Abstract
Male reproductive success is dependent on insemination success and reproductive output. During mating, male mosquitoes transfer not just sperm, but also seminal fluid proteins that may have profound effects on mated female biology and behavior. In this study, we investigated the role of male body size and mating history on semen depletion, female longevity and reproductive success in Aedes aegypti L. Small and large males were mated in rapid succession with up to five females. Our results indicate that large males had greater mating capacity than small males. A reduction in fecundity by more than 50% was observed in females that were fourth to mate with small males in comparison to females that mated earlier in sequence. For females mated to large males, this reduction became evident for females that mated fifth in sequence. No loss of fertility (measured as hatch rate) was observed in females that were 3rd-5th in mating sequence compared to females mated to virgin males. When females were maintained on a low-quality (5% sucrose) diet, those mated to virgin males had a greater longevity compared to females mated third in sequence. We conclude that small males experience more rapid seminal depletion than large males, and discuss the role of semen depletion in the mated female. Our results contribute towards a better understanding of the complexity of Ae. aegypti mating biology and provide refined estimates of mating capacity for genetic control efforts.
Keywords: Aedes aegypti, male mating history, depletion, fecundity, longevity
Aedes aegypti L. is the principal vector of the four serotypes of dengue virus that cause dengue and dengue hemorrhagic fever throughout the tropics (Gubler 2002). The only way to combat dengue is through vector control because there is no licensed vaccine or antiviral treatment currently available (Swaminathan and Khanna 2009, Webster et al. 2009). Classic methods of vector control focus on reduction of larval breeding sites (WHO 1997) and use of insecticides; however, these approaches are not always successful in reducing disease burden (Ooi et al. 2006, Knox and Scott 2009). Genetic control strategies to target disease vectors have received considerable attention over recent years (James 2007, Knols et al. 2007, Speranca and Capurro 2007, Aldridge 2008, Marshall and Taylor 2009), and Ae. aegypti is one of the candidate species for which genetic strategies are being devised (Olson et al. 2006, Phuc et al. 2007, Medlock et al. 2009, Fu et al. 2010). The success of genetic control strategies depends on the ability of released males to mate successfully with females in the local population. Consequently, a thorough knowledge of mosquito mating biology is required to determine baseline levels which genetically modified males will need to attain in order to be successful and the variables most important for evaluating success (Ferguson et al. 2005, Scott et al. 2008, Magori et al. 2009, Ferguson et al. 2010).
Male fitness is dependent on the number of females he can inseminate during his lifetime (Bateman 1948), and the total number of viable progeny those females produce. Males can be limited in the number of inseminations they achieve on a given day due to the depletion of semen (Wedell 2007). Previous mating experiments with Ae. aegypti indicate that males are capable of inseminating 4–6 females consecutively (Gwadz and Craig 1970, Jones 1973, Foster and Lea 1975). In these studies, insemination was confirmed by the presence of sperm in spermathecae, but female reproductive success after mating was not determined. In addition, the influence of male factors on mating success was not assessed, despite the fact that conditions such as body size may impact on male fitness.
Variation in Ae. aegypti body size is primarily the result of larval conditions, and, in the field, a wide range of sizes are observed (Schneider et al. 2004). Greater body size is associated with increased male mating success in swarms of Anopheles freeborni (Yuval et al. 1993), however, in swarms of An. gambiae and An. funestus no such relationship is found (Charlwood et al. 2002, Charlwood et al. 2003). Ae. aegypti have been observed to mate in close proximity to the host (Hartberg 1971, Yuval 2006), but factors associated with Ae. aegypti male mating success in the field have not been determined. Males are considered to be polygynous, potentially mating with multiple females within one day, while females are considered to be generally monandrous over their lifetime (Clements 1999). Normal sex ratios observed in the field are 50:50 with average numbers of males per house ranging from 0–20 and varying considerably by house and region of the world (Harrington et al. unpublished data from Thailand, Mexico, and Puerto Rico, (Scott et al. 2000, Garcia-Rejon et al. 2008)). Data collected from Ae. aegypti in the laboratory demonstrate the role of body size as a determinant of total sperm number in males (Ponlawat and Harrington 2007), and the number of sperm transferred to females during mating (Ponlawat and Harrington 2009).
Besides sperm, males in many species of arthropods transfer a large number of seminal fluid proteins (Sfps) to females that can induce a wide range of behaviors (Gillott 2003, Wolfner 2007). In Drosophila, where the majority of the work has been conducted, Sfps enhance egg production, reduce receptivity to remating, alter immune responses and feeding behavior, facilitate sperm storage and use, and affect longevity (reviewed by Ravi Ram and Wolfner (2007)). Depletion of Sfps was demonstrated in Drosophila, where reduced amounts of two specific proteins were transferred to females with each successive mating (Sirot et al. 2009). Previous experiments in Ae. aegypti established the role of partially purified male accessory gland extracts in female sexual refractoriness (Fuchs et al. 1968, Fuchs et al. 1969, Fuchs and Hiss 1970), and the role of accessory gland secretions on fertility (Adlakha and Pillai 1975). In addition, blood digestion, ovarian development, oviposition, and feeding behavior are all influenced by female mating status (Lavoipierre 1958, Leahy and Craig 1965, Edman 1970, Downe 1975, Klowden and Lea 1979, Klowden and Chambers 1991). Recently, a large number of putative Sfps have been identified in Ae. aegypti (Sirot et al. 2008) and An. gambiae (Dottorini et al. 2007, Rogers et al. 2009). Many of the Ae. aegypti Sfps are now confirmed to be transferred to females during mating (Sirot et al. in review).
Sfps can influence female life span, and, in some fly species, longevity is negatively affected. In the Mediterranean fruit fly Ceratitis capitata, virgin females live longer than non-virgin females (Chapman et al. 1998), and female Drosophila that mate at high frequencies have shortened life spans (Fowler and Partridge 1989). In contrast, in the fruit fly Anastrepha striata, females that were first to copulate with a virgin male had a higher longevity than his subsequent mates (Pérez-Staples and Aluja 2004), and a similar finding was observed in the housefly Musca domestica (Arnqvist and Andrés 2006) and the butterfly Jalmenus evagoras (Hughes et al. 2000). In other insects, e.g. some monandrous butterflies, no effect of mating on female life span is observed (Svärd and Wiklund 1991, Ward and Landolt 1995, Lauwers and van Dyck 2006).
Here, we investigated Ae. aegypti male mating capacity and the effect of male mating history on female reproductive success and longevity. Male body size was manipulated by altering larval conditions to determine the role of body size on depletion, and it was anticipated that small males would be depleted sooner than large males. We predicted that semen depletion would result in a reduction of female’s fecundity or fertility, and would affect female life span.
Material and Methods
Mosquitoes
A Mexican strain of Ae. aegypti L. was used for this study. These mosquitoes originated from the Tapachula area (14° 54’N, 92° 15’W) and have been in colony since 2006. The strain is augmented yearly with wild mosquitoes from the collection site. Males were reared under standardized crowding and diet conditions to obtain small and large body sizes (for rearing details see Ponlawat and Harrington (2007)). Females were reared to obtain medium body sizes (200 larvae/ liter). Pupae were placed in individual vials for emergence and separated by sex after eclosion. Mosquitoes were maintained in cages (5 liter) with constant access to a 10% sucrose solution until experiments commenced. Mosquitoes were initially maintained at 23.1 ± 2.7 °C, 79.2 ± 8.8% RH, and a photoperiod of 10D: 10L with a two hour simulated dusk and dawn period. On day 45 in experiment I, climate conditions were changed to 25.9 ± 0.6 °C with 71.9 ± 9.5%) RH, and these conditions remained for all other experiments.
Matings
Five experiments using different cohorts of mosquitoes were performed. Females from these experiments were used for longevity studies, reproductive studies or both (Table 1). Mating experiments were conducted at 24.3 ± 1.9 (SD) °C and 35.8 ± 2.7% RH.
Table 1.
Overview of experimental design conducted to determine effects of male mating history and body size class on female fecundity and longevity. Five separate experiments were performed where large (LM) or small males (SM) were mated in rapid succession with up to 5 females within 8 h.
| Exp | Male body size class |
♂ wing length (age) |
♀ wing length (age) |
Females evaluated for: R = reproduction, L= longevity |
Treatments |
|---|---|---|---|---|---|
| I | LM | 2.34 ± 0.02A (8–9) |
2.61 ± 0.02c (3–5) |
R, L | LM-1, LM-3, LM-4 |
| SM | 1.88 ± 0.01a (8–10) |
2.59 ± 0.02c (4–7) |
R, L | SM-1, SM-3, SM-4 | |
| II | LM | 2.28 ± 0.01B (6–7) |
2.66 ± 0.03c (3–6) |
R, L | LM-1, LM-3, LM-4, LM-5 |
| III | LM | 2.35 ± 0.01A (5–6) |
2.79 ± 0.02a,b (6–7) |
R, L | LM-1, LM-4, LM-5 |
| SM | 1.86 ± 0.01a (5) |
2.69 ± 0.02b,c (8–10) |
R, L | SM-1, SM-3, SM-4 | |
| IV | LM | 2.34 ± 0.02A (6–8) |
2.82 ± 0.03a (4–7) |
L (Sugar) | LM-1, LM-2, LM-3, LM-4 |
| V | SM | 1.85 ± 0.02a (7–11) |
2.76 ± 0.02a,b (9–12) |
L (Sugar) | SM-1, SM-2, SM-3 |
Male and female wing lengths ± SEM (mm) and age in days at the start of each mating experiment are indicated. Females were either used for reproductive studies (R), longevity (L) studies, or both; treatments used for each are indicated.
Letters indicate significant differences at the P < 0.05 level for each wing length column. For males, capital letters indicate significant differences between large males (LM), and lower case letters between small males (SM).
Fifty males (5–11 d; Table 1) of one body size (large or small) were placed in mating cage 1 (5 liter; treatment 1). Virgin females were introduced one or two at a time and mating pairs that formed copulas for a minimum of 6 s were removed with an aspirator at the end of copulation (as indicated by a subtle leg movement of the female (Spielman 1964)). Female age at the start of mating was between 3–12 d; however, within each experiment, female age range was between 1–3 d (Table 1). Copulation time was recorded and categorized in time intervals as follows, 6–8 s, 8–14 s, 15–20 s, and > 20 s. Mated females were either transferred to a cage or an individual carton depending on the type of experiment (longevity or reproductive study, see below), and mated males were transferred to the next mating cage until 50 mating pairs had been collected (i.e. within 2–3 h). Subsequently, virgin females were introduced to the second cage (treatment 2) and again couples were removed as described above; with mated males transferred to the third cage (treatment 3). This procedure was continued until males had mated 4 times (cage four, treatment 4) for small males. For large males in experiments II and III, the experiment was terminated after males had mated 5 times (cage 5, treatment 5). All matings for a given male body size (i.e. small or large males) from first to last mating occurred within an 8 h interval. Across all experiments we observed 1,147 copulations. It was not always possible to remove both sexes before the couple disengaged from copulation, and in a small percentage of observations (5.1%) across all treatments, the male was lost in the cage.
Male wing length data were analyzed using non-parametric tests (Mann-Whitney U or Kruskal-Wallis). Wing length measurements confirmed that small males (1.86 ± 0.01 (SEM) mm) were significantly smaller than large males (2.33 ± 0.01 mm) for all experiments (U = 4.0, df = 1, P < 0.01). Wing lengths among small males was similar for all experiments (χ2 = 5.9, df = 2, P > 0.05). Wing length data of large males was significantly different between experiments (χ2 = 23.8, df = 3, P < 0.01), due to the slightly smaller size of large males in experiment II (Table 1).
Reproductive success
Twenty-five females from treatment 1 (i.e., inseminated by a virgin male), from treatment 3 (inseminated by a male mated twice previously), from treatment 4 (inseminated by a male mated three times previously), and from treatment 5 (inseminated by a male mated four times previously; only for large male experiments), were selected for continuation in reproduction or longevity studies (Table 1). We excluded females from treatment 2 to focus our efforts on the transition into a depleted state for small and large males. Females from these treatments will be referred to throughout the text as follows: treatment 1 females mated to large males (LM) or small males (SM)= LM-1, SM-1; treatment 3 females= LM-3, SM-3; treatment 4 females= LM-4, SM-4; and treatment 5 females= LM-5.
Females were transferred to individual containers and offered blood from the forearm of the authors (MEHH/LCH, in accordance with Cornell University’s Institutional Review Board approved protocol #0906000050). Females that did not take a blood meal on day 1 were offered blood again the following day; non-fed females were discarded. Females from each treatment were divided approximately evenly between both human hosts with the exception of females mated to small males in experiment III that fed exclusively on MEHH. Female individual containers consisted of a 0.5 liter paper carton with a mesh lid containing an oviposition vessel (90 ml cup lined with oviposition paper). Ten (experiment I and II) or eleven (experiment III) blood meal opportunities were offered every 2–4 d for 5 min to each female over the course of the experiments. Blood feeding frequency was recorded after each meal. Water present in the oviposition vessel was offered ad libitum. After each blood feeding opportunity, females were offered a 5% sucrose solution on cotton wicks for 2 d. Eggs were removed regularly when blood was offered. After the blood feeding ended, any remaining eggs were removed when the female died. Sucrose (5%) was offered continuously after the last blood meal. Mortality was scored regularly (i.e., daily during blood feeding and then every 2–3 d thereafter) until around day 75 when the experiment was terminated and the few remaining alive females were censored for analysis.
Female wing lengths were analyzed with GLMs. No differences were observed in female body size between treatments for each experiment (Exp I: F8,131 = 0.96, P > 0.05; Exp II: F3,21 = 1.51, P > 0.05, Exp III: F3,78 = 0.38, P > 0.05, Exp IV: F3,48 = 0.08, P > 0.05; Exp V: F3,51 = 0.19, P > 0.05). However, in experiment III, females used in the small male experiments were significantly smaller than females used in the large male experiment (F1,78 = 6.60, P < 0.05). Between experiments, some significant differences in female size were observed but differences were small (F5,350 = 20.01, P < 0.01; Table 1).
Longevity
The life span of mated females from experiments I–III used in the reproduction studies was monitored. In addition, females mated to males in experiments IV and V were used to determine longevity on a low-quality diet (low sugar concentration, i.e. 5% sucrose; Table 1). Females were transferred to cages and daily mortality was recorded until all died.
Fertility
We used egg hatch rate as a measure of fertility. Individual egg batches from females in experiment II and III were conditioned under high humidity and stored until hatching. To determine hatch rate, individual egg papers were soaked for 1–3 d, and subsequently vacuum hatched for 30 min. A small amount of food was added on day 1 to stimulate hatching, and eggs were allowed to hatch for seven days. Hatched larvae were removed, egg papers were dried, submersed in water the following day, and another small aliquot of food was added. Six- seven days later, a subsample of eggs (on average 59%) from each individual egg batch were examined for evidence of hatch (operculum open or closed) under a stereomicroscope. A total of 12,845 eggs from experiments II and III were screened. Only those egg batches with >25% of eggs screened were used in the analysis. In experiment II, egg batches from the first egg collection were improperly stored and were excluded from analysis. Any egg batches laid after egg collection was terminated were not included in hatch rate estimates.
Statistical analysis
All data were analyzed using SPSS v17 (SPSS Inc, Chicago, IL, USA). Prior to analysis, data were checked for normality. When possible, transformations were used to normalize the data. Bonferroni corrections were applied when multiple comparisons were made.
Female fecundity was determined per blood meal by dividing a female’s total lifetime egg production by the number of blood meals taken. In experiment I females from treatment 2 were dissected to determine insemination status; six out of 71 females dissected (9%) were not inseminated indicating a low rate of error in classifying true copulations. This error rate is likely consistent across experiments since the same two observers were involved. Because it was impossible to distinguish copulations that did not result in insemination and egg production from true depletion events, all females were included in the fecundity analysis (but see discussion). Transformations of fecundity data did not result in normality and nonparametric tests were employed (Mann-Whitney U and Kruskal-Wallis). Treatments were compared for each body class between experiments and when no statistical differences were observed they were combined.
Several life-table parameters including survival (lx), expected number of daughters (mx) assuming a 1:1 sex ratio, net reproductive rate R0 (= Σlxmx), and generation time Tc (= Σxlxmx/R0) were calculated for each experiment and treatment (Begon et al. 1996). The intrinsic rate of increase (r) was calculated iteratively by solving Euler’s equation (Begon et al. 1996). A small number of females escaped or were accidently killed (i.e. 7%), and these were not included in the analysis. Because eggs were not collected daily, the number of eggs collected was averaged over the number of days included in the collection interval.
The number of blood meals each female took was divided by the total number of blood meals offered to obtain blood feeding frequencies. Only females that survived for all blood feeding opportunities were included in the analysis. Data were analyzed with non-parametric tests (Kruskal-Wallis). Mating duration categorical data were analyzed using cross tabulation with χ2 tests.
Fertility data (i.e. hatch rate) from individual egg batches were arcsine square root transformed, and the effects of male mating history and body class on hatch rates were analyzed with General Linear Models (GLMs).
Longevity of females maintained on blood and sugar during the reproduction studies was analyzed with Cox regression survival analysis (Cox 1972) . Data were analyzed separately for each male size class to determine if mating history had an effect on longevity, and all covariates (i.e., treatment, experiment, blood feeding frequency, and host type) were initially entered in the model and then non-significant terms were removed. To compare longevity of females between male size classes, a separate model including all data was run. Survival curves were derived from Kaplan-Meier analysis. Longevity of females in the sugar only treatments was analyzed using Kaplan-Meier analysis for each male size class, and compared between treatments using Mantel-Cox Log-rank tests. Longevity data were not compared between diet regimes because of the time-dependent covariate blood feeding frequency.
Results
Female reproduction and fitness
Fecundity
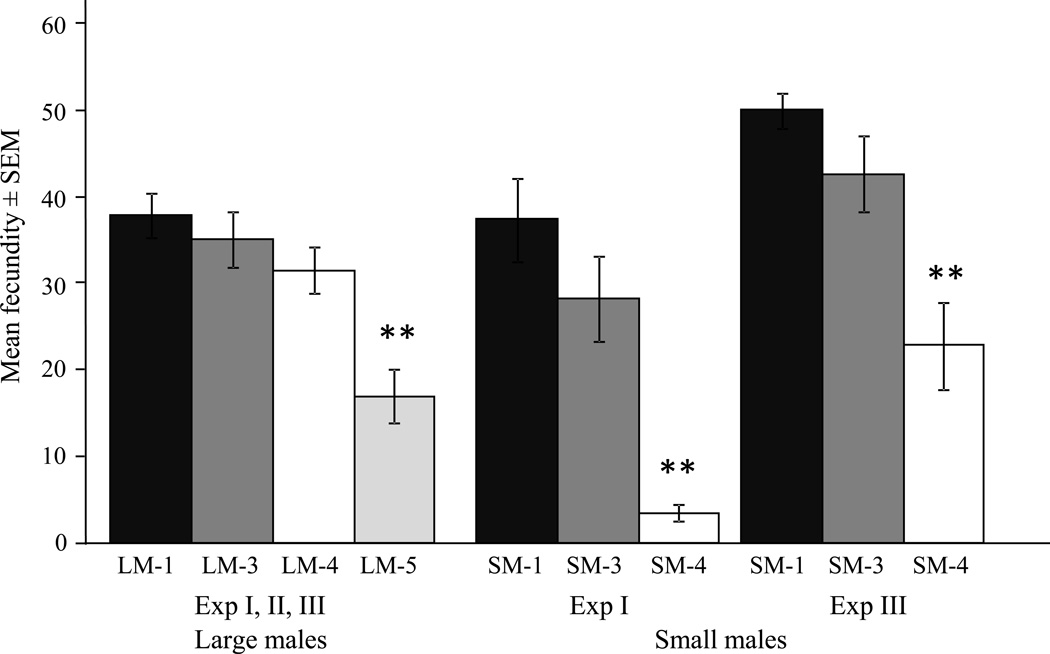
Male mating history had a significant impact on female fecundity (number of eggs/ number of blood meals) for both male size classes (Fig. 1). There we no significant differences in fecundity of females mated to large males between experiments for the various treatments and data were combined. Females that mated fifth in sequence (LM-5) had significantly reduced fecundity compared to females mated earlier in sequence (χ2 = 24.97, df = 3, P < 0.01; Fig. 1), suggesting semen depletion for large males mating a fifth time. Significant differences in fecundity of females mated to small males were observed between experiments I and III, and data were analyzed separately. Females mated to small males fourth in sequence (SM-4) experienced a significant reduction in fecundity compared to females mated earlier in sequence in both experiments (Exp I: χ2 = 23.20, df = 2, P < 0.01, Exp III: χ2 = 18.08, df = 2, P < 0.01; Fig. 2). Male body size played a significant role in the rate of depletion and small males became depleted earlier than large males (Fig. 1).
Fig. 1.
Mean fecundity ± SEM (number of eggs/ number of blood meals) of females mated to males of differing mating history (i.e. LM/SM-1: females inseminated by a virgin male, LM/SM-2: females inseminated by a male mated once previously, LM/SM-3: females inseminated by a male mated twice previously; LM/SM-4: females inseminated by a male mated three times previously; LM-5: females inseminated by a male mated four times previously). Large male (LM) experiments were combined, for small males (SM) results are presented for both experiments as they were significantly different. Asterisks indicate significant differences between treatments for each set at P < 0.01 level.
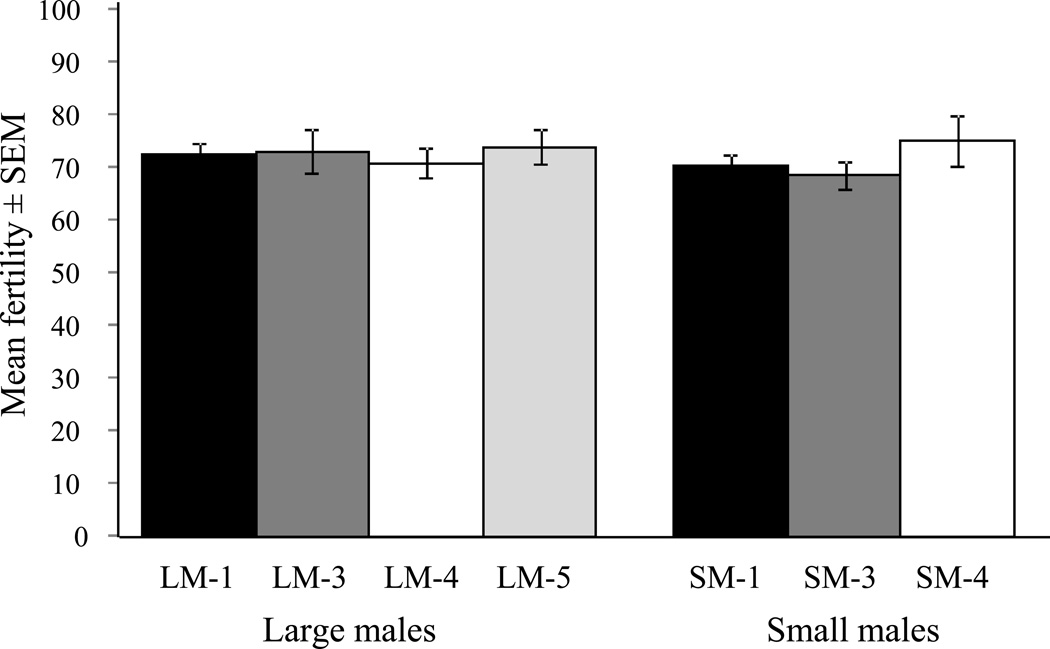
Fig. 2.
Mean fertility (i.e. hatch rate) ± SEM of eggs from females mated to small or large males of differing mating history. No significant differences (P < 0.05) in fertility were observed between treatments, or body size classes.
Cumulative net reproductive rate (R0) followed the same trend as the fecundity data, with females mated fifth in sequence to large males showing a large reduction in R0, and the same was observed for females mated to small males 4th in sequence. Correspondingly the intrinsic rate of increase (r) was reduced in treatments with low R0 and generation time increased (Table 2).
Table 2.
Life-table characteristics for females mated to small or large males of varying mating history.
| Exp | Parameters | Females mated to large males | Females mated to small males | |||||
|---|---|---|---|---|---|---|---|---|
| LM-1 | LM-3 | LM-4 | LM-5 | SM-1 | SM-3 | SM-4 | ||
| I | R0 | 52.3 | 44.6 | 48.4 | - | 44.0 | 44.9 | 7.0 |
| Tc | 20.2 | 18.4 | 18.4 | - | 19.9 | 19.5 | 26.3 | |
| r | 0.6 | 0.6 | 0.7 | - | 0.7 | 0.5 | 0.1 | |
| II | R0 | 80.4 | 66.6 | 59.0 | 18.0 | - | - | - |
| Tc | 19.6 | 20.4 | 20.5 | 22.7 | - | - | - | |
| r | 0.9 | 0.9 | 0.8 | 0.2 | - | - | - | |
| III | R0 | 61.6 | - | 72.2 | 41.0 | 107.1 | 84.2 | 44.3 |
| Tc | 23.2 | - | 22.6 | 24.7 | 21.0 | 20.9 | 26.1 | |
| r | 0.8 | - | 0.7 | 0.5 | 1.3 | 1.1 | 0.4 | |
Net reproductive rate (R0), generation time (Tc), and intrinsic rate of increase (r) are displayed for each experiment. Dashes indicate data were not collected.
There were no significant differences in the percentage of females blood feeding in two out of three experiments for females mated to males with different mating history or body size class (Exp I: 29 ± 1% (SEM), χ2 = 3.97, df = 5, P > 0.05, Exp III: 39 ± 1%, χ2 = 4.04, df = 5, P > 0.05). In experiment II, reduced feeding frequencies for females mated as the fifth mate (LM-5, 26 ± 3%) were observed compared to females mated earlier in sequence (35 ± 2 %; χ2 = 8.50, df = 3, P < 0.05). Host blood source impacted fecundity in two out of three experiments, when blood from one of the hosts imparted higher fecundity (Exp I: U = 1,774.5, df = 1, P < 0.01, Exp III (LM only): U = 423, df = 1, P < 0.01).
Male mating history had no effect on mating duration, and similar copulation times were recorded between treatments for most of the experiments (Exp II: χ2 = 9.90, df = 12, P > 0.05, Exp III: χ2 = 5.23, df = 8, P > 0.05, Exp V: χ2 = 9.83, df = 6, P > 0.05). Male body size had no effect on mating duration, and similar times were recorded for small or large males (χ2 = 4.05, df = 3, P > 0.05).
Fertility
For females that laid eggs, no loss of fertility was associated with mating history for small or large males, and egg hatch rates were similar between treatments (F3,452= 0.12, P > 0.05), experiments (F1,452= 0.89, P > 0.05) and male body size classes (F1,452= 0.77, P > 0.05; Fig 2).
Longevity
Sugar only
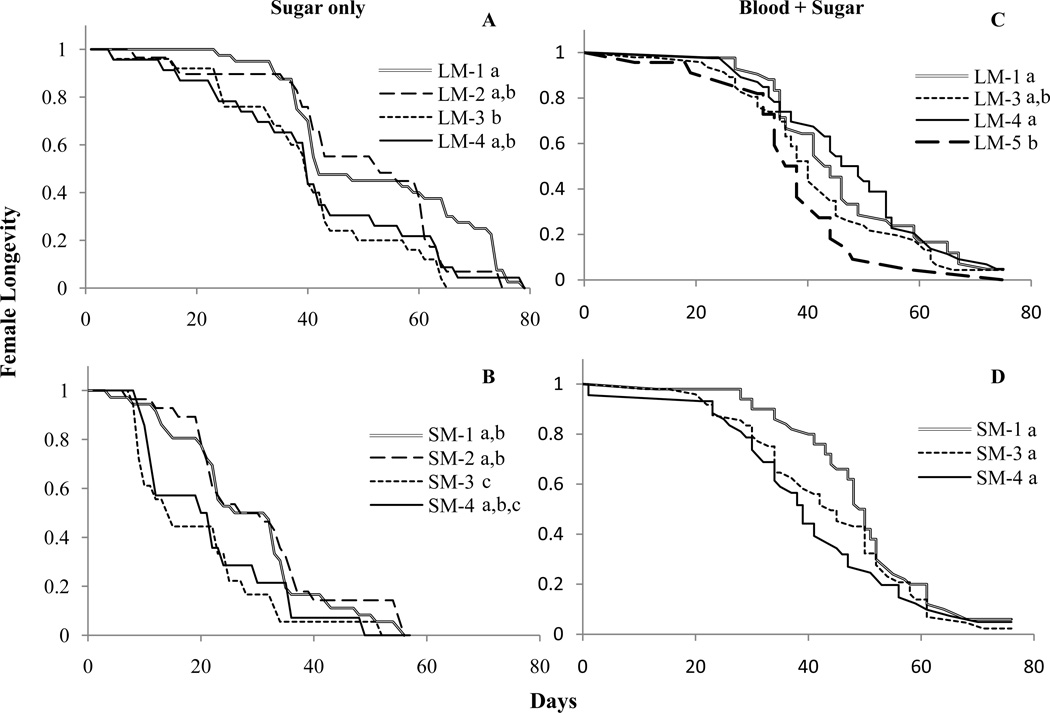
When females were maintained on sugar only, being a later female reduced longevity when the male was large or small. Females mated to virgin large males (LM-1) experienced increased longevity compared to females mated third in sequence (LM-3, Fig. 3a). A similar result was observed for small males, where females mated as first or second mates had increased longevity compared to females mated third in sequence (Fig. 3b). Females mated to small males had significantly lower overall longevity compared to females mated to large males (χ2 = 92.72, P < 0.01), which could be a result of females older age at the start of the experiment (Table 1).
Fig. 3.
Longevity of females maintained on 5% sucrose only (A, B) and females maintained on a diet of blood and sugar (C, D). Females were mated to large (A, C) or small (B, D) males with differing mating history. Letters indicate significant differences at P < 0.05 level between survival curves of treatments for each graph.
Blood and sugar
When females were maintained on blood and sugar significant effects of mate order on female life span were observed only for females mated to large males (Fig 3c). Females mated to virgin males (LM-1) and females mated 4th in sequence (LM-4) had significantly higher longevity than females mated 5th in sequence (LM-5; Fig 3c). The hazard of females dying in LM-1 and LM-4 was only 66% of the hazard rate females experienced when mated to a depleted male (LM-5; Table 3). Longevity of females mated third in sequence was similar to all other treatments. There were no significant differences in the life span of females mated to small males with varying mating history (Table 3, Fig. 3d), although a trend towards increased longevity of females mated to virgin males compared to females mated at the end of the sequence (SM-4) was present at the α = 0.10 level. When data were combined, there were no significant differences in life span of females mated to males of either body size class (B= −0.06, W= 0.10, df=1, P > 0.05).
Table 3.
Cox regression survival analysis results for females mated to large or small males of varying mating history.
| Male size class |
Survival of females mated to small or large males in fecundity studies | |||
|---|---|---|---|---|
| Covariates | B ± SEM | Wald (df) | Hazard Rate (95% CI) | |
| Large | Blood (T COV)† | −0.003 ± 0.00 | 8.98** (1) | 1.00 (1.00–1.00) |
| Treatment | 8.24* (3) | |||
| LM-1 | −0.414 ± 0.20 | 4.40* (1) | 0.66 (0.45–0.97) | |
| LM-3 | −0.039 + 0.21 | 0.03 (1) | 0.96 (0.63–1.46) | |
| LM-4 | −0.420 ± 0.19 | 4.69* (1) | 0.66 (0.45–0.96) | |
| Small | Treatment | 3.50 (2) | ||
| SM-1 | −0.398 ± 0.22 | 3.35 (1) | 0.67 (0.44–1.03) | |
| SM-3 | −0.148 ± 0.22 | 0.45 (1) | 0.86 (0.56–1.33) | |
Females were maintained on a diet of blood and sugar. For each male size class a separate model was run. Non-significant covariates were removed from the model.
and indicate significant differences at the P < 0.05, or P < 0.01 level, respectively.
Treatments are compared to the final treatment (i.e. LM-5 or SM-4).
Blood feeding was used as a time-dependent covariate.
Discussion
Our study indicates that a sequence of matings over an eight-hour period depletes components of Ae. aegypti seminal fluid that affect female fecundity and longevity. Females mated to males at the end of the mating sequence experienced greater than fifty percent reduction in fecundity compared to females mated early in the sequence. Our results are consistent with previous laboratory observations in Ae. aegypti with regard to the number of females that males could consecutively inseminate before reduced numbers of sperm were observed in spermathecae (Gwadz and Craig 1970, Jones 1973, Foster and Lea 1975). The effect of male mating history on female fecundity has been investigated in other insects with varying results. In the majority of Lepidopteran species studied, reproductive output of females mated to virgin males is higher compared to those that mated with non-virgin males. This observation is especially frequent for polyandrous species (reviewed in Torres-Vila and Jennions (2005)). Conversely, in the hide beetle Dermestes maculatus, no differences in female fecundity are observed with male mating history (Jones and Elgar 2004). Male mating history has no effect on female fecundity in the tephritid fly A. obliqua (Pérez-Staples et al. 2008), probably as a result of replenished ejaculate since males of this species only mate once each day.
As expected, male body size significantly affected depletion rate, and females mated to small males in sequence experienced a more rapid loss of fecundity than females mated to large males. Body size in our study was manipulated by altering larval densities. Therefore, the resulting size differences reflect the average effect of density and food, and besides size, both groups of males will differ in other characteristics such as physiology and nutrient stores. Based on the average number of sperm present in males of each size class and the average number of sperm transferred during a first mating (see Ponlawat and Harrington (2007, 2009)), small or large males should have had enough sperm to fertilize four or five females, respectively, and sustain lifetime egg production of their mates at levels comparable to females mated to virgin males. However, data from other insects suggest that females store only a proportion of sperm transferred (Eady 1994, Bernasconi et al. 2002) and data from our laboratory support this finding in Ae. aegypti (Harrington, unpublished data). In the majority of experiments performed, small males were slightly older than large males. It is unlikely though this influenced our conclusions as the greatest increase in sperm occurs in the first days of a male’s life (Ponlawat and Harrington 2007), while males in our study were all above 5 days of age.
Even though we observed an effect of male depletion on female egg production, fertility of sperm transferred by depleted males was not compromised, and females laid egg batches with similar hatch rates as females mated to virgin males. Similar observations were made in the hide beetle D. maculatus where male mating history did not impact fertility (Jones and Elgar 2004). In contrast, in the butterfly Pararge aegeria, females mated to virgin males produced eggs with a higher fertility than females mated to a once mated male (Lauwers and van Dyck 2006), and also in A. obliqua fertilization success decreased with mating order (Pérez-Staples et al. 2008). Seminal fluid proteins (Sfps) play an important role in sperm storage, retention and release in Drosophila (Neubaum and Wolfner 1999, Ravi Ram and Wolfner 2007). Based on the results of one study, Sfps appear to be important for normal sperm function in Ae. aegypti as well; when females were mated to males with surgically removed accessory glands, they produced non-fertilized eggs although sperm was present in their spermathecae (Adlakha and Pillai 1975). Previous work also indicated the importance of Sfps in oviposition behavior; when virgin Ae. aegypti females are injected or implanted with accessory glands, they oviposit infertile eggs at levels similar or higher to mated females (Leahy and Craig 1965, Hiss and Fuchs 1972). Based on this work, it is possible that the observed reduction in fecundity but not in fertility was the result of depletion of specific Sfps responsible for inducing oviposition, but not fertility, or a shortage of sperm. Future studies are planned to quantify the amount of sperm and Sfps transferred by individual males to females over multiple matings to determine their relative impact on female fecundity.
Sfps mediate female life span in several Dipterans. In this study, females mated to virgin males had increased longevity over females mated to depleted males when maintained under low-quality (i.e. 5% sucrose) diet conditions. The same trend was visible for females maintained on blood and sugar, but some contradictory findings were also observed and longevity of females mated fourth in sequence to large males was significantly higher than longevity of females mated third. This was surprising as we would have expected to find a unidirectional effect of depletion. One explanation for this result may be our sample size, which could have prevented us from seeing a more distinct relationship between longevity and mating history. These differences are typically subtle and require large numbers of insects to be observed (Chapman et al. 1998). In addition, we do not know to what extent individual male variation plays a role. Previous studies in Ae. aegypti showed that mated females had greater longevity compared to unmated ones (Lavoipierre 1958, Liles and DeLong 1960). In A. striata, females that were first to copulate with a virgin male had a higher longevity than his subsequent mates (Pérez-Staples and Aluja 2004). In other Dipterans, mating results in survival costs. In C. capitata, virgin females live longer than non-virgin females (Chapman et al. 1998), while female Drosophila that mate at high frequencies have shortened life spans (Fowler and Partridge 1989), largely due to the transfer of toxic Sfps (Chapman et al. 1995). Both C. capitata and Drosophila females mate multiply (Markow 1996, Kraaijeveld and Chapman 2004) and it has been suggested that the reduced female longevity after mating is a side-effect of evolutionary conflict between the sexes (Chapman et al. 1995). Ae. aegypti females on the other hand are considered to be largely monandrous ((Clements 1999); although monandry has never been assessed in the field), and male-male competition within the female reproductive tract is therefore expected to be rare. In other monandrous species, the transfer of Sfps can enhance female life span (i.e. M. domestica; (Arnqvist and Andrés 2006)), or no effect on female longevity is observed (i.e. some monandrous butterflies ((Svärd and Wiklund 1991, Ward and Landolt 1995, Lauwers and van Dyck 2006) but see (Hughes et al. 2000)). Based on the above studies, it appears that for monandrous insects, mating has a neutral or positive effect on female longevity. This suggests that female life span may be mediated by the receipt of male-derived nutrients in the ejaculate, which become depleted after multiple matings. This idea is supported by the observation that male mating status had a greater impact on longevity of females maintained on a no-protein diet compared to females that could derive nutrients from blood. Although we could have expected to see a trade-off between egg production and longevity as has been observed in other organisms (Sgro and Partridge 1999, Eady et al. 2007, Carey et al. 2008, Vilela et al. 2008), females in the depleted treatments that invested fewer resources in egg production, did not have increased longevity compared to females from the non-depleted treatments that invested more.
Direct observation was used in this study to detect mating events, and a low error rate in determining true copulations was observed. Considering that these events were equally likely to occur in all treatments, and all females were included for analysis, we do not believe this influenced our conclusions. Furthermore, no differences were observed in the mating behavior of more depleted males compared to males mated earlier in the sequence, and identical copulation times were recorded. If females with zero fecundity were excluded from the analysis, similar trends in fecundity were observed. However, results were not always significant due to the large number of females with zero fecundity towards the end of the mating sequence; most likely because males became depleted. Our fertility estimates were not affected by this error rate since virgin females of this strain do not readily lay eggs. A drawback of the design was that when mating en masse, a few males were not captured immediately after mating and could not be removed.
In conclusion, our results indicate that females mated by males depleted in seminal fluid proteins and/or sperm suffered a cost in fecundity and longevity, while fertility (i.e. hatch rate) was not affected. While it is not clear if males mate with the same frequency in nature as we observed in our study, our results suggest that smaller males could be at a competitive disadvantage in nature if polygyny is common. Future studies in the field are needed to understand the importance of semen depletion. The results reported in this study contribute to a better understanding of Ae. aegypti mating capacity and dynamics, which is of importance to the development of genetic control strategies. Knowledge of the number of females a male can inseminate will inform modeling exercises to estimate the optimal number of males to release. In addition, this data will advance knowledge of basic reproductive biology and the potential for male and female conflict and cooperation in this medically important species.
Acknowledgments
We are thankful to all members of the Harrington laboratory at Cornell for their support, and constructive comments on the manuscript. Elizabeth Glennon assisted with egg collection and counting, and Francoise Vermeylen provided excellent statistical advice. We appreciate the constructive comments of the reviewers which significantly improved the manuscript. This study was supported by the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (GC7 #316) and National Institutes of Health Grant 5R21AI0176828.
References Cited
- Adlakha V, Pillai MK. Involvement of male accessory gland substance in the fertility of mosquitoes. J. Insect. Physiol. 1975;21:1453–1455. doi: 10.1016/0022-1910(75)90207-3. [DOI] [PubMed] [Google Scholar]
- Aldridge S. Genetically modified mosquitoes. Nat. Biotechnol. 2008;26:725. doi: 10.1038/nbt0708-725a. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Andrés JA. The effects of experimentally induced polyandry on female reproduction in a monandrous mating system. Ethology. 2006;112:748–756. [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Begon M, Townsend CR, Harper JL. Ecology: from individuals to ecosystems. Oxford: Blackwell; 1996. [Google Scholar]
- Bernasconi G, Hellriegel B, Heyland A, Ward PI. Sperm survival in the female reproductive tract in the fly Scathophaga stercoraria (L.) J. Insect Physiol. 2002;48:197–203. doi: 10.1016/s0022-1910(01)00164-0. [DOI] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Müller H, Wang J, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata . Proc. Biol. Sci. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Thompson R, Madsen H. Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malaria J. 2003;2:2. doi: 10.1186/1475-2875-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, do Rosario VE. Male size does not affect mating success (of Anopheles gambiae in Sao Tome) Med.Vet. Entomol. 2002;16:109–111. doi: 10.1046/j.0269-283x.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Wallingford: CABI Publishing; 1999. [Google Scholar]
- Cox DR. Regression Models and Life Tables. J. Roy. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. PNAS. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downe AE. Internal regulation of rate of digestion of blood meals in the mosquito, Aedes aegypti . J. Insect Physiol. 1975;21:1835–1839. doi: 10.1016/0022-1910(75)90250-4. [DOI] [PubMed] [Google Scholar]
- Eady PE. Sperm transfer and storage in relation to sperm competition in Callosobruchus maculatus. Behav. Ecol. Sociobiol. 1994;35:123–129. [Google Scholar]
- Eady PE, Hamilton L, Lyons RE. Copulation, genital damage and early death in Callosobruchus maculatus . Proc. Biol. Sci. 2007;274:247–252. doi: 10.1098/rspb.2006.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JD. Rate of digestion of vertebrate blood in Aedes aegypti (L.). Effect of age, mating, and parity. Am. J. Trop. Med. Hyg. 1970;19:1031–1033. doi: 10.4269/ajtmh.1970.19.1031. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, John B, Ng’habi K, Knols BG. Redressing the sex imbalance in knowledge of vector biology. Trends Ecol. Evol. 2005;20:202–209. doi: 10.1016/j.tree.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WA, Lea AO. Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. J. Insect Physiol. 1975;21:1083–1090. doi: 10.1016/0022-1910(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760–761. [Google Scholar]
- Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P, Berendonk TU, White-Cooper H, Scaife S, Kim Phuc H, Marinotti O, Jasinskiene N, James AA, Alphey L. Female-specific flightless phenotype for mosquito control. PNAS. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs MS, Hiss EA. The partial purification and separation of the protein components of matrone from Aedes aegypti. J. Insect Physiol. 1970;16:931–939. doi: 10.1016/0022-1910(70)90223-4. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Craig GB, Jr, Hiss EA. The biochemical basis of female monogamy in mosquitoes. I. Extraction of the active principle from Aedes aegypti . Life Sci. 1968;7:835–839. doi: 10.1016/0024-3205(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Craig GB, Despommier DD. The protein nature of the substance inducing female monogramy in Aedes aegypti. J. Insect Physiol. 1969;15:701–709. [Google Scholar]
- Garcia-Rejon J, Lorono-Pino MA, Farfan-Ale JA, Flores-Flores L, Del Pilar Rosado-Paredes E, Rivero-Cardenas N, Najera-Vazquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am. J. Trop. Med. Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Gwadz RW, Craig GB., Jr Female polygamy due to inadequate semen transfer in Aedes aegypti . Mosq. News. 1970;30:355–360. [Google Scholar]
- Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull. World Health Organ. 1971;45:847–850. [PMC free article] [PubMed] [Google Scholar]
- Hiss EA, Fuchs MS. The effect of matrone on oviposition in the mosquito, Aedes aegypti. J. Insect Physiol. 1972;18:2217–2227. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- Hughes L, Siew-Woon Chang B, Wagner D, Pierce NE. Effects of mating history on ejaculate size, fecundity, longevity, and copulation duration in the ant-tended lycaenid butterfly, Jalmenus evagoras. Behav. Ecol. Sociobiol. 2000;47:119–128. [Google Scholar]
- James AA. Preventing the spread of malaria and dengue fever using genetically modified mosquitoes. JoVE. 2007:231. doi: 10.3791/231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC. A study on the fecundity of male Aedes aegypti . J. Insect Physiol. 1973;19:435–439. doi: 10.1016/0022-1910(73)90118-2. [DOI] [PubMed] [Google Scholar]
- Jones TM, Elgar MA. The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc. R. Soc. Lond. B. Biol. Sci. 2004:271. doi: 10.1098/rspb.2004.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden M, Chambers G. Male accessory gland substances activate egg development in nutritionally stressed Ae. aegypti mosquitoes. J. Insect Physiol. 1991;37:721–726. [Google Scholar]
- Klowden MJ, Lea AO. Humoral inhibition of host-seeking in Aedes aegypti during oocyte maturation. J. Insect Physiol. 1979;25:231–235. doi: 10.1016/0022-1910(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, Bossin HC, Mukabana WR, Robinson AS. Transgenic mosquitoes and the fight against malaria: managing technology push in a turbulent GMO world. Am. J. Trop. Med. Hyg. 2007;77:232–242. [PubMed] [Google Scholar]
- Knox TB, Scott TW. Advances in Human Vector Control, ACS Symposium Series. Am. Chem. Soc.; 2009. Vector control for prevention of dengue- current status and future strategies; pp. 39–57. [Google Scholar]
- Kraaijeveld K, Chapman T. Effects of male sterility on female remating in the mediterranean fruitfly, Ceratitis capitata. Proc. Biol. Sci. 2004;271(Suppl 4):S209–S211. doi: 10.1098/rsbl.2003.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers K, van Dyck H. The cost of mating with a non-virgin male in a monandrous butterfly: experimental evidence from the speckled wood, Pararge aegeria . Behav. Ecol. Sociobiol. 2006;60:69–76. [Google Scholar]
- Lavoipierre MM. Biting behavior of mated and unmated females of an African strain of Aedes aegypti. Nature. 1958;181:1781–1782. doi: 10.1038/1811781a0. [DOI] [PubMed] [Google Scholar]
- Leahy M, Craig G. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and Ae. albopictus. Mosq. News. 1965;25:448–452. [Google Scholar]
- Liles JM, DeLong DM. The longevity and productivity of adult male and female Aedes aegypti when reared separately and together on three different diets. Ann. Entom. Soc. Amer. 1960;53:277–280. [Google Scholar]
- Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, Gould F. Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS NTD. 2009;3:e508. doi: 10.1371/journal.pntd.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA. Evolution of Drosophila mating systems. Evol. Biol. 1996;29:73–106. [Google Scholar]
- Marshall JM, Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6:e20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock J, Luz PM, Struchiner CJ, Galvani AP. The impact of transgenic mosquitoes on dengue virulence to humans and mosquitoes. Am. Nat. 2009;174:565–577. doi: 10.1086/605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KE, Alphey L, Carlson JO, James AA. Genetic approaches in Aedes aegypti for control of dengue: an overview. In: Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht: Springer; 2006. [Google Scholar]
- Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg. Infec. Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Staples D, Aluja M. Anastrepha striata (Diptera: Tephritidae) females that mate with virgin males live longer. Ann. Entomol. Soc. Am. 2004;97:1336–1341. [Google Scholar]
- Pérez-Staples D, Aluja M, Macías-Ordóñez R, Sivinski J. Reproductive tradeoffs from mating with a successful male: the case of the tephritid fly Anastrepha obliqua. Behav. Ecol. Sociobiol. 2008;62:1333–1340. [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, Fu G, Condon KC, Scaife S, Donnelly CA, Coleman PG, White-Cooper H, Alphey L. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2007;44:422–426. doi: 10.1603/0022-2585(2007)44[422:aabsim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti . Am. J. Trop. Med. Hyg. 2009;80:395–400. [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007;3:e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J. Med. Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- Scott TW, Harrington LC, Knols BG, Takken W. Applications of mosquito ecology for successful insect transgenesis-based disease prevention programs. Adv. Exp. Med. Biol. 2008;627:151–168. doi: 10.1007/978-0-387-78225-6_13. [DOI] [PubMed] [Google Scholar]
- Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J. Med. Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–4. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Buehner NA, Fiumera AC, Wolfner MF. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: an ELISA-based method for tracking individual ejaculates. Behav. Ecol. Sociobiol. 2009;63:1505–1513. doi: 10.1007/s00265-009-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Poulson RL, McKenna MC, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem. Mol. Biol. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski MEH, Kimura M, Deewathanawong P, Wolfner MF, Harrington LC. Towards an ejaculatome of the yellow fever mosquito: Protein identification and potential functions. doi: 10.1371/journal.pntd.0000989. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranca MA, Capurro ML. Perspectives in the control of infectious diseases by transgenic mosquitoes in the post-genomic era- a review. Mem. Inst. Oswaldo Cruz. 2007;102:425–433. doi: 10.1590/s0074-02762007005000054. [DOI] [PubMed] [Google Scholar]
- Spielman A. The mechanics of copulation in Aedes aegypti . Biol. Bull. 1964;127:324–344. [Google Scholar]
- Svärd L, Wiklund C. The effect of ejaculate mass on female reproductive output in the european swallowtail butterfly, Papilio machaon (L.) (Lepidoptera: Papilionidae) J. Insect Behav. 1991;4:33–41. [Google Scholar]
- Swaminathan S, Khanna N. Dengue: recent advances in biology and current status of translational research. Curr. Mol. Med. 2009;9:152–173. doi: 10.2174/156652409787581592. [DOI] [PubMed] [Google Scholar]
- Torres-Vila LM, Jennions MD. Male mating history and female fecundity in the Lepidoptera: do male virgins make better partners? Behav. Ecol. Sociobiol. 2005;57:318–326. [Google Scholar]
- Vilela A, Cariaga R, González-Paleo L, Ravetta D. Trade-offs between reproductive allocation and storage in species of Oenothera L. (Onagraceae) native to Argentina. Acta Oecol. 2008;33:85–92. [Google Scholar]
- Ward KE, Landolt PJ. Influence of multiple matings on fecundity and longevity of female cabbage looper moths (Lepidoptera: Noctuidae) Ann. Entom. Soc. Am. 1995:88. [Google Scholar]
- Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect. Dis. 2009;9:678–687. doi: 10.1016/S1473-3099(09)70254-3. [DOI] [PubMed] [Google Scholar]
- Wedell N. Sperm competition and ejaculate evolution. In: Roldan ERS, Gomendio M, editors. Spermatology. Nottingham: Nottingham University Press; 2007. [Google Scholar]
- WHO. Vector surveillance and control Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. Geneva: WHO; 1997. [Google Scholar]
- Wolfner MF. "S.P.E.R.M." (seminal proteins (are) essential reproductive modulators): the view from Drosophila. Soc. Reprod. Fertil. Suppl. 2007;65:183–199. [PubMed] [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Ann. Rev. Entomol. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- Yuval B, Wekesa JW, Washino RK. Effects of body size on swarming behavior and mating success of male Anopheles freeborni (Diptera: Culicidae) J. Insect Behav. 1993;6:333–342. [Google Scholar]