Abstract
Objective
With improved survival of HIV-infected persons on antiretroviral therapy and growing prevalence of non-AIDS diseases, we asked whether the VACS Index, a composite measure of HIV-associated and general organ dysfunction predictive of all-cause mortality, predicts hospitalization and medical intensive care unit (MICU) admission. We also asked whether AIDS and non-AIDS conditions increased risk after accounting for VACS Index score.
Methods
We analyzed data from the Veterans Aging Cohort Study (VACS), a prospective study of HIV-infected Veterans receiving care between 2002–2008. Data were obtained from the electronic medical record, VA administrative databases and patient questionnaires, and were used to identify comorbidities and calculate baseline VACS Index scores. The primary outcome was first hospitalization within 2 years of VACS enrollment. We used multivariable Cox regression to determine risk factors associated with hospitalization and logistic regression to determine risk factors for MICU admission, given hospitalization.
Results
1141/3410 (33.5%) patients were hospitalized within 2 years; 203/1141 (17.8%) included a MICU admission. Median VACS Index scores were 25 (no hospitalization), 34 (hospitalization only) and 51 (MICU). In adjusted analyses, a 5-point increment in VACS Index score was associated with 10% higher risk of hospitalization and MICU admission. In addition to VACS Index score, Hispanic ethnicity, current smoking, hazardous alcohol use, chronic obstructive pulmonary disease, hypertension, diabetes and prior AIDS-defining event predicted hospitalization. Among those hospitalized, VACS Index score, cardiac disease and prior cancer predicted MICU admission.
Conclusions
The VACS Index predicted hospitalization and MICU admission as did current smoking, hazardous alcohol use, and AIDS and certain non-AIDS diagnoses.
Keywords: HIV, hospitalization, medical intensive care unit (MICU), aging, VACS Index, comorbidity
INTRODUCTION
Hospitalizations, particularly those that require medical intensive care unit (MICU) admission, are difficult for patients and their caregivers, can contribute to increasing frailty and mortality risk, and result in substantial costs among those without HIV infection.1–10 Overall, hospitalization rates for HIV-infected individuals have decreased since the introduction of combination antiretroviral therapy (ART).11–16 However, MICU admissions have been at least stable and may be increasing.11; 12; 17
Patients typically want to avoid hospitalization and are fearful of treatments and interventions commonly used during MICU admission. Healthcare systems want to avoid these admissions and will often assign case managers to patients at particularly high risk.18 Nevertheless, hospitalizations, particularly those that require MICU admission, may become more common in HIV-infected patients due to treatment toxicities and increasingly prevalent non-AIDS conditions.11; 12; 19–27 Non-AIDS conditions such as cardiovascular disease, pulmonary disease, chronic liver disease, and cancer account for the majority of hospitalizations and MICU admissions.11–13; 19–27 Many of these are considered "HIV-associated non-AIDS (HANA) conditions" 11; 12; 19–27 as they occur with increased frequency among HIV-infected patients and may progress more rapidly compared to demographically similar uninfected individuals.20; 28–32
Several HANA conditions have been identified as risk factors for hospitalization13–16 and are strongly associated with age and with lower CD4 count and/or higher HIV-1 RNA levels. However, age, CD4 count and HIV-1 RNA considered individually may not accurately predict risk of hospitalization and MICU admission since the majority of these events are for non-AIDS conditions.11; 13; 23; 33; 34 While risk factors for MICU mortality have been identified in HIV-infected patients, risk factors for MICU admission have not been identified. Finally, risk factors for hospitalization and MICU admission have not been organized into a weighted index in the current treatment era.
Therefore, we sought to identify risk factors for hospitalization and MICU admission among HIV infected patients in the current ART era. We hypothesized that the VACS Risk Index (VACS Index), a measure of HIV-specific and general organ injury, 35; 36 could be used to predict risk for hospitalization and need for MICU admission. The VACS Index incorporates age and 8 routine clinical laboratory measures, namely: CD4 count, HIV RNA, hemoglobin, aspartate and alanine aminotransferase (AST and ALT), creatinine, and hepatitis C sero status. Variables are weighted to produce a composite score for individuals. 22; 37; 38 (Supplemental Digital Content Table 1) Substantial work has been completed developing and validating the VACS Index for all-cause mortality in European and North American cohorts.22; 39 We first asked whether the VACS Index predicts 2-year risk of hospitalization and MICU admission. We then determined whether history of common AIDS-defining events (ADEs), prevalent HANA conditions, smoking, and alcohol use increased predictive ability after adjusting for VACS Index scores.
METHODS
Patients
The Veterans Aging Cohort Study (VACS) has been described in detail elsewhere and is approved by all participating institutional review boards.40; 40 Written informed consent was obtained from patients prior to enrollment. VACS is a prospective, observational cohort study conducted at 8 Veterans Administration (VA) Medical Centers’ infectious disease and general medicine clinics located in Atlanta, Baltimore, the Bronx, Houston, Los Angeles, Manhattan/Brooklyn, Pittsburgh, and Washington, D.C. Enrollment began in 2002 and is ongoing. HIV-infected patients are matched by age, race, and site-of-care to HIV-uninfected patients. Analyses in this study were restricted to HIV-infected patients enrolled between 2002–2008.
Data and sources used
Data were obtained from self-administered patient questionnaires completed at study entry and extracts from the electronic medical record (EMR), including demographics, International Classification of Diseases, 9th Revision (ICD-9) codes, laboratory tests and medications. Patients were considered on ART, defined as at least 3 antiretroviral medications (other than boosted ritonavir), if they were receiving it at enrollment.
Smoking status and hazardous alcohol use
Smoking status was defined based on questionnaires. 28; 41–43 Current smokers reported smoking any amount in the preceding 4 weeks. Former smokers reported quitting smoking 4 or more weeks prior to questionnaire administration. Hazardous alcohol use was a composite variable defined by the presence of at least one of the following: ICD-9 code for alcohol abuse/dependence; binge drinking; or hazardous drinking (Alcohol Use Disorders Identification Test (AUDIT-C) score ≥4 for men or ≥3 for women at baseline questionnaire completion).31; 40; 44
ADEs and HANA conditions
History of common ADEs and HANA conditions were identified by at least one inpatient or two outpatient ICD-9 codes for the condition within 12 months prior or 7 days after study enrollment, an approach shown to improve the specificity of these codes.40 Individual ICD-9 codes utilized for different diseases are available on the study website, at www.vacohort.org. We defined history of common ADEs as previously described,45 with the exception that history of bacterial pneumonia was defined as one or more episodes (not necessarily recurrent) within 12 months. Common HANA conditions included were history of non-AIDS associated cancer (excluding squamous cell or basal cell carcinoma of the skin), hypertension, coronary artery disease (CAD) and congestive heart failure (CHF) as a combined cardiac disease variable (CAD/CHF), chronic obstructive pulmonary disease (COPD), and diabetes mellitus (DM).
VACS Risk Index
The variables required to calculate the VACS Index were retrieved within 6 months prior to or 7 days after VACS enrollment, and were available for 95% of patients. Prognostic factors in the VACS index include age, CD4 count, HIV-1 RNA and laboratory measurements of hemoglobin, AST, ALT, platelets, creatinine and HCV status. Composite markers of liver and renal injury (FIB-4 and estimated glomerular filtration rate (eGFR)) are computed. FIB-4, composed of AST, ALT, platelets, and age, is a validated indicator of liver fibrosis. eGFR, based on the Modification of Diet in Renal Disease (MDRD) equation, is a validated indicator of impaired renal function. HCV infection status was based on documented diagnosis, a positive antibody test or detectable plasma HCV-RNA. Variables of the VACS Index were combined via a previously established point system based on hazard ratios (HR) to calculate a score.22 (Supplemental Digital Content Table 1)
Hospitalization and MICU admission
Our primary outcome was first VA-based hospitalization for any indication occurring >7 days after VACS enrollment. Patients admitted to the hospital were identified from the EMR and were then stratified based on whether or not the hospitalization included admission to the MICU. MICU admission was identified by bedsection code (12). MICU admissions include cardiac intensive care unit admissions, as there was not a separate bedsection code. Surgical ICU (SICU) admissions were not included. Time-to-admission (in days) was defined starting from the date of VACS enrollment until the date of hospitalization, date of last follow-up, or date of death, whichever occurred first. Mortality was determined from combined sources including inpatient mortality, social security data, and national death benefits data, a method previously shown to provide excellent mortality ascertainment.46
Statistical analysis
We compared patient characteristics at baseline stratified by subsequent admission status in the 2 years following VACS enrollment: no hospitalization, hospitalization but no MICU admission, or hospitalization with MICU admission. Test for trend (Cochran-Armitage for 2×k variables and Jonckheere-Terpstra for n×k variables, where n and k >2) were performed to compare baseline demographic variables, HIV-specific variables including history of common ADEs and prevalence of HANA conditions as well as smoking and hazardous alcohol use stratified by outcome group. VACS Index scores were categorized into quartiles such that there were an equal number of admissions over the 2-year period per quartile. Kaplan-Meier estimates were generated to compare time-to-first-admission by VACS Index quartiles.
Multivariable regression models were used to assess the association between VACS Index score and outcomes. Cox proportional hazards models were used to determine adjusted hazard ratios (HR) with 95% confidence interval (95% CI) for first hospitalization within 2 years of study enrollment. We used logistic regression models to determine odds ratios (OR) with 95% CI for MICU admission among those with hospitalization. Candidate variables in addition to the VACS Index included race, gender, combination ART use, VACS Index score, history of common ADEs, prior bacterial pneumonia, smoking status, hazardous alcohol use, cancer history, hypertension, CAD/CHF, COPD and DM. We initially included all variables from bivariate analyses that were significant at p<0.05. In our final parsimonious multivariable model we retain only variables that remained significant. Variables not included did not add to model fit nor change our inference. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA), with the exception of C-statistics which were calculated using Stata 11 (StataCorp; College Station, TX). Statistical significance was defined as p < 0.05.
RESULTS
From 2002–2008, 3,410 HIV-infected patients were enrolled in VACS 8. The majority of patients were male (97%) and African American (67%). During the first two years after study enrollment, 1141/3410 (33.5%) patients were hospitalized within any VA facility; 203/1141 (17.8%) of these had a concurrent MICU admission. The median time to hospitalization was 264 days and 159 days in those without and with MICU admission, respectively. Median length of hospitalization was 4 days and median length of MICU stay was 6 days.
VACS Index scores were higher among patients with hospitalization, particularly those that included MICU admission (Table 1). The median and interquartile ranges were 25 (16,39) in those with no hospitalization, 34 (21,52) in those with hospitalization without MICU and 51 (34,70) in those with MICU admission (p for trend < 0.001). This reflects older age, lower CD4 count, higher HIV RNA and higher prevalence of abnormal hemoglobin, FIB-4 and eGFR and hepatitis C coinfection among those hospitalized. History of common ADEs, bacterial pneumonia and prevalence of HANA conditions including cancer, CAD/CHF, hypertension, and COPD also varied by admission status. Median VACS Index scores were higher in patients who were current or former smokers compared with never smokers and in those who had prior ADEs, hypertension and COPD (data not shown).
Table 1.
Characteristics of patients at study enrollment by subsequent hospital admission, MICU admission or neither (N=3,410)
| Characteristic | No admission (n=2269) |
Hospitalization (n=1141) |
p for trend | |
|---|---|---|---|---|
| No MICU (n=938) |
MICU (n=203) |
|||
| Age in years, mean (SD) | 49 (9) | 49 (9) | 52 (8) | <0.001 |
| Male Gender, % | 97 | 98 | 99 | 0.30 |
| Race/ethnicity, % | 0.001 | |||
| White | 22 | 16 | 14 | |
| Black | 65 | 70 | 71 | |
| Hispanic | 9 | 10 | 10 | |
| Other | 4 | 4 | 4 | |
| CD4+ T-cell count in cells/mm3, median (IQR) | 394 (241, 579) | 316 (167, 514) | 277 (124, 494) | <0.001 |
| HIV RNA non-detectable (<500 copies/ mm3), % | 53 | 45 | 44 | <0.001 |
| HIV RNA in copies/ml, median (IQR) | 400 (75, 11000) |
1190 (75, 30240) |
1460 (75, 51063) |
<0.001 |
| ART use at baseline, % | 74 | 71 | 74 | 0.31 |
| History of any common AIDS-defining event*, % | 6 | 14 | 20 | <0.001 |
| Smoking status (%) | 0.54 | |||
| Never | 25 | 18 | 17 | |
| Current | 49 | 61 | 63 | |
| Former | 26 | 21 | 19 | |
| Hazardous alcohol use, % | 39 | 49 | 45 | <0.001 |
| Comorbid condition | ||||
| Hemoglobin, g/dl, % | <0.001 | |||
| < 10 | 1 | 4 | 12 | |
| 10–11.9 | 9 | 15 | 24 | |
| 12–13.9 | 38 | 44 | 39 | |
| ≥ 14 | 52 | 38 | 25 | |
| FIB-4, % | <0.001 | |||
| <1.45 | 60 | 52 | 35 | |
| 1.45–3.25 | 33 | 35 | 42 | |
| ≥ 3.25 | 7 | 14 | 24 | |
| Estimated glomerular filtration rate ml/min, % | <0.001 | |||
| <30 | 1 | 3 | 11 | |
| 30–45 | 0.6 | 1 | 5 | |
| 45–60 | 3 | 5 | 11 | |
| ≥ 60 | 95 | 91 | 73 | |
| Hepatitis C coinfection, % | 43 | 58 | 58 | <0.001 |
| VACS Risk Index Score, median (IQR) | 25 (16, 39) | 34 (21, 52) | 51 (34, 70) | <0.001 |
| Cancer history**, % | 3 | 5 | 10 | <0.001 |
| Hypertension, % | 18 | 25 | 38 | <0.001 |
| CAD/CHF, % | 3 | 4 | 13 | <0.001 |
| Chronic obstructive pulmonary disease, % | 3 | 7 | 12 | <0.001 |
| Diabetes mellitus, % | 9 | 12 | 13 | 0.02 |
| Bacterial pneumonia, % | 3 | 7 | 10 | <0.001 |
| Time-to-event in days (IQR) | ||||
| Time to hospitalizations, median (IQR) | N/A | 264 (121, 466) | 159 (56, 355) | <0.001 |
| Time to MICU, median (IQR) | N/A | N/A | 325 (175, 485) | n/a |
| Time to death, median (IQR) | 1089 (566, 1622) | 864 (473, 1271) | 573 (350, 838) | <0.001 |
IQR=interquartile range; 25% and 75% quartiles included.
ART=antiretroviral therapy
CAD/CHF=cardiovascular disease/congestive heart failure
MICU=medical intensive care unit
Any common AIDS-defining event variable derived from Mocroft et al.(25); composite variable including mild AIDS-defining events (HIV wasting syndrome, pulmonary and extrapulmonary tuberculosis, Pneumocystis pneumonia, esophageal candidiasis, cytomegalovirus, Kaposi sarcoma, and herpes simplex disease); moderate AIDS-defining events (Cryptococcus, cerebral toxoplasmosis, AIDS dementia complex, and disseminated mycobacterial avium infection); and severe AIDS-defining events (non-Hodgkin lymphoma only because no patient in this cohort had progressive multifocal leukencephalopathy).
Excludes ICD9 codes for AIDS-related cancers (non-Hodgkin lymphoma, Kaposi sarcoma, and cervical cancer) and squamous and basal cell carcinoma of the skin.
Mortality rates varied with hospitalization status. The mortality rate over the two years of observation for those without hospitalization was 15 per 1000 person-years (PY) compared with 58 per 1000 PY in patients with hospitalization only and 236 per 1000 PY in patients with hospitalization and MICU admission (p for trend <0.001). (Table 1)
Risk factors associated with hospitalization in unadjusted analyses
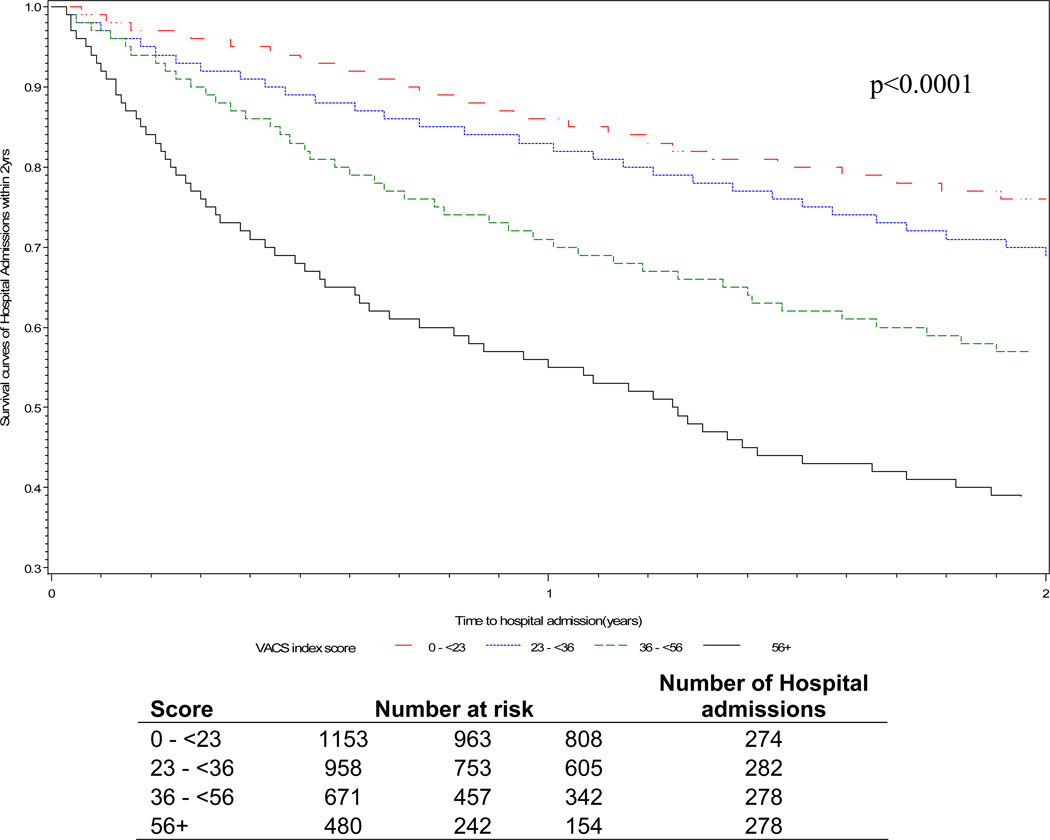
In unadjusted analysis, VACS Index score was associated with increased risk of hospitalization (Table 2). All individual components of the VACS Index were associated with increased risk of hospitalization (Supplemental digital content Table 2). Black and Hispanic race/ethnicity (reference white), current smoking, hazardous alcohol use, history of ADEs and bacterial pneumonia, COPD, cancer, CAD/CHF, hypertension, and DM were also associated with increased risk for hospital admission (p≤0.01 for all). Combination ART use and former cigarette smoking were not directly associated with hospitalization. Kaplan-Meier plots (Figure 1) show a strong gradient of risk by quartiles of VACS Index score. For those in the highest quartiles (scores ≥ 56), more than 40% were hospitalized by one year and 60% by two years after enrollment compared with those in the lowest quartiles (score < 36) who had less than 20% of patients hospitalized within one year and less than 30% within 2 years.(Figure 1)
Table 2.
Models for risk of hospitalization within 2 years of enrollment.
| Unadjusted bivariate models (n=3262) |
Adjusted multivariable model* (n=3251) |
|||
|---|---|---|---|---|
| Characteristic | HR | (95% CI) | HR | (95%CI) |
| VACS Index score/5 point change** | 1.12 | (1.10, 1.13) | 1.10 | (1.09, 1.12) |
| Race/ethnicity, ref white | ||||
| Black | 1.44 | (1.22, 1.69) | 1.13 | (0.96, 1.34) |
| Hispanic | 1.38 | (1.09, 1.75) | 1.28 | (1.01, 1.62) |
| Other | 1.33 | (0.96, 1.84) | 1.22 | (0.88, 1.70) |
| Smoking status, ref never | ||||
| Current smoker | 1.61 | (1.38, 1.88) | 1.40 | (1.19, 1.65) |
| Former smoker | 1.09 | (0.91, 1.32) | 0.92 | (0.76, 1.12) |
| Hazardous alcohol use | 1.39 | (1.23, 1.56) | 1.23 | (1.09, 1.39) |
| COPD | 2.62 | (2.10, 3.26) | 2.06 | (1.65, 2.58) |
| Hypertension | 1.57 | (1.38, 1.79) | 1.39 | (1.21, 1.59) |
| Diabetes mellitus | 1.25 | (1.05, 1.50) | -- | -- |
| Cancer history | 1.70 | (1.32, 2.19) | -- | -- |
| CAD/CHF | 1.69 | (1.33, 2.16) | -- | -- |
| ART use at baseline | 0.90 | (0.79, 1.02) | -- | -- |
| Previous bacterial pneumonia | 2.01 | (1.61, 2.51) | -- | -- |
| History of ADE | 2.30 | (1.96, 2.72) | 1.90 | (1.61, 2.25) |
ADE=AIDS-defining event
Final model includes only variables that were significant in multivariable model. All levels of multi-variables were included if any level had significant association with hospitalization.
Figure 1.
Kaplan-Meir curves for hospitalization-free survival within two years of baseline VACS Index scores. VACS Index score at study enrollment separated into quartiles with approximately equal number of admissions per group.
Risk factors associated with hospitalization in adjusted analyses
In adjusted multivariable analysis the VACS Index remained significantly associated with risk of hospitalization (Table 2). A 5-point increment in VACS Index score was associated with 10% increased risk of hospital admission within two years after study enrollment (HR per 5-points =1.10; 95% CI [1.09, 1.12]; p<0.001). After adjustment for the VACS Index score the effect of other predictors was attenuated. COPD remained the strongest risk factor for hospitalization (HR=2.06; 95% CI [1.65, 2.58]; p<0.001) followed by history of common ADEs, current smoking, hypertension, hazardous alcohol use and DM. Risk factors varied between patients without and with detectable viral load.(Supplemental Digital Content Table 2) Current smoking, hypertension and hazardous alcohol use were significant risk factors for hospitalization in patients without detectable viral load, while intermediate levels of renal dysfunction and CD4 count 100–299 were risk factors only in those with a detectable viral load. COPD and history of common ADEs remained significant risk factors for hospitalization in patients without and with detectable viral load.
Among those hospitalized, higher VACS Index score predicted MICU admission (Table 3), with a 5-point increment in VACS Index score associated with 11% increased risk (Odds ratio (OR) per 5-points=1.11; 95% CI [1.08, 1.15]; p<0.001). After adjustment for the VACS Index, only CAD/CHF (OR 2.88) and non-AIDS cancer (OR 2.06) remained associated with risk of MICU admission. While cancer diagnoses combined were significant, the number of patients with any specific cancer was too small to allow further analysis by cancer type. The three most frequent types of cancer among MICU patients were male genital system (n=12), prostate (n=11), and lymphoma (n=9).
Table 3.
Multivariable model for odds of MICU admission within 2 years of enrollment among those hospitalized.* (n=1141)
| Characteristic | Odds Ratio | (95% CI) |
|---|---|---|
| VACS Index score/5 point change | 1.11 | (1.08, 1.15) |
| Hypertension | 1.38 | (0.97, 1.96) |
| CAD/CHF | 2.88 | (1.66, 5.01) |
| History of any cancer | 2.06 | (1.15, 3.68) |
Final model includes only variables that were significant in multivariable analysis.
Discrimination using the VACS Index score (weighted for all cause mortality risk) was as good as using the components of the VACS Index (fitted to hospitalization risk), based on similar C-statistics. For hospitalization, the Cox model including all significant covariates c-stat = 0.67 (95% CI [0.66, 0.69]) for the VACS Index score and 0.68 (95% CI [0.67, 0.70]) for the VACS Index components. For MICU admission, given hospitalization, the logistic regression model including all significant covariates c-stat = 0.70 (95% CI [0.66, 0.74]) for the Index score and 0.69 (95% CI [0.65, 0.74]) for the components. We chose to report the VACS Index score for simplicity.
DISCUSSION
In this cohort of HIV-infected Veterans, hospitalization and MICU admission were common and were associated with substantially increased risk of mortality. Hospitalized patients admitted to the MICU had more advanced HIV disease, higher VACS Index scores, and increased prevalence of HANA conditions. A five-point increment in VACS Index score conferred an approximate 10% increased risk of hospitalization, and given hospitalization, an approximate 10% increased risk of MICU admission (p<0.0001). Additionally, prior ADEs and several HANA conditions, specifically, current smoking, hazardous alcohol use, COPD, hypertension, and DM, substantially increased the risk of subsequent hospitalization among HIV-infected Veterans. Among those hospitalized, we found CAD/CHF and history of non-AIDS cancer were associated with increased risk for MICU admission.
While risk factors for hospitalization have been identified in the current ART era,13–16 this study is the first to identify risk factors for MICU admission in a longitudinal cohort of HIV-infected patients admitted to the hospital and to organize these into a weighted index. The VACS Index has also been shown to predict short and long term all-cause mortality, but this is the first study reporting its ability to predict major morbidity events such as hospitalization and MICU admission.22 Of note, we used the same variables and weights as those used in the prior studies of all-cause mortality. In models of the VACS Index components and HANA conditions, the weights for the components of the VACS Index were stable and performed similarly in the overall model and in models stratified by detectable viral load; the weights for other conditions varied in the stratified models (See Supplemental Digital Content Table 2). Allowing individual components of the Index to be fit to the outcome of hospitalization did not substantially improve prediction. This suggests that the VACS Index is a robust predictor of both morbidity and mortality.
We note that while the VACS Index was the single most important predictor of hospitalization and MICU admission, other factors were independently associated with these outcomes. This underscores two observations. First, factors that predict mortality may be different from factors that predict morbidity yet both are of great importance. Second, risk indices have to be developed and weighted for a particular outcome. It is logical to do this for all-cause mortality. Nevertheless it is important to understand what factors may predict other patient-relevant outcomes. Our findings regarding current smoking, hazardous alcohol use, COPD, hypertension, and diabetes suggest potential areas for interventions. The VACS Index could be used to motivate patients to change behaviors aimed at decreasing hospitalization risk. Given the strong association between age and many of these conditions, our findings may be increasingly important as individuals living with HIV infection age.
The VACS Index could be used to identify patients at greatest risk for hospitalization and MICU admission which can contribute to increasing frailty.9; 10; 47 The VACS Index is higher in patients with certain HANA conditions such as hypertension and COPD and thus, partially captures the impact of these conditions on hospitalization risk. The VACS Index could be valuable for healthcare systems to employ case managers for high-risk patients. The VACS Index may be a clinically important tool particularly for patients at high risk for these outcomes due to HANA conditions, despite adequately suppressed viral loads and appropriate CD4 counts. For example, recent guidelines for laboratory monitoring suggest less frequent monitoring for adherent patients with suppressed viral loads and stable CD4 counts.48 The VACS Index measures organ dysfunction earlier than what may commonly be acted upon clinically and provides an aggregate score that could be used to trigger more intensive follow up for patients with higher scores. For example, men are not considered anemic unless their hemoglobin is < 13, yet the VACS Index indicates increased risk of death and 10 points of VACS Index score when hemoglobin is < 14. For patients who are hospitalized, the VACS Index, as an earlier composite marker of subtle organ dysfunction in these patients, could be useful for informing triage decision such as MICU admission. Other uses could include informing thresholds for blood transfusion, earlier identification of patients at increased risk of delirium, and for prognosis. Future work is needed to determine how the VACS Index on MICU admission could be used for management of critically ill HIV-infected patients.
In terms of MICU admission, our findings complement epidemiologic studies describing MICU admission diagnoses in HIV-infected patients in the combination ART era. In other studies, non-AIDS cardiovascular and respiratory diseases are the acute indications for more than half of MICU admissions.11; 12; 17; 24; 26; 33; 34; 49; 50 While we did not have data to determine indication for MICU admission in these analyses, CAD/CHF and COPD were significantly more prevalent among hospitalized and MICU admitted patients. Similar to other studies from the current ART era,11; 11; 19; 19; 24; 24; 26; 26; 49; 49 MICU admission was associated with substantial mortality in our study. Hospitalization and MICU admission may indicate increased frailty among those aging with HIV infection who are also likely to have organ dysfunction and HANA conditions.
VACS has several features that make it a unique cohort to examine risk factors for hospitalization and MICU admission in HIV-infected patients. First, this is one of the largest cohort studies to investigate HANA conditions and risk of hospitalization and MICU admission in HIV-infected patients in care. In prior epidemiologic studies of critical illness in HIV infection, non-AIDS conditions were examined for their impact on MICU mortality rather than on their risk for hospitalization or MICU admission. Using validated EMR data to reflect prevalence of non-AIDS conditions prior to admission,40 we prospectively examined those conditions that conferred the greatest risk for subsequent admission, allowing us to assess factors that may be amenable for future intervention. This is also one of the first studies of hospital and MICU admissions in HIV-infected patients in a multi-center, racially diverse cohort.
There are several limitations to this study. First, it likely underestimates hospital and MICU admission rates because patients may be admitted to non-VA hospitals, especially patients with higher severity of acute critical illness and in areas where non-VA facilities are more easily accessible. However, this limitation implies a bias toward the null suggesting that we may have underestimated prognostic associations. In addition, there may be significant case mix differences between VA and non-VA hospitals and MICUs; for example, VA MICUs generally include cardiac ICU and neurologic ICU patients. Similarly, we cannot assess how the threshold for hospital and MICU admission varies according to clinicians and institutions. However, VACS Index scores did not vary significantly by site among those with hospitalization and MICU admission suggesting that these thresholds did not vary systematically by site. In addition, we only considered the prognostic importance of baseline values for future admission. We did not analyze time-updated values for CD4 count, HIV viral load, hemoglobin, eGFR or FIB4 and we did not collect reason for the hospital or MICU admission. We also recognize that hospitalization and MICU admission do not necessarily equate to acute illness and patients may be admitted for other reasons such as awaiting long-term placement, hospital policy, or bed availability. Thus, it is perhaps not surprising that the C-statistic for the VACS Index for hospitalization is not as strong as that previously reported for all-cause mortality. Finally, nearly all patients in VACS8 were men. Prediction of hospital and MICU admission with the VACS Index needs to be evaluated in non-Veteran populations that include a representative proportion of women. Since the VACS Index has been shown to accurately predict mortality among women in other validation cohorts,39 we would expect that the association reported with hospital and MICU admission will also be generalizable to women.
CONCLUSION
Hospitalization and MICU admission are common in aging HIV-infected patients. Hospitalization and especially MICU admission suggest increased frailty and substantially increased risk of mortality.21 The VACS Index, representing age and bone marrow, renal, liver and immunologic dysfunction, identifies HIV-infected patients at high risk for hospitalization and MICU admission. The VACS Index could be a useful clinical tool for providers to target modifiable mediators among those at higher risk for hospitalization or MICU admission. Interventions might include smoking cessation, reduction in hazardous alcohol use and optimization of HIV and HANA conditions.
Supplementary Material
Acknowledgments
Funding support: Association of Subspecialty Physicians and CHEST Foundation of the American College of Chest Physicians T. Franklin Williams Award (KMA); National Institute of Health K24 Hl087713 (LH); National Institute on Alcohol Abuse and Alcoholism 5U01AA013566-05 (AJ); National Institutes of Health, National Heart, Lung, and Blood Institute R01 HL090342 (KC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen M. Akgün, Department of Internal Medicine and General Internal Medicine, VA Connecticut Healthcare System, West Haven, CT, USA; and Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Kirsha Gordon, Department of Internal Medicine, VA Connecticut Healthcare System, West Haven, CT, USA; and Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Margaret Pisani, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Terri Fried, Department of Internal Medicine, VA Connecticut Healthcare System, West Haven, CT, USA; and Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Kathleen A. McGinnis, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Janet P. Tate, Department of Internal Medicine, VA Connecticut Healthcare System, West Haven, CT, USA; and Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Adeel A. Butt, Department of Medicine, University of Pittsburgh School of Medicine and VA Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Cynthia L. Gibert, Department of Medicine, VAMC & George Washington University -Washington, DC, USA.
Laurence Huang, Department of Medicine, University of California, San Francisco, CA, USA.
Maria C. Rodriguez-Barradas, Infectious Diseases Section, Michael E. DeBakey VAMC and Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
David Rimland, Department of Medicine, Atlanta VA & Emory University- Decatur, GA, USA.
Amy C. Justice, VA Connecticut Healthcare System, West Haven, CT, USA; and Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA.
Kristina Crothers, Department of Medicine, University of Washington School of Medicine, Seattle, WA, USA.
Reference List
- 1.Chatila W, Kreimer DT, Criner GJ. Quality of life in survivors of prolonged mechanical ventilatory support. Crit Care Med. 2001;29:737–742. doi: 10.1097/00003246-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RL, Campion EW, Thibault GE, Mulley AG, Skinner E. Functional outcomes following medical intensive care. Crit Care Med. 1986;14:783–788. doi: 10.1097/00003246-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Konopad E, Noseworthy TW, Johnston R, Shustack A, Grace M. Quality of life measures before and one year after admission to an intensive care unit. Crit Care Med. 1995;23:1653–1659. doi: 10.1097/00003246-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Rimachi R, Vincent JL, Brimioulle S. Survival and quality of life after prolonged intensive care unit stay. Anaesth Intensive Care. 2007;35:62–67. doi: 10.1177/0310057X0703500108. [DOI] [PubMed] [Google Scholar]
- 5.Wu AW, Damiano AM, Lynn J, et al. Predicting future functional status for seriously ill hospitalized adults. The SUPPORT prognostic model. Ann Intern Med. 1995;122:342–350. doi: 10.7326/0003-4819-122-5-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Williams TA, Dobb GJ, Finn JC, Webb SA. Long-term survival from intensive care: a review. Intensive Care Med. 2005;31:1306–1315. doi: 10.1007/s00134-005-2744-8. [DOI] [PubMed] [Google Scholar]
- 7.Williams TA, Dobb GJ, Finn JC, et al. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36:1523–1530. doi: 10.1097/CCM.0b013e318170a405. [DOI] [PubMed] [Google Scholar]
- 8.Williams TA, Ho KM, Dobb GJ, Finn JC, Knuiman M, Webb SA. Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth. 2010;104:459–464. doi: 10.1093/bja/aeq025. [DOI] [PubMed] [Google Scholar]
- 9.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill TM, Gahbauer EA, Han L, Allore HG. The Relationship Between Intervening Hospitalizations and Transitions Between Frailty States. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasimhan M, Posner AJ, DePalo VA, Mayo PH, Rosen MJ. Intensive care in patients with HIV infection in the era of highly active antiretroviral therapy. Chest. 2004;125:1800–1804. doi: 10.1378/chest.125.5.1800. [DOI] [PubMed] [Google Scholar]
- 12.Nickas G, Wachter RM. Outcomes of intensive care for patients with human immunodeficiency virus infection. Arch Intern Med. 2000;160:541–547. doi: 10.1001/archinte.160.4.541. [DOI] [PubMed] [Google Scholar]
- 13.Berry SA, Fleishman JA, Moore RD, Gebo KA. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. 2012;59:368–375. doi: 10.1097/QAI.0b013e318246b862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, Gebo KA. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr. 2010;53:397–404. doi: 10.1097/QAI.0b013e3181bcdc16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22:1345–1354. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 16.Crum-Cianflone NF, Grandits G, Echols S, et al. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr. 2010;54:248–257. doi: 10.1097/qai.0b013e3181c8ef22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coquet I, Pavie J, Palmer P, et al. Survival trends in critically ill HIV-infected patients in the highly active antiretroviral therapy era. Crit Care. 2010;14:R107. doi: 10.1186/cc9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko NY, Lai YY, Liu HY, et al. Impact of the nurse-led case management program with retention in care on mortality among people with HIV-1 infection: a prospective cohort study. Int J Nurs Stud. 2012;49:656–663. doi: 10.1016/j.ijnurstu.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Dickson SJ, Batson S, Copas AJ, Edwards SG, Singer M, Miller RF. Survival of HIV-infected patients in the intensive care unit in the era of highly active antiretroviral therapy. Thorax. 2007;62:964–968. doi: 10.1136/thx.2006.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC. HIV and aging: time for a new paradigm. Curr HIV /AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 22.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of 'non-HIV' biomarkers. HIV Med. 2010;11:143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris A, Creasman J, Turner J, Luce JM, Wachter RM, Huang L. Intensive care of human immunodeficiency virus-infected patients during the era of highly active antiretroviral therapy. Am J Respir Crit Care Med. 2002;166:262–267. doi: 10.1164/rccm.2111025. [DOI] [PubMed] [Google Scholar]
- 24.Powell K, Davis JL, Morris AM, Chi A, Bensley MR, Huang L. Survival for patients With HIV admitted to the ICU continues to improve in the current era of combination antiretroviral therapy. Chest. 2009;135:11–17. doi: 10.1378/chest.08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schein RM, Fischl MA, Pitchenik AE, Sprung CL. ICU survival of patients with the acquired immunodeficiency syndrome. Crit Care Med. 1986;14:1026–1027. doi: 10.1097/00003246-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Vincent B, Timsit JF, Auburtin M, et al. Characteristics and outcomes of HIV-infected patients in the ICU: impact of the highly active antiretroviral treatment era. Intensive Care Med. 2004;30:859–866. doi: 10.1007/s00134-004-2158-z. [DOI] [PubMed] [Google Scholar]
- 27.Wachter RM, Luce JM, Turner J, Volberding P, Hopewell PC. Intensive care of patients with the acquired immunodeficiency syndrome. Outcome and changing patterns of utilization. Am Rev Respir Dis. 1986;134:891–896. doi: 10.1164/arrd.1986.134.5.891. [DOI] [PubMed] [Google Scholar]
- 28.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 29.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 30.Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50:299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freiberg MS, McGinnis KA, Kraemer K, et al. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53:247–253. doi: 10.1097/QAI.0b013e3181c6c4b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casalino E, Wolff M, Ravaud P, Choquet C, Bruneel F, Regnier B. Impact of HAART advent on admission patterns and survival in HIV-infected patients admitted to an intensive care unit. AIDS. 2004;18:1429–1433. doi: 10.1097/01.aids.0000131301.55204.a7. [DOI] [PubMed] [Google Scholar]
- 34.Nuesch R, Geigy N, Schaedler E, Battegay M. Effect of highly active antiretroviral therapy on hospitalization characteristics of HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002;21:684–687. doi: 10.1007/s10096-002-0792-3. [DOI] [PubMed] [Google Scholar]
- 35.Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 36.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 37.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tate JP and Justice AC for the VACS Project Team. Change in a prognostic index for survival in HIV infection after one year on cART by level of adherence. [abstract] 48th Annual Meeting of the Infectious Disease Society of America. 2010 [Google Scholar]
- 39.Brown S, Kyriakides T, Kirkwood K, Halodniy M, Cameron DW, Angus B, Tate J, Goulet J, Justice AC. External validation for mortality and discrimination among treatment arms in OPTIMA. [abstract] International AIDS Society Annual Meeting. 2010 [Google Scholar]
- 40.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crothers K, Griffith TA, McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21:40–53. doi: 10.1521/aeap.2009.21.3_supp.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 45.Mocroft A, Sterne JA, Egger M, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis. 2009;48:1138–1151. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 47.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15:301. doi: 10.1186/cc9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2012 [serial online] [Google Scholar]
- 49.Barbier F, Coquet I, Legriel S, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. 2009;35:1678–1686. doi: 10.1007/s00134-009-1559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palacios R, Hidalgo A, Reina C, de la Torre M, Marquez M, Santos J. Effect of antiretroviral therapy on admissions of HIV-infected patients to an intensive care unit. HIV Med. 2006;7:193–196. doi: 10.1111/j.1468-1293.2006.00353.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.