Abstract
The Hedgehog (Hh) signaling pathway is aberrantly activated in a wide variety of human cancers, and recent clinical studies have demonstrated that pathway inhibitors are effective in advanced basal cell carcinoma (BCC). The majority of these agents have been designed to target SMOOTHENED (SMO), a transmembrane regulator of Hh signaling, but subsequent mutations in SMO have been found to generate drug resistance. In other cancers, oncogenic events that bypass SMO may activate canonical Hh signaling, and SMO antagonists have not demonstrated significant activity in several diseases. Therefore, alternative strategies targeting the Hh pathway downstream of SMO may have clinical utility. Liver X Receptors (LXRs) regulate cholesterol and fatty acid homeostasis, and LXR activation can inhibit the Hh pathway in normal mouse embryonic fibroblasts. We examined the effects of LXR activation on Hh signaling in human multiple myeloma (MM) cells and found that LXR agonists inhibited Hh pathway activity and clonogenic tumor growth in vitro. LXR activation also inhibited putative MM cancer stem cells in vivo leading to the loss of tumor initiating and self-renewal potential. Finally, Hh signaling was inhibited downstream of SMO, suggesting that LXR agonists may represent a novel strategy to target pathogenic Hh signaling as well as treat MM.
Keywords: Liver X Receptors, Hedgehog pathway, multiple myeloma, cancer stem cells
INTRODUCTION
The Hedgehog (Hh) signaling pathway is highly conserved and directs cell fate decisions during normal embryonic development (1). The pathway is subsequently silenced in most post-natal tissues, but aberrant reactivation has been identified in many human cancers (2). The widespread nature of aberrant Hh pathway activity in cancer has led to the development of several Hh pathway antagonists, and the recent approval of the novel inhibitor vismodegib for the treatment of advanced basal cell carcinoma (BCC) has validated the pathway as a therapeutic target in clinical oncology (3, 4).
Mammalian Hh signal transduction involves binding of one of the three extracellular ligands Sonic (SHH), Indian (IHH) and Desert (DHH) Hedgehog, to the cell surface receptor PATCHED (PTCH), a twelve-transmembrane protein that normally inhibits the seven-transmembrane protein SMOOTHENED (SMO) and renders the pathway inactive in the absence of ligand (5). Ligand binding inactivates PTCH, which in turn, de-represses SMO and modulates the interaction between SUPRESSOR OF FUSED (SUFU) and the three GLI proteins that act as transcriptional regulators and downstream effectors of Hh signaling.
In cancer, aberrant Hh signaling may occur by several mechanisms (6). Mutations in PTCH1 and SMO that lead to Hh ligand-independent pathway activation have been described in basal cell carcinoma (BCC) and medulloblastoma. In other malignancies, pathway activation may be driven by increased levels of Hh ligands secreted by either tumor cells or non-malignant cells in the microenvironment that directly or indirectly enhance cell proliferation and survival. Similar to its effects on normal stem cells and progenitors during development, increased Hh signaling may also enhance the tumorigenic potential and self-renewal of putative cancer stem cells (CSCs) in several malignancies (7), including glioblastoma, colorectal carcinoma, and chronic myeloid leukemia (8–11). In the plasma cell malignancy multiple myeloma (MM), Hh signaling induces the expansion of MM precursors that enhances their clonogenic growth potential, whereas pathway inhibition induces terminal tumor cell differentiation and the loss of self-renewal (12). Therefore, strategies to inhibit pathogenic Hh signaling may be effective across several cancer types as well as against multiple tumor cell subpopulations.
The vast majority of clinical strategies targeting the Hh pathway, including vismodegib, have been designed to inhibit SMO (13). However, secondary SMO mutations resulting in drug-resistance may emerge (14–16), and specific oncogenic events, such as mutated RAS and increased TGF-β signaling, may activate GLI transcription factors in a SMO independent manner (17). Therefore, agents acting downstream of SMO may represent novel anti-cancer approaches.
Oxysterols are oxidized cholesterol molecules capable of both activating and inhibiting Hh signaling (18–20). Specific oxysterols may activate the Hh pathway by directly interacting with SMO through a putative sterol-sensing domain (18, 21). In addition, oxysterols also act as ligands for Liver X Receptors (LXR) that are members of the nuclear receptor superfamily of transcriptional regulators and regulate lipid and cholesterol homeostasis by inducing the expression of several cellular factors involved in cholesterol efflux and fatty acid and triglyceride synthesis (22). Both oxysterols and synthetic non-steroidal LXR ligands have been found to inhibit Hh signaling in normal embryonic fibroblasts suggesting that these agents may serve as novel Hh pathway antagonists (20).
The impact of LXRs on Hh signaling within cancer cells is unknown, therefore, we examined the effects of LXR agonists on Hh signaling and the growth of MM cells. Similar to embryonic fibroblasts, LXR activation inhibited Hh signaling in MM cells. LXR agonists also inhibited the tumorigenic potential of MM cells both in vitro and in vivo and acted downstream of SMO suggesting that they may have broader applicability than current clinically available Hh pathway inhibitors.
MATERIALS AND METHODS
Cell lines, clinical specimens, and cell culture
The human MM cell lines NCI-H929, U266, NCI-H929, and MM1.S were obtained from the American Type Culture Collection (Manassas, VA) and KMS-11 and KMS-12 from the DSMZ (Brunswick, Germany) and authenticated by short tandem repeat profiling at the Johns Hopkins Genetic Resources Core Facility (Baltimore, MD). All cell lines were obtained in 2012, expanded and frozen down in several aliquots. Each aliquot was thawed and used for no more than 6 months. Cells were cultured in advanced RPMI (Invitrogen, Carlsbad, CA) containing 1% fetal bovine serum (FBS, Sigma, St. Louis, MO), 2 mM L-glutamine, 10 mM Hepes, 50 U/mL penicillin, and 50 μg/mL streptomycin. Primary bone marrow samples were obtained from newly diagnosed MM patients granting informed consent as approved by the Johns Hopkins Medical Institutes Institutional Review Board. Bone marrow mononuclear cells (BMMCs) were isolated by density centrifugation (Ficoll-Paque; Pharmacia, Piscataway, NJ), and plasma cells were isolated using anti-human CD138 magnetic beads (Miltenyi Biotech, Auburn, CA). Cells were treated with 22(R)-hydroxycholesterol, 22(S)-hydroxycholesterol, T0901317, GW3965, all obtained from Sigma and used at a concentration of 1 μM or ethanol as a vehicle control. Cyclopamine was obtained from Sigma and used at 5 μM (12). Hh signaling was induced with supernatant collected from 293 cells stably transfected to produce SHh ligand and titered to provide equivalent Hh luciferase reporter activity as 100 ng/ml recombinant N-SHh (R & D Systems, San Francisco, CA) using NIH 3T3 Light II cells (ATCC) (23, 24). Supernatant from non-transfected 293 cells was used as a control.
Tumor cell colony formation in methylcellulose was used to quantify in vitro clonogenic growth according to our previously published methods (25, 26). Briefly, MM cell lines (1 × 105 cells/ml) were treated for 96 hours, washed twice with phosphate buffered saline (PBS), then plated in triplicate into 35 mm2 tissue culture dishes containing 1.2% methylcellulose, 30% FBS, 1% bovine serum albumin (BSA), 10−4 M 2-mercaptoethanol, and 2 mM L-glutamine. For clinical specimens, mononuclear cells were isolated from primary clinical bone marrow aspirates, depleted of CD34+ and CD138+ cells using magnetic microbeads, then plated (5 ×105/ml) in methylcellulose cultures containing 10% lymphocyte conditioned media as a source of growth factors. Following 10–21 days of culture at 37°C and 5% CO2, tumor colonies consisting of more than 40 cells were quantified using an inverted microscope.
Reverse transcriptase PCR
Following 48 hours of treatment cellular RNA was isolated with the RNeasy Plus Mini Kit (Qiagen, Germantown, MD), reverse transcribed using Superscript II polymerase and oligo-dT according to the manufacture’s protocol (Invitrogen, Grand Island, NY). Real-time quantitative RT-PCR (qPCR) was performed using the TaqMan Universal Master Mix (Applied Biosystems) and the following FAM-based Taqman primer/probes sets (Applied Biosystems): LXRα (Hs00172885_m1), LXRβ (Hs01027215_g1), ABCA1 (Hs001059118_m1), ABCG1 (Hs00245154_m1), SREBP-1c (Hs00550338_m1), PTCH1 (Hs00181117_m1), GLI1 (Hs00171790_m1), HHIP (Hs00368450_m1), β-Actin (huACTB, #4352935E) on an I-Cycler thermocycler (Biorad, Hercules, CA). Expression levels were normalized to β-actin and compared using the ΔΔCT method.
Transient transfections
U266 cells (2 × 106) were transfected with DNA (2 μg) or siRNA (500 nM) in Solution C using the Amaxa Nucleofector (Program X-05, Lonza, Gaithersburg, MD). The expression of Gli1 or the constitutively active SMO mutant Smo-M2 was induced by transfection of pSR1a-Gli1 or pEGFP-C1-SmoM2, respectively (27). Gene knock-down was carried out using ON-TARGET plus SMARTpool and/or Trilencer-27 siRNA against LXRα, LXRβ, SUFU, ABCA1, ABCG1, SREBP-1c, or a scrambled siRNA control (Dharmacon, Chicago, IL and OriGene, Rockville, MD). Following transfection, target gene knock down was quantified by qPCR and found to be >80% for each gene 24 hours following transfection (data not shown). Hh pathway activity was quantified using a Gli-responsive firefly luciferase reporter plasmid (pGli8-Luc, 1.8 μg) co-transfected with a constitutive Renilla luciferase expression vector (0.4 μg, pRL-CMV; Promega, Madison, WI) to correct for transfection efficiency. Cells were collected 24 hours after transfection and examined for luciferase activity using the Dual Luciferase Reporter Assay according to the manufacturer’s instructions (Promega).
Flow cytometry and cell sorting
Cells were stained with a mouse anti-human CD138 antibody conjugated to fluourescein isothyocyanate (FITC) or allophycocyanin (APC) (BD Pharmingen, San Diego, CA) for 30 min at 4°C, washed and resuspended in PBS containing 0.2% BSA and 1mg/ml propidium iodide (PI; Sigma), and analyzed on a FACSCalibur flow cytometer (BD, Mountain View, CA). Aldehyde dehydrogenase (ALDH) activity was detected using the Aldefluor kit (Stem Cell Technologies, Vancouver, Canada) and ALDH+ cells were identified based on a control reaction containing the ALDH inhibitor diethylaminobenzaldehyde (DEAB). CD138+ and CD138neg cells were isolated by FACS following staining for surface CD138 expression and the exclusion of dead cells using 7-AAD. The lowest and highest 5% of cells expressing CD138 were collected using a FACSAria II cell sorter (BD Biosciences, Bedford, MA).
Animal studies
All animal studies were approved by the Johns Hopkins Medical Institutes Animal Care Committee. Immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were bred and maintained in the Johns Hopkins animal core facility. For ex vivo treatment studies, U266 cells were treated with vehicle or T0901317 for two weeks then washed twice with PBS and injected (1 × 106 cells) via the tail vein into 8–10 week old mice. Following the development of hind limb paralysis or after 6 months, engraftment was examined by detection of human CD138+ cells within the bone marrow by flow cytometry. For in vivo T0901317 treatment studies, NSG mice were injected with U266 cells (1 × 106) followed by feeding ad libitum with Teklad Global 16% Protein Diet (Harlan, Madison, WI) containing or lacking T0901317 (0.013%) to approximate 25 mg/kg/day (28). Secondary transplants were carried out by determining the concentration of human CD138+ cells within the bone marrow of primary recipients by flow cytometry then injecting whole bone marrow cells containing 1 × 106 human CD138+ cells into secondary recipients.
Statistical analysis
Results are presented as the mean ± SEM. Comparisons between groups were performed using a 2-tailed, paired Student t test. Kaplan-Meier analysis was carried out using a log-rank test. P values <0.05 were considered significant.
RESULTS
Multiple myeloma cells express LXRs and are responsive to LXR agonists
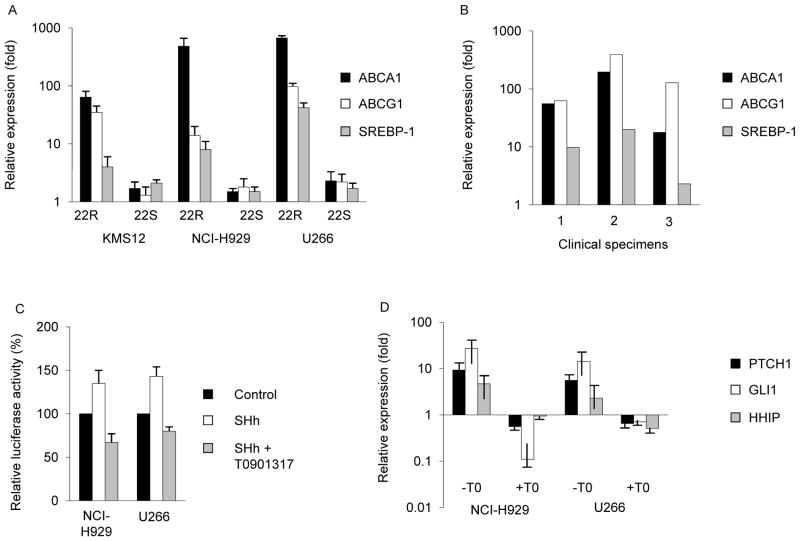
We initially examined the expression and activity of LXRs in MM cells. LXRs consist of two isoforms with high levels of LXRα (NR1H3) in the liver, gut, spleen, and macrophages whereas LXRβ(NR1H2) is broadly expressed in all adult tissues. We studied the human MM cell lines U266, NCI-H929, and KMS12, and detected the expression of both LXRα and LXRβ by RT-PCR (Supplemental Fig. 1). Ligand-dependent activation of LXRs by oxysterols induces the expression of specific target genes involved in cholesterol and lipid metabolism including the ATP binding cassette transporters ABCA1 and ABCG1 as well as the transcriptional regulator of lipogenesis, sterol regulatory binding protein 1c (SREBP-1c) (22). To determine whether LXRs are active in MM cells, we treated MM cells with the naturally-occurring LXR ligand 22(R)-hydroxycholesterol (22R) or the non-steroidal synthetic LXR agonists T0901317 and GW3965 and found that each compound significantly induced the expression ABCA1 and ABCG1 in all three cell lines by 8–750 fold and SREBP-1c by 1.4–40 fold over vehicle treated control cells similar to findings in other cell types (Fig. 1A and Supplemental Fig. 2; P<0.05) (29, 30). In contrast, the stereo-isomer of 22R, 22(S)-hydroxycholesterol (22S) that can act as an LXR antagonist in some cell types failed to significantly induce LXR target genes in any of the MM cell lines studied (Fig. 1B). We also examined the activity of LXRs in MM plasma cells isolated from three distinct primary clinical bone marrow specimens and found a similar induction of LXR target gene expression following treatment with T0901317 compared to control treated cells (Fig.. 1B; P<0.05). Therefore, LXRs are present and active within MM cells.
Figure 1. LXR activation modulates Hh signaling in MM cells.
A. KMS12, NCI-H929, and U266 MM cells were treated with 22(R) or 22(S)-hydroxycholesterol for 96 hours followed by quantification of LXR target genes by qPCR. B. Isolated MM plasma cells from 3 primary clinical specimens were treated with T0901317 for 96 hours then evaluated for LXR target gene expression. C. NCI-H929 and U266 cells were transfected with a Hh-responsive luciferase reporter plasmid then treated with SHh-containing (SHh) or control conditioned media ± T0901317 for 48 hours followed by quantification of relative firefly luciferase activity. D. MM cell lines were treated with SHh-containing with or without T0901317 for 96 hours followed by quantification of Hh target gene expression. For A, C, and D, values represent relative expression or reporter activity compared to vehicle treated control cells and the mean ± SEM of >3 independent experiments.
LXR activation inhibits Hedgehog signaling in multiple myeloma
In murine embryonic fibroblasts, LXR activation inhibits Hh pathway signaling (20). We examined whether this cross-talk was conserved in human cancer cells by treating NCI-H929 and U266 MM cells with T0901317 following transfection with a GLI-responsive reporter construct. Compared to vehicle treated control cells, exogenous SHh ligand increased baseline reporter activity that was subsequently inhibited by approximately 50% following treatment with T0901317 (Fig. 1C; P<0.01). We also examine the expression of canonical Hh target genes and found that T0901317 significantly inhibited the expression of PTCH1, GLI1, and HHIP induced by SHh ligand compared to vehicle treated control cells (Fig. 1D; P<0.03). Therefore, LXR activation inhibits Hh pathway activity in human MM cells similar to its effects on normal murine fibroblasts.
LXR activation inhibits clonogenic MM growth in vitro
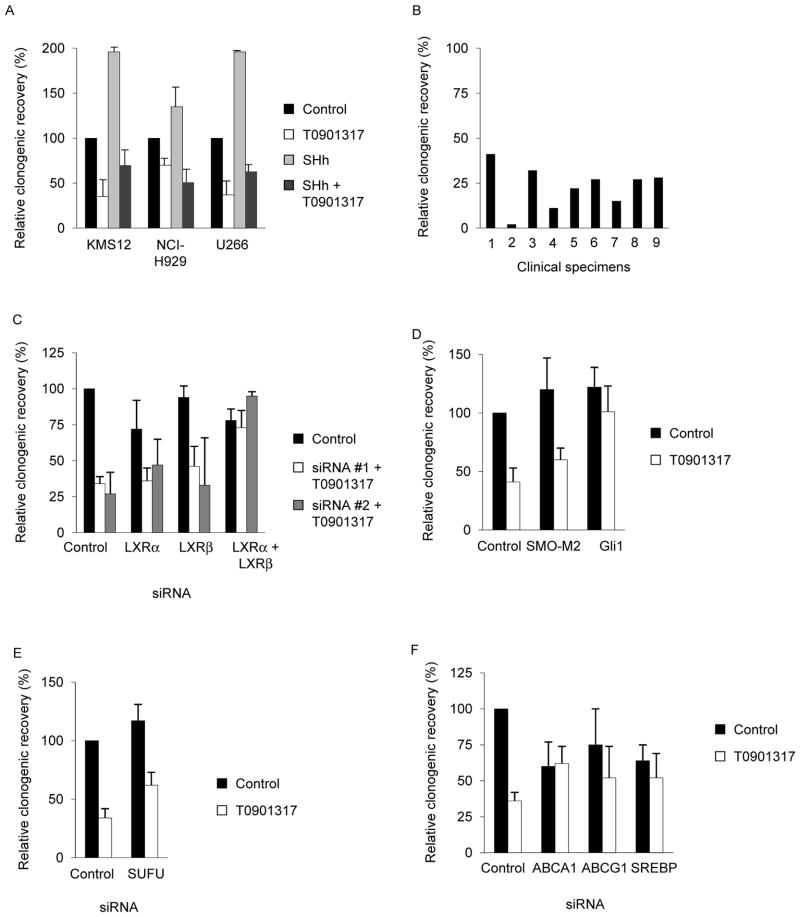
We previously demonstrated that Hh signaling enhances MM growth, and to determine the impact of LXR activation on clonogenic MM growth, we treated KMS12, NCI-H929, and U266 cells with T0901317 and quantified colony forming potential in methylcellulose. Compared to vehicle controls, treatment with T0901317, GW3965, or 22R significantly inhibited tumor cell colony formation by 30–65% in all 3 cell lines (Fig. 2A and Supplemental Fig. 3; P<0.01). Furthermore, T0901317 inhibited colony formation to a similar degree both at baseline as well as in the presence of exogenous SHh ligand to maximize Hh pathway activity (Fig 2A; P>0.2). This inhibition of clonogenic MM growth was not due to changes in cell proliferation or the induction of apoptosis as treatment with T0901317 did not significantly impact the number of total or viable cells compared to vehicle control cells (Supplemental Fig. 4; P>0.9). We also studied 9 primary clinical bone marrow specimens collected from patients with MM and found that T0901317 similarly inhibited colony formation by 59–98% (Fig. 2B) similar to what we previously observed with the SMO antagonist cyclopamine (12). We repeated these studies over a range of T0901317 concentrations and found that Hh pathway inhibition and clonogenic growth closely mirrored LXR transcriptional activity suggesting that these activities are linked (Supplemental Fig. 5). Although T0901317 has been used to investigate the function of LXRs in a wide variety of experimental systems, it may activate other nuclear receptors including the Farnesyl X (FXR) and Pregnane X (PXR) receptors in addition to LXRs. In order to demonstrate that LXR activation was primarily responsible for the effects of T0901317 on clonogenic MM growth, we transfected cells with siRNA against LXRα and LXRβ prior to treatment. Combined knock-down of LXRα and LXRβ using two distinct sets of siRNA, but neither LXR isoform alone, rescued U266 cells from the inhibitory effects of T0901317 (Fig. 2C, Supplemental Fig. 6), therefore, the effects of T0901317 on clonogenic MM growth by is dependent on the expression of both LXRα and LXRβ.
Figure 2. T0901317 inhibits clonogenic MM growth in a Hh-dependent manner.
A. KMS12, NCI-H929, and U266 cells were treated with SHh-containing (SHh) or control conditioned media ± T0901317 for 96 hours followed by the quantification of relative tumor colony formation compared to cells treated with control media and drug vehicle. B. Primary clinical MM specimens were treated with T0901317 for 96 hours then plated in methylcellulose. Values represent relative tumor colony formation compared to vehicle control treated cells. U266 cells were transected with siRNA against LXRα and/or LXRβ (C), expression vectors for SMO-M2 and Gli1 (D), siRNA against SUFU (E), or siRNA against ABCA1, ABCG1, SREBP1-c (F) then treated for 96 hours with or without T0901317 and plated in methylcellulose. Values represent mean ± SEM colony formation compared to control transfected (scrambled siRNA or pGL3basic) and vehicle treated control cells derived from 3 independent experiments.
LXR activation inhibits Hh signaling epistatic to SUFU
Pathogenic Hh pathway activation may occur at several levels of the signal transduction cascade including mutations in PTCH1, SMO, and SUFU, as well as amplification of GLI1 (7). Therefore, we examined the level at which LXR activation impacts Hh signaling in MM cells. Hh signaling is initiated by ligand binding to PTCH that leads to the de-repression of SMO. In order to determine whether T901317 inhibits Hh signaling at or above the level of SMO, we transfected U266 MM cells with a constitutively active SMO mutant, Smo-M2 (31), and found that its expression did not significantly affect the ability of T0901317 to inhibit colony formation compared to cells transfected with a control expression vector (Fig. 2D; P>0.02 compared to vehicle control). In contrast, the over-expression of GLI1 in U266 cells increased clonogenic MM growth that was unaffected by treatment with T0901317 (Fig. 2D; P>0.3). SUFU acts down-stream of SMO and mediates the post-transcriptional inactivation of the GLI transcription factors, and SUFU inactivation leads to aberrant Hh pathway activation. The transfection of U266 cells with siRNA against SUFU resulted in increased colony formation compared to cells transfected with control scrambled siRNA, and this increased clonogenic growth was significantly, albeit not completely, inhibited by subsequent treatment with T0901317 (66% vs. 38% inhibition compared to siRNA transfected cells, Fig. 2E; P<0.05). Therefore, T0901317 appears to impact the Hh signal transduction pathway at a level epistatic to SUFU.
We also investigated the requirement for specific LXR target genes on the effects of T0901317 and quantified the clonogenic growth of U266 cells following gene knockdown. Compared to control siRNA transfected cells, the loss of ABCA1 and SREBP1-c, but not ABCG1, expression inhibited baseline colony formation (Fig. 2F; P<0.04). Moreover, loss of ABCA1, ABCG1, or SREBP1-c failed to completely rescue the inhibitory effects of T0901317 suggesting that each of these LXR target genes contributes to its activity.
LXR activation inhibits MM tumor initiating cells
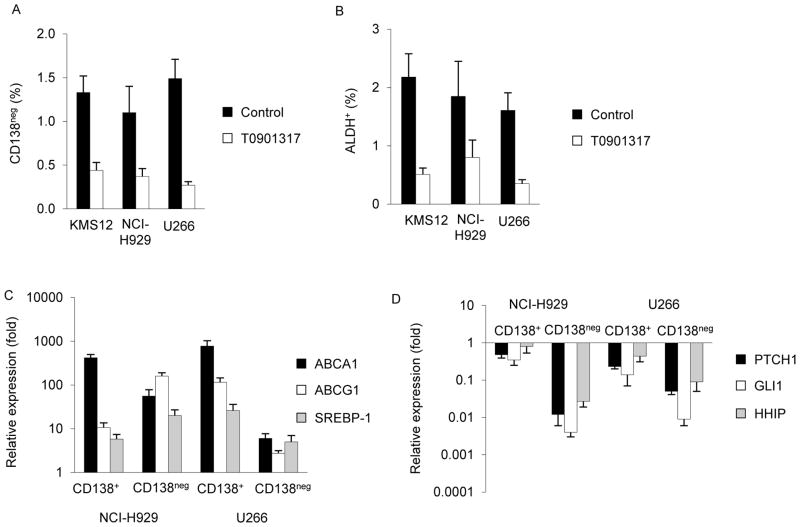
We previously demonstrated that Hh pathway inhibition induces the terminal differentiation of CD138neg MM tumor initiating cells (TICs) that results in the loss of clonogenic growth potential. In order to determine whether LXR activation similarly inhibits MM precursors, we treated KMS12, NCI-H929, and U266 cells with T0901317 or 22R and quantified CD138 expression by flow cytometry. Compared to vehicle control treated cells, treatment with T0901317 or 22R significantly decreased the proportion of CD138neg cells within each cell line by 67–82% (Fig. 3A, Supplemental Fig. 7A; P<0.01). Since CD138 may be lost by mature MM plasma cells during apoptosis, we examined Aldehyde dehydrogenase (ALDH) expression that also enriches for MM TICs and found that T0901317 or 22R treatment significantly decreased the proportion of ALDH+ KMS12, NCI-H929, and U266 cells by 57–77% (Fig. 3B, Supplemental Fig. 7B; P<0.02). We also examined the impact of LXR activation on the differentiation of isolated CD138neg U266 cells and found that CD138 expression was induced by T0901317 to a similar extent as cyclopamine (Supplemental Fig. 7C).
Figure 3. T0901317 inhibits MM TICs.
MM cells were treated with or without T0901317 then evaluated for CD138 expression (A) and ALDH (B) by flow cytometry. Values represent the mean ± SEM for 3 independent experiments. Quantification of LXR (C) and Hh (D) target gene expression in isolated CD138+ and CD138neg cells following treatment with T0901317. Values represent the mean ± SEM expression relative to vehicle control treated cells from 3 independent experiments.
We previously found that Hh pathway activity is relatively increased within CD138neg precursors, and we examined the relative effects of T0901317 on CD138+ and CD138neg MM cells. We isolated each subpopulation from U266 and NCI-H929 cells by FACS and quantified the expression of LXR and Hh target genes following treatment with T0901317. In NCI-H929 cells, T0901317 induced the expression of LXR target genes (i.e., ABCA1, ABCG1, and SREBP-1c) to a similar extent in CD138+ and CD138neg cells (Fig. 3C), but inhibited Hh signaling (i.e., PTCH and GLI1) to a greater degree in CD138neg than CD138+ cells (Fig. 3D). In U266 cells, LXR target genes were induced to higher levels in CD138+ cells compared to CD138neg cells (Fig. 3C), however, T0901317 treatment inhibited PTCH1 and GLI1 expression levels to a significantly greater degree in CD138neg than CD138+ cells (Fig 3D; P<0.03). Therefore, LXR activation may preferentially target Hh signaling within CD138neg TICs.
LXR activation inhibits clonogenic MM growth in vivo
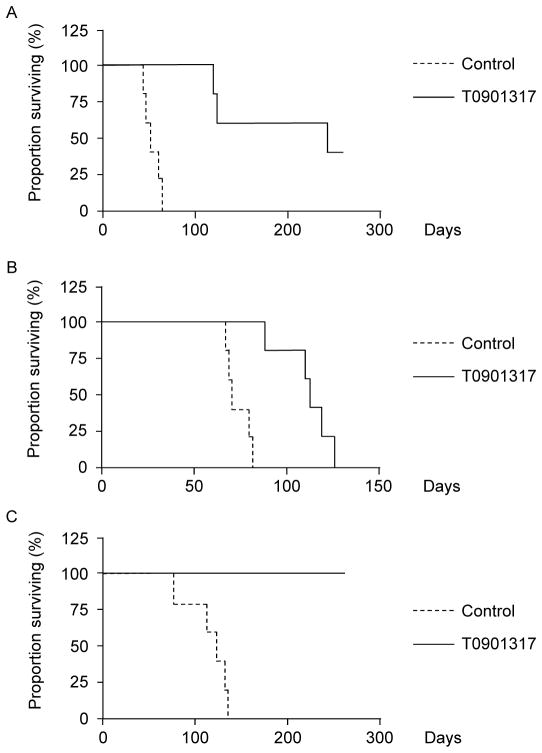
In order to examine the impact of LXR activation on the tumorigenic potential of MM cells, we treated U266 MM cells ex vivo with T0901317 for 2 weeks followed by injection into NSG mice. Compared to vehicle control cells, mice injected with T0901317 treated cells displayed a significantly prolonged survival (11.6 vs. 29.6 weeks, Fig. 4A; P<0.002). We also examined the impact of in vivo T0901317 treatment by injecting NSG mice with U266 cells then providing feed containing or lacking T0901317, a method that significantly induced the expression of the LXR target genes ABCA1, ABCG1, and SREBP-1c in the bone marrow of mice receiving T0901317 (5–84 fold compared to control treated animals, Supplemental Fig. 8; P<0.02). All mice receiving control or T0901317 containing feed engrafted with MM evidenced by human CD138+ MM cells within the bone marrow (data not shown) and eventually succumbed, but mice treated with T0901317 survived significantly longer compared to control treated mice (16 vs. 10 weeks, Fig. 4B; P<0.002). In order to examine the impact of in vivo LXR treatment on MM self-renewal, we carried out secondary transplants by injecting equal numbers of tumor cells into naïve recipients without further treatment. All mice injected with cells derived from control treated animals demonstrated MM engraftment and survived a median of 18.6 weeks. In contrast, none of the animals receiving an equivalent numbers of MM cells from T0901317-treated animals had evidence of tumor engraftment or died by 40 weeks (Fig. 4C; P<0.002).
Figure 4. T0901317 limits MM tumor initiation and self-renewal in vivo.
A. Survival of NSG mice following injection of U266 MM cells treated ex vivo with T0901317 or vehicle control. B. Survival of NSG mice following injection of U266 cells and in vivo treatment with T0901317. C. Survival of secondary recipient NSG mice receiving tumor cells, but no further treatment, from mice depicted in B.
DISCUSSION
Aberrant Hh pathway activation in human cancers has led to the development of specific pathway antagonists, such as vismodegib, with significant clinical activity in BCC (4, 32). Secondary mutations in SMO may lead to therapeutic resistance (14), therefore, novel Hh pathway inhibitors may be clinically beneficial. We have found that LXR activation significantly inhibits Hh signaling in MM cells and results in the inhibition of TICs and clonogenic growth. Moreover, the LXR agonist T0901317 was effective in cells expressing SMO-M2 suggesting that LXR activation may represent an alternative approach to inhibiting pathogenic Hh signaling.
Despite the success of Hh inhibitors in BCC, SMO inhibitors have not been effective in other tumor types (6). In BCC, mutations in PTCH or SMO lead to aberrant Hh pathway activity, but similar mutations are rarely found in other tumor types. Other events such as mutations in SUFU, the EWS-FLI fusion gene characteristic of Ewing sarcoma, and RAS mutations can also increase GLI transcriptional activity in a SMO independent manner (7). Several preclinical strategies have identified agents that target Hh pathway activation downstream of SMO by disrupting the intracellular processing and translocation of pathway components (Hh pathway inhibitors 1–4, arsenic, itraconazole), GLI1 function (GANT-61), or the formation of primary cilia (33–37). We found that LXR agonists are also capable of inhibiting MM clonogenic growth in cells with decreased levels of SUFU but not over-expression of GLI1. Therefore, LXR agonists appear to interact with Hh signaling at or downstream of SUFU.
Although our studies do not reveal the precise mechanism for Hh pathway inhibition, it is unlikely that it directly acts on GLI1 transcriptional activity as the over-expression of GLI1 rescued U266 cells from T0901317 mediated inhibition. Moreover, it is unlikely that the disruption of primary cilia is involved, as these cellular structures have not been described in lympho-hemtopoietic cells. Oxysterols serve as ligands for LXRs that regulate cholesterol metabolism, and both oxysterols and cholesterol have been found to impact Hh pathway activity. Specific oxysterols, such as 20(S)-hydroxycholesterol, can directly bind to and activate SMO (18), but we found that the effects of T0901317 were dependent on the expression of LXRα and LXRβ suggesting that it does not directly target SMO. T0901317 induced the expression of ABCA1 and ABCG1, two ATP-binding cassette (ABC) transporters that mediate cholesterol efflux, and the inhibition of ABCA1 and SREBP1-c expression inhibited colony formation by U266 MM cells. Decreased intracellular cholesterol levels have been found to inhibit Hh signaling (38), possibly by modulating the accumulation of SMO at the plasma membrane (39), and it is also possible that T0901317 acts through this mechanism.
Several reports have demonstrated that T0901317 can inhibit the proliferation and induce the apoptosis of prostate and breast cancer cells (40, 41). We found that T0901317 did not induce cell death, but rather inhibited clonogenic MM growth and self-renewal. T0901317 has also been found to induce the differentiation of human AML cell lines and primary clinical specimens in combination with Retinoid X Receptors agonists (42), and we previously found that Hh pathway inhibition induces the differentiation of MM precursors (12). T0901317 and 22R also decreased the relative proportion of MM CSCs defined as CD138neg or ALDH+ cells. Since MM CSCs are relatively resistant to several anti-MM agents including the thalidomide analogue lenalidomide and the proteasome inhibitor bortezomib (26, 43), LXR agonists may represent novel strategies to target drug-resistant CSCs.
Supplementary Material
Acknowledgments
Financial support: This study was supported by grants from the NIH (R01CA127574 and R21CA155733 to W. Matsui, K08CA154975 to A. Merchant, R01CA113669 to W. Matsui and Z. Rasheed, P01CA015396 to I. Borrello and C.A. Huff, R01AR059794 to F. Parhami). J.R. Agarwal was supported by a postdoctoral fellowship from the German Research Foundation. W. Matsui is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
The authors thank Lisa Marx for administrative assistance.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 3.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Hoff D, Lorusso P, Rudin C, Reddy J, Yauch R, Tibes R, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009 doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 5.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan R, Matsui W. Molecular pathways: the Hedgehog signaling pathway in cancer. Clin Cancer Res. 2012;18:4883–8. doi: 10.1158/1078-0432.CCR-11-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009:1. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierks C, Beigi R, Guo G-R, Zirlik K, Stegert MR, Manley P, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–49. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proceedings of the National Academy of Sciences. 2007;104:4048–53. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin TL, Matsui W. Hedgehog pathway as a drug target: Smoothened inhibitors in development. OncoTargets and therapy. 2012;5:47. doi: 10.2147/OTT.S21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–44. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 17.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernández-Zapico ME, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103:8408–13. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–68. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 20.Kim W-K, Meliton V, Park KW, Hong C, Tontonoz P, Niewiadomski P, et al. Negative regulation of Hedgehog signaling by liver X receptors. Mol Endocrinol. 2009;23:1532–43. doi: 10.1210/me.2008-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–20. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233–40. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 23.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen CL, Hsu P-P, Glienke J, Rubanyi GM, Brooks AR. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer. 2004;4:43. doi: 10.1186/1471-2407-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–7. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 28.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and Liver X Receptor agonists in mice with selective deficiency of Sterol Regulatory Element-binding Protein-1c. J Biol Chem. 2002;277:9520–8. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 29.Repa J, Turley S, Lobaccaro J-M, Medina J, Li L, Lustig K, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 30.Sparrow CP, Baffic J, Lam M-H, Lund EG, Adams AD, Fu X, et al. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J Biol Chem. 2002;277:10021–7. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 31.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 32.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–6. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci U S A. 2009;106:14132–7. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Lee J, Gardner D, Beachy P. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci U S A. 2010;107:13432–7. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Tang J, Gong R, Kim J, Lee J. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–99. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauth M, Bergström A, Shimokawa T, Toftgård R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–13. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 39.Riobo NA. Cholesterol and its derivatives in Sonic Hedgehog signaling and cancer. Curr Opin Pharmacol. 2012;12:736–41. doi: 10.1016/j.coph.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pommier AJC, Alves G, Viennois E, Bernard S, Communal Y, Sion B, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–23. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 41.Vedin L-L, Lewandowski SA, Parini P, Gustafsson J-A, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–9. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez PV, Glantz ST, Scotland S, Kasner MT, Carroll M. Induced differentiation of acute myeloid leukemia cells by activation of retinoid X and liver X receptors. Leukemia. 2013 doi: 10.1038/leu.2013.202. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, et al. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.