Abstract
CGL (Congenital generalized lipodystrophy) is a genetic disorder characterized by near complete loss of adipose tissue along with increased ectopic fat storage in other organs including liver and muscle. Of the four CGL types, BSCL2 (Berardinelli–Seip Congenital lipodystrophy type 2), resulting from mutations in the BSCL2/seipin gene, exhibits the most severe lipodystrophic phenotype with loss of both metabolic and mechanical adipose depots. The majority of Seipin mutations cause C-terminal truncations, along with a handful of point mutations. Seipin localizes to the ER and is composed of a conserved region including a luminal loop and two transmembrane domains, plus cytosolic N- and C-termini. Animal models deficient in seipin recapitulate the human lipodystrophic phenotype. Cells isolated from seipin knockout mouse models also exhibit impaired adipogenesis. Mechanistically, seipin appears to function as a scaffolding protein to bring together interacting partners essential for lipid metabolism and LD (lipid droplet) formation during adipocyte development. Moreover, cell line and genetic studies indicate that seipin functions in a cell-autonomous manner. Here we will provide a brief overview of the genetic association of the CGLs, and focus on the current understanding of differential contributions of distinct seipin domains to lipid storage and adipogenesis. We will also discuss the roles of seipin-interacting partners, including lipin 1 and 14-3-3β, in mediating seipin-dependent regulation of cellular pathways such as actin cytoskeletal remodelling.
Keywords: adipocyte, lipid droplet, lipin, lipolysis, metabolism, obesity
Abbreviations: AGPAT, 1-acylglycerol-3-phosphate-O-acyl-transferase; BSCL, Berardinelli–Seip congenital lipodystrophy; C/EBP, CCAAT/enhancer binding protein; CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; Cav1, Caveolin-1; CGL, congenital generalized lipodystrophy; ER, endoplasmic reticulum; HCV, hepatitis C virus; IL-6, interleukin-6; LD, lipid droplet; LPA, lysophosphatidic acid; MEF, mouse embryonic fibroblasts; NFAT, nuclear factor of activated T cells; nSREBP1c, nuclear SREBP1c; PA, phosphatidic acid; Pio, Pioglitazone; PKA, protein kinase A; PPARγ, peroxisome proliferator-activated receptor gamma; PTRF, polymerase I and transcript release factor; TAG, triacylglycerol; TMDs, transmembrane domains; TNFα, tumor necrosis factor alpha; WAT, white adipose tissue
INTRODUCTION
Lipodystrophy is characterized by the selective loss of adipose tissue, with manifestation of clinically heterogeneous phenotypes. Since a key function of adipose tissue is the storage of excess energy in the form of lipids, extreme loss of fat in lipodystrophy results in hypertriglyceridemia and ectopic accumulation of TAG (triacylglycerol) in other organs, particularly liver, leading to a severe metabolic condition. Not surprisingly, lipodystrophy is often accompanied by insulin resistance and its complications of diabetes mellitus, hepatic steatosis, acanthosis nigricans and dyslipidaemia [1]. Other major manifestations include muscular hypertrophy and alterations of the reproductive system (increase in external genitalia size) and renal disorder [2]. There are two types of lipodystrophy: acquired and inherited. Acquired lipodystrophy, more common than inherited lipodystrophy, is not genetically predisposed, and can be categorized as HIV-infected lipodystrophy, acquired partial lipodystrophy, acquired generalized lipodystrophy and localized lipodystrophy. Clinical features include the aforementioned as well as low levels of leptin and adiponectin in serum [1,3]. Inherited lipodystrophies are genetically predisposed from carrier parents and arise from the specific gene mutations. Inherited lipodystrophies include BSCL (Berardinelli–Seip congenital lipodystrophy) type 1–4 [4], familial partial lipodystrophy associated with mutations in PPARγ (peroxisome proliferator-activated receptor gamma) and other genes, Mandibuloacral dysplasia-type A lipodystrophy and-type B lipodystrophy.
BSCL is a rare autosomal recessive disorder, with an estimated prevalence of 1 in 10 million worldwide, discovered by Berardinelli and Seip in 1954 and 1959, respectively [5,6]. Prominent clinical features include almost complete loss of adipose tissue and a muscular appearance from birth. Affected patients exhibit accelerated growth in infancy, progressive bone ageing, fatty liver and hepatomegaly. Each BSCL type is identified by mutations in one of the four genes; AGPAT2 (BSCL1), Seipin (BSCL2), CAV1 (BSCL3) and PTRF (polymerase I and transcript release factor)/Cavin (BSCL4), with majority of patients (~95%) studied thus far possessing mutations in BSCL1 or BSCL2. Here we will summarize the current knowledge on each of the causative genes, and focus on the mechanistic understanding of BSCL2 in this review.
ETIOLOGIES OF BSCL
BSCL1
Loss of ‘metabolically active’ adipose tissue is observed in BSCL1 patients, specifically at subcutaneous, intermuscular, intra-abdominal, intrathoracic and bone marrow areas [7,8]. BSCL1 patients exhibit phenotypes, including lipodystrophy, acanthosis nigricans, hepatosplenomegaly and hepatic steatosis as mentioned above. No mutations are found to be associated with neuronal dysfunction or intellectual impairment, although 10% of BSCL1 subjects (17 families/21 affected subjects) tested by Maldergem et al. showed mild or moderate intellectual impairment [9].
Aberrant expression of the AGPAT2 (1-acylglycerol-3-phosphate-O-acyl-transferase 2) gene, on chromosome 9q34, was identified as the cause of BSCL1 [10,11]. Expression of AGPAT2 is tissue specific, high in liver, pancreas, skeletal muscle and small intestine and highest in adipose tissue [10,12]. AGPAT2 has 278 amino acids, shows up to 48% homology with AGPAT1, lower with AGPAT3-5, and catalyses the acylation of LPA (lysophosphatidic acid) to PA (phosphatidic acid) during phospholipid and TAG synthesis [13,14]. Agarwal et al. discovered ten aberrant expressions of AGPAT2 (G106fsX188, R68X, 221delGT, Q196fsX228, 140delF, L228P, L126fsX146, G136R, V167fsX183 and A239V) in 17 families [10]. Nine other genetic mutations (F60fsX102, W65X, delL165-Q196, Q172K, F109fsX452, K216X, F109fsX452, Q226X and A238G) were subsequently identified in 38 BSCL1 patients [15]. Haque et al. studied eight of the above mutants in CHO cells and reported that seven mutants exhibited significant reduction in enzyme activities, i.e. decreased acylation of LPA to PA, which could result in impaired lipogenesis [3]. However, the 8th lipodystrophy-associated A239V mutation had only mild effect on AGPAT2 enzyme activity (90% of wild-type) [3], suggesting the involvement of additional factors. Phenotypic analysis of the AGPAT2−/− mouse model revealed reduced amount of WAT (white adipose tissue), mimicking observations in human counterparts [16]. Taken together, loss of AGPAT2 activity likely is the cause of BSCL1.
BSCL2
BSCL2 patients exhibit the most severe phenotype of lipodystrophy among the four BSCLs. In addition to the loss of metabolically active adipose tissue, BSCL2 patients also exhibit loss of mechanical adipose tissue, which serves protective or supportive functions of body parts. Loss of these mechanical adipose tissue was observed in the palms, soles, scalp, orbits (around eyeball) and periarticular regions (around the joints) [7,8]. So far, predisposition of seipin mutations is not linked to a specific ethnic type, and BSCL2 patients studied originate from various races, including white ethnicities of Europeans, Mediterranean and Middle Eastern Arabs [9,15,17], African descendants [18] and Japanese [19].
Through a genome-wide analysis of linkage and homozygosity mapping, Magre et al. first identified seipin to be the responsible gene of BSCL2 in 2001 [4]. Coincidentally, in the same year Patel et al. identified a variant on chromosome 11q12–q14 in several families exhibiting a motor neuronal disorder through linkage analysis, which was termed ‘SPG17’ [20]. Windpassinger et al. then further mapped ‘SPG17’ to 11q13 and subsequently identified mutations, N88S and S90L, in BSCL2 causing the Silver syndrome or distal hereditary motor neuropathy type V (collectively named Seipinopathy) [21,22]. In short, mutations in seipin are involved in two seemingly distinct disorders: lipodystrophy and motor neuropathy. It is worth noting that there is higher prevalence of cardiomyopathy and intellectual impairment in BSCL2 versus BSCL1 patients [9,23]. In fact, 78% of BSCL2 subjects (45 affected subjects in 24 families) observed by Maldergem et al. exhibit mild or moderate intellectual impairment [9], suggesting that seipin may have a direct role in the regulation of neuronal functions. Two recent studies from our laboratory support this notion by demonstrating the involvement of seipin in neurotransmission [24,25].
Although seipin is ubiquitously expressed, lipodystrophic mutations in seipin are considered loss-of-function, whereas mutations associated with motor neuropathies are deemed gain-of-function. It was originally thought that specific mutations in seipin are explicit to either lipodystrophy or seipinopathy. However, a recent finding of a novel c.985C>T nonsense mutation that results in Y289LfsX64 (tyrosine 289 mutated to leucine with 64 amino acid frameshift including stop codon) revealed a lipodystrophic phenotype, severe neuronal damage and early death at 7–8 years of age in both compound heterozygous (n=4) and homozygous children (n=2) [26]. Physiological defects include failed motor skills, myoclonic seizures and impaired cognitive development that were attributed to atrophy of the caudate nucleus (within the basal ganglia controlling motor control), posterior corpus callosum and parasagital parietal cortex. Intriguingly, cause of death was respiratory related in four out of the six subjects, either involving infection or status epilepticus. One subject was only 3.5 years of age and the cause of death of the 6th subject was not available. This study highlights that specific mutations in seipin are not necessarily explicit to lipodystrophy or Seipinopathy, but that certain mutations may cause both lipodystrophic and neuropathic manifestations.
The majority of identified mutations in seipin are nonsense, producing truncated protein products, with only a handful being missense. As such, it is reasonable that literatures to date have generally associated aberrant seipin expression to be loss-of-function mutations.
BSCL3
A recent study by Kim et al. linked BSCL mutation of the scaffolding protein Cav1 (Caveolin-1) [27]. The nonsense mutation p.Glu38X in Cav1 (7q31), caused by the 112G◇T nucleotide change, was found in a homozygous Brazilian female patient [27]. Intriguingly only homozygous mutations in CAV1 exhibit a typical BSCL phenotype, with the exception of one heterozygous patient carrying the -88delC frameshift mutation, who exhibits partial lipodystrophy of upper body subcutaneous fat [28]. As no mutation of AGPAT2 or seipin was found in the subject, CAV1 was suggested as the causative gene of BSCL3. The affected patient exhibits near total loss of subcutaneous and visceral adipose tissue depots, muscular hypertrophy, hypertriglyceridemia, hepatosplenomegaly, hepatic steatosis and severe insulin resistance, typical of BSCL phenotype. CAV1 is a major structural component of caveolae, flask-shaped invaginations in the plasma membrane involved in endocytosis, lipid regulation and signal transduction [29]. The ability of CAV1 to bind to fatty acids and associate with LDs (lipid droplets) can potentially reduce uptake of fatty acids into cells and LD size if dysfunctional [30–33]. Reminiscent of patient clinical features, CAV1 null mice show reduced subcutaneous and intra-abdominal fat, adipocyte hypertrophy and interestingly, high susceptibility to tumourigenesis [34–36]. These mice also exhibit acquired lipodystrophy-like phenotype with hypertriglyceridemia, insulin resistance and resistance to diet-induced obesity [37,38]
BSCL4
BSCL4 [39–41] was identified in consanguineous Oman families and caused by mutations in the PTRF/cavin-1 gene essential for caveolae formation [41,42]. Four patients were genotyped as p.K233fs homozygous while one was heterozygous for p.E176fs mutation in PTRF. All patients exhibit typical generalized lipodystrophy, muscular hypertrophy and elevated serum creatine kinase, but vary with other phenotypes such as hepatosplenomegaly and acanthosis nigricans [42]. What is intriguing is the observation of muscular dystrophy on top of muscular hypertrophy, a potential indication of multiple roles of PTRF [39]. Causative genes of both BSCL3 and BSCL4 are somewhat related in that both CAV1, a structural protein, and PTRF/cavin-1, a transcription factor, are essential players of caveolae formation [43,44].
MOLECULAR MECHANISMS OF SEIPIN FUNCTIONS
In the following sub-sections, we will discuss the current understanding of the molecular functions of seipin and potential pathogenic mechanisms of seipin in lipodystrophy.
Molecular structure of seipin
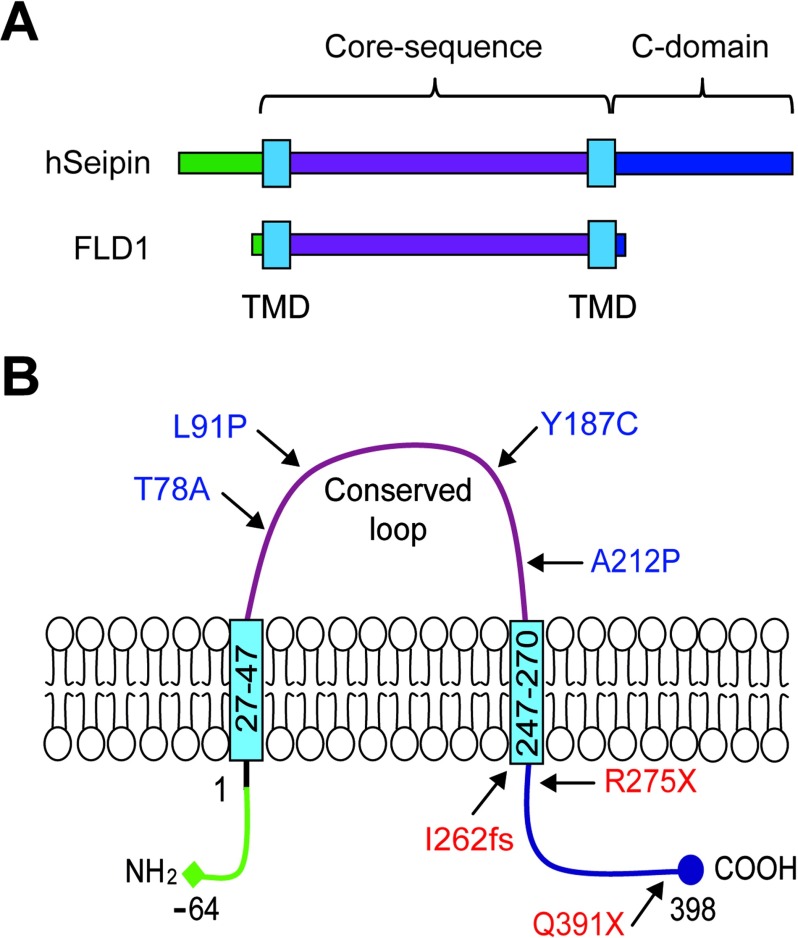
The structure of seipin has been well reviewed by Cartwright and Goodman [45]. Seipin is located on chromosome 11q13 and exists in three isoforms. Isoform 1 has 11 exons and translates to 462 amino acids. Isoform 2 lacks exon 1 and translates a shorter product of 398 amino acids without the 64 amino acids at the N-terminus, whereas isoform 3 splices out exon 7 and generates an even smaller 287 amino acid product with a shortened C-terminus [45]. All three isoforms contain two hydrophobic amino acid regions predicted as TMDs (transmembrane domains), indicating that seipin can be membrane-anchored via these sites (Figure 1A) [4]. Indeed, seipin was shown to be a resident protein of the ER (endoplasmic reticulum) where two TMDs are embedded in the ER membrane, with an evolutionary conserved domain loop in the ER lumen, and a N- and C- terminal domains exposed in the cytoplasm (Figure 1B) [22,46–48]. Orthologues of seipin in mammals, fruit fly and yeast contain a conserved central domain, including the TMDs and the central loop, of about 230 amino acids [45]. Although human and mouse orthologues exhibit high similarity in sequence, the yeast counterpart YLR404W/FLD1 has only 20% similarity to human and mouse seipin, and lacks both N- and C- termini (Figure 1A) [48].
Figure 1. Schematic diagrams of seipin structures.
(A) Domain structure of human seipin and yeast orthologue FLD1 and (B) protein structure of human seipin with both N- and C- termini in the cytosol, two TMDs embedded in the ER membrane and a conserved loop localized in the ER lumen.
The majority of seipin mutations associated with BSCL2 are nonsense or frameshift mutations that result in early truncated non-functional products, except for Q391X [18], I262fs [49] and R275X [19] in isoform 2 (Figure 1B). Q391X results in partial C-terminal truncation, whereas I262fs and R275X cause near complete loss of the C-terminal end. Importantly, these mutants retain the conserved loop region and the two TMDs. Several seipin point mutants due to missense mutations may cause misfolding of the secondary structure of the proteins. The few missense mutations involved in BSCL2 include A212P [4], T78A [18], L91P [18] and Y187C [50]. Two other missense mutations, N88S and S90L [22] are implicated in Seipinopathy. To date, most in vivo and in vitro studies investigating the function of seipin utilize wild-type, null or A212P mutant expression, with the exception of recent publication using T78A and L91P in vitro [51]. Seipin was proposed to be a homo-oligomer consisting of nine subunits, at least in yeast, and the wild-type protein is able to interact with its missense (N88S, S90L and A212P) mutants [52,53].
Role of seipin in adipogenesis
As lipodystrophy is characterized by loss of adipose depots, it is necessary to study seipin in the context of adipose development. Adipogenesis is the differentiation process of a lineage committed pre-adipocyte to a fully matured adipocyte that functions as an energy storage depot in the form of TAG in LDs, governed by various adipogenic and lipogenic genes, including PPARγ, C/EBPα and SREBP1c. The involvement of seipin was suggested by its concordant increase in mRNA expression levels during the adipogenesis process in 3T3-L1 murine preadipocytes and C3H10T1/2 murine multipotent stem cells [54,55]. To decipher the molecular mechanism of seipin, Chen et al. isolated MEF (mouse embryonic fibroblasts) from BSCL2−/− mice, which exhibit lipodystrophy, and subjected them to adipogenesis. Unexpectedly these MEF cells were able to initiate early phase adipogenesis and LD formation, and showed elevated expression of adipogenic genes PPARγ and C/EBPα in the absence of seipin. However, they lacked the ability to sustain the developmental process to fully maturate into functional adipocytes [56]. This was evident from the drastic drop in mRNA and protein expression of the adipocyte marker, aP2 and loss of LD in mutant MEF at the terminal stage of adipocyte differentiation. The latter phenotype is consistent with other studies including ours that used seipin knockdown in C3H10T1/2 cells or the 3T3-L1 cell line, and demonstrated significant adipogenic defects accompanied by reduced mRNA levels of PPARγ C/EBPα and SREBP1c at the late stage of adipogenic induction [48,54,55]. Unexpectedly, treatment with the PPARγ agonist Pio (Pioglitazone) failed to rescue this defect in seipin knockout MEF cells [56]. This is in contrast with the previous finding showing partial restoration of adipogenesis in vitro and in vivo with treatment of thiazolidinediones compounds, rosiglitazone or Pio [55,57]. When overexpressed in 3T3-L1 cells, the seipin A212P mutant also showed inhibition of adipogenesis similar to seipin knockdown, and the impaired adipogenesis was partially restored with Pio treatment [58]. The conflict results may be due to the indirect association of PPARγ with seipin-related cellular pathways.
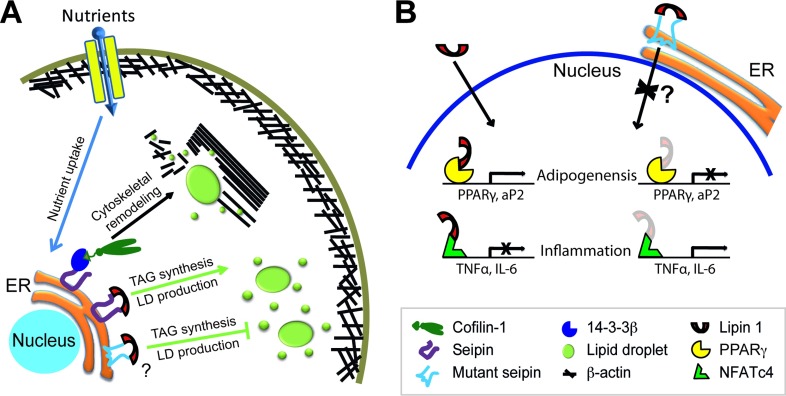
It appears that the C-terminus is indispensible for seipin to regulate adipogenesis, in cooperation with the evolutionarily conserved core sequence as a potential lipid sensor [48]. This regulation was shown to involve the direct interaction of seipin C-terminus with 14-3-3β, which in turn recruits cofilin-1 to remodel actin cytoskeleton during adipocyte development (Figure 2A) [59]. This model explains how C-terminal truncation leads to lipodystrophy, such as in the case of patients with I262fs, R275X and Q391X mutations [18,19,49]. However, it is unclear why the missense mutations in the loop region lead to lipodystrophy, such as A212P, which still retain the C-terminal sequence (Figure 1B). One possible mechanism is that the A212P point mutation may be associated with activation of inflammation pathways during pre-adipocyte stage [58] (see below for more). An alternative model may be that the A212P mutation leads to disruption of secondary protein structure and thus inability of the core sequence to sense lipid level, as the mutant protein fails to inhibit LD formation in preadipocytes [48,59]. It is interesting to note that A212P inhibition of adipogenesis was also suggested to be an overexpression artifact [60]. However, we observed no significant inhibition in adipogenesis when seipin mutants unrelated to lipodystrophy, such as Seipinopathy mutants N88S and S90L, were overexpressed in 3T3-L1 cells. Both N88S and S90L mutations, which disrupt N-glycosylation of seipin, interact with the ER chaperone calnexin due to mutations in a glycosylation site and was proposed to activate ER stress response or autophagy [61,62]. The difference in adipogenic effects by A212P versus N88S/S90L warrants further investigation to determine to what extent the structural disruption of mutant proteins can account for the lipodystrophic effects.
Figure 2. Hypothetical models of seipin functions.
(A) Uptake of excess nutrients leads to sensing of lipid levels by seipin and its recruitment of 14-3-3β and subsequently cofilin-1, inducing cytoskeleton remodelling of the cell towards adipogenesis and LD expansion. In addition, interaction with lipin 1 at the ER is involved in TAG synthesis and storage in LDs. (B) Activities of lipin 1 in the nucleus in adipogenesis and inflammation. Seipin point mutants such as A212P, still retaining interactive capability to lipin 1 and 14-3-3β, mis-localize to the nuclear region. The complex may sequester lipin 1 co-activator activity with PPARγ or 14-3-3β-binding partner cofilin-1 and suppress adipogenesis, and/or sequester lipin 1 co-repressor activity with NFATc4, causing up-regulated expression of inflammatory cytokines TNFα and IL-6.
Role of seipin in LD formation and lipid homoeostasis
To understand the functions of seipin and the underlying molecular mechanisms, it is critical to differentiate the studies on lipogenesis and LD formation from those on adipogenesis. Although lipogenesis and LD formation can happen in many different cell types, adipogenesis is only applicable to adipocytes that are specialized in storing lipids. Here we discuss the functions of seipin in non-adipocytes (including yeast, hepatocytes and undifferentiated 3T3-L1 and other preadipocytes treated with oleate) and adipocytes (preadipocytes treated with adipogenic cocktail and mature adipocytes).
In non-adipocytes
In 2008, Fei et al. showed that in yeast, depletion of a putative seipin orthologue FLD1 (FLD1Δ) exhibited LD-associated defects [63]. These defects include altered quantity and size of LDs in FLD1Δ cells, with ~30% of mutant cells containing one or a couple of ‘supersized’ LD (0.5–1.5 μm as opposed to 0.2–0.4 μm in wild-type cells), suggesting fusion of small droplets in FLD1-deficient cells. Higher amounts of TAG and sterol esters were also accumulated in the LDs of FLD1Δ cells, consistent with inhibition of lipolysis in FLD1Δ yeast reported by another group [64]. Interestingly, the defects observed in yeast could be rescued by expression of human Seipinopathy mutants N88S and S90L, but not lipodystrophy-associated A212P mutant [63]. However, when 3T3-L1 cells expressing N88S and S90L mutants were treated with oleate to induce LD formation and lipid accumulation, small LDs were observed instead, a phenotype reminiscent of seipin knockdown [53]. Similar to FLD1Δ yeast cells, seipin knockdown 3T3-L1 preadipocytes exhibited enhanced TAG synthesis. The observed increase in TAG synthesis in non-adipocytes was further confirmed in seipin knockout mice on chow diet, which exhibited elevated TAG content in the liver [56,57]. In contrast, A212P overexpression did not result in such an enhanced LD formation and exhibited a phenotype similar to normal 3T3-L1 cells [53]. Consistent with the latter study, we did not observe defective LD formation when A212P seipin expressing 3T3-L1 cells were overloaded with oleate, suggesting that there is no defect in TAG storage. Surprisingly, overexpression of wild-type seipin specifically inhibits TAG synthesis and LD formation in non-adipocytes, which may not involve enhanced lipolysis [48,53,59]. Since seipin is composed of a conserved loop in the ER lumen and the C-terminus in the cytoplasm, we truncated seipin either by removing the C-terminus [ΔCT] or the loop domain [CT] [26,48]. Importantly, we found significant inhibition of LD formation when ΔCT or wild-type seipin overexpressing cells were subjected to oleate treatment. This is in stark contrast to the effect of overexpressing CT or A212P seipin where LD formation was unaffected. Although seipin A212P plays an inhibitory role in adipogenesis programming, this mutation does not seem to affect TAG accumulation in non-adipocytes, which may account for the observation of TAG storage in non-adipose organs in BSCL2 human patients. As mentioned above, the A212P mutant may function as a dominant negative by interacting with seipin-binding partners through its cytosolic N- or C-termini, thus sequester and prevent the binding partners from interacting with endogenous wild-type seipin when A212P mutant is overexpressed.
A recent interesting study examined the relations of seipin and HCV (hepatitis C virus). By overexpressing wild-type seipin in a hepatocellular carcinoma cell line Huh7, the authors showed increased size and reduced number of LDs per cell. This led to reduced total outer surface area of LDs, which was correlated with reduced production of HCV particles [65]. This finding suggests a possibility that expression of seipin mutants may affect HCV, at least in vitro, and potentially increase the risk for liver cirrhosis and hepatocellular carcinoma in BSCL2 patients.
In adipocytes
In BSCL2−/− MEF cells during adipogenesis, phosphorylation of hormone-sensitive lipase and perilipin 1A, target substrates of PKA (protein kinase A), is increased [56]. It was proposed that this enhanced lipolysis activity is the result of failed adipogenesis, although the underlying mechanism is unclear. Indeed, treatment of H89 (PKA inhibitor) or E600 (TAG lipase inhibitor) could rescue the adipogenic defect in BSCL2−/− MEF cells, as evidenced by elevated levels of C/EBPα, PPARγ and aP2 [56]. Another potential mechanistic model is the plausible reduction in TAG synthesis through interaction and modulation of lipin 1, a PA phosphatase, by the C-terminus of seipin [66]. Levels of PA, a substrate of lipin 1, were significantly increased upon seipin knockdown, and decreased by seipin overexpression in differentiating 3T3-L1 cells, indirectly signifying the reduced and enhanced TAG synthesis, respectively. Besides its enzymatic activity in TAG synthesis, lipin 1 also regulates transcriptional activities as a co-activator or co-repressor of transcription factors. Lipin 1 activity is indispensable in 3T3-L1 adipogenic programming through the co-activation of the transcription factor PPARγ, as evidenced by reduced TAG storage and suppressed expression of adipogenic markers upon its knockdown [67]. Considering the observation that seipin mutations L91P and A212P both retain the binding capability to lipin 1 and mis-localize to the nuclear envelope, it is reasonable to propose that both of these mutants may contribute to the transcription activity of lipin 1 leading to suppression of its target PPARγ (Figure 2B) [51,54].
Three in vivo models thus far have highlighted the differential roles of seipin in LD formation in non-adipocytes and adipocytes. Knockdown of seipin leads to (1) enhanced TAG synthesis and LD formation in non-adipocytes, and (2) dysfunction of adipocyte development and LD formation through enhanced lipolysis or inhibition of TAG production. While the absence of seipin expression evidently inhibits LD development during adipogenesis, excess expression of wild-type seipin appears to have a similar detrimental effect. In a mouse model overexpressing 150% more seipin in adipose tissue (aP2-seipin), MRI and histological analysis showed significant reduction in subcutaneous and intra-abdominal fat and up to 50% increase in TAG storage in the liver [68]. Instead of adipocyte development, excessive expression of seipin increased the rate of lipolysis similar to the lipolysis model found in knockout mice above [56,68]. The precise mechanism underlying the phenotype remains elusive.
Taken into consideration of our recent studies that seipin regulates lipid homoeostasis by preventing lipid overloading in non-adipocytes while promoting lipid storage in adipocytes [48], and that seipin binds to 14-3-3β through the cytosolic N- and C-termini of seipin [59], a plausible model for the function of seipin in adipogenesis starts to emerge: excess nutrients stimulate the uptake of lipids into preadipocytes during adipogenesis, and the accumulation of lipids in the ER is sensed by seipin; this energy storage signal is transduced to the cytoplasm via seipin-interacting partner 14-3-3β, which mediates the recruitment of cofilin-1, thereby coordinating the remodelling of actin cytoskeleton to accommodate LD formation and expansion in adipocytes (Figure 2A). Presumably there are other binding partners associated with 14-3-3β in regulating multiple cellular pathways to promote adipogenesis. Interestingly, a recent study showed that seipin binds and modulates activity of SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) that transport calcium from cytosol to ER lumen [69]. Although intracellular calcium gradient appears to signal for lipid storage, further studies are necessary to understand how this cooperates with the above proteins and contributes to adipogenesis in mammalian cells.
Seipin and inflammation
Among potential pathological features of CGL/BSCL is the inflammatory activation. So far no report has yet to tie BSCL disorders to inflammation, although there was a report of elevated circulating TNFα (tumour necrosis factor α) levels in patients of HIV-associated lipodystrophy [70]. Additionally, in a relatively new syndrome identified as CANDLE (‘chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature’) four patients exhibited lipodystrophic phenotype similar to that seen in acquired partial lipodystrophy [71]. Systemic inflammation was observed, with an elevated erythrocyte sedimentation rate (an indirect measure of inflammation), increased C-reactive protein (produced by liver in response to inflammatory cytokines) and dermal infiltration of mature neutrophils and mononuclear myeloid cells. Although preliminary, the authors speculated that lipodystrophy could be a result of elevated secretion of inflammatory cytokines IL-6 (interleukin-6) and TNFα. Recent findings from our laboratory revealed a significant elevation of inflammatory pathways involving TNFα, IL-6, iNOS (inducible nitric oxide synthase), COX2 (cyclooxygenase-2) and MCP-1 (monocyte chemoattractant protein-1) when A212P seipin overexpressing 3T3-L1 cells were subjected to adipogenesis [58]. Similarly, elevated circulating and mRNA levels of TNFα, IL-6 and MCP-1 in WAT and brown adipose tissue were found in a transgenic mouse model overexpressing nSREBP1c (nuclear SREBP1c) that mimics lipodystrophy [72]. MCP-1 recruits macrophages to adipose tissue and induces an inflammatory response, which was observed in this nSREBP1c lipodystrophy model.
Adipose tissue macrophage infiltration and inflammatory response are often associated with an obesity phenotype, where enlarged adipocytes release free fatty acids through lipolysis and pro-inflammatory cytokines TNFα, IL-6 and MCP-1, resulting in development of insulin resistance. For instance, IL-6 inhibits insulin receptor substrate 1, impairs insulin stimulated glucose transport, and causes insulin resistance [73,74]. Results from our laboratory showed that treatment of TUDCA (tauroursodeoxycholic acid), a stabilizer of protein misfolding, or Indomethacin, an anti-inflammatory drug, could significantly rescue the adipogenic defects in seipin A212P expressing 3T3-L1 cells [58]. Although the precise mechanism was not clear, IL-6 was significantly reduced by TUDCA treatment in the mutant cells, thus providing a potential therapeutic approach of BSCL2. While the inflammatory mechanism of lipodystrophy is relatively a new idea, overlapping aetiology between lipodystrophy and obesity might indicate that a platform of obesity treatment may well be applied to BSCL2 treatment. Indeed administration of chemical chaperons, TUDCA and 4-PBA in mice fed with high-fat diet showed significant reduction in both ER stress and inflammation in adipose tissue and reversal of impaired insulin signalling induced by the diet [75]. A hypothetical inflammatory model may be again linked to lipin 1. As mentioned above, lipin 1 serves as a co-activator of PPARγ transcription activity. Conversely, lipin 1 can additionally act as a co-repressor of the transcription factor NFATc4 (nuclear factor of activated T cells c4), and down-regulate expression of inflammatory cytokines IL-6 and TNFα in 3T3-L1 adipocytes [76]. Furthermore, MCP-1 expression and secretion increased when lipin 1 expression was suppressed in 3T3-L1 adipocytes, presumably via NFATc4 activities [77]. Together, it is plausible that mutant seipin mislocalization to the nuclear envelope along with its association with lipin 1 may inhibit the suppressive activity of lipin 1 on NFATc4, leading to subsequent activation of inflammation in lipodystrophy, as also observed in obesity associated inflammation (Figure 2B) [76]
CONCLUDING REMARKS
The role of BSCL2/seipin seems to be complex and studies to date suggested that this enigmatic protein may differentially regulate lipid homoeostasis by inhibiting LD formation in non-adipocytes while promoting adipogenesis to store excess lipid. Depletion of seipin leads to increased TAG accumulation in non-adipocytes but inhibition of adipogenesis. However, expression of the seipin mutant, specifically A212P, has no effect on TAG accumulation in non-adipocytes yet inhibits adipogenesis. Mechanistically, the C-terminus of seipin appears to play an essential role in both LD biogenesis and adipogenic programming, shedding light on the pathogenesis of non-functional truncated protein due to nonsense mutations, such as R275X, I262fs and Q391X, which lack the C-terminus. However, other point mutations, T78A, L91P and A212P, seem to retain the C-terminal region. In our model (Figure 2), the conserved core sequence has its distinct function in sensing lipid levels, either preventing lipid accumulation in non-adipocytes or facilitating lipid accumulation in adipocytes. This scenario may possibly explain why A212P and other missense mutants with C-terminus cause lipodystrophy. Nevertheless, seipin mutations in the loop domain may lead to other cellular responses to inhibit adipogenesis. Our study proposes another possible mechanism of inflammatory activation as seen in HIV-lipodystrophy and CANDLE. Further studies are necessary to address validity of these scenarios by identifying other novel molecular players and delineating cellular pathways that differentially regulate LD biogenesis and lipogenesis in non-adipocytes and adipocytes.
FUNDING
Research in the Laboratory of Metabolic Medicine was supported by A*STAR Biomedical Research Council.
References
- 1.Garg A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 2.Gomes K. B., Pardini V. C., Fernandes A. P. Clinical and molecular aspects of Berardinelli–Seip Congenital Lipodystrophy (BSCL) Clin. Chim. Acta. 2009;402:1–6. doi: 10.1016/j.cca.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Haque W. A., Shimomura I., Matsuzawa Y., Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 2002;87:2395–2398. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 4.Magre J., Delepine M., Khallouf E., Dahl T., Jr, Van Maldergem L., Sobel E., Papp J., Meier M., Megarbane A., Bachy A., et al. Identification of the gene altered in Berardinelli–Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 5.Berardinelli W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J. Clin. Endocrinol. Metab. 1954;14:193–204. doi: 10.1210/jcem-14-2-193. [DOI] [PubMed] [Google Scholar]
- 6.Seip M. Lipodystrophy and gigantism with associated endocrine manifestation: a new diencephalic syndrome? Acta Paediatr. 1959;48:555–574. [PubMed] [Google Scholar]
- 7.Simha V. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J. Clin. Endocrinol. Metab. 2003;88:5433–5437. doi: 10.1210/jc.2003-030835. [DOI] [PubMed] [Google Scholar]
- 8.Garg A., Fleckenstein J. L., Peshock R. M., Grundy S. M. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J. Clin. Endocrinol. Metabol. 1992;75:358–361. doi: 10.1210/jcem.75.2.1639935. [DOI] [PubMed] [Google Scholar]
- 9.Maldergem L. V., Magre J., Khallouf T. E., Dahl T., Jr, Delepine M., Trygstad O., Seemanova E., Stephenson T., Albott C. S., Bonnici F., et al. Genotype-phenotype relationships in Berardinelli–Seip congenital lipodystrophy. J. Med. Genet. 2002;39:722–733. doi: 10.1136/jmg.39.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A. K., Arioglu E., De Almeida S., Akkoc N., Taylor S. I., Bowcock A. M., Barnes R. I., Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 11.Garg A., Wilson R., Barnes R. I., Arioglu E., Zaidi Z., Gurakan F., Kocak N., O’Rahilly S., Taylor S. I., Patel S. B., Bowcock A. M. A gene for congenital generalized lipodystrophy maps to human chrmosome 9q34. J. Clin. Endocrinol. Metab. 1999;84:3390–3394. doi: 10.1210/jcem.84.9.6103. [DOI] [PubMed] [Google Scholar]
- 12.Eberhardt C., Gray P. W., Tjoelker L. W. Human lysophosphatidic acid acyltransferase. cDNA cloning, expression, and localization to chromosome 9q34.3. J. Biol. Chem. 1997;272:20299–20305. doi: 10.1074/jbc.272.32.20299. [DOI] [PubMed] [Google Scholar]
- 13.Leung D. W. The structure and functions of human lysophosphatidic acid acyltransferases. Front. Biosci. 2001;6:D944–D953. doi: 10.2741/Leung. [DOI] [PubMed] [Google Scholar]
- 14.Lewin T. M., Wang P., Coleman R. A. Analysis of Amino Acid Motifs Diagnostic for the sn -Glycerol-3-phosphate Acyltransferase Reaction. Biochemistry. 1999;38:5764–5771. doi: 10.1021/bi982805d. [DOI] [PubMed] [Google Scholar]
- 15.Magre J., Delepine M., Maldergem L. V., Robert J.-J., Maassen J. A., Meier M., Panz V. R., Kim C. A., Tubiana-Rufi N., Czernichow P., et al. Prevalence of mutations in AGPAT2 among human lipodystrophies. Diabetes. 2003;52:1573–1578. doi: 10.2337/diabetes.52.6.1573. [DOI] [PubMed] [Google Scholar]
- 16.Vogel P., Read R., Hansen G., Wingert J., Dacosta C. M., Buhring L. M., Shadoan M. Pathology of congenital generalized lipodystrophy in Agpat2−/− mice. Vet. Pathol. 2011;48:642–654. doi: 10.1177/0300985810383870. [DOI] [PubMed] [Google Scholar]
- 17.Cristancho A. G., Lazar M. A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda D. M., Wajchenberg B. L., Calsolari M. R., Aguiar M. J., Silva J. M., Ribeiro M. G., Fonseca C., Amaral D., Boson W. L., Resende B. A., De Marco L. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with Berardinelli–Seip congenital generalized lipodystrophy syndrome. Clin. Endocrinol. (Oxf.) 2009;71:512–517. doi: 10.1111/j.1365-2265.2009.03532.x. [DOI] [PubMed] [Google Scholar]
- 19.Ebihara K. Gene and phenotype analysis of congenital generalized lipodystrophy in Japanese: a novel homozygous nonsense mutation in seipin gene. J. Clin. Endocrinol. Metab. 2004;89:2360–2364. doi: 10.1210/jc.2003-031211. [DOI] [PubMed] [Google Scholar]
- 20.Patel H., Hart P. E., Warner T. T., Houlston R. S., Patton M. A., Jeffery S., Crosby A. H. The Silver syndrome variant of hereditary spastic paraplegia maps to chromosome 11q12–q14, with evidence for genetic heterogeneity within this subtype. Am. J. Hum. Genet. 2001;69:209–215. doi: 10.1086/321267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windpassinger C., Wagner K., Petek E., Fischer R., Auer-Grumbach M. Refinement of the Silver syndrome locus on chromosome 11q12–q14 in four families and exclusion of eight candidate genes. Hum. Genet. 2003;114:99–109. doi: 10.1007/s00439-003-1021-6. [DOI] [PubMed] [Google Scholar]
- 22.Windpassinger C., Auer-Grumbach M., Irobi J., Patel H., Petek E., Horl G., Malli R., Reed J. A., Dierick I., Verpoorten N., et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat. Genet. 2004;36:271–276. doi: 10.1038/ng1313. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal A. K. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J. Clin. Endocrinol. Metab. 2003;88:4840–4847. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- 24.Wei S., Soh S. L., Xia J., Ong W. Y., Pang Z. P., Han W. Motor neuropathy-associated mutation impairs Seipin functions in neurotransmission. J. Neurochem. 2014;129:328–338. doi: 10.1111/jnc.12638. [DOI] [PubMed] [Google Scholar]
- 25.Wei S., Soh S. L., Qiu W., Yang W., Seah C. J., Guo J., Ong W. Y., Pang Z. P., Han W. Seipin regulates excitatory synaptic transmission in cortical neurons. J. Neurochem. 2013;124:478–489. doi: 10.1111/jnc.12099. [DOI] [PubMed] [Google Scholar]
- 26.Guillen-Navarro E., Sanchez-Iglesias S., Domingo-Jimenez R., Victoria B., Ruiz-Riquelme A., Rabano A., Loidi L., Beiras A., Gonzalez-Mendez B., Ramos A., et al. A new seipin-associated neurodegenerative syndrome. J. Med. Genet. 2013;50:401–409. doi: 10.1136/jmedgenet-2013-101525. [DOI] [PubMed] [Google Scholar]
- 27.Kim C. A., Delepine M., Boutet E., El Mourabit H., Le Lay S., Meier M., Nemani M., Bridel E., Leite C. C., Bertola D. R., et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli–Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 28.Cao H., Alston L., Ruschman J., Hegele R. A. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parton R. G., Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 30.Trigatti B. L., Anderson R. G. W., Gerber G. E. Identification of caveolin-1 as a fatty acid binding protein. Biochem. Biophys. Res. Commun. 1999;255:34–39. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- 31.Martin S., Parton R. G. Caveolin, cholesterol, and lipid bodies. Semin. Cell. Dev. Biol. 2005;16:163–174. doi: 10.1016/j.semcdb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Pol A., Martin S., Fernandez M. A., Ferguson C., Carozzi A., Luetterforst R., Enrich C., Parton R. G. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol. Biol. Cell. 2004;15:99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meshulam T., Simard J. R., Wharton J., Hamilton J. A., Pilch P. F. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry. 2006;45:2882–2893. doi: 10.1021/bi051999b. [DOI] [PubMed] [Google Scholar]
- 34.Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 35.Razani B., Engelman J. A., Wang X. B., Schubert W., Zhang X. L., Marks C. B., Macaluso F., Russell R. G., Li M., Pestell R. G., et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 36.Williams T. M., Lisanti M. P. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 37.Cohen A. W., Razani B., Wang X. B., Combs T. P., Williams T. M., Scherer P. E., Lisanti M. P. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell Physiol. 2003;285:C222–C235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 38.Razani B., Combs T. P., Wang X. B., Frank P. G., Park D. S., Russell R. G., Li M., Tang B., Jelicks L. A., Scherer P. E., Lisanti M. P. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 39.Rajab A., Heathcote K., Joshi S., Jeffery S., Patton M. Heterogeneity for congenital generalized lipodystrophy in seventeen patients from Oman. Am. J. Med. Genet. 2002;110:219–225. doi: 10.1002/ajmg.10437. [DOI] [PubMed] [Google Scholar]
- 40.Heathcote K., Rajab A., Magre J., Syrris P., Besti M., Patton M., Delepine M., Lathrop M., Capeau J., Jeffery S. Molecular analysis of Berardinelli–Seip congenital lipodystrophy in Oman-evidence for multiple loci. Diabetes. 2002;51:1291–1293. doi: 10.2337/diabetes.51.4.1291. [DOI] [PubMed] [Google Scholar]
- 41.Rajab A., Straub V., McCann L. J., Seelow D., Varon R., Barresi R., Schulze A., Lucke B., Lutzkendorf S., Karbasiyan M., et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6:e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi Y. K., Matsuda C., Ogawa M., Goto K., Tominaga K., Mitsuhashi S., Park Y. E., Nonaka I., Hino-Fukuyo N., Haginoya K., et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Pilch P. F. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 45.Cartwright B. R., Goodman J. M. Seipin: from human disease to molecular mechanism. J. Lipid Res. 2012;53:1042–1055. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irobi J., Van den Bergh P., Merlini L., Verellen C., Van Maldergem L., Dierick I., Verpoorten N., Jordanova A., Windpassinger C., De Vriendt E., et al. The phenotype of motor neuropathies associated with BSCL2 mutations is broader than Silver syndrome and distal HMN type V. Brain. 2004;127:2124–2130. doi: 10.1093/brain/awh232. [DOI] [PubMed] [Google Scholar]
- 47.Lundin C., Nordstrom R., Wagner K., Windpassinger C., Andersson H., von Heijne G., Nilsson I. Membrane topology of the human seipin protein. FEBS Lett. 2006;580:2281–2284. doi: 10.1016/j.febslet.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Yang W., Thein S., Guo X., Xu F., Venkatesh B., Sugii S., Radda G. K., Han W. Seipin differentially regulates lipogenesis and adipogenesis through a conserved core sequence and an evolutionarily acquired C-terminus. Biochem. J. 2013;452:37–44. doi: 10.1042/BJ20121870. [DOI] [PubMed] [Google Scholar]
- 49.Huang H.-H., Chen T.-H., Hsiao H.-P., Huang C.-T., Wang C.-C., Shiau Y.-H., Chao M.-C. A taiwanese boy with congenital generalized lipodystrophy caused by homozygous Ile262fs mutation in the BSCL2 gene. The Kaohsiung J. Med. Sci. 2010;26:615–620. doi: 10.1016/S1607-551X(10)70094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishiyama A., Yagi M., Awano H., Okizuka Y., Maeda T., Yoshida S., Takeshima Y., Matsuo M. Two Japanese infants with congenital generalized lipodystrophy due to BSCL2 mutations. Pediatr. Int. 2009;51:775–779. doi: 10.1111/j.1442-200X.2009.02863.x. [DOI] [PubMed] [Google Scholar]
- 51.Sim M. F., Talukder M. M., Dennis R. J., O’Rahilly S., Edwardson J. M., Rochford J. J. Analysis of naturally occurring mutations in the human lipodystrophy protein seipin reveals multiple potential pathogenic mechanisms. Diabetologia. 2013;56:2498–2506. doi: 10.1007/s00125-013-3029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binns D., Lee S., Hilton C. L., Jiang Q. X., Goodman J. M. Seipin is a discrete homooligomer. Biochemistry. 2010;49:10747–10755. doi: 10.1021/bi1013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fei W., Li H., Shui G., Kapterian T. S., Bielby C., Du X., Brown A. J., Li P., Wenk M. R., Liu P., Yang H. Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. J. Lipid Res. 2011;52:2136–2147. doi: 10.1194/jlr.M017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Payne V. A., Grimsey N., Tuthill A., Virtue S., Gray S. L., Dalla Nora E., Semple R. K., O’Rahilly S., Rochford J. J. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes. 2008;57:2055–2060. doi: 10.2337/db08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W., Yechoor V. K., Chang B. H., Li M. V., March K. L., Chan L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150:4552–4561. doi: 10.1210/en.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W., Chang B., Saha P., Hartig S. M., Li L., Reddy V. T., Yang Y., Yechoor V., Mancini M. A., Chan L. Berardinelli–Seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol. Cell Biol. 2012;32:1099–1111. doi: 10.1128/MCB.06465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prieur X., Dollet L., Takahashi M., Nemani M., Pillot B., Le May C., Mounier C., Takigawa-Imamura H., Zelenika D., Matsuda F., et al. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia. 2013;56:1813–1825. doi: 10.1007/s00125-013-2926-9. [DOI] [PubMed] [Google Scholar]
- 58.Qiu W., Wee K., Takeda K., Lim X., Sugii S., Radda G. K., Han W. Suppression of adipogenesis by pathogenic seipin mutant is associated with inflammatory response. PLoS ONE. 2013;8:e57874. doi: 10.1371/journal.pone.0057874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang W., Thein S., Wang X., Bi X., Ericksen R. E., Xu F., Han W. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum. Mol. Genet. 2014;23:502–513. doi: 10.1093/hmg/ddt444. [DOI] [PubMed] [Google Scholar]
- 60.Dollet L., Magre J., Cariou B., Prieur X. Function of seipin: new insights from Bscl2/seipin knockout mouse models. Biochimie. 2014;96:166–172. doi: 10.1016/j.biochi.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Ito D., Suzuki N. Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann. Neurol. 2007;61:237–250. doi: 10.1002/ana.21070. [DOI] [PubMed] [Google Scholar]
- 62.Guo J., Qiu W., Soh S. L., Wei S., Radda G. K., Ong W. Y., Pang Z. P., Han W. Motor neuron degeneration in a mouse model of seipinopathy. Cell Death Dis. 2013;4:e535. doi: 10.1038/cddis.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P., Brown A. J., Wenk M. R., Parton R. G., Yang H. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 2008;180:473–482. doi: 10.1083/jcb.200711136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolinski H., Kolb D., Hermann S., Koning R. I., Kohlwein S. D. A role for seipin in lipid droplet dynamics and inheritance in yeast. J. Cell Sci. 2011;124:3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 65.Clement S., Fauvelle C., Branche E., Kaddai V., Conzelmann S., Boldanova T., Bartosch B., Minehira K., Negro F. Role of seipin in lipid droplet morphology and hepatitis C virus life cycle. J. Gen. Virol. 2013;94:2208–2214. doi: 10.1099/vir.0.054593-0. [DOI] [PubMed] [Google Scholar]
- 66.Sim M. F., Dennis R. J., Aubry E. M., Ramanathan N., Sembongi H., Saudek V., Ito D., O’Rahilly S., Siniossoglou S., Rochford J. J. The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol. Metab. 2012;2:38–46. doi: 10.1016/j.molmet.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koh Y. K., Lee M. Y., Kim J. W., Kim M., Moon J. S., Lee Y. J., Ahn Y. H., Kim K. S. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J. Biol. Chem. 2008;283:34896–34906. doi: 10.1074/jbc.M804007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui X., Wang Y., Meng L., Fei W., Deng J., Xu G., Peng X., Ju S., Zhang L., Liu G., et al. Overexpression of a short human seipin/BSCL2 isoform in mouse adipose tissue results in mild lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 2012;302:E705–E713. doi: 10.1152/ajpendo.00237.2011. [DOI] [PubMed] [Google Scholar]
- 69.Bi J., Wang W., Liu Z., Huang X., Jiang Q., Liu G., Wang Y. Seipin promotes adipose tissue fat storage through the ER Ca(2)(+)-ATPase SERCA. Cell Metab. 2014;19:861–871. doi: 10.1016/j.cmet.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 70.Mynarcik D. C., McNurlan M. A., Steigbigel R. T., Fuhrer J., Gelato M. C. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J. Acquir. Immune Defic. Syndr. 2000;25:312–321. doi: 10.1097/00126334-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 71.Torrelo A., Patel S., Colmenero I., Gurbindo D., Lendinez F., Hernandez A., Lopez-Robledillo J. C., Dadban A., Requena L., Paller A. S. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J. Am. Acad. Dermatol. 2010;62:489–495. doi: 10.1016/j.jaad.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 72.Herrero L., Shapiro H., Nayer A., Lee J., Shoelson S. E. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senn J. J., Klover P. J., Nowak I. A., Mooney R. A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 74.Rotter V., Nagaev I., Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 75.Kawasaki N., Asada R., Saito A., Kanemoto S., Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H. B., Kumar A., Wang L., Liu G. H., Keller S. R., Lawrence J. C., Jr, Finck B. N., Harris T. E. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol. Cell Biol. 2010;30:3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi N., Yoshizaki T., Hiranaka N., Suzuki T., Yui T., Akanuma M., Oka K., Kanazawa K., Yoshida M., Naito S., et al. Suppression of lipin-1 expression increases monocyte chemoattractant protein-1 expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2011;415:200–205. doi: 10.1016/j.bbrc.2011.10.060. [DOI] [PubMed] [Google Scholar]