Abstract
Adolescents living with human immunodeficiency virus (HIV) comprise approximately 12% of the HIV-positive population worldwide. HIV-positive adolescents experience a higher rate of clinical depression, a greater risk of sexual and drug abuse behaviors, and a decreased adherence to highly active antiretroviral therapies (HAART). Using adolescent HIV-1 transgenic rats (HIV-1 tg) that display related immune response alterations and pathologies, this study tested the hypothesis that developmental expression of HIV-1-related proteins induces a depressive-like phenotype that parallels a decrease in hippocampal cell proliferation and an increase in pro-inflammatory cytokine expression in the hippocampus. Consistent with this hypothesis, adolescent HIV-1 tg rats demonstrated a depressive-like behavioral phenotype, had decreased levels of cell proliferation, and exhibited elevated expression of monocyte chemotactic protein-1 (Mcp-1) in the hippocampus relative to controls. Subsequently, we tested the ability of meloxicam, a selective COX-2 inhibitor, to attenuate behavioral deficits via inflammatory mechanisms. Daily meloxicam treatments did not alter the behavioral profile despite effectively reducing hippocampal inflammatory gene expression. Together, these data support a biological basis for the co-morbid manifestation of depression in HIV-positive patients as early as in adolescence and suggest that modifications in behavior manifest independent of inflammatory activity in the hippocampus.
Introduction
Successful highly active anti-retroviral therapy (HAART) has led to the unexpected consequence of aviremic complications of human immunodeficiency virus (HIV). In addition to the high frequency of reported HIV-Associated Dementia [1], it is estimated that 30–50% of HIV-positive patients have a mood disorder such as depression, compared to 6.5% of HIV-negative individuals [2], [3]. Furthermore, adolescents living with HIV are at an even higher risk of developing a mood disorder than adults living with HIV, and adolescents in general experience a greater incidence of mental health problems, including high rates of depression [4]. Depression in HIV-positive adolescents can become life-threatening as these individuals exhibit decreased adherence to HAART and other treatment plans [4]–[6]. Depression has also been proposed to accelerate neurocognitive impairment in HIV-positive patients [7], which is substantially higher (67%) in perinatally infected young adults, even those on successful HAART, compared to older HIV-positive individuals (19%; [8]).
While the cause of the higher incidence of depression among HIV-positive adolescents is unknown, the stigma of being HIV-positive and the burden of lifelong medication have been proposed to account for the increased incidence of depression [9]. Despite this relationship, similar associations between external stimuli and depression have been proposed and refuted in cases of late-life depression and post-stroke depression, implicating primary changes in neuronal function [10]. We hypothesize that alterations in cerebral function may also contribute to the increased incidence of depression in HIV-positive patients. One approach that allows us to disassociate the psychosocial influences of HIV infection and the environment from the biological influences of HIV is the use of model animals.
The HIV-1 transgenic (tg) rat model is a non-replicating model of HIV infection that mimics the pathogenesis of HIV-infected patients under long-term HAART control with no detectable levels of viral replication. These rats can serve as a model of HAART-controlled HIV and are used extensively to assess the effects of HIV on various biological systems especially when the focus is on vertical transmission, as expression occurs from embryogenesis [11], [12]. HIV-1 tg rats contain a gag-pol-deleted HIV-1 provirus regulated by the viral long terminal repeat. As a result, no infectious virus particles are produced, but tissues express HIV-1 proteins gp120, tat, rev, and nef in lymphoid tissues with elevated concentrations in the spleen detectable by two months of age [13]. Spleen concentrations decrease with age as HIV protein concentrations increase in other tissues, including the brain [11], [13]. Viral gp120 is expressed in macrophages, B and T cells, and shed into the peripheral blood reaching concentrations of 100–200 pg/ml by the time rats develop AIDS-like pathology beyond 7 months of age [14]. The HIV-1 tg rat has proven useful for the study of neurocognitive deficits [15]–[18] and neuroimmune dysregulation [14], [19] in adult males and females [18].
While studying the underlying neurobiology of depression in the presence of HIV is important, understanding the mechanisms related to depression in females is particularly critical. The occurrence of depression and anxiety disorders in females is two times the rate in males during adulthood and as high as two and a half times the rate observed in males during adolescence [20]. Given the potential for vertical transmission by women who are not on successful HAART and the negative impact of depression on HAART compliance [21], understanding the mechanisms that mediate depression in HIV-positive adolescent females is of serious importance and has the potential for trans-generational impact. Although psychosocial factors may account for the increased incidence of depression in adolescent females living with HIV, the potential role of biological changes has not been assessed and therefore cannot be ruled out. Here, we use the HIV-1 tg rat to directly assess the hypothesis that HIV proteins can independently precipitate depressive-like behaviors in adolescent female rats. Specifically, we assess the impact of HIV proteins on two established mediators of depressive-like behaviors: cell proliferation and neuroinflammation. Furthermore, via the use of a commonly prescribed COX-2 preferential inhibitor, we determine the extent to which inflammation mediates deficits in affective behavior in the presence of HIV-1 related proteins.
Materials and Methods
Animals
Wild-type (WT; n = 9) and HIV-1 transgenic (HIV-1 tg; n = 12) rats were bred on site (HIV-1 tg breeder males obtained from Harlan Laboratories, Indianapolis, IN) and offspring were group housed with siblings after weaning. Female rats were used for all behavioral and histological assessments. No more than two pups per genotype per litter were used in each condition. Animals were kept in an AAALAC-approved temperature- and humidity-controlled facility and maintained on a 12∶12 light:dark cycle with lights on at 7 AM. Food and water were available ad libitum and rats were individually-housed beginning 24 hours prior to behavioral testing. Rat body mass was monitored throughout the duration of study and food consumption was recorded over a two day period. All experiments were approved and performed in accordance with the Institutional Animal Care and Use Committee of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The HIV-1 tg rat (as previously described in [13]) is hemizygous NLF-3Δgag/pol and contains functional deletions of gag in the 3′ region and pol in the 5′ region. HIV-1 tg rats manifest clinical signs and symptoms of HIV, including neurological changes, respiratory difficulty, and cataracts. Rats develop normally; however, by six months of age the rats experience poor weight gain and muscle atrophy that continues over time. The studies conducted here were completed well before 6 months of age and prior to the onset of the documented pathologies. Because these rats are hemizygous, WT littermates can be used as controls. An equal number of pups from each litter were included in all groups. Our examinations of developmental behavior and cell proliferation began at post-natal day (PND) 48 and were completed by PND 58.
Behavioral Testing
Each genotype was assessed using an extensive behavioral phenotyping schedule which included primary, secondary, and tertiary indices of behavior (adapted from Urbach et al. [22]). Primary behaviors were comprised of assessments of general health (appearance and weight), neurological reflexes, neuromuscular strength, and sensory function. Secondary behaviors included motor function and social behavior. Tertiary behavioral paradigms examined depressive- and anxiety-like behaviors. All behavioral observations took place during the animals’ light cycle and were performed in order of increasing anxiogenesis: primary behaviors (Day 1), sucrose consumption test (Days 1–3), open field paradigm (Day 4), social interaction (Day 5), and the Porsolt forced swim test (Day 6). All rats were tested in a room separate from other animals. Behavioral tests and scoring rubric were modified from Korenova et al [23].
Primary Behaviors
Surface righting: Rats were placed in a supine position and the latency to correct itself was measured (Latency <1 second = 0 points; latency ≥1 second = 1 point). Prehensile traction: Rats were allowed to grasp onto a wire cage lid at which point the lid is slowly inverted and the latency to fall was measured (latency >15.1 seconds = 0 points, 10.1–15 seconds = 1 point; 6.1–10 seconds = 2 points; 4.1–6.0 seconds = 3 points; 2.1–4.0 seconds = 4 points; <2 seconds = 5 points). Balance: Rats were placed in an empty cage that is rapidly moved from side to side and up and down. Normal posture is to extend all four legs and maintain an upright and balanced position (normal = 0, abnormal = 1). Audition: Two metal rods were tapped and startle response was observed (startle = 0, no startle = 1). Points for each behavior were averaged by group and compared.
Secondary Behaviors
Overall activity: Distance traveled and velocity were measured over 10 minutes in an open field arena measuring 75 cm×75 cm and scored by Cleversys automated tracking system (Reston, VA). Social interaction: The test began with the placement of the experimental rat in the center of the arena. Each rat was allowed to explore a 75 cm×75 cm arena containing a same sex and age matched stimulus rat. Latency to first interaction as well as total time interacting were measured [24].
Tertiary Behaviors
Sucrose consumption: Rats were given equal access to bottles containing a 0.9% sucrose solution or tap water. Following a 24-hour habituation, we assessed sucrose consumption by recording the volume (extrapolated from grams) of solution consumed over a 24 hour period. Presentation of bottles was reversed after the first 24 hours to prevent a side bias. A decrease in sucrose consumption relative to control rats is used as an index of anhedonic-like behaviors [25]. Open field: Rats were allowed to explore a 75 cm×75 cm arena for 10 minutes. Time spent in the center of the arena versus the periphery was used as a measure of anxiety-like behavior [26]. The test began on Day 4 with the placement of the experimental rat in the center of the arena. Behavior was videotaped and scored by Cleversys automated behavioral software. Porsolt forced swim: Rats were placed in a swim tank for 10 minutes during which time the amount of time spent struggling (defined as breaking the surface of the water with the forepaws) and time spent immobile (defined as limbs stationary for at least 2 seconds) was assessed as an index of depressive-like behaviors [27]. Latency to first bout of immobility was also recorded. As with open field, behavior was videotaped and scored automatically.
Cell Proliferation
Twenty-four hours following the last behavioral test, all rats were anesthetized and transcardially perfused with 4% paraformaldehyde. Brains were removed and stored in 4% paraformaldehyde at 4°C for at least 24 hours prior to sectioning. Brains were hemisected and one hemisphere was vibratome sectioned at 40 µm throughout the entire rostrocaudal extent of the dentate gyrus. Every 12th serial section was mounted on clear Superfrost Plus Microscope Slides (Fisher Brand Scientific, Pittsburgh, PA) and allowed to dry overnight. Slides were then incubated in a 0.1 mol/L citric acid for 40 minutes at 90°C. After a 10 minute incubation in a 3% H2O2 solution, slides were rinsed with phosphate buffered saline (PBS, pH 7.4) and incubated at room temperature overnight in primary (1∶100; mouse monoclonal anti-Ki67 [Vector Laboratories, Burlingame, CA] in 0.3% Triton X-100 and 2% normal horse serum in PBS). Twenty-four hours later, slides were rinsed in PBS before entering secondary incubation (1∶200; biotinylated horse anti-mouse, Vector Laboratories) for 1 hour. Slides were again rinsed in PBS and then reacted with a mouse Vectastain ABC Elite kit (Vector Laboratories) solution for 30 minutes according to manufacturer’s instructions (Vector Laboratories) followed by a 10 minute 0.01% diaminobenzidine with 0.003% H2O2 (Sigma-Aldrich, St. Louis, MO) incubation. Slides were then rinsed in PBS, dried, counterstained with cresyl violet, cleared in xylene, and coverslipped with Permount (Fisher Brand Scientific).
Gene Expression
A separate cohort of rats raised under the same conditions (WT n = 10; HIV-1 tg n = 9), was used for gene expression analysis. Animals were rapidly decapitated, brain tissue was dissected frozen under RNAse-free conditions, and the hippocampus was isolated for analysis. Samples were then homogenized using the Qiagen RNeasy Mini Kit (Valencia, CA) according to manufacturer’s instructions. RNA samples were reverse transcribed using Applied Biosystem’s High Capacity cDNA Reverse Transcription Kit. Resulting cDNA was then quantified and normalized using the PicoGreen method (Invitrogen, Grand Island, NY). The TaqMan gene expression system was used for the detection of monocyte chemotactic protein-1 (Mcp-1; Rn00580555_m1), interleukin-1β (Il-1β; Rn00580432_m1), nuclear factor-kappa-β inhibitor, α (Nf-κBiα; RN01473654_g1), tumor necrosis factor (Tnf; Rn00562055_m1), and standardized to β-actin (Rn00667869_m1). All samples were prepared in triplicate using 1 µg of sample and carried out on an Applied Biosystems HT7900 Fast Real-Time PCR system. Data were averaged by group and analyzed via the ΔΔCt method.
Meloxicam Administration
A third cohort of animals (WT n = 5; HIV-1 tg n = 9) received oral meloxicam (1 mg/kg) daily starting at weaning (PND 25) and continuing throughout the duration of the study. Behavioral testing and hippocampal gene expression of Mcp-1 were measured as described above. Because several studies have demonstrated that daily administration of COX-2 inhibitors does not augment cell proliferation [28]–[30], expression of Ki-67 was not assessed in these animals.
Data Analysis
For all analyses, data were averaged by genotype and compared via one-tailed unpaired t-tests. Statistical outliers were identified by the Grubbs’ outlier test and removed. Group sizes varied slightly due to the presence of statistical outliers as well as errors with video recording and/or scoring software.
For secondary and tertiary behaviors, group totals were averaged by genotype and are presented as percent of control. Rt-PCR results were averaged by genotype and analyzed via the 2−ΔΔCt method. Specifically, fold change was calculated, standardized to a housekeeping gene (β-actin), and normalized to WT or vehicle treated animals.
For quantification of Ki67, slides were coded prior to data collection. The code was broken after analyses were completed. Ki67-labeled cells in the granule cell layer, subgranular zone, and hilus of the hippocampal dentate gyrus were exhaustively counted at 100× on a Zeiss Primo Star light microscope (Thornwood, NY). One hemisphere of each brain was analyzed and counts were multiplied by 24 (to account for opposite hemisphere and sampling fraction) to obtain estimates of Ki67-labeled cells in the dentate gyrus per brain.
All statistical tests were performed using GraphPad Prism 6, and differences were considered statistically significant if p<0.05.
Results
HIV-1 tg rats have minimal differences in general health and primary behaviors
Body mass differed between HIV-1 tg rats and WT litter-mate controls such that transgenic rats weighed less than controls (t[15] = 7.12, p<0.05); however, total food consumed over a two-day period did not differ between WT and HIV-1 tg rats (p>0.05; Table 1). Nominal scale results reveal few errors in HIV-1 tg rats’ balance and no detected errors in surface righting or audition. Further, indices of grip strength (prehensile tension) were normal, suggesting behavioral effects were not due to signs of early muscle wasting (summarized in Table 1).
Table 1. Summary and mean scores of primary behaviors.
| Body Mass (g) | Food Consumption (g) | Balance (0–1) | Surface Righting (0–1) | Audition (0–1) | Prehensile Traction (0–5) | |
| WT | 120.0±4.163 | 10.0±1.472 | 0 | 0 | 0 | 0 |
| HIV-1 tg | 90.4±1.945* | 8.25±0.250 | 0.2 | 0 | 0 | 0 |
Wild-type (WT) and HIV-1 transgenic (HIV-1 tg) rats were assessed using an extensive behavioral phenotyping schedule of primary, secondary, and tertiary behaviors. Mean body mass and food consumption are shown for adolescent WT and HIV-1 tg rats. Mean scores of neurologic tests, muscular strength, and sensory function are shown. Compared to WT controls, HIV-1 tg rats had decreased body mass at post-natal day 48, or the first day of behavioral testing (*p<0.05). Despite this, no differences were detected in any other measure.
HIV-1 tg adolescent rats exhibit elevated levels of depressive-like behaviors
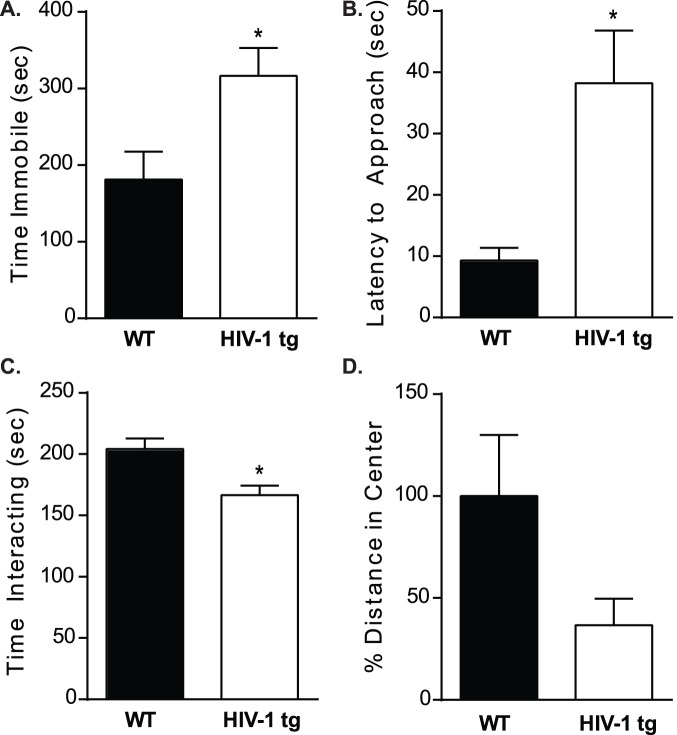
HIV-1 tg rats had similar latencies to first bout of floating as WT littermates (p>0.05); however, HIV-1 tg rats spent more time immobile than WT controls (t[12] = 2.420, p<0.05; Figure 1A). In the social interaction test, HIV-1 tg rats exhibited an increased latency to interact with a novel animal (t[13] = 2.682, p<0.05; Figure 1B). Furthermore, HIV-1 tg rats spent less time interacting with the novel stimulus rat as compared WT controls (t[16] = 3.237, p<0.05; Figure 1C).
Figure 1. Rats were assessed in a battery of behavioral tests of depressive- and anxiety-like behaviors.
A,B) In the forced swim test, latency to first immobile bout was not different between WT and HIV-1 tg rats (p>0.05); but HIV-1 tg rats spent significantly more time immobile in the test relative to WT controls. To measure social interaction, WT and HIV-1 tg rats were presented with an age-matched stimulus conspecific and allowed to interact for 10 minutes. C,D) HIV-1 tg rats had a greater latency to approach the stimulus animal (*p<0.05) and spent less total time interacting, relative to WT controls. The open field paradigm was used to assess anxiety-like behaviors over a 10 minute period. Total distance traveled as well as distance traveled in the center was recorded. E) Total activity was unchanged between WT and HIV-1 tg rats as measured in 75 cm×75 cm arena during the open field test (p>0.05). F) Compared to WT controls, HIV-1 tg rats showed decreased activity in the center of the open field suggestive of increased anxiety-like behavior. For all, *p<0.05 and data are presented as mean ± SEM.
Despite a depressive-like phenotype in the Porsolt forced swim and social interaction tests, when corrected for body mass, WT and HIV-1 tg rats consumed similar volumes of sucrose over a 24-hour test period in the sucrose consumption test (WT: 0.182±0.021 g of sucrose/g of animal; HIV-1 tg: 0.196±0.009 g of sucrose/g of animal; p>0.05).
HIV-1 tg rats exhibit normal activity but a trend towards increased anxiety-like behavior
Total distance traveled and central tendency were measured during the 10 minute open field test. WT and HIV-1 tg adolescent rats had similar levels of activity (WT: 34.16±6.81 m; HIV-1 tg: 37.21±3.17 m); however, HIV-1 tg rats exhibit a trend towards decreased activity in the center of the open field when compared to litter-mate controls (t[14] = 2.062, p = 0.07; Figure 1D).
HIV-1 tg rats have decreased levels of hippocampal cell proliferation
Ki67 reactivity in the dentate gyrus was used as an index of cell proliferation. Data reveal that HIV-1 tg rats had significantly fewer Ki67-positively stained cells relative to WT controls (t[18] = 3.728, p<0.05; Figure 2A).
Figure 2. WT and HIV-1 tg brains were sectioned and stained for Ki-67 reactivity as a measure of cell proliferation.
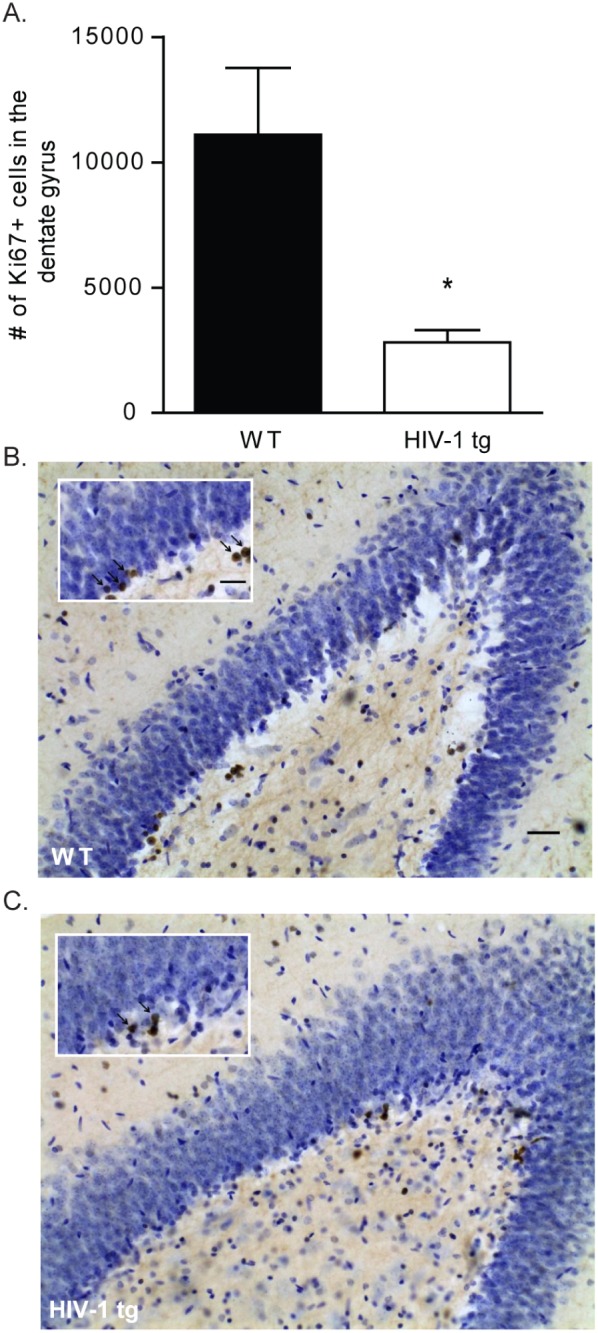
(A) HIV-1 tg brains had significantly decreased levels of cell proliferation in the dentate gyrus of the hippocampus as compared to WT brains (*p<0.05). Data are presented as mean ± SEM. (B) Photomicrograph of hemisection containing Ki-67 stained cells in the dentate gyrus of a WT rat. Inset highlights dense region of Ki-67 positive cells, marked with arrows. (C) Photomicrograph of Ki-67 stained cells in a HIV-1 tg rat. Again, insert highlights region of Ki-67 stained cells marked with arrows. Images captured at 20x, scale bar equals 50 µM.
HIV-1 tg rats display increased Mcp-1 gene expression in the hippocampus
Post-mortem assessment of gene expression for inflammatory markers indicated that hippocampal expression of Mcp-1 was significantly increased in HIV-1 tg rats as compared to WT controls (t[15] = 2.015, p<0.05; Figure 3A). No group differences were detected in gene expression levels of Il-1β (fold change: 0.968±0.144), NF-κBia (fold change: 1.475±0.271), and Tnf (fold change: 1.007±0.353) in the hippocampus (p>0.05; data not shown).
Figure 3. The effect of HIV related proteins on hippocampal inflammation was measured in adolescent WT and HIV-1 tg rats.
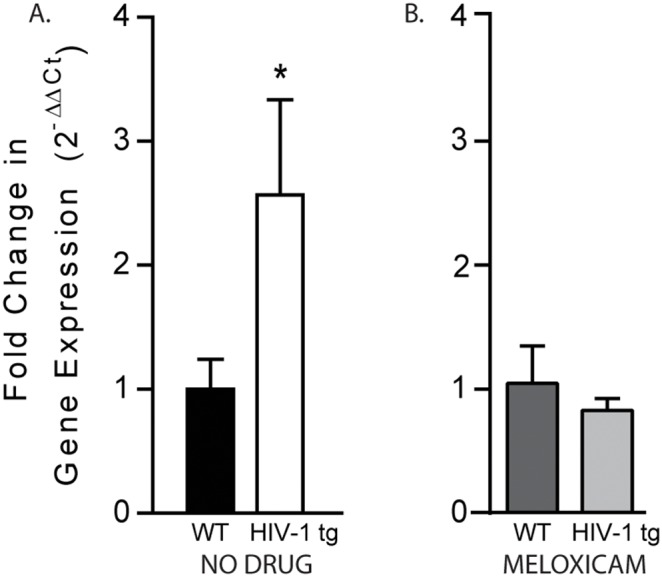
A) Analysis of hippocampal gene expression of inflammatory markers showed increased expression of the potent chemokine, Mcp-1, in non-drug treated HIV-1 tg rats compared to WT controls (*p<0.05). B) In contrast, once daily meloxicam administration attenuated Mcp-1 expression in HIV-1 tg rats, normalizing inflammatory gene expression to WT levels (p>0.05). Data are presented as mean ± SEM.
Meloxicam treatment reduces Mcp-1, but does not rescue behavior
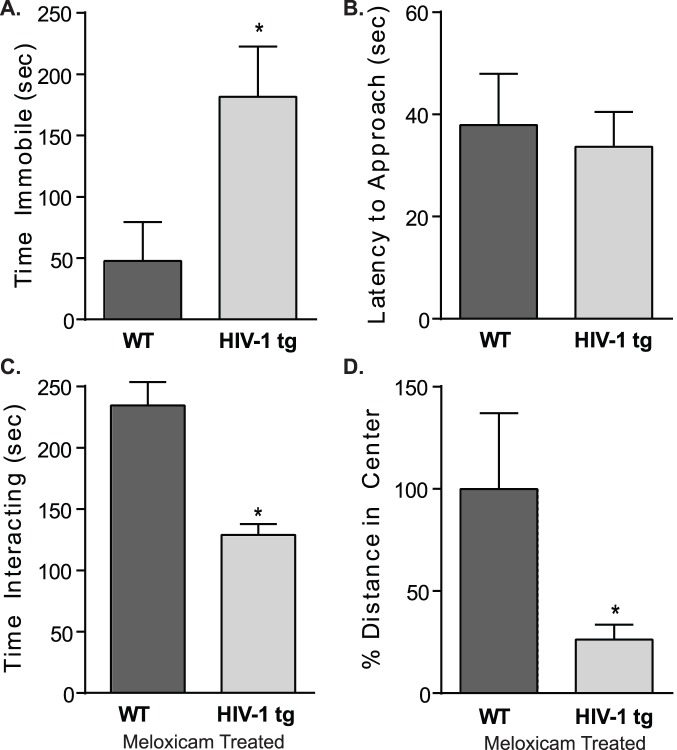
Importantly, meloxicam administration attenuated expression of Mcp-1 in the hippocampus of female adolescent HIV-1 tg rats. A t-test of Mcp-1 gene expression revealed no difference between HIV-1 tg rats given meloxicam as compared to WT meloxicam-treated rats (t[15] = 0.718, p>0.05; Figure 3B). Once daily meloxicam treatment did not alter body mass in WT (126.0±3.567 g) or HIV-1 tg (103.5±2.678 g) rats.
Conversely, daily meloxicam administration did not attenuate the behavioral deficits in the HIV-1 tg rats. Latencies to first float were comparable between WT and HIV-1 tg rats in the forced swim test (WT: 108.4±55.56 sec; HIV-1 tg: 172.1±53.03 sec); however, overall, HIV-1 tg rats spent more time immobile when compared to WT meloxicam-treated rats (t[12] = 2.207, p<0.05; Figure 4A). WT and HIV-1 tg rats had similar latencies to approach a novel conspecific (Figure 4B); but, HIV-1 tg rats spent less overall time interacting compared to meloxicam-treated WT controls (t[12] = 2.564, p<0.05; Figure 4C).
Figure 4. Depressive- and anxiety-like behaviors were measured in WT and HIV-1 tg rats receiving daily oral dosing of meloxicam.
A,B) In the Porsolt forced swim test, meloxicam treated WT and HIV-1 tg rats showed similar latencies to first float (p>0.05); however, HIV-1 tg rats spent more time immobile compared to WT controls (*p<0.05). C,D) Similarly, in the social interaction test, meloxicam treated WT and HIV-1 tg rats showed no differences in their time to approach a stimulus rat (p>0.05); however, overall, meloxicam-treated HIV-1 tg rats spend less time interacting (*p<0.05). E,F) In the open field test, meloxicam treated HIV-1 tg rats showed no differences in overall locomotion (p>0.05), but did show a significant decrease in distance traveled in the center of the open field, as compared to WT rats (*p<0.05). Data are presented as mean ± SEM.
In terms of anxiety-like behaviors, total distance traveled did not statistically differ between WT and HIV-1 tg meloxicam-treated rats (WT: 26.15±5.39 m; HIV-1 tg: 15.77±2.99 m; p>0.05); however, meloxicam-treated rats traveled less in the center of the open field compared to WT controls (t[11] = 2.859, p<0.05; Figure 4D).
Discussion
Expression of HIV-1 proteins triggered depressive-like and anxiety-like behaviors in adolescent female rats and reduced cell proliferation in the dentate gyrus of the hippocampus, concomitant with increased expression of Mcp-1. Given that Mcp-1 is increased in the hippocampi of HIV-1 tg adolescent female rats, and given the potential role of neuroinflammation in altered affective-like behavior, we tested the hypothesis that affective-like behavior was driven by inflammatory mechanisms. Meloxicam, frequently prescribed for osteoarthritis, was selected to block inflammation in the current set of experiments due to its highly potent inhibition of prostaglandin synthetase and low level of gastrointestinal toxicity as demonstrated in both humans and model animal systems [31], [32]. While inhibition of Mcp-1 in the hippocampus of HIV-1 tg rats by oral meloxicam treatment does not attenuate the presence or degree of behavioral disruption, future work is necessary to determine if there is a functional link between the observed decreased basal rate of cell proliferation and the increased depressive- and anxiety-like behavior in the HIV-1 tg rat. At present, we can conclude that expression of HIV-1 proteins during development is sufficient, in the absence of stress or other psychosocial and environmental factors, to precipitate increased depressive-like and anxiety-like behaviors in adolescent females. This finding is important because it draws attention to a potential neurobiological basis for the increased incidence of depression in HIV-positive individuals. Furthermore, the HIV-1 tg rat may provide a model system in which novel antidepressant therapies can be assessed.
Although rodents are incapable of experiencing depression to the same extent as humans, core symptoms of the disease can be manipulated and assessed in a rodent model. Social withdrawal is a hallmark symptom of clinical depression and is an ethologically relevant metric of depressive-like behaviors in rodent models [33], [34]. When presented with a stimulus animal in the social interaction task, HIV-1 tg rats exhibit a greater latency to approach a novel conspecific, in addition to a decrease in the total time interacting with the novel conspecific. Importantly, this behavior is not confounded by an overall deficit of motor ability or activity. Furthermore, this social withdrawal is consistent with performance in the Porsolt forced swim test, the most widely used paradigm of depressive-like behaviors [35], in which HIV-1 tg rats spent more time immobile compared to WT controls. The same pattern is apparent for rats receiving daily meloxicam administration. However, it may be important to note that meloxicam-treated rats show an insignificant decrease in overall activity, suggestive of an interaction between drug treatment and locomotor ability, despite no change in body mass compared to non-treated animals and reports that meloxicam is well tolerated by rodents [36]. Finally, depressive behaviors in the social interaction test and Porsolt forced swim paradigm were apparent in the absence of altered sucrose consumption, which may be due to differences in metabolic rates between WT and HIV-1 tg rats. When corrected for weight, HIV-1 tg rats consumed similar volumes of a sucrose solution compared to WT rats and similar amounts of chow in the home cage, despite a more than 30 g difference in body weight between WT and HIV-1 tg rats.
In adulthood, HIV-1 tg rats develop pathology consistent with human AIDS [13]; however, in the current study, increases in depressive- and anxiety-like behavior are detectable before such pervasive alterations in general health and mobility occur. Our testing began on PND 48, and at this time, neurological reflexes, neuromuscular strength, and sensory function were indistinguishable from WT litter-mate controls. Therefore, these data support the sufficiency of HIV-1 related proteins, in the absence of additional stressors or active virus replication, to precipitate deficits in affective-like behavior.
Increased peripheral circulating inflammatory biomarkers are well established in major depressive disorders as well as other disease states [37], [38]. Similarly, given that HIV in humans is marked by a pronounced inflammatory response in the brain and periphery that is believed to contribute to the neuropathology of the disease [39], [40] and HIV-associated dementias [41], we examined inflammatory activity in the hippocampus as a potential mechanism driving behavior. Measures of inflammatory cytokines in the hippocampi of adolescent HIV-1 tg rats reveals increased expression of Mcp-1, a pro-inflammatory biomarker produced by macrophages and endothelial cells that is elevated in cases of major depression [42]–[44]. However, no differences were observed in the expression of Il-1β, Nf-κBia, or Tnf in the hippocampus. Despite the involvement of proinflammatory cytokines in HIV-1 related pathology, depressive disorders, [42], [43], [45] and decreased cell proliferation [30], [46], daily administration of meloxicam, a selective COX-2 inhibitor, had no effect on affective behavior notwithstanding the attenuation of increased expression of Mcp-1 in the hippocampus. These findings suggest that the manifestation of altered affective-like behaviors in the presence of HIV-1 related proteins does not stem from inflammatory mechanisms and that dysregulation of other systems may play a larger role in the HIV-1 related behavioral phenotype. Previous groups have associated inflammatory activity in the presence of HIV-1 proteins, either in adult whole brain lysate immediately following tat administration [47] or in frontal cortex brain lysate of the adult male tg rat [48]. With this said, inflammatory activation in adolescent HIV-1 tg rats appears to be limited to the expression of Mcp-1 and rampant HIV-related inflammation may not be apparent at this early stage. As such, Mcp-1 may serve as an early indicator of HIV-1 associated disease progression independent of behavior. Alternatively, it has been hypothesized that inflammatory activation in HIV rodent models is due to the neurotoxic effects of gp120 and tat on neurons and astrocytes and is not a result of HIV-1 related pathology [48]. However, due to the limited inflammatory response observed in adolescent HIV-1 tg rats and sustained altered affective-like behavior in the absence of Mcp-1 activation, it is unlikely that inflammation drives behavioral change in the HIV-1 tg rat.
Consistent with the manifestation of depressive-like behaviors observed in adolescent HIV-1 tg rats, the current study demonstrates that developmental expression of HIV-1 related proteins results in decreased levels of cell proliferation in the dentate gyrus of the hippocampus. Ki67 is present in almost all phases of the cell cycle, with the exception of G0, making it a suitable marker for proliferating progenitor cells [49], a precursor for neurogenesis in regions of the hippocampus [50]. Decreases in cell proliferation, as indicated by Ki67, have been recorded in other instances of depressive-like states in rodents, including in dams who have experienced repeated separation from their pups [50] and in rats who have experienced chronic stress [51]. Furthermore, morphologic and volumetric differences observed in the hippocampi of patients with major depressive disorders [52]–[54] are paralleled by a decrease of adult hippocampal neurogenesis [55]. The neurogenesis hypothesis of major depression suggests that decreases in cell proliferation may underlie structural alterations within the adult brain, an effect that has been reversed by the administration of classic anti-depressant treatments in the dentate gyrus of the human, rodent, and non-human primate brain [56], [57].
The rates of clinical depression and depressive symptomatology among the adult HIV-positive population are higher than observed in the general population [58] and adolescents living with HIV-1 are at a higher risk of psychiatric illness and risk-taking behaviors [59]. We show here that the developmental expression of HIV-1 related proteins is sufficient to induce depressive-like behaviors in adolescent rats, cause a decrease in cellular proliferation in the dentate gyrus of the hippocampus, and lead to increased expression of hippocampal Mcp-1. Further, blockade of Mcp-1 in the adolescent female HIV-1 tg rat fails to attenuate the presence or degree of depressive-like behavioral disruption. These findings are important because they demonstrate that depressive-like behaviors, decreased cell proliferation, and increased neuroinflammation can be induced by the presence of HIV proteins in the absence of active virus and that behavioral despair is not driven by Mcp-1 activation. These data suggest that HIV-associated depression may have a neurobiological basis that is separate from either the psychosocial impact of the disease or the rampant neuroinflammation characteristic of neuroAIDS.
Acknowledgments
The authors would also like to thank Sean D. Kelly and Leah Chisholm for their assistance with this work.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors would like to acknowledge the financial support of The Department of Physiology, Emory University, Atlanta, GA. Additionally, this research was supported by the Creative and Novel Ideas in HIV Research Program (CNIHR) through a supplement to the University of Alabama at Birmingham (UAB) Center For AIDS Research funding (P30 AI027767-24). This funding was made possible by collaborative efforts of the Office of AIDS Research, the National Institutes of Allergies and Infectious Diseases, and the International AIDS Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Simioni S, Cavassini M, Annoni J-M, Rimbault A, Bourquin I, et al. (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS: 1243–1250. 10.1097/QAD.0b013e3283354a7b [DOI] [PubMed] [Google Scholar]
- 2. Hinkin C, Castellon S, Durvasula R, Hardy D, Lam M, et al. (2002) Medication adherence among HIV+ adults. Neurology 59: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopes M (2012) Gender, HIV Status, and Psychiatric Disorders: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. Psychiatry Interpers Biol Process: 384–391. 10.4088/JCP.10m06304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams PL, Leister E, Chernoff M, Nachman S, Morse E, et al. (2010) Substance use and its association with psychiatric symptoms in perinatally HIV-infected and HIV-affected adolescents. AIDS Behav 14: 1072–1082 10.1007/s10461-010-9782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellins CA, Tassiopoulos K, Malee K, Moscicki A-B, Patton D, et al. (2014) Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDS 25: 413–422 10.1089/apc.2011.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, et al. (2009) Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav 13 Suppl 182–91 10.1007/s10461-009-9546-x [DOI] [PubMed] [Google Scholar]
- 7. Bragança M, Palha A (2011) Depression and neurocognitive performance in Portuguese patients infected with HIV. AIDS Behav 15: 1879–1887 10.1007/s10461-011-9973-3 [DOI] [PubMed] [Google Scholar]
- 8. Paramesparan Y, Garvey L, Ashby J, Foster C, Fidler S, et al. (2010) High Rates of Asymptomatic Neurocogntivie Impairment in Vertically Acquired HIV-1-Infected Adolescents Survivng to Adulthood. J Acquir Immune Defic Syndr 55: 132–136. [DOI] [PubMed] [Google Scholar]
- 9. Chaudoir SR, Norton WE, Earnshaw VA, Moneyham L, Mugavero MJ, et al. (2011) Coping with HIV Stigma: Do Proactive Coping and Spiritual Peace Buffer the Effect of Stigma on Depression? AIDS Behav 16: 2382–2391 10.1007/s10461-011-0039-3 [DOI] [PubMed] [Google Scholar]
- 10. Neigh GN, Karelina K, Zhang N, Glasper ER, Owens MJ, et al. (2009) Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J Cereb Blood Flow Metab 29: 1673–1682 10.1038/jcbfm.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng J, Vigorito M, Liu X, Zhou D, Wu X, et al. (2010) The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol 218: 94–101 10.1016/j.jneuroim.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 12. Ray P, Liu X-H, Robinson L, Reid W, Xu L, et al. (2003) A novel HIV-1 transgenic rat model of childhood HIV-1– associated nephropathy. Kidney Int 63: 2242–2253. [DOI] [PubMed] [Google Scholar]
- 13. Reid W, Sadowska M, Denaro F, Rao S, Foulke J, et al. (2001) An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. P Natl Acad Sci USA 98: 9271–9276 10.1073/pnas.161290298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reid W, Abdelwahab S, Sadowska M, Huso D, Neal A, et al. (2004) HIV-1 transgenic rats develop T cell abnormalities. Virology 321: 111–119 10.1016/j.virol.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 15. Vigorito M, LaShomb AL, Chang SL (2007) Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol 2: 319–328 10.1007/s11481-007-9078-y [DOI] [PubMed] [Google Scholar]
- 16. Lashomb AL, Vigorito M, Chang SL (2009) Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol 15: 14–24 10.1080/13550280802232996 [DOI] [PubMed] [Google Scholar]
- 17. Moran L, Aksenov M, Booze R, Webb K, Mactutus C (2013) Adolescent HIV-1 transgenic rats: Evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res 10: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moran LM, Booze RM, Webb KM, Mactutus CF (2013) Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol 239: 139–147 10.1016/j.expneurol.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao JS, Kim H-W, Kellom M, Greenstein D, Chen M, et al. (2011) Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflamm 8: 101 10.1186/1742-2094-8-101 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.National Survey on Drug Use and Health (2012) Depression triples between the ages of 12 and 15 among adolescent girls.
- 21. Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, et al. (2010) Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living study. J Acquir Immune Defic Syndr 53: 266–272 10.1097/QAI.0b013e3181b720e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbach YK, Bode FJ, Nguyen HP, Riess O, Hörsten S Von (2010) Rat Genomics. Methods 597. doi:10.1007/978-1-60327-389-3. [DOI] [PubMed]
- 23. Korenova M, Zilka N, Stozicka Z, Bugos O, Vanicky I, et al. (2009) NeuroScale, the battery of behavioral tests with novel scoring system for phenotyping of transgenic rat model of tauopathy. J Neurosci Methods 177: 108–114 10.1016/j.jneumeth.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 24. File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364. [DOI] [PubMed] [Google Scholar]
- 26. Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463: 3–33 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- 27. Porsolt R, Anton G, Blavet N, Jalfre M (1978) Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391. [DOI] [PubMed] [Google Scholar]
- 28. Goncalves MB, Williams E-J, Yip P, Yáñez-Muñoz RJ, Williams G, et al. (2010) The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br J Pharmacol 159: 1118–1125 10.1111/j.1476-5381.2009.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung K-H, Chu K, Lee S-T, Kim J, Sinn D-I, et al. (2006) Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23: 237–246 10.1016/j.nbd.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 30. Russo I, Amornphimoltham P, Weigert R, Barlati S, Bosetti F (2011) Cyclooxygenase-1 is involved in the inhibition of hippocampal neurogenesis after lipopolysaccharide-induced neuroinflammation. Cell Cycle 10: 2568–2573 10.4161/cc.10.15.15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmed M, Khanna D, Furst DE (2005) Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol 1: 739–751 10.1517/17425255.1.4.739 [DOI] [PubMed] [Google Scholar]
- 32. Engelhardt G (1996) Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol 35 Suppl 14–12. [DOI] [PubMed] [Google Scholar]
- 33. Fischer CW, Liebenberg N, Elfving B, Lund S, Wegener G (2012) Isolation-induced behavioural changes in a genetic animal model of depression. Behav Brain Res 230: 85–91 10.1016/j.bbr.2012.01.050 [DOI] [PubMed] [Google Scholar]
- 34. Derntl B, Seidel E-M, Eickhoff SB, Kellermann T, Gur RC, et al. (2011) Neural correlates of social approach and withdrawal in patients with major depression. Soc Neurosci 6: 482–501 10.1080/17470919.2011.579800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121: 66–72. [DOI] [PubMed] [Google Scholar]
- 36. Bourque SL, Adams MA, Nakatsu K, Winterborn A (2010) Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49: 617–622. [PMC free article] [PubMed] [Google Scholar]
- 37. Miller AH (2010) Depression and immunity: a role for T cells? Brain Behav Immun 24: 1–8 10.1016/j.bbi.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4: 47 10.1186/1750-1326-4-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zanin V, Delbue S, Marcuzzi A, Tavazzi E, Del Savio R, et al. (2012) Specific protein profile in cerebrospinal fluid from HIV-1-positive cART-treated patients affected by neurological disorders. J Neurovirol 18: 416–422 10.1007/s13365-012-0109-y [DOI] [PubMed] [Google Scholar]
- 40. Merrill JE, Chen ISY (1991) HIV-1, macrophages, glial cells and cytokines in AIDS nervous system disease. FASEB J 5: 2391–2397. [DOI] [PubMed] [Google Scholar]
- 41. Kipnis J, Derecki NC, Yang C, Scrable H (2008) Immunity and cognition: what do age-related dementia, HIV-dementia and “chemo-brain” have in common? Trends Immunol 29: 455–463 10.1016/j.it.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 42. Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, et al. (2009) Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry 10: 313–323 10.3109/15622970802573246 [DOI] [PubMed] [Google Scholar]
- 43. Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suarez EC (2003) The Relation of Severity of Depressive Symptoms to Monocyte-Associated Proinflammatory Cytokines and Chemokines in Apparently Healthy Men. Psychosom Med 65: 362–368 10.1097/01.PSY.0000035719.79068.2B [DOI] [PubMed] [Google Scholar]
- 45.Brabers NACH, Nottet HSLM (2006) Role of the pro-inflammatory cytokines TNF- α and IL-1 β in HIV-associated dementia. Eur J Clin Invest: 447–458. [DOI] [PubMed]
- 46. Taupin P (2008) Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci 5: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lawson MA, Kelley KW, Dantzer R (2011) Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: A possible mechanism for AIDS comorbid depression. Brain Behav Immun 25: 1569–1575 10.1016/j.bbi.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Royal W, Zhang L, Guo M, Jones O, Davis H, et al. (2012) Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol 247: 16–24 10.1016/j.jneuroim.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scholzen T, Gerdes J (2000) The Ki-67 protein: From the known and the unknown. J Cell Phys 182: 311–322. [DOI] [PubMed] [Google Scholar]
- 50. Sung Y-H, Shin M-S, Cho S, Baik H-H, Jin B-K, et al. (2010) Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett 470: 86–90 10.1016/j.neulet.2009.12.063 [DOI] [PubMed] [Google Scholar]
- 51. Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ (2004) Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci 19: 131–144 10.1046/j.1460-9568.2003.03100.x [DOI] [PubMed] [Google Scholar]
- 52. Campbell S, Macqueen G (2004) The role of the hippocampus in the pathophysiology of major depression. J Psych Neurosci 29: 417–426. [PMC free article] [PubMed] [Google Scholar]
- 53. Sheline YI, Gado MH, Kraemer HC (2004) Untreated depression and hippocampal volume loss. Am J Psychiatry 161: 1309–1310. [DOI] [PubMed] [Google Scholar]
- 54. Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, et al. (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psych 56: 640–650 10.1016/j.biopsych.2004.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kempermann G, Kronenberg G (2003) Depressed new Neurons?–Adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psych 54: 499–503 10.1016/S0006-3223(03)00319-6 [DOI] [PubMed] [Google Scholar]
- 56. Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, et al. (2009) Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34: 2376–2389 10.1038/npp.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duman RS, Nakagawa S, Malberg J (2001) Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25: 836–844 10.1016/S0893-133X(01)00358-X [DOI] [PubMed] [Google Scholar]
- 58. Walkup J, Wei W, Sambamoorthi U, Crystal S (2008) Antidepressant treatment and adherence to combination antiretroviral therapies among patients with AIDS and diagnosed depression. Psychiatr Q 79: 43–53 10.1007/s11126-007-9055-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koenig LJ, Nesheim S, Abramowitz S (2011) Adolescents with perinatally acquired HIV: emerging behavioral and health needs for long-term survivors. Curr Opin Obstet Gynecol 23: 321–327 10.1097/GCO.0b013e32834a581b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.