** | 1.56 (1.46–1.66)**** |
| 3 | 1.57 (1.48–1.66)**** | 1.40 (1.32–1.48)**** | 1.41 (1.33–1.49)**** | 1.41 (1.33–1.50)**** |
| 4 | 1.39 (1.31–1.47)**** | 1.31 (1.24–1.39)**** | 1.32 (1.24–1.40)**** | 1.32 (1.24–1.40)**** |
| 5 (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
Model 1: adjusted for age (years), hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Model 2: adjusted Model 1 plus history of stroke, heart disease, and renal failure.
*P<0.05,
**P<0.01,
***P<0.001,
****P<0.0001.
Table 5. Odds ratios for the association between the healthy lifestyle score and prevalent non-restorative sleep in women (N = 146,705).
| Healthy lifestyle score | Unadjusted | Age-adjusted | Model 1 | Model 2 |
| 0 | 3.43 (2.09–5.65)**** | 2.76 (1.68–4.56)**** | 2.88 (1.74–4.76)**** | 2.88 (1.74–4.75)**** |
| 1 | 2.91 (2.59–2.26)**** | 2.43 (2.16–2.73)**** | 2.48 (2.21–2.79)**** | 2.47 (2.20–2.78)**** |
| 2 | 2.25 (2.14–2.37)**** | 2.00 (1.90–2.11)**** | 2.04 (1.94–2.15)**** | 2.03 (1.93–2.14)**** |
| 3 | 1.84 (1.77–1.91)**** | 1.71 (1.65–1.78)**** | 1.74 (1.67–1.81)**** | 1.73 (1.67–1.80)**** |
| 4 | 1.50 (1.45–1.55)**** | 1.44 (1.39–1.49)**** | 1.45 (1.40–1.50)**** | 1.44 (1.39–1.49)**** |
| 5 (ref) | 1.00 | 1.00 | 1.00 | 1.00 |
Model 1: adjusted for age (years), hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Model 2: adjusted Model 1 plus history of stroke, heart disease, and renal failure.
*P<0.05,
**P<0.01,
***P<0.001,
****P<0.0001.
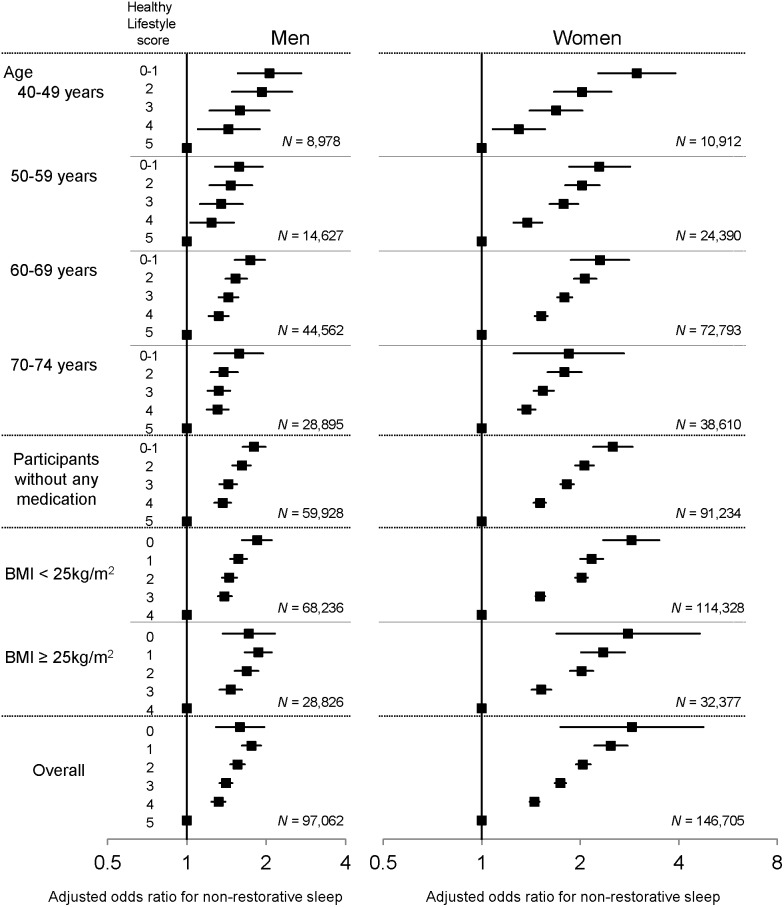
When stratified by age categories, the association between a healthy lifestyle score and prevalence of NRS was similar when compared with the entire study population for both men and women (Figure 4). Among participants who currently took no medications for diabetes, hypertension, or hypercholesterolemia, a similar association was also observed for both sexes. Furthermore, the associations were consistent among obese and non-obese participants.
Figure 4. Subgroup analysis.
Forest plot shows odds ratio with 95% confidence interval for the association between a healthy lifestyle score and prevalent non-restorative sleep in subgroups and in the entire study population. All analyses were adjusted for the following covariates: age (in years), hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Discussion
Our findings support the hypothesis that a combination of unhealthy lifestyle factors is associated with inadequate sleep. A combination of healthy lifestyle factors was associated with a decreased prevalence of NRS in both men and women of any age, even after adjusting for comorbidities such as hypertension, diabetes mellitus, hypercholesterolemia, chronic kidney disease, and history of stroke, heart disease, and renal failure. Although further study is needed to strengthen the association between the combined lifestyle factors and inadequate sleep, these findings raise the possibility that a healthy lifestyle could not only reduce the risk of developing several diseases but also improve sleep quality. If healthy lifestyle factors can provide restorative sleep, gaining satisfying rest would be a strong motivator for lifestyle modification.
Insomnia is an important public health issue [36] characterized by nighttime sleep problems. These may be manifest as difficulties in initiating or maintaining sleep, NRS, a combination of these complaints, or daytime symptoms [37]. Although there is a lack of consistency in the definition of NRS, and reports on NRS have been less extensive compared with other symptoms of primary insomnia (i.e., sleep latency and total sleep time), mounting evidence suggests that NRS is a frequent symptom observed in the general population. NRS prevalence was reported to be 10.8% in the non-institutionalized general population in seven European countries (France, the United Kingdom, Germany, Italy, Portugal, Spain, and Finland) [38], 35% in the participants of the Atherosclerosis Risk in Community Study in the United States [39], and 14.8% in the general South Korean population [40]. Although our methodology and questionnaire were not the same as those used in these studies, our findings were consistent in that NRS is frequently observed in the general Japanese population. The prevalence of NRS was higher in women than in men and decreased with age in our study. These results are in line with previous studies from Europe [38], the United States [36], [39], South Korea [40], as well as an international survey conducted in Finland, Greece, Jordan and Lebanon, Morocco, Mexico, the Philippines, Portugal, Sweden, and Switzerland [41]. Although the prevalence of insomnia differs from one country to another [40], a decrease in NRS prevalence with age is commonly observed. This suggests that some common risk factors may be shared among young people with different racial, ethnic, cultural, and environmental background.
With regard to individual lifestyle factors, we found associations between NRS and physical inactivity or unhealthy eating patterns. Both physical activity and a healthy diet are known to be associated with sleep quality. A high level of exercise is related to better sleep patterns such as higher sleep quality, shortened sleep latency, and fewer awakenings during the night [42], while lack of habitual exercise is associated with more reported sleep complaints [43]–[44]. Meanwhile, skipping breakfast and a regular habit of snacking are more common in individuals with short sleep duration than in those with normal sleep duration [45]. A randomized crossover study showed that skipping breakfast in a nocturnal lifestyle, i.e., sleeping at 1∶30 a.m. and waking at 8∶30 a.m., was associated with decreased secretion of melatonin and leptin [46], suggesting that those lifestyle factors might be both a cause and a consequence of inadequate sleep. Although information is limited about NRS and physical inactivity or eating patterns, our findings are in line with these studies.
We found no association between NRS and BMI. Although obesity is associated with sleep apnea, little is known about its connection to NRS. Previous literature has shown that BMI is not associated with the prevalence of NRS, except in underweight individuals (BMI<20 kg/m2) who had a higher prevalence of NRS than those with normal BMI (20–24 kg/m2) [40]. A possible reason for this discrepancy is that use of a binary variable in our analysis did not detect an association between being underweight and having NRS that might only be observed when analyzed quantitatively.
Notably, sex differences were observed for smoking and alcohol consumption. Although smoking was associated with increases in NRS in women, the opposite relationship was observed in men, and adequate alcohol consumption was associated with increases in NRS among only male participants. It has generally been found that both alcohol consumption and smoking are associated with increases in sleep disorders [47]–[49], but associations between NRS and alcohol consumption or smoking are still controversial. Smoking has been associated with NRS in some studies [40], [48] but not in another [39]. While alcohol consumption is not associated with NRS [39], some studies have found that alcohol abuse [50] and alcohol dependence [40] are connected to NRS. These inconsistencies may be primarily due to the different definitions of NRS, which only represents a single dimension or item of sleep symptoms [36]. It is also possible that using the binary variable in our study influenced results. Although associations between NRS and alcohol or smoking differed between men and women, the combined effect of healthy lifestyle factors on NRS were similar for both, suggesting that lifestyle factors cooperate with one another and are important for restorative sleep. Furthermore, a comprehensive analysis of healthy lifestyle may capture influence of individual factors better than analyses based on a single factor, given the complexity and multiple dimensions of habitual health behaviors. Further investigation is needed to determine whether critical thresholds exist for each lifestyle factor and to establish combined effects and interaction effects as well as individual effects.
Patients with NRS were recently reported to have higher C-reactive protein (CRP) levels, a marker for systemic inflammation, than those without NRS [51]. Elevated CRP has also been associated with lifestyle risk factors such as obesity [52], physical inactivity [53], cigarette smoking [54], and alcohol consumption [55], [56]. These findings together suggest that participants with NRS have higher CRP levels due to unhealthy lifestyle behaviors, although no information about CRP was available in our study. It is possible that sleep deprivation [57], [58] or stress [59] leads to increased CRP levels. Further investigation is warranted to clarify these aspects of the relationship between NRS and lifestyle factors.
Our study has several limitations. First, a selection bias of subjects might exist. Because participants in this cohort received annual physical checkups, they might be more health-conscious than the average Japanese population. Second, NRS was determined solely based on self-reported information and may not be accurate. A single retrospective item has limitations that need to be addressed, such as recall bias and demand characteristics. In addition, frequency (i.e., more than three times per week) and sleep duration were not included in the questionnaire used to assess NRS. However, there is no reliable and well-validated patient-reported outcome instrument currently available for evaluating NRS [27]. Third, we cannot exclude the possibility that residual confounding factors exist, which were not measured in the present study, such as marital status [38], educational level [36], [50], employment status [38], work schedule [50], level of stress [50], psychiatric disorders [38], and use of sleep medications. Whether these factors affect the relationship between lifestyle factors and NRS should be assessed in the future. Fourth, we gave equal weight to each lifestyle factor to achieve the main purpose of the study. That may have resulted in conservative estimates for multiple lifestyle factors. Fifth, the nutritive content in diet could not be evaluated due to lack of information. Evidence regarding the associations between diet and NRS is scarce, although a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) from the United States has shown that NRS is positively associated with butanoic acid, moisture, cholesterol, and negatively associated with calcium, vitamin C, and water [60]. Sixth, odds ratios do not approximate well to the relative risk when the effect sizes are large and the prevalence of the outcome of interest is high [61]. In our study, the prevalence of NRS was relatively high, whereas the effect sizes were not large. In addition, qualitative judgments based on interpreting odds ratios as though they were relative risks are unlikely to be seriously in error [61]. Therefore, we consider that our results demonstrate important effects. Finally, the cross-sectional study design limited our ability to determine the direction of the association or causality. NRS might be the cause, rather than the consequence, of one or more unhealthy lifestyle factors.
Our study also has several strengths. First, it was a large-scale cross-sectional study with participants from all over Japan. Second, this is the first report, to the best of our knowledge, to demonstrate the prevalence of NRS in the general Japanese population and assess the association between combined lifestyle factors and NRS.
In conclusion, a combination of healthy lifestyle factors was associated with an increase in restorative sleep independently of age, sex, and comorbidities in the general Japanese population. Further studies are needed to establish whether general lifestyle modification improves restorative sleep.
Supporting Information
Clinical characteristics of participants included and excluded in the analysis. Numbers in the table are means (standard deviation) for continuous variables except triglycerides (median and interquartile range) or numbers (percentages) for categorical variables. Note that the percentages are computed based only on the total number of non-missing cases. LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
(DOC)
Funding Statement
This study was supported by a Health and Labor Sciences Research Grant for “Design of a comprehensive health care system for chronic kidney disease (CKD) based on individual risk assessment by Specific Health Checkups” (H24-intractible(renal)-ippan-006) from the Ministry of Health, Labor, and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC (2000) Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 343: 16–22. [DOI] [PubMed] [Google Scholar]
- 2. Chiuve SE, McCullough ML, Sacks FM, Rimm EB (2006) Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 114: 160–167. [DOI] [PubMed] [Google Scholar]
- 3. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, et al. (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345: 790–797. [DOI] [PubMed] [Google Scholar]
- 4. Kurth T, Moore SC, Gaziano JM, Kase CS, Stampfer MJ, et al. (2006) Healthy lifestyle and the risk of stroke in women. Arch Intern Med 166: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 5. Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, et al. (2011) Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA 306: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakasugi M, Kazama JJ, Yamamoto S, Kawamura K, Narita I (2013) A combination of healthy lifestyle factors is associated with a decreased incidence of chronic kidney disease: a population-based cohort study. Hypertens Res 36: 328–333. [DOI] [PubMed] [Google Scholar]
- 7. Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, et al. (2000) Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 11: 579–588. [DOI] [PubMed] [Google Scholar]
- 8. Jiao L, Mitrou PN, Reedy J, Graubard BI, Hollenbeck AR, et al. (2009) A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 169: 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, et al. (2012) Combined impact of five lifestyle factors and subsequent risk of cancer: the Japan Public Health Center Study. Prev Med 54: 112–116. [DOI] [PubMed] [Google Scholar]
- 10. van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB (2008) Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 337: a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, et al. (2012) Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 125: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shochat T (2012) Impact of lifestyle and technology developments on sleep. Nature Science Sleep 4: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peuhkuri K, Sihvola N, Korpela R (2012) Diet promotes sleep duration and quality. Nutrition Research 32: 309–319. [DOI] [PubMed] [Google Scholar]
- 14. Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, et al. (2004) The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 27: 661–666. [DOI] [PubMed] [Google Scholar]
- 15. Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB (2005) Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 28: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 16. Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB (2006) Association between reduced sleep and weight gain in women. Am J Epidemiol 164: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaput JP, Després JP, Bouchard C, Tremblay A (2008) The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 31: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, et al. (2003) A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26: 380–384. [DOI] [PubMed] [Google Scholar]
- 19. Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, et al. (2007) Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 30: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, et al. (2006) Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47: 833–839. [DOI] [PubMed] [Google Scholar]
- 21. Kaneita Y, Uchiyama M, Yoshiike N, Ohida T (2008) Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep 31: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, et al. (2003) A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med 163: 205–209. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto R, Nagasawa Y, Iwatani H, Shinzawa M, Obi Y, et al. (2012) Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis 59: 343–355. [DOI] [PubMed] [Google Scholar]
- 24. Chang ET (2009) The impact of daytime naps on the relation between sleep duration and cardiovascular events. Arch Intern Med 169: 717 doi:10.1001/archinternmed.2009.29 [DOI] [PubMed] [Google Scholar]
- 25. Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG (2010) The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep 33: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM (2011) Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep 34: 1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vernon MK, Dugar A, Revicki D, Treglia M, Buysse D (2010) Measurement of non-restorative sleep in insomnia: A review of the literature. Sleep Med Rev 14: 205–212. [DOI] [PubMed] [Google Scholar]
- 28. Kohro T, Furui Y, Mitsutake N, Fujii R, Morita H, et al. (2008) The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J 49: 193–203. [DOI] [PubMed] [Google Scholar]
- 29. Tsuruya K, Yoshida H, Nagata M, Kitazono T, Hirakata H, et al. (2014) Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: analysis in a large Japanese population. Atherosclerosis. 233: 260–267. [DOI] [PubMed] [Google Scholar]
- 30.The Office for Lifestyle-Related Diseases Control, General Affairs Division, Health Service Bureau, Ministry of Health, Labour and Welfare of Japan (2006) Exercise and Physical Activity Guide for Health Promotion 2006 - To Prevent Lifestyle-related Diseases - <Exercise Guide 2006>Prepared in August, 2006. Available: http://www.nih.go.jp/eiken/programs/pdf/exercise_guide.pdf. Accessed 13 September 2011.
- 31. The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus (2010) Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 53: 450–467. [Google Scholar]
- 32.American Diabetes Association (2011) Diagnosis and classification of diabetes mellitus. Diabetes Care (Suppl 1): S62–S69. [DOI] [PMC free article] [PubMed]
- 33. National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 (Suppl 1)S1–S266. [PubMed] [Google Scholar]
- 34. Harrison NA, Rainford DJ, White GA, Cullen SA, Strike PW (1989) Proteinuria: What value is the dipstick? Br J Urol 63: 202–208. [DOI] [PubMed] [Google Scholar]
- 35. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 36.Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, et al. (2013) Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 9: 897–905; 905A–905D. [DOI] [PMC free article] [PubMed]
- 37. Roth T, Zammit G, Lankford A, Mayleben D, Stern T, et al. (2010) Nonrestorative sleep as a distinct component of insomnia. Sleep 33: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohayon MM (2005) Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med 165: 35–41. [DOI] [PubMed] [Google Scholar]
- 39. Phillips B, Mannino D (2005) Correlates of sleep complaints in adults: the ARIC study. J Clin Sleep Med 1: 277–283. [PubMed] [Google Scholar]
- 40. Kim BS, Jeon HJ, Hong JP, Bae JN, Lee JY, et al. (2012) DSM-IV psychiatric comorbidity according to symptoms of insomnia: a nationwide sample of Korean adults. Soc Psychiatry Psychiatr Epidemiol 47: 2019–2033. [DOI] [PubMed] [Google Scholar]
- 41. Léger D, Partinen M, Hirshkowitz M, Chokroverty S, EQUINOX (Evaluation of daytime QUality Impairment by Nocturnal awakenings in Outpatient’s eXperience) Survey Investigators, et al (2010) Characteristics of insomnia in a primary care setting: EQUINOX survey of 5293 insomniacs from 10 countries. Sleep Med. 11: 987–998. [DOI] [PubMed] [Google Scholar]
- 42. Brand S, Gerber M, Beck J, Hatzinger M, Pühse U, et al. (2010) High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J Adolesc Health 46: 133–141. [DOI] [PubMed] [Google Scholar]
- 43. Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R (2000) An epidemiological study of insomnia among the Japanese general population. Sleep 23: 41–47. [PubMed] [Google Scholar]
- 44. Morgan K (2003) Daytime activity and risk factors for late-life insomnia. J Sleep Res 12: 231–238. [DOI] [PubMed] [Google Scholar]
- 45. Kim S, DeRoo LA, Sandler DP (2011) Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr 14: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin LQ, Li J, Wang Y, Wang J, Xu JY, et al. (2003) The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 73: 2467–2475. [DOI] [PubMed] [Google Scholar]
- 47. Phillips BA, Danner FJ (1995) Cigarette smoking and sleep disturbance. Arch Intern Med 155: 734–737. [PubMed] [Google Scholar]
- 48. Wetter DW, Young TB (1994) The relation between cigarette smoking and sleep disturbance. Prev Med 23: 328–334. [DOI] [PubMed] [Google Scholar]
- 49. Brower KJ (2003) Insomnia, alcoholism and relapse. Sleep Med Rev 7: 523–539. [DOI] [PubMed] [Google Scholar]
- 50. Ohayon MM, Hong SC (2002) Prevalence of insomnia and associated factors in South Korea. J Psychosom Res 53: 593–600. [DOI] [PubMed] [Google Scholar]
- 51. Zhang J, Lamers F, Hickie IB, He JP, Feig E, et al. (2013) Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep 36: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi J, Joseph L, Pilote L (2013) Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 14: 232–244. [DOI] [PubMed] [Google Scholar]
- 53. Kasapis C, Thompson PD (2005) The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol 45: 1563–1569. [DOI] [PubMed] [Google Scholar]
- 54. Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK (2003) Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med 138: 891–897. [DOI] [PubMed] [Google Scholar]
- 55. Albert MA, Glynn RJ, Ridker PM (2003) Alcohol consumption and plasma concentration of C-reactive protein. Circulation 107: 443e7. [DOI] [PubMed] [Google Scholar]
- 56. Averina M, Nilssen O, Arkhipovsky VL, Kalinin AG, Brox J (2006) C-reactive protein and alcohol consumption: Is there a U-shaped association? Results from a population-based study in Russia. The Arkhangelsk study. Atherosclerosis 188: 309–315. [DOI] [PubMed] [Google Scholar]
- 57. van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, et al. (2009) Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 4: e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, et al. (2004) Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 43: 678–683. [DOI] [PubMed] [Google Scholar]
- 59. Johnson TV, Abbasi A, Master VA (2013) Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Mol Diagn Ther. 17: 147–164. [DOI] [PubMed] [Google Scholar]
- 60. Grandner MA, Jackson N, Gerstner JR, Knutson KL (2014) Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 23: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davies HT, Crombie IK, Tavakoli M (1998) When can odds ratios mislead? BMJ. 316: 989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of participants included and excluded in the analysis. Numbers in the table are means (standard deviation) for continuous variables except triglycerides (median and interquartile range) or numbers (percentages) for categorical variables. Note that the percentages are computed based only on the total number of non-missing cases. LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
(DOC)