Abstract
Colorectal cancer (CRC) is a leading cause of cancer related death in Europe and the USA. There is no universally accepted effective non-invasive screening test for CRC. Guaiac based faecal occult blood (gFOB) testing has largely been superseded by Faecal Immunochemical testing (FIT), but sensitivity still remains poor. The uptake of population based FOBt testing in the UK is also low at around 50%. The detection of volatile organic compounds (VOCs) signature(s) for many cancer subtypes is receiving increasing interest using a variety of gas phase analytical instruments. One such example is FAIMS (Field Asymmetric Ion Mobility Spectrometer). FAIMS is able to identify Inflammatory Bowel disease (IBD) patients by analysing shifts in VOCs patterns in both urine and faeces. This study extends this concept to determine whether CRC patients can be identified through non-invasive analysis of urine, using FAIMS. 133 patients were recruited; 83 CRC patients and 50 healthy controls. Urine was collected at the time of CRC diagnosis and headspace analysis undertaken using a FAIMS instrument (Owlstone, Lonestar, UK). Data was processed using Fisher Discriminant Analysis (FDA) after feature extraction from the raw data. FAIMS analyses demonstrated that the VOC profiles of CRC patients were tightly clustered and could be distinguished from healthy controls. Sensitivity and specificity for CRC detection with FAIMS were 88% and 60% respectively. This study suggests that VOC signatures emanating from urine can be detected in patients with CRC using ion mobility spectroscopy technology (FAIMS) with potential as a novel screening tool.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer related death in Europe and the USA [1], [2]. At present there is a lack of effective, non-invasive screening tests for CRC. Current methods utilise guaiac based faecal occult blood (gFOB) testing, however, this has now largely been replaced by Faecal Immunochemical Testing (FIT). Whilst this is an improvement, FIT still shows relatively low sensitivity for CRC, 66–88%, depending on the cut off values for haemoglobin (50–200 ng/ml), with a specificity of 87–96% [3]–[5]. The sensitivity for advanced adenoma is even lower at 27–41%, with a specificity of 91–97% [5]. The uptake of screening utilising faecal samples is also an issue, with approximately 50% of invited participants not accepting population based FOBt screening in our locality.
Non-invasive testing of cancers, using Volatile Organic Compounds (VOCs) and gases that emanate from urine, breath, stool and blood, has received growing interest and has been an expanding area of research in recent years. This work initially started from the use of canines to detect cancers, which showed a marked ability to discriminate cancer patients from healthy individuals [6], [7], [8]. However, more recently a number of groups have indicated that it is possible to use gas phase analytical instruments, specifically gas chromatography and mass spectrometry (GC-MS), selective-ion flow mass spectrometer (SIFT) and the electronic nose (e-nose), to detect lung, breast, bladder and prostate cancers [9], [10], [11], [12]. For a detailed review on gas phase biomarkers in Gastroenterology, please see Arasaradnam et al [13].
In direct relation to colorectal cancer (CRC), recent work has shown that it is possible to discriminate cancer from non-cancer patients, but can also be used for the discrimination of lung, breast, prostate and colorectal cancer from each other by analysing breath samples [14]. This is further supported by recent work that has also shown that CRC can be distinguished from controls with over 75% accuracy using GC-MS, again employing breath analysis [15]. VOCs present in urine have also been shown to distinguish CRC patients from control groups and other cancers (leukaemia and lymphoma), with GC-MS [16]. The electronic nose (Cyrano 320, Sensigent, USA) has also been shown to discriminate between CRC and healthy controls when the VOCs profile in faeces are analysed (85% sensitivity and 87% specificity). Additionally the e-nose was able to discriminate between advanced adenomas and healthy controls with 62% sensitivity and 86% specificity [5]. These studies support the existence of putative gas phase bio-markers within biological output media for detecting CRC and thus could be the basis of a rapid screening tool.
VOCs can exist in the gaseous phase and are present in exhaled air, sweat, urine and faeces [17], [18]. The mechanism for the generation of VOCs is the subject of current research but they are perturbed in many physiological and pathological states - affected by diet and disease states. It is believed that the generation of VOCs within the bowel are the result of colonic bacteria undergoing fermentation of non-starch polysaccharides – fibre consumed by the host. As such, they represent the complex interaction of colonic cells, human gut microflora and invading pathogens [19], [20]. The study of the resultant products of fermentation which we have termed ‘the fermentome’ [17], [18], [21], [22] can be measured in urine. The latter is presumed possible due to the altered gut permeability afforded in certain gut diseases [23]. We believe that VOCs represent a bio-signature that represents the sum of the multifactorial influences (genetics, environmental factors including diet and disease states) affecting an individual. The aim of this study was to test the potential of FAIMS – a novel highly sensitive technology to differentiate between CRC and healthy controls using only urine samples.
Materials and Methods
2.1 Subjects
One hundred and thirty three individuals were recruited prospectively for this study. Eighty-three of these patients had histologically confirmed colorectal cancer and fifty were healthy individuals who had a recent normal colonoscopy. The mean age of the CRC patients was 60 years (SD 17 years), 53 (64%) were male. The demographics of the subjects are shown in Table 1.
Table 1. Demographic data for CRC and control patients.
| N = 133 | CRC | Controls |
| Number | 83 | 50 |
| Mean Age (years) | 60 (17) | 47 (16) |
| Sex: M/F | 53∶30 | 21∶29 |
| Mean BMI | 27 (7) | 26 (5) |
| Current Smokers (% of whole population) | 6.0% | 1.5% |
| Alcohol: Greater than recommended units/week (% of whole population) | 5.3% | 3.8% |
Figures in parenthesis are standard deviations (SD).
2.2 Study Design
This was a case control study where patients were recruited from outpatient clinics at University Hospital Coventry & Warwickshire, UK. Urine was then collected in standard universal Sterilin specimen containers (Newport, UK) and frozen immediately to −80°C, for subsequent batch analysis.
2.3 Analysis
Samples were thawed at room temperature overnight, aliquoted into appropriate sample bottles and analysed using the FAIMS experimental methods described below.
2.3.1 FAIMS
For FAIMS analysis, a commercial instrument was utilised (Lonestar, Owlstone, UK, employing an ATLAS sampling system and split flow box). This system achieves separation of chemical components on the basis of differences in the electric field dependence of ionised chemical mobilities. FAIMS allows gas molecules to be separated and analysed at atmospheric pressure and room temperature, unlike similar traditional analytical techniques. After a sample is ionised, it is composed of ions of various sizes and types. These are introduced between two metal plates and an asymmetric high voltage waveform is applied to these plates, subjecting the ionized molecules to high electric fields. The difference in movement of these molecules within this high electric field can be measured, thus resulting in a separation of the complex mixture.
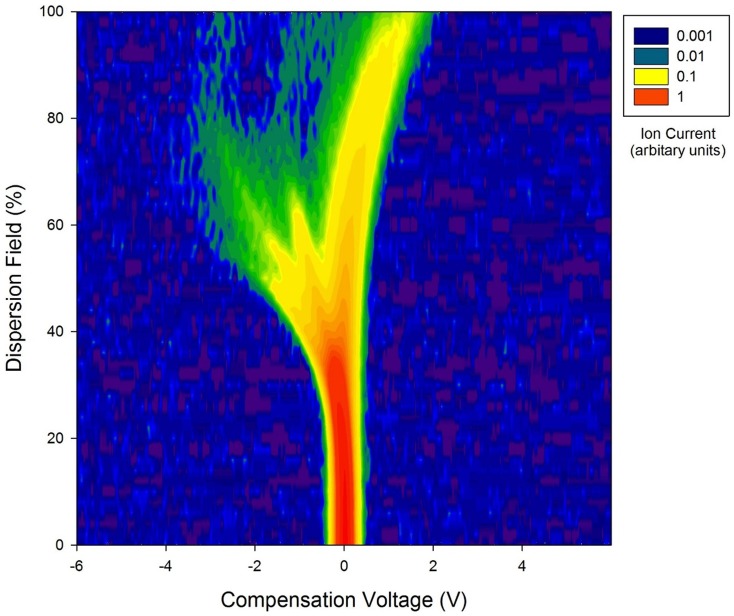
A sample of 5 ml of urine is aliquoted into a 20 ml glass vial and placed inside the ATLAS sampler. The sampler heats the sample to (in our case) 40±0.1°C, when the sample reaches the correct temperature (typically 10 min), clean synthetic air is passed over the sample and into the Lonestar FAIMS instrument. The flow rate over the sample is 500 ml/min and increased to 2 L/min by additional clean air before being passed into the instrument. The Lonestar is set up to scan between 0 and 90% dispersion field (the dispersion field represents the magnitude of the electric field) in 51 steps and a compensation voltage of between −6 V and +6 V in 512 steps. The compensation voltage is used to remove the effect of the drift produced by the high electric field, thus only molecules that have a specific mobility exit the plates at that point. Figure 1 shows a typical FAIMS ‘plume’ produced from a CRC patient's urine sample plotted on a log scale.
Figure 1. Log of raw data from the FAIMS instrument for a colorectal cancer patient.
Intensity is in arbitrary units of ion count.
2,3.2 GC-MS
For GC-MS analysis, separate aliquots of 5 ml are taken from each sample to run through a Bruker Scion SQ GC-MS instrument. Each aliquot was agitated and heated to 60°C for 5 minutes, before the contents of its headspace are extracted using a Combi-PAL ITEX automated pre-concentrator system. The volatiles contained in the ITEX were then released by heating to 250°C, and injected into the instrument at a split ratio of 1∶20 with helium carrier gas. The Restek Rxi-624Sil column (20 m length, 0.18 mm ID, 1.0 um df) fitted to the GC was kept at a constant 50°C for 1 minute before being increased up to 280°C at a rate of 20°C/min, separating out the constituent VOCs in terms of molecular weight and polarity. After an initial detection to produce a chromatogram, the VOC molecules are fragmented by the mass spectrometer and detected to produce corresponding mass spectra. These are correlated with the GC chromatogram peaks, and checked against a National Institute of Standards and Technology library (NIST 2013) of chemical compounds. This was perfrimed in order to gain additional insight into the specific chemical groups that make up the volatile headspace of the urine samples in those with CRC.
2.4 Statistical Methods
Data analysis for FAIMS results is performed using Fisher Discriminant Analysis. This allows for the simple interpretation of complex data to determine if differences in groups can be detected. The data was processed in Matlab (Mathworks Inc., USA, R2013b). For analysis, both the positive and negative ion count matrices of each sample were concatenated into a single 52,224 element vector (or 1D array). These were then wavelet transformed using a Daubechies D4 wavelet, a technique commonly used in data compression and has the ability to separate out subtle signals within a dataset. Data points within the 52,224 elements suitable for discrimination are then identified. This is achieved by calculating the within class scatter (Σσi) and the between class scatter [(σμ)2/(Σσi)2] for each point within the vector (thus the same datapoints (or variable) from all the sample datasets are examined and the within class as well as between class scatter calculated across all the samples), to generate two further 1D arrays, again formed of 52,224 data points. Different thresholds are then set for within class and between class scatter and the variables that are within these thresholds are then used for data processing by fisher discriminant analysis (FDA; a pre-classified linear technique). To test the validity of the FDA, five samples from each group (CRC and controls) were removed before the FDA was performed. Then, based on the FDA results of the remnant, a prediction is made on the group of the unknown samples. This prediction is based on a KNN (k-nearest neighbor) method. This process is repeated ten times for each pair threshold values until optimum thresholds, and thus set of variables, are identified. This variable group is then used for the remainder of the analysis process to calculate sensitivity and specificity. For further details on analyses described in detail, please see Covington et al [23].
2.5 Ethics
Scientific and ethical approval was obtained from local Research & Development Department and Warwickshire Ethics Committee 09/H1211/38. Written informed consent was obtained from all patients who participated in the study.
Results
The demographic data of the cancer and non-cancer group are described in Table 1. No statistically significant difference between the groups was noted however, as expected there was male gender predominance. Details of the tumour staging for the 83 CRC patients are shown in Table 2.
Table 2. Staging for CRC patients using the tumour, nodal and metastases classification (TNM).
| N = 83 | T1 | T2 | T3 | T4 | ||||
| N0 | N1/2 | N0 | N1/2 | N0 | N1/2 | N0 | N1/2 | |
| Non-metastatic (M0) | 3 | 1 | 6 | 5 | 19 | 19 | 5 | 7 |
| Metastatic (M1) | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 4 |
N.B. 9 CRC patients could not be fully staged (inoperable).
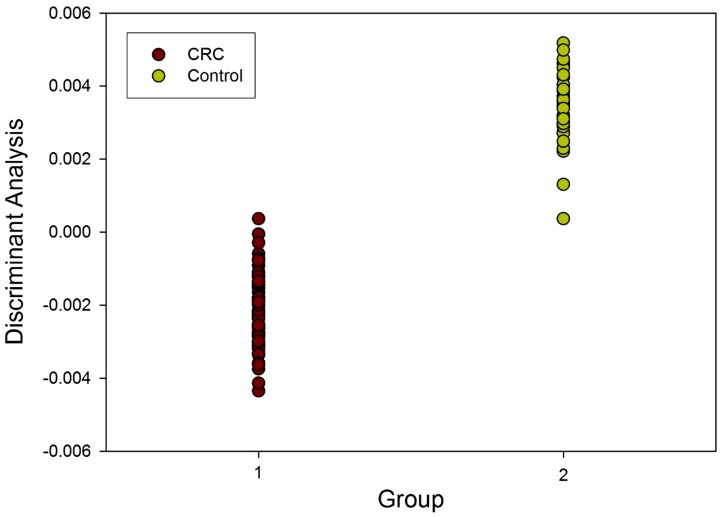
The analysis of the FAIMS data for CRC patients and controls was carried out using Fisher Discriminant Analysis, as described above, and the results from the identified variables are shown in Figure 2. Reclassification for CRC was correct in 74% of cases (p<0.001). The sensitivity and specificity of the FAIMS analysis to detect CRC were 88% and 60% respectively.
Figure 2. Fisher Disciminant Analysis (FDA) of FAIMS data in patients with CRC and controls.
The CRC patients' urine was also analysed by GC-MS, the results can be found in table 3, along with a list of corresponding chemicals from the National Institute for Standards and Technology (NIST) database. No unique chemical was identified in those with CRC compared with controls.
Table 3. GC-MS peaks for the CRC patients and corresponding National Institute of Standards and Technology (NIST) targets for these peaks.
| Average GC Retention Time (min) | Major MS Peaks | Common NIST targets |
| 1.4 | 42, 43, 45, 56, 58 | Acetaldehyde, Ethylene Oxide, Oxalic Acid |
| 1.53 | 42, 44, 55, 67 | Dimethyl Diazene, Cyclobutyl Amine, Oxepane |
| 1.75 | 42, 43, 58 | Acetone |
| 2.95 | 39, 43, 44, 58, 71, 86 | 2-Pentanone, 3-methyl-2-Butanone, 2,3-Butanedione |
| 4.56 | 43, 58, 71 | 4-Heptanone, 3-Heptanone, 2,4-dimethyl-3-Pentanone |
| 4.7 | 44, 51, 63, 78, 104 | Acetyloxime-Pyridine Carboxaldehyde, Hydrocinnamoyl-Bezene-ethanamine, Styrene, Dimethyl-Thiourea |
| 4.77 | 39, 40, 60, 72, 99 | Allyl Isothiocyanate, Isothiocyanato-cyclopropane, 2-cyano-acetamide |
| 5.31 | 42, 44, 56, 75, 94, 118, 133, 151 | Methoxy-phenyl-oxime, Ethylbenzoic acid (pentyl ester), Carbamic acid (methyl ester) |
| 5.38 | 41, 43, 44, 57, 72 | Hexen-1-ol, 4-methyl-1-hexene, Hexanal |
Discussion
This study is the first to our knowledge to report the utility FAIMS analyses for CRC detection in urine and supports previous work by others using electronic nose [5] and GCMS [16]. This has been achieved by investigating how gases and vapours (VOCs) emanating from urine samples are disparate in those with CRC compared with controls. Our findings also support previous work where, VOC signature differences were noted in CRC patients within different biological materials (faeces, breath and urine) [5], [15], [16]. Our CRC cohort had a male sex predominance, as would be expected for a population of CRC patients, whilst our control group had a slight female predominance. This could raise the prospect of sex bias within the cohort, however, whilst the pattern of VOCs and by association, the fermentome, could theoretically be affected by sex and age, data from our previous published studies of IBD, bile acid malabsorption, pelvic cancer and coeliac disease have not shown any propensity for age and sex affecting the VOC signals [23], [24], [25], [26], [27].
De Meij et al showed that the e-nose could discriminate CRC from healthy controls with 85% sensitivity and 87% specificity, and could also distinguish advanced adenomas from healthy controls with 62% sensitivity and 86% specificity [5]. Our study has shown using urine specimens rather than faeces – with FAIMS demonstrating a sensitivity of 88% and a specificity of 60%. This has importance especially in our local population where uptake of screening is poor due to the requirement to produce faecal samples.
Ion mobility has a number of advantages over both GCMS and e-nose: for example it is undertaking a physical measurement of molecules instead of a chemical interaction (as would a traditional e-nose) and secondly, the sensitivity is much higher i.e. parts per billion to parts per trillion. This makes it an ideal platform for a future screening tool especially as the sensitivity is high. Our findings expand on previous research describing how both e-nose and FAIMS technologies can be used to distinguish effects of radiation in pelvic cancers [25] and inflammatory conditions e.g. between Crohn's disease and ulcerative colitis [26], in addition to Bile acid malabsorption and Coeliac disease [23], [27].
Genetic stool markers have received interest as a potential non-invasive screening target for CRC. Lidgard et al [28] performed a study of automated stool DNA analysis for β-actin, mutant kRAS, aberrantly methylated BMP3 and NDRG4, and faecal haemoglobin. This showed a sensitivity of 98% and 90% specificity for CRC and 57–83% sensitivity for advanced adenomas depending on size. Our study shows comparable sensitivity results, but at a much lower process cost, and with urine rather than faecal sampling.
The unique chemical fingerprint or ‘bio-odorant fingerprint’ produced by the different disease states, and healthy individuals, shows the potential of this technology to screen for, and aid, in the diagnosis of CRC. It also has the potential to aid in further investigation of individuals with other gastrointestinal diseases. Gases and vapours are thought to be produced by the process of colonic fermentation involving a complex interaction between the colonocyte cells, human faecal flora, mucosal integrity and invading pathogens [17], [18]. VOCs emitted from bodily fluids thus have huge potential as putative biomarkers for use in the assessment of gastrointestinal diseases. Alterations in the pattern of VOCs are thought to reflect changes in the gastrointestinal environment. This suggests a possible role for gut microflora dysbiosis in the pathophysiology of CRC [29].
Conclusions
This study has shown that the VOC signature present in the urine of patients with CRC, can be distinguished from healthy controls using FAIMS. The sensitivity and specificity of FAIMS is 88% and 60% respectively for CRC. Whilst this is lower than the gold standard of colonoscopy it is comparable with current faecal stool testing including the guaiac and immunohistochemical methods. The UK uptake for screening is low; 62%, 57% and 59% uptake in the first, second and third rounds of the national screening programme [30], and around 50% currently in the local population. One of the reasons for this is the nature of the biological sample required. Offering an alternative and less intrusive option, such as urine rather than faeces, is likely to be far more acceptable to patients, and can be incorporated into screening pathways for the future.
Acknowledgments
We would like to thank WPH, BRET and the Eveson Trust who provided funding for this research.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included within the paper.
Funding Statement
The work was supported by Bardhan Research and Education Charity (BRET) charity, Warwickshire Private Hospital charity, Eveson trust - provided funding for materials. BRET was established by Professor Bardhan but he had no input into the funding decision made by the grant awarding committee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62 (1) 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46: 765–81. [DOI] [PubMed] [Google Scholar]
- 3. Brenner H, Tao S (2013) Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 49 (14) 3049–54. [DOI] [PubMed] [Google Scholar]
- 4. Imperiale TF (2012) Non-invasive screening tests for colorectal cancer. Dig Dis 30 Suppl 2: 16–26. [DOI] [PubMed] [Google Scholar]
- 5. De Meij TG, Ben Larbi I, van der Schee MP, Lentferink YE, Paff T, et al. (2014) Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: proof of principle study. Int J Cancer 134 (5) 1132–1138. [DOI] [PubMed] [Google Scholar]
- 6. Lippi G, Cervellin G (2012) Canine olfactory detection of cancer versus laboratory testing: myth or opportunity? Clin Chem Lab Med 50 (3) 435–9. [DOI] [PubMed] [Google Scholar]
- 7. Sonoda H, Kohnoe S, Yamazato T, Satoh Y, Morizono G, et al. (2011) Colorectal cancer screening with odour material by canine scent detection. Gut 60 (6) 814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arasaradnam RP, Nwokolo CU, Bardhan KD, Covington JA (2011) Electronic nose versus canine nose: clash of the titans. Gut 60 (12) 1768. [DOI] [PubMed] [Google Scholar]
- 9. Westhoff M, Litterst P, Maddula S, Bödeker B, Rahmann S, et al. (2010) Differentiation of chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control group by breath analysis using ion mobility spectrometry. International Journal for Ion Mobility Spectrometry 13 (3–4) 131–139. [Google Scholar]
- 10. Phillips M, Cataneo RN, Ditkoff BA, Fisher P, Greenberg J, et al. (2003) Volatiles markers of breast cancer in the breath. The Breast J 9: 184–191. [DOI] [PubMed] [Google Scholar]
- 11. Khalid T, White P, De Lacy Costello B, Persad R, Ewen R, et al. (2013) A Pilot Study Combining a GC-Sensor Device with a Statistical Model for the Identification of Bladder Cancer from Urine Headspace. PLoS ONE 8 (7) e69602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernabei M, Pennazza G, Santonico M, Corsi C, Roscion C, et al. (2008) A preliminary study on the possibility to diagnose urinary tract cancers by an electronic nose. Sens Actuat B Chem 131: 1–4. [Google Scholar]
- 13. Arasaradnam RP, Covington JA, Harmston C, Nwokolo CU (2014) Next generation diagnostic modalities in gastroenterology – gas phase volatile compound biomarker detection. Alimentary Pharmacology and Therapeutics 39 (8) 780–789. [DOI] [PubMed] [Google Scholar]
- 14. Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, et al. (2010) Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 103 (4) 542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, et al. (2013) Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 100 (1) 144–50. [DOI] [PubMed] [Google Scholar]
- 16. Silva CL, Passos M, Câmara JS (2011) Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer 105 (12) 1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, et al. (2009) Volatile organic compunds as diagnostic biomarkers in gastronintestinal and liver disease. J Gastroninestin Liver Dis 18: 337–43. [PubMed] [Google Scholar]
- 18. Arasaradnam RP, Pharaoh MW, Williams GJ, Nwokolo CU, Bardhan KD, et al. (2009) Colonic fermentation–more than meets the nose. Med Hypotheses 73 (5) 753–6. [DOI] [PubMed] [Google Scholar]
- 19. Buszewski B, Kesy M, Ligor T, Amann A (2007) Human exhaled air analytics: biomarkers of disease. Biomed Chromatogr 21: 533–66. [DOI] [PubMed] [Google Scholar]
- 20. Garner CE, Smith S, de Lacy Costello B, White P, et al (2007) Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J 21: 1675–88. [DOI] [PubMed] [Google Scholar]
- 21. Arasaradnam RP, Quraishi N, Kyrou I, Nwokolo CU, Joseph M, et al. (2011) Insights into ‘Fermentonomics’: Evaluation of volatile organic compounds (VOCs) in human disease using an Electronic ‘e’ Nose. J Med Eng Technol 35 (2) 87–91. [DOI] [PubMed] [Google Scholar]
- 22. Arasaradnam RP, Ouaret N, Thomas MG, Gold P, Quraishi MN, et al. (2012) Evaluation of gut bacterial populations using an electronic e-nose and field asymmetric ion mobility spectrometry: further insights into ‘fermentonomics’. J Med Eng Technol 36 (7) 333–7. [DOI] [PubMed] [Google Scholar]
- 23. Covington JA, Westenbrink EW, Ouaret N, Harbord R, Bailey C, et al. (2013) Application of a novel tool for diagnosing bile acid diarrhoea. Sensors (Basel) 13 (9) 11899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arasaradnam RP, Bardhan KD (2010) Bioactive foods and Extracts – Cancer treatment and prevention. Taylor Francis, New York. [Google Scholar]
- 25. Covington JA, Wedlake L, Andreyev J, Ouaret N, Thomas MG, et al. (2012) The detection of patients at risk of gastrointestinal toxicity during pelvic radiotherapy by electronic nose and FAIMS: a pilot study. Sensors (Basel) 12 (10) 13002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arasaradnam RP, Ouaret N, Thomas MG, Quraishi N, Heatherington E, et al. (2013) A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm Bowel Dis 19 (5) 999–1003. [DOI] [PubMed] [Google Scholar]
- 27. Arasaradnam R, Westinbrink E, McFarlane M, Harbord R, Chambers S, et al. (2014) Differentiating Coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis – a pilot study. PLOS One (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, et al. (2013) Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol 11 (10) 1313–8. [DOI] [PubMed] [Google Scholar]
- 29. Pagnini C, Corleto VD, Mangoni ML, Pilozzi E, Torre MS, et al. (2011) Alteration of local microflora and α-defensins hyper-production in colonic adenoma mucosa. J Clin Gastroenterol 45 (7) 602–10. [DOI] [PubMed] [Google Scholar]
- 30. Moss SM, Campbell C, Melia J, Coleman D, Smith S, et al. (2012) Performance measures in three rounds of the English bowel cancer screening pilot. Gut 61 (1) 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included within the paper.