Abstract
Background
The sagittal abdominal diameter (SAD) measured in supine position is an alternative adiposity indicator that estimates the quantity of dysfunctional adipose tissue in the visceral depot. However, supine SAD’s distribution and its association with health risk at the population level are unknown. Here we describe standardized measurements of SAD, provide the first, national estimates of the SAD distribution among US adults, and test associations of SAD and other adiposity indicators with prevalent dysglycemia.
Methods and Findings
In the 2011–2012 National Health and Nutrition Examination Survey, supine SAD was measured (“abdominal height”) between arms of a sliding-beam caliper at the level of the iliac crests. From 4817 non-pregnant adults (age ≥20; response rate 88%) we used sample weights to estimate SAD’s population distribution by sex and age groups. SAD’s population mean was 22.5 cm [95% confidence interval 22.2–22.8]; median was 21.9 cm [21.6–22.4]. The mean and median values of SAD were greater for men than women. For the subpopulation without diagnosed diabetes, we compared the abilities of SAD, waist circumference (WC), and body mass index (BMI, kg/m2) to identify prevalent dysglycemia (HbA1c ≥5.7%). For age-adjusted, logistic-regression models in which sex-specific quartiles of SAD were considered simultaneously with quartiles of either WC or BMI, only SAD quartiles 3 (p<0.05 vs quartile 1) and 4 (p<0.001 vs quartile 1) remained associated with increased dysglycemia. Based on continuous adiposity indicators, analyses of the area under the receiver operating characteristic curve (AUC) indicated that the dysglycemia model fit for SAD (age-adjusted) was 0.734 for men (greater than the AUC for WC, p<0.001) and 0.764 for women (greater than the AUC for WC or BMI, p<0.001).
Conclusions
Measured inexpensively by bedside caliper, SAD was associated with dysglycemia independently of WC or BMI. Standardized SAD measurements may enhance assessment of dysfunctional adiposity.
Introduction
The body mass index (BMI, weight/height2) is recommended for clinical and epidemiological assessments of human adiposity [1], [2], but BMI cannot distinguish between lean mass and depots of adipose tissue (AT). Dependence on the categorical BMI has sometimes misclassified health risk, leading commentators to call for the exploration of alternative, low-cost, adiposity metrics [3]. A candidate alternative indicator is the sagittal abdominal diameter (SAD) which, when measured externally in the supine position (“abdominal height”), estimates the volume of visceral (intra-abdominal) AT [4], [5]. As demonstrated by expensive imaging technologies, it is primarily the visceral depot of AT (as opposed to subcutaneous depots) that correlates with cardiometabolic risk [6]–[8]. Associations have been found between SAD and chronic-disease risk factors or outcomes, but these reports depended on selected research populations and employed varying methods and positions for measuring SAD [9]–[16]. Wider use of the SAD would benefit from a standardized measurement protocol and the availability of SAD normative reference values.
This paper describes a simple, inexpensive protocol for SAD measurement and estimates the distribution of SAD values in the US adult population examined during 2011–2012. It also demonstrates how the use of SAD measurements could improve upon BMI or waist circumference (WC) for the recognition of impaired glucose regulation (“dysglycemia”).
Methods
Participants and their clinical measurements
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative, cross-sectional survey of the resident civilian, non-institutionalized, US population. Participants in NHANES underwent home interviews followed by standardized anthropometric and laboratory assessments in mobile examination centers. The complex, multistage-probability, sampling design of NHANES requires sample weights for each participant so that characteristics of the US population can be estimated. In the 2011–2012 NHANES, of 5560 interviewed adults (≥20 years old), 5319 were examined, and 4817 had SAD measurement data. Since pregnant women (n = 57) were not eligible for SAD measurement, the participation rate for SAD was 88% among non-pregnant interviewees. A general description of NHANES has been published elsewhere [17].
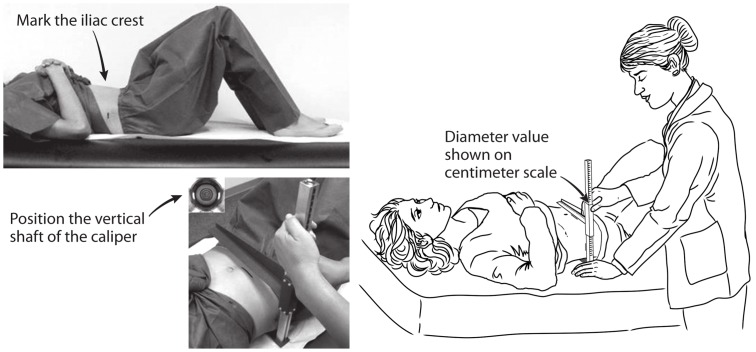
The SAD was measured using a sliding-beam, abdominal caliper (Holtain, Ltd, Wales, UK). Supine participants rested on a lightly padded exam table with their hips in a relaxed, flexed position as the examiner marked the level of their iliac crests with a wax pencil. The lower arm of the caliper was then inserted under the small of the back, and the upper arm was raised above the belly in alignment with the transverse pencil mark (Figure 1). After confirming that the caliper shaft was vertical, the examiner asked the participant to inhale gently, slowly let the air out, and then pause (“… rest,… relax…”). The examiner then slid down the caliper’s upper arm, letting it lightly touch the abdomen but without compressing it. The SAD value was read directly from the centimeter scale on the caliper shaft and recorded to the nearest 0.1 cm [18]. Then, after raising the caliper’s upper arm and repeating breathing instructions, a second SAD measurement was recorded. If the two SAD values differed by >0.5 cm, third and fourth measurements were obtained. For this report we defined each participant’s SAD as the mean of 2 initial measurements or of up to 4 measurements as specified in the online, analytic notes from NHANES [19]. Weight, height and a standing-position WC were measured by established methods [18].
Figure 1. Measurement of the sagittal abdominal diameter by use of a sliding-beam caliper in NHANES, 2011–2012.
Within our analytic sample we identified adults with diagnosed diabetes by their affirmative answer to the question “have you ever been told by a doctor or other health professional that you have diabetes or sugar diabetes?” For those without diagnosed diabetes we defined categorical dysglycemia by a glycated hemoglobin (HbA1c) concentration ≥5.7% (≥39 mmol/mol). This is a common threshold value that points to an increased risk of cardiovascular disease [20] as well as to “prediabetes” or undiagnosed diabetes [21]. Assays of HbA1c in NHANES were performed on whole-blood hemolysate presented to a high-performance liquid chromatography column (Fairview Medical Center Laboratory, University of Minnesota, Minneapolis).
Ethics Statement
The NHANES protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics; participants provided informed consent.
Statistical analyses
All analyses accounted for the sampling weights and sample design using SAS (release 9.3 [SAS Institute Inc., Cary, NC], SUDAAN (release 11.1) [RTI International, Research Triangle Park, NC]) or the ‘survey’ package in R [22], [23]. We estimated the distribution of SAD values in 2011–2012 among US adults overall and by sex and age group (20–34, 35–49, 50–64, and 65+ years). The means, quartiles, and their corresponding Wald 95% confidence intervals were calculated using the DESCRIPT procedure of SUDAAN.
For the subpopulation not diagnosed with diabetes, we then assessed the utility of SAD compared to other adiposity indicators (WC or BMI) for identifying prevalent dysglycemia. Our first approach examined the relation of sex-specific quartiles of SAD, WC and BMI to this outcome of interest. Predictive margins from age-adjusted logistic regression models were estimated to provide prevalence ratios (PRs) relative to the lowest quartile; each model’s goodness of fit was estimated as R2 (Cox & Snell method). We examined ordinal quartiles for each adiposity indicator individually, as well as the independent effect of SAD quartiles in models that also included either BMI quartiles or WC quartiles.
The three adiposity indicators were highly correlated with each other, and collinearity might complicate interpretation of the individual regression coefficients in models that simultaneously contained SAD and another adiposity indicator. Therefore, we also calculated receiver operator characteristic curves for each indicator and compared the areas under these curves (AUCs) as indices of fit for the various models. These sex-specific logistic regression models included age and an adiposity indicator modeled as continuous variables using natural splines with three knots to allow for non-linearity. They also included a term for sex when the sample included both men and women. Each model’s goodness of fit was estimated as R2 (Nagelkerke method). We assessed the difference in the AUCs between models using jackknife resampling [24] with the ‘withReplicates’ function in R [22] to estimate the standard error of the difference between models.
Results
SAD means and selected percentile values for US adults are presented in Table 1. These estimates for calendar years 2011–2012 were derived from 4817 examined adults (irrespective of metabolic status or other anthropometry; not pregnant) who represented the US non-institutionalized, civilian, population of approximately 224 million at age ≥20 years. The mean and median values of SAD were greater for men than women. In both sexes the means and medians of SAD increased with age at least through 64 years.
Table 1. Population mean and median (50th percentile) values with selected percentiles of the sagittal abdominal diameter in US adults, from NHANES 2011–2012.
| Population Percentiles, cm | ||||||||
| Sex | Age, y | Sample N | Mean (95% CI), cm | 5th | 25th | 50th (95% CI) | 75th | 95th |
| Both | ≥20 | 4817 | 22.5 (22.2−22.8) | 16.4 | 19.2 | 21.9 (21.6−22.4) | 25.2 | 30.5 |
| 20–34 | 1292 | 21.0 (20.6−21.5) | 15.9 | 17.8 | 20.1 (19.7−20.8) | 23.4 | 29.1 | |
| 35–49 | 1240 | 22.5 (22.0−23.0) | 16.4 | 19.4 | 21.9 (21.3−22.5) | 25.0 | 30.8 | |
| 50–64 | 1311 | 23.5 (23.0−24.0) | 17.3 | 20.3 | 23.1 (22.4−23.7) | 26.1 | 31.9 | |
| ≥65 | 974 | 23.4 (22.9−23.8)* | 17.5 | 20.5 | 23.2 (22.6–23.5) | 25.9 | 30.4 | |
| Men | ≥20 | 2450 | 23.2 (22.8–23.6) | 17.3 | 20.1 | 22.7 (22.2–23.2) | 25.8 | 31.2 |
| 20–34 | 696 | 21.5 (20.9–22.0) | 16.3 | 18.3 | 20.8 (19.9–21.4) | 23.7 | 29.1 | |
| 35–49 | 610 | 23.3 (22.9–23.7) | 17.9 | 20.4 | 22.8 (22.3–23.4) | 25.3 | 31.1 | |
| 50–64 | 638 | 24.4 (23.8–24.9) | 17.9 | 21.3 | 23.7 (23.2–24.6) | 26.9 | 32.4 | |
| ≥65 | 506 | 24.3 (23.7–24.9)* | 18.6 | 21.7 | 23.8 (23.4–24.5) | 26.7 | 31.6 | |
| Women | ≥20 | 2367 | 21.8 (21.5–22.1)† | 15.9 | 18.5 | 21.1 (20.8–21.5) | 24.7 | 30.0 |
| 20–34 | 596 | 20.6 (20.1–21.0)‡ | 15.3 | 17.1 | 19.6 (18.8–20.0) | 22.8 | 29.1 | |
| 35–49 | 630 | 21.6 (21.0–22.2)† | 15.7 | 18.2 | 20.8 (20.2–21.8) | 24.4 | 30.2 | |
| 50–64 | 673 | 22.7 (22.1–23.3)† | 16.9 | 19.4 | 21.8 (21.2–23.5) | 25.3 | 30.6 | |
| ≥65 | 468 | 22.5 (21.9–23.2)* † | 16.9 | 19.3 | 22.3 (21.3–23.2) | 25.4 | 29.2 | |
* p<0.001 for age trend.
p<0.001 compared to men.
p<0.01 compared to men.
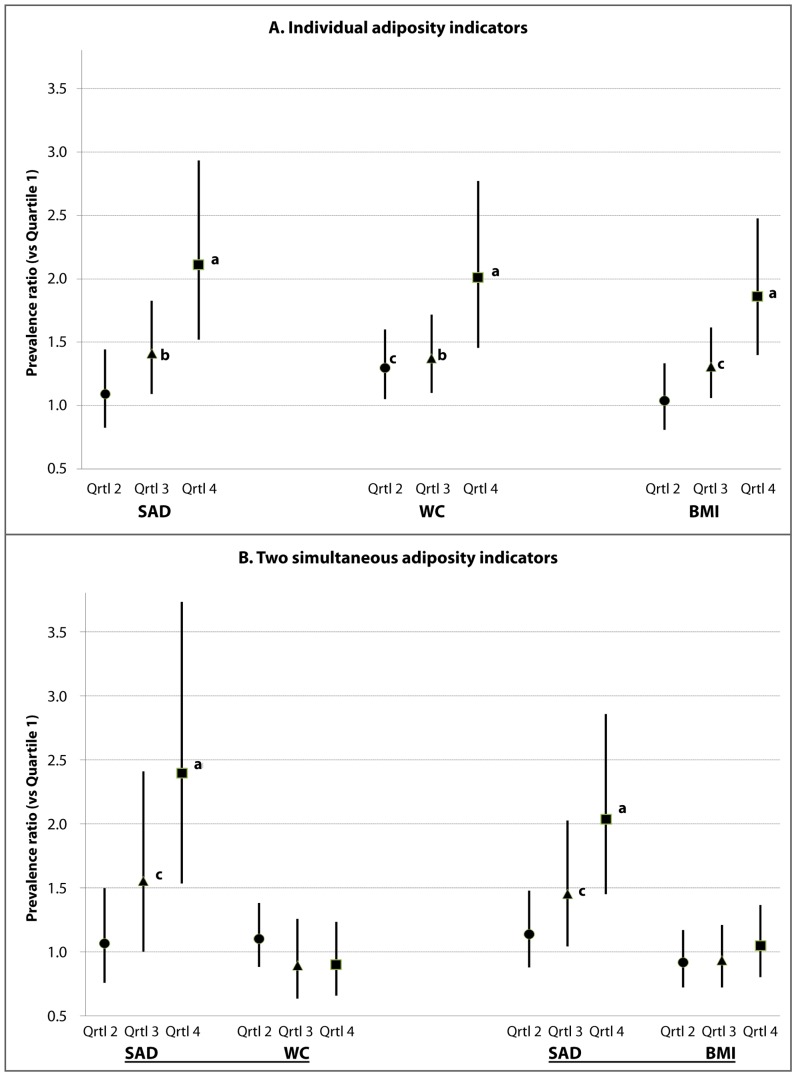
Among adults without a diabetes diagnosis who were evaluated for prevalent dysglycemia, the analytic subpopulation (subsample n = 4037; excluding participants without information on HbA1c, WC, or BMI) included dysglycemic persons with prediabetes or undiagnosed diabetes. For our initial assessment of how dysglycemia would be identified by the 3 adiposity indicators, the sex-specific quartile cutoffs for SAD, WC and BMI are shown in Table 2. The dimensions describing abdominal size (SAD and WC) had cutoff values for men consistently larger than those for women. However, for the indicator of generalized relative weight (BMI), there was no consistent sex distinction for the quartile cutoff values. The overall crude prevalence of dysglycemia in this subpopulation was 26.4%, similar for men (25.9% [95% confidence interval 23.5–28.3]) and women (26.8% [23.8–29.9]). The crude dysglycemia prevalence estimates across the ordinal quartiles demonstrated an increasing trend (p<0.01) of each adiposity indicator (Table 3). In age-adjusted logistic models, the ordinal quartiles of each adiposity indicator were likewise associated with an increasing prevalence of dysglycemia (Figure 2, panel A). The explained variations in dysglycemia (multiple R2) for these quartile-based models were 0.133 for SAD, 0.123 for WC, and 0.125 for BMI. When SAD quartiles were simultaneously considered with quartiles of WC in the model, SAD quartile 3 (PR 1.55; p<0.05) and quartile 4 (PR 2.39; p<0.001) remained significantly different from SAD quartile 1 (Figure 2, panel B). When quartiles of SAD were simultaneously considered with quartiles of BMI in the same model, dysglycemia prevalence of SAD quartile 3 (PR 1.45; p<0.05 vs quartile 1) and quartile 4 (PR 2.03; p<0.001 vs quartile 1) likewise remained significantly elevated. However, for both of these models that tested simultaneous indicators, the competing quartiles 3 and 4 of BMI or WC were not significantly associated with dysglycemia.
Table 2. Subpopulation quartile cutoffs of adiposity indicators in US adults ages ≥20 years without diagnosed diabetes, estimated from NHANES 2011–2012.
| Quartile cutoffs (95% confidence interval) | |||||
| Indicator | Sex | Subsample n | 25th percentile | 50th percentile | 75th percentile |
| SAD, cm | Total | 4,037 | 19.0 (18.6–19.4) | 21.6 (21.3–22.0) | 24.8 (24.2–25.2) |
| Men | 2,035 | 19.9 (19.5–20.4) | 22.4 (22.0–22.9) | 25.4 (24.8–25.9) | |
| Women | 2,002 | 18.3 (17.9–18.7) | 20.8 (20.3–21.3) | 24.0 (23.5–24.6) | |
| WC, cm | Total | 4,037 | 86.0 (84.6–87.9) | 96.0 (95.1–97.3) | 106.5 (105.4–107.8) |
| Men | 2,035 | 89.6 (87.5–91.7) | 99.0 (97.5–100.6) | 108.8 (107.7–110.5) | |
| Women | 2,002 | 82.9 (81.6–84.6) | 93.4 (91.8–94.9) | 104.1 (102.2–105.6) | |
| BMI, kg/m2 | Total | 4,037 | 23.8 (23.5–24.4) | 27.2 (26.8–27.7) | 31.3 (30.8–31.9) |
| Men | 2,035 | 24.3 (23.8–24.8) | 27.5 (27.0–27.9) | 31.0 (30.5–31.7) | |
| Women | 2,002 | 23.4 (23.0–23.9) | 26.9 (26.3–27.5) | 31.8 (31.0–32.5) | |
Table 3. Crude prevalence (%) of dysglycemia by quartiles of adiposity indicators in US adults ages ≥20 years without diagnosed diabetes, estimated from NHANES 2011–2012.
| Crude prevalence (95% confidence interval) of dysglycemia, percentage | |||||
| Indicator | Sex | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile |
| SAD | Total | 14.4 (10.8–18.9) | 19.9 (16.3–24.0) | 28.9 (25.6–32.5) | 42.0 (37.4–46.7) ∥ |
| Men | 13.8 (10.1–18.7) | 21.5 (16.4–27.7) | 26.5 (21.2–32.5) | 41.4 (36.1–46.9) ∥ | |
| Women | 14.9 (10.4–20.7) | 18.3 (14.1–23.3) | 31.3 (25.8–37.3) | 42.5 (35.4–50.0) ∥ | |
| WC | Total | 14.1 (10.7–18.4) | 23.9 (21.1–27.0) | 28.1 (23.9–32.7) | 39.1 (34.6–43.7) ∥ |
| Men | 14.4 (10.5–19.4) | 22.7 (19.4–26.5) | 26.4 (21.5–32.0) | 39.6 (33.9–45.7) ∥ | |
| Women | 13.8 (10.0–18.8) | 25.0 (19.4–31.6) | 29.7 (24.0–36.1) | 38.5 (33.0–44.3) § | |
| BMI | Total | 18.3 (14.4–22.9) | 21.7 (17.3–26.7) | 28.4 (24.6–32.6) | 36.7 (32.0–41.7) ∥ |
| Men | 19.6 (14.6–25.7) | 21.6 (17.1–26.7) | 26.9 (22.1–32.2) | 35.1 (29.0–41.7) ∥ | |
| Women | 17.0 (12.0–23.5) | 21.7 (16.1–28.7) | 29.9 (24.7–35.8) | 38.3 (32.0–45.1) ∥ | |
p<0.001 for quartile trend.
p<0.01 for quartile trend.
Figure 2. Panel A: Dysglycemia prevalence ratios by quartiles of sagittal abdominal diameter (SAD), waist circumference (WC) or body mass index (BMI).
Panel B: Prevalence ratios when SAD is considered simultaneously with WC (left side) or with BMI (right side). In age-adjusted models, the relative prevalence of dysglycemia (HbA1c ≥5.7% [≥39 mmol/mol]) is displayed in association with the second (circle), third (triangle), and fourth (square) quartiles (with reference to first quartile) of each indicator. Error bars indicate 95% confidence intervals. a p<0.001; b p<0.01; c p<0.05.
When our age-adjusted models with competing quartiles (“SAD vs WC” or “SAD vs BMI”) were restricted to either sex, SAD quartiles 3 and 4 again provided elevated point estimates although their confidence intervals did not always exclude one. For men (subsample n = 2035), when competing with WC quartiles, the SAD quartile 3 had PR 1.57 [0.89–2.76] and SAD quartile 4 had PR 2.31 [1.36–3.92]; when SAD competed with BMI quartiles, the men’s SAD quartile 3 had PR 1.57 [0.92–2.68] and SAD quartile 4 had PR 2.28 [1.49–3.49]. For women (subsample n = 2002), when competing with WC quartiles, the women’s SAD quartile 3 had PR 1.60 [1.00–2.56] and SAD quartile 4 had PR 2.52 [1.56–4.06]; when SAD competed with BMI quartiles, the SAD quartile 3 had PR 1.35 [0.84–2.16] and SAD quartile 4 had PR 1.83 [1.15–2.92]. In these sex-specific models all the quartiles of BMI or WC had weaker, non-significant associations with dysglycemia (PRs <1.21).
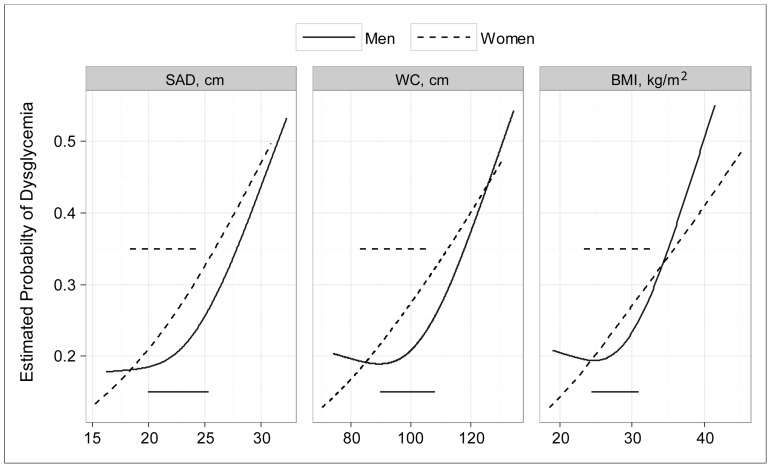
In the assessment of how well the continuous adiposity indicators identified dysglycemia (our second approach), the competing models adjusted for age and sex tended to confirm that continuous SAD explained a greater proportion of dysglycemia than continuous WC or BMI. Multiple R2 values for these continuous models were 0.201 for SAD, 0.195 for WC, and 0.198 for BMI. The differences between these AUCs were non-significant for the models in which both sexes were analyzed together (Table 4), but sex interactions were found for all three adiposity indicators. In sex-stratified analyses the men’s AUC for SAD was greater (p<0.001) than the AUC for WC (but not greater than the AUC for BMI); the women’s SAD area was greater (p<0.001) than the AUC for either WC or BMI. In sex-specific, age-adjusted curves we found for each of the adiposity indicators that the relationship with dysglycemia was curvilinear for men (J-shaped) but nearly linear for women (Figure 3).
Table 4. Areas under the curve (AUCs) of the receiver operating characteristic (ROC) for identification of dysglycemia by an adiposity indicator adjusted for age; comparisons of SAD with WC or BMI.
| AUCs (areas under the ROC curve) | p-value for difference in areas | ||||||
| Population | Subsample n | SAD | WC | BMI | SAD vs. WC | SAD vs. BMI | |
| Total* | 4037 | 0.748 | 0.741 | 0.744 | NS | NS | |
| Sex | Men | 2035 | 0.734 | 0.728 | 0.732 | <0.001 | NS |
| Women | 2002 | 0.764 | 0.757 | 0.758 | <0.001 | <0.001 | |
* Model includes adjustment for sex.
NS, p>0.10.
Figure 3. Probability of prevalent dysglycemia estimated by continuous sagittal abdominal diameter, waist circumference or body mass index.
In these age-adjusted plots prepared by restricted cubic splines, the horizontal lines represent the interquartile range (p25 to p75) in the sex-specific population distributions of each adiposity indicator.
Discussion
Adult SAD measurements obtained in NHANES 2011–2012 demonstrate the feasibility and utility of assessing abdominal adiposity with a portable, sliding-beam caliper. Identical or very similar anthropometric protocols have been used previously in studies of diabetes, incident coronary heart disease and several cardiometabolic risk factors among selected adults [11]–[14], [16], [25]–[29] and in a Finnish national survey of persons ≥30 years [30]. The choice of the iliac crests (approximating lumbar interspace L4–L5) as the most effective measurement landmark has been recommended by reports that also evaluated alternative sites such as the L3–L4 interspace, the umbilical level, or the highest point on the abdomen [31]–[33].
The historical rationale for measuring SAD has been the presumption that variation in this simple dimension would reflect increases in the amount primarily of visceral AT. An early proponent of the SAD pointed out that visceral AT would tend to ‘pump up’ the abdomen in the sagittal direction of supine subjects [34], and later investigators confirmed that the surrounding subcutaneous AT would tend to flow out at the flanks [35]. Recent advances in AT imaging, however, have demonstrated that subcutaneous AT contains distinct deep and superficial sub-compartments, each with its own histologic and physiologic characteristics. Deep abdominal subcutaneous AT may be located primarily near the anatomic midline (as inferred from cross-sectional abdominal images). This deep subcutaneous sub-compartment is associated, notably among men, with increased levels of circulating HbA1c [36] and other cardiometabolic risk variables [37]. Superficial abdominal subcutaneous AT is relatively more prominent at the sides of the abdomen, and its physiologic correlates are relatively benign. If the SAD incorporates primarily the deep (midline) subcutaneous AT but less of the superficial (lateral) AT, this might explain why prior research reported the SAD, when compared to the area of visceral AT alone, was more strongly associated with the metabolic syndrome and other cardiometabolic risk variables [14], [38], [39].
The distinction between deep and superficial subcutaneous AT may help to explain also why men, but not women, have a J-shaped relation of adiposity to dysglycemia prevalence (Figure 3). A deficit of superficial subcutaneous AT may be considered a marker of metabolic dysfunction since adipocytes in this sub-compartment are capable of safely storing energy during positive caloric balance. Compared to women, men have lesser amounts of superficial subcutaneous AT in the abdominal region [36], [37]. Some men with low levels of generalized adiposity may have so little superficial subcutaneous AT that any net excess of energy intake will result in an overflow to less benign AT depots or to ectopic sites such as the liver, skeletal muscle or pancreas. Others have previously commented on metabolic dysfunctions that occur when subcutaneous AT, irrespective of its sub-compartments, fails to expand sufficiently in response to metabolic overload [40], [41]. Given that the anthropometric methods of NHANES cannot directly distinguish between the deep and superficial components of subcutaneous AT, this speculative explanation of the J-shaped relationship to dysglycemia cannot be tested in our dataset.
Type 2 diabetes has been related to adiposity phenotypes that have an increased volume of visceral AT or elevations of hepatic fat content [6], [7], [42]. An enlarged visceral adipose depot and hepatic steatosis both represent forms of ectopic fat deposition. Since the SAD is associated with visceral AT volume [34] it is reasonable to expect that this easily measured external dimension would be associated also with dysglycemia and with an increased risk of diabetes. Direct assessments of hepatic fat content could likewise provide correlations with dysglycemia and cardiometabolic risk, but such assessments depend on liver biopsy or technologies (e.g., multi-slice magnetic resonance or tomographic imaging, magnetic resonance spectroscopy) that carry substantial costs in time, money, and possibly radiation.
Our finding that SAD was associated with dysglycemia in the general US adult population, independently of age and of WC or BMI, confirms smaller studies of SAD restricted to obese adults [13], [28]. Hyperinsulinemia, a marker of insulin resistance, has likewise been associated with SAD among young adults [12] and among older men without diabetes [13]. A prospective comparison from Finland of four adiposity indicators measured at ages ≥30 years has reported recently that the co-occurrence of high BMI and high SAD, but not high WC or high waist-to-hip ratio, was associated with the highest incidence of type 2 diabetes [30].
The absence of prospective, follow-up information is a major limitation of our study. Current survey data from NHANES are necessarily cross-sectional, although some earlier waves of NHANES examinations have been followed by re-contact [43] or mortality reviews [44]. Measurements of SAD within NHANES did not begin, however, until 2011. The Finnish national survey mentioned above was conducted in 2000–2001, and it employed an SAD protocol nearly identical to that used by NHANES. Smaller studies based on selected populations have reported prospectively on mortality [45]–[47] and incident dementia [48] in association with the SAD, but their anthropometric protocols differed substantially from that of SAD in NHANES. With regard to our participants who reported not having diabetes, another possible limitation of our study is the dependence on an assay of HbA1c to define the metabolic outcome of interest. However, the common limitations of HbA1c interpretation [49] are likely to be minimized in our analyses since all HbA1c assays for NHANES were performed by a single, highly standardized laboratory.
Consistent with physiologic and anatomic principles, the SAD stands as a credible alternative to the conventional WC or BMI for the clinical assessment of adiposity. As validated in this nationally representative sample, SAD could inexpensively augment the understanding of abdominal AT and its associated health risks. The public-use NHANES data will provide opportunities to test cross-sectional associations between SAD and many biomarkers or clinical conditions. Future studies employing a prospective design could expand on these findings and explore the associations of this adiposity indicator with medical outcomes and mortality.
Acknowledgments
The authors acknowledge the participants in 2011–2012 NHANES, and the efforts of the NHANES field staff and laboratory personnel. Margaret D. Carroll, MSPH, of NHANES shared her statistical expertise, and Kyung M. Park, BA, provided assistance with graphics.
All original data described in this paper are available for public use through the website http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx. Investigators should consult http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf for important information on conducting analyses.
The findings and conclusions in this article are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. As described under Acknowledgments, current NHANES survey data can be found at http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx.
Funding Statement
The authors have no support or funding to report.
References
- 1.World Health Organization (2011) Waist circumference and waist–hip ratio: Report of a WHO expert consultation, Geneva, 8–11 December, 2008. Nutrition Publications: 39 pages. Geneva, Switzerland. Available: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf. Accessed 1 May 2014.
- 2. Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, et al. (2011) Assessing adiposity: A scientific statement from the American Heart Association. Circulation 124: 1996–2019. [DOI] [PubMed] [Google Scholar]
- 3. Ahima RS, Lazar MA (2013) Physiology. The health risk of obesity–better metrics imperative. Science 341: 856–858. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom L, Kvist H (1988) Regional body fat measurements with CT-scan and evaluation of anthropometric predictions. Acta Med Scand Suppl 723: 169–177. [DOI] [PubMed]
- 5. Jensen MD, Kanaley JA, Reed JE, Sheedy PF (1995) Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61: 274–278. [DOI] [PubMed] [Google Scholar]
- 6. Gastaldelli A (2011) Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract 93 Suppl 1: S60–65. [DOI] [PubMed] [Google Scholar]
- 7. Neeland LJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, et al. (2012) Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tchernof A, Despres JP (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404. [DOI] [PubMed] [Google Scholar]
- 9. Houmard JA, McCulley C, Roy LK, Bruner RK, McCammon MR, et al. (1994) Effects of exercise training on absolute and relative measurements of regional adiposity. Int J Obes Relat Metab Disord 18: 243–248. [PubMed] [Google Scholar]
- 10. Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, et al. (1994) Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 73: 460–468. [DOI] [PubMed] [Google Scholar]
- 11. Kahn HS, Austin H, Williamson DF, Arensberg D (1996) Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol 49: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 12. Gustat J, Elkasabany A, Srinivasan S, Berenson GS (2000) Relation of abdominal height to cardiovascular risk factors in young adults: the Bogalusa heart study. Am J Epidemiol 151: 885–891. [DOI] [PubMed] [Google Scholar]
- 13. Riserus U, Arnlov J, Brismar K, Zethelius B, Berglund L, et al. (2004) Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care 27: 2041–2046. [DOI] [PubMed] [Google Scholar]
- 14. Valsamakis G, Chetty R, Anwar A, Banerjee AK, Barnett A, et al. (2004) Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet Med 21: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 15. Iribarren C, Darbinian JA, Lo JC, Fireman BH, Go AS (2006) Value of the sagittal abdominal diameter in coronary heart disease risk assessment: cohort study in a large, multiethnic population. Am J Epidemiol 164: 1150–1159. [DOI] [PubMed] [Google Scholar]
- 16. Ehrlich AC, Smith DA (2011) Abdominal diameter index and 12-year cardiovascular disease incidence in male bridge and tunnel workers. Int J Obes (Lond) 35: 409–415. [DOI] [PubMed] [Google Scholar]
- 17.CDC/National Center for Health Statistics (2013) About the National Health and Nutrition Examination Survey. Hyattsville, MD. Available: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 1 May 2014.
- 18.National Center for Health Statistics (2013) Anthropometry Procedures Manual - National Health and Nutrition Examination Survey (NHANES). Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Anthropometry.pdf. Accessed 1 May 2014.
- 19.National Center for Health Statistics (2013) NHANES 2011–2012 Data Documentation, Codebook, and Frequencies – Body Measures (BMX_G) Hyattsville, MD. Available: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/BMX_G.htm#Analytic_Notes. Accessed 1 May 2014.
- 20. Ackermann RT, Cheng YJ, Williamson DF, Gregg EW (2011) Identifying adults at high risk for diabetes and cardiovascular disease using hemoglobin A1c–National Health and Nutrition Examination Survey 2005–2006. Am J Prev Med 40: 11–17. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes A (2014) Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1: S14–80. [DOI] [PubMed] [Google Scholar]
- 22.Lumley T (2013) survey: analysis of complex survey samples. (R package version 3.29-5). Available: http://cran.r-project.org/web/packages/survey/index.html. Accessed 30 May 2014.
- 23.R Development Core Team (2014) The R Project for Statistical Computing. Vienna, Austria. Available: http://www.r-project.org/. Accessed 30 May 2014.
- 24.WesVar Development Team (2007) WesVar 4.3 User’s Guide. Rockville, MD. Available: http://www.westat.com/westat/pdf/wesvar/wv_4-3_manual.pdf. Accessed 30 May 2014.
- 25. Snijder MB, Visser M, Dekker JM, Seidell JC, Fuerst T, et al. (2002) The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord 26: 984–993. [DOI] [PubMed] [Google Scholar]
- 26. Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, et al. (2004) Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care 27: 2948–2953. [DOI] [PubMed] [Google Scholar]
- 27.Andersson K, Karlstrom B, Freden S, Petersson H, Ohrvall M, et al.. (2008) A two-year clinical lifestyle intervention program for weight loss in obesity. Food Nutr Res 52. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2596731/. Accessed 1 May 2014. [DOI] [PMC free article] [PubMed]
- 28. Gletsu-Miller N, Kahn HS, Gasevic D, Liang Z, Frediani JK, et al. (2013) Sagittal abdominal diameter and visceral adiposity: Correlates of beta-cell function and dysglycemia in severely obese women. Obes Surg 23: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Souza NC, de Oliveira EP (2013) Sagittal abdominal diameter shows better correlation with cardiovascular risk factors than waist circumference and BMI. J Diabetes Metab Disord 12: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pajunen P, Rissanen H, Laaksonen MA, Heliovaara M, Reunanen A, et al. (2013) Sagittal abdominal diameter as a new predictor for incident diabetes. Diabetes Care 36: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L (1988) Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr 48: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 32. Paula HA, Ribeiro Rde C, Rosado LE, Abranches MV, Franceschini Sdo C (2012) Relationship between waist circumference and supine abdominal height measured at different anatomical sites and cardiometabolic risk factors in older women. J Hum Nutr Diet 25: 563–568. [DOI] [PubMed] [Google Scholar]
- 33.Anunciacao PC, Ribeiro RC, Pereira MQ, Comunian M (2014) Different measurements of waist circumference and sagittal abdominal diameter and their relationship with cardiometabolic risk factors in elderly men. J Hum Nutr Diet. Available: http://onlinelibrary.wiley.com/enhanced/doi/10.1111/jhn.12201/. Accessed 1 May 2014. [DOI] [PubMed]
- 34. Sjostrom L (1991) A computer-tomography based multicompartment body composition technique and anthropometric predictions of lean body mass, total and subcutaneous adipose tissue. Int J Obes 15 Suppl 2: 19–30. [PubMed] [Google Scholar]
- 35. Kullberg J, von Below C, Lonn L, Lind L, Ahlstrom H, et al. (2007) Practical approach for estimation of subcutaneous and visceral adipose tissue. Clin Physiol Funct Imaging 27: 148–153. [DOI] [PubMed] [Google Scholar]
- 36. Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, et al. (2012) Abdominal superficial subcutaneous fat–A putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 35: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, et al. (2014) Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 37: 821–829. [DOI] [PubMed] [Google Scholar]
- 38. Guzzaloni G, Minocci A, Marzullo P, Liuzzi A (2009) Sagittal abdominal diameter is more predictive of cardiovascular risk than abdominal fat compartments in severe obesity. Int J Obes (Lond) 33: 233–238. [DOI] [PubMed] [Google Scholar]
- 39. Hoenig MR (2010) MRI sagittal abdominal diameter is a stronger predictor of metabolic syndrome than visceral fat area or waist circumference in a high-risk vascular cohort. Vasc Health Risk Manag 6: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, et al. (2013) Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 21: E439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebbert JO, Jensen MD (2013) Fat depots, free fatty acids, and dyslipidemia. Nutrients 5: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, et al. (2010) The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 53: 882–889. [DOI] [PubMed] [Google Scholar]
- 43.National Center for Health Statistics (2010) NHANES I Epidemiologic Followup Study (NHEFS). Hyattsville, MD. Available: http://www.cdc.gov/nchs/nhanes/nhefs/nhefs.htm. Accessed 1 May 2014.
- 44.National Center for Health Statistics (2013) NCHS Data Linked to Mortality Files. Hyattsville, MD. Available: http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm. Accessed 1 May 2014.
- 45. Seidell JC, Andres R, Sorkin JD, Muller DC (1994) The sagittal waist diameter and mortality in men: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord 18: 61–67. [PubMed] [Google Scholar]
- 46. Empana JP, Ducimetiere P, Charles MA, Jouven X (2004) Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: the Paris Prospective Study I. Circulation. 110: 2781–2785. [DOI] [PubMed] [Google Scholar]
- 47. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752. [DOI] [PubMed] [Google Scholar]
- 48. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, et al. (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 49. Lenters-Westra E, Schindhelm RK, Bilo HJ, Slingerland RJ (2013) Haemoglobin A1c: Historical overview and current concepts. Diabetes Res Clin Pract 99: 75–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. As described under Acknowledgments, current NHANES survey data can be found at http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx.