Abstract
Background
The pathophysiological changes before the presentation of clinical symptoms in parkinsonism are unclear. In this study, we investigated neural network modulations in persons in the preclinical stage of familial parkinsonism, and how the network interactions change at the clinical stage.
Methods
We performed functional MRI in a family with SCA2 mutation, including 9 asymptomatic carriers and 10 mutation carriers with parkinsonian symptoms. Functional connectivity from the posterior putamen bilaterally and rostral supplementary motor area was used to explore network interactions in the subjects.
Results
Both the asymptomatic carriers and patients had decreased connectivity within the basal ganglia-thalamus-cortical motor loop compared to controls. The asymptomatic carriers showed extensively increased connectivity compared to controls, including the cortico-cortical motor, cortico-cerebellar, cortico-brainstem, and part of the basal ganglia-thalamus-cortical motor circuits. In contrast, the connectivity of most of these networks was decreased in the patients. These abnormalities were relatively normalised after levodopa administration.
Conclusions
In the preclinical stage of SCA2 parkinsonism, the connectivity of a part of the basal ganglia motor loop is weakened as a consequence of dopaminergic deficits; meanwhile, the connectivity of other large-scale brain networks is strengthened presumably to compensate for the dysfunction of the basal ganglia to maintain brain function in the early stage of dopaminergic deficits. The simultaneous effects of progressive disruption of basal ganglia motor circuits and failure of compensatory mechanisms as dopaminergic dysfunction progresses may contribute to the onset of clinical symptoms.
Keywords: parkinsonism, SCA2 mutation, Functional connectivity, Basal ganglia motor circuits, Compensation
1. Introduction
The neurodegenerative process in parkinsonism begins several years before the onset of any clinical symptoms [1]. The pathophysiological changes of preclinical parkinsonism remain unclear. An approach is to use asymptomatic genetic mutation carriers to study preclinical parkinsonism, because they are associated with a latent nigrostriatal dysfunction, and have an increased risk to develop clinical parkinsonism. Neuroimaging studies have found additional recruitment of premotor areas in some types of asymptomatic mutation carriers, e.g., Parkin, PINK1, compared to healthy controls during motor tasks, and have suggested that this might reflect a compensatory mechanism to maintain motor function [2–4]. However, it remains unclear whether there is dysfunction of basal ganglia (BG)-thalamo-cortical circuits at the preclinical stage, what the nature of compensatory processes might be, and how such compensatory processes differ in the preclinical and clinical stages.
Since parkinsonism affects large-scale brain networks, examination of network interactions may provide more valuable information in understanding pathophysiological changes than simply investigating local brain activity [5–7]. With this method, it has been observed that the cingulate motor area (CMA) has a stronger influence on activity in the BG in asymptomatic Parkin mutation carriers compared to controls during motor performance [2]. However, this finding only showed neural network modulation in a specific motor condition. Recent years, a resting state functional MRI (fMRI) method has been developed to detect neural connectivity [8]. Compared to the conventional task-based method, resting state fMRI can circumvent task-related confounds, increase signal to noise ratio, expand patient populations [9], and can explore BG or cortical motor network modulations in parkinsonism patients [10–12].
CAG trinucleotide repeat expansion within the spinocerebellar ataxia type 2 (SCA2) gene is a cause of parkinsonism, especially in Chinese [13–15]. A striatal dopaminergic deficit has been demonstrated in both familial parkinson patients and asymptomatic carriers with SCA2 mutations [16,17]. In the current study, we used the asymptomatic and symptomatic SCA2 mutation carriers to investigate the resting state functional connectivity of brain networks in the preclinical and clinical stages. We hypothesized that there is a pattern of modulations of neural networks in preclinical parkinsonism that may help to maintain normal motor behavior despite dopaminergic deficits. We further hypothesized that this pattern of modulations would not be maintained in the clinical stage, and that this loss would contribute to the onset of clinical symptoms.
2. Methods
2.1. Subjects
We studied 10 patients and 9 asymptomatic mutation carriers from a large kindred, who carried a heterozygous CAG expansion with repeat numbers varying from 37 to 40 in the SCA2 gene. The repeat number was slightly variable among siblings but stable between generations. The normal alleles for all participants contained 22 repeats. Sequence analyses suggested that the normal allele contained two CAA interruptions and displayed a configuration of (CAG)8CAA(CAG)4CAA(CAG)8, while the expanded alleles only contained a reserved 3’-CAA interruption and displayed a configuration as (CAG)nCAA(CAG)8. Two patients had participated in a previous study, and had been examined with [11C]-CFT (2-b-carbomethoxy-3b-(4-fluorophenyl)tropane) positron emission tomography (PET) showing bilateral decrement of 11C-CFT uptake in the stratum [16].
We chose these participants because all SCA2 mutation carriers in this kindred developed parkinsonian symptoms at around 40 years old (38.30 ± 5.50), and the genetic mutations were consistent across them. Additionally, PET results indicated that the patients had dopaminergic deficits [16]. Therefore, it is logical to consider that these asymptomatic SCA2 mutation carriers were at the preclinical stage of parkinsonism and provide a good dataset to investigate neural modulations at preclinical and clinical stage.
The asymptomatic carriers consisted of 4 males and 5 females (mean age 25.33 ± 7.04, 17 – 36 years old). The patients included 4 males and 6 females (mean age 46.10 ± 4.12, 40 – 53 years old). All participants underwent a detailed neurological examination. Symmetrical bradykinesia and rigidity were the leading symptoms in every patient, and all patients had a good response to L-dopa treatment. Only one patient had slight ataxia, whilst none of them showed slow saccades. No definite cerebellar or brainstem atrophy was observed in any patient. Patients were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS) [18], and the Hoehn and Yahr disability scale [19] while off their medications (Table 1). All asymptomatic carriers had no motor signs or other parkinsonian symptoms or any neurological problems upon careful clinical examination, and had no history of a previous neurological or psychiatric disease nor had they previously received dopaminergic or other antiparkinsonian drug treatment.
Table 1.
Clinical details of symptomatic patients
| Patient | Age (years) |
Gender | Age at onset |
Disease duration (years) |
Ataxia | Slow Saccades |
Parkinsonism | Clinical scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | B | R | PI | UPDRS | H-Y | |||||||
| 1 | 53 | M | 38 | 15 | − | − | + | +++ | +++ | ++ | 75 | 3 |
| 2 | 40 | F | 36 | 4 | − | − | + | +++ | ++ | + | 63 | 2.5 |
| 3 | 47 | M | 32 | 15 | − | − | − | + | + | − | 33 | 2 |
| 4 | 46 | F | 36 | 10 | − | − | − | ++ | − | − | 13 | 2 |
| 5 | 44 | F | 36 | 8 | + | − | − | ++ | ++ | − | 38 | 2 |
| 6 | 41 | M | 36 | 5 | − | − | + | ++ | +++ | + | 58 | 2.5 |
| 7 | 52 | M | 48 | 4 | − | − | − | ++ | − | − | 14 | 2 |
| 8 | 45 | F | 44 | 1 | − | − | − | ++ | + | ++ | 44 | 3 |
| 9 | 47 | F | 32 | 15 | − | − | + | ++ | +++ | ++ | 57 | 3 |
| 10 | 46 | F | 45 | 1 | − | − | + | ++ | + | + | 40 | 2.5 |
| Mean (SD) | 46.10 (4.12) | 38.30 (5.50) | 7.80 (5.67) | 43.50 (20.33) | 2.45 (0.44) | |||||||
RT=rest tremor; B=Bradykinesia; R=rigidity; PI=posture instability; Sym: symmetric; UPDRS: unified Parkinson’s disease rating scale; H-Y: Hoehn-Yahr stage; MMSE: mini-mental state examination.
Two groups of healthy controls were included. One group had 9 healthy volunteers, age- and sex-matched (mean age 25.33 ± 7.04 years, 4 male) as the controls for the asymptomatic carriers (young controls). Another group had 10 healthy volunteers, age- and sex-matched (mean age 46.10 ± 4.12 years, 4 male) as the controls for the patients (aged controls). Controls did not have SCA2, Parkin, DJ-1, PINK1, or LRRK2 mutations. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects gave their written informed consent for the study.
2.2. fMRI procedure
fMRIs were performed on a 3T Siemens Sonata scanner. High resolution axial T1- and T2- weighted images were obtained in every participant. Blood-oxygen-level dependent (BOLD) data were acquired with gradient-echo echo-planar sequences (TR = 2000 ms, TE = 30 ms, 33 axial slices, 3.5 mm thickness, FOV = 220 mm). During the resting fMRI scanning, subjects were instructed to keep their eyes closed, to remain motionless, and to not to think of anything in particular.
We additionally asked the subjects to perform a right index finger tapping task, to help to define the regions of interest (ROIs) for functional connectivity analysis in the resting state. This session was block-designed, and included two states, “movement” and “resting”, alternately. In the movement state, the subjects were asked to perform a self-paced motor task, during which they briskly tapped their right index finger at an interval of 2 s. Each state lasted for 20 sec and repeated 6 times.
Healthy controls were scanned once, whereas the patients and asymptomatic carriers were scanned twice, both with and without medication. Patients were first scanned after a minimum period of 12 hours since their last dose of anti-parkinson medication (off state). The asymptomatic carriers were also first scanned without levodopa administration. After the first scan, levodopa was administered orally as 250 mg Madopar (200 mg levodopa/50 mg benserazide, Roche company, Shanghai) in all patients and asymptomatic carriers. The second scan was performed 60 min after oral levodopa had been given, approximately when plasma levodopa achieve the highest level.
2.3. Data analysis
Preprocessing of fMRI data was carried out using Data Processing Assistant for Resting-State fMRI (DPARSF) V2.0 (http://www.restfmri.net). After slice-timing, realignment, and normalization, all images were resampled into voxels that were 3×3×3 mm in size, and smoothed with a 6 mm Gaussian smoothing kernel. Then, linear drift was removed. A temporal filter (0.01 Hz < f < 0.08 Hz) was applied to remove very-low-frequency drifts and physiological high-frequency noise [8].
Data from the motor task were analyzed for each subject separately on a voxel-by-voxel basis using the general linear model approach for the time series. A one-sample t-test was used to identify brain activity across all subjects. As leading symptoms in our patients were motor problems, we focused on connectivity of motor circuits. The putamen is critical in the BG-thalamo-cortical motor circuit [20], and is divided into anterior and posterior portions by the anterior commissure. The posterior putamen connects to the primary motor cortex (M1) and supplementary motor area (SMA) [21], and is the region where dopamine is most heavily depleted [22]. Because the motor symptoms were presented symmetrically in our patients, we chose both sides of the posterior putamen as ROIs. Additionally, as bradykinesia was a leading symptom in our patients, and the rostral SMA (pre-SMA) is critical in initiating movements [23], we chose the pre-SMA as a ROI.
These three ROIs were centered at the voxels that showed the maximum magnitude of activation from the results of movement. The ROI from the pre-SMA had a radius of 5 mm. To avoid having the putaminal ROI extending to the adjacent regions, a radius of 3 mm was used for the ROIs of the posterior putamen bilaterally [24]. Functional connectivity was analyzed with Resting-State fMRI Data Analysis Toolkit (REST, http://www.restfmri.net). Nine nuisance covariates were regressed, including: the white matter signal, cerebrospinal fluid signal, mean global signal, and six head motion parameters. Correlation analysis was carried out between the seed reference and the whole brain in a voxel-wise manner.
A random effect one-sample t-test was applied to determine brain regions showing significant connectivity with each ROI within each group. Random effect two-sample t-tests were used to identify the differences between asymptomatic carriers and controls, and between patients and controls. Then, we compared the results between the clinical and preclinical stages. As the asymptomatic carriers were younger than the patients, to exclude aging effect, a one-way ANOVA including the patients, aged controls, asymptomatic carriers, and young controls group was employed. We used a contrast of (1, −1, −1, 1), and a contrast of (−1, 1, 1, −1) to show increased or decreased connectivity in patients compared to asymptomatic carriers, respectively.
In addition, the differences before and after levodopa administration in asymptomatic carriers and patients were examined by paired t-tests. Finally, a correlation analysis of connectivity results with the UPDRS scores was performed in patients in the off state to examine whether the network connectivity related with the disease severity. A false discovery rate (FDR) corrected threshold of p < 0.05 was used for all above statistical analysis. Extent threshold was 10 voxels.
Results
The voxels that showed the maximum magnitude of activation during motor task across the subjects and were used as the center of the ROIs were pre-SMA: −2, 8, 56; left putamen: −30, −6, −2; and right putamen: −29, −4, −6 (MNI coordinates). Because the results of such movement in parkinsonism patients or healthy subjects have been extensively reported, the details of brain activity are not shown here.
The results from the left and right posterior putamen were similar; thus, we only present findings from the right posterior putamen here (Fig. 1). In asymptomatic carriers compared to controls, the posterior putamen showed decreased connectivity with the anterior putamen, caudate nucleus, globus pallidus, insula, temporal cortex, and pre-SMA, and had increased connectivity with the M1, postcentral gyrus, precuneus, superior parietal lobule, inferior parietal lobule, anterior cingulate cortex, prefrontal cortex, and pons (Fig. 2A, Supplementary Table 1). The patients compared to controls had decreased connectivity with the anterior putamen, caudate nucleus, globus pallidus, thalamus, insula, temporal cortex, postcentral gyrus, pre-SMA, SMA-proper, CMA), and pons, but there was no increased connectivity (Fig. 2B, Supplementary Table 2). In patients compared with asymptomatic carriers, the putamen had less connectivity with the M1, postcentral gyrus, pre-SMA, PMC, superior parietal lobule, thalamus, precuneus, insula, and pons, but without any increased connectivity (Fig. 2C, Supplementary Table 3).
Figure 1. Functional connectivity in the right posterior putamen.
Brain regions showing significantly functional connectivity in the right posterior putamen in the resting state in healthy controls (A), asymptomatic SCA2 mutation carriers (B), and familiar parkinsonism patients with SCA2 mutation carriers (C). The results are shown across each group (one-sample t-test, p < 0.05, FDR corrected).
Figure 2. Differences of functional connectivity with the right posterior putamen between the groups.
Hot and cold colors indicate increases and decreases of connectivity with the posterior putamen in asymptomatic carriers compared to healthy controls (A), in familiar parkinsonism patients compared to healthy controls (B), and in familiar parkinsonism patients compared to asymptomatic carriers (C), respectively.
Abbreviations: L, left; R, right; ACC: anterior cingulate cortex; CN: caudate nucleus; GP: globus pallidus; IPL: inferior parietal lobule; LL: limbic lobe; M1, primary sensorimotor cortex; MTG: middle temporal gyrus; PCG: postcentral gyrus; PMC, premotor cortex; pre-SMA, rostral supplementary motor area; SMA: caudal supplementary motor area; SPL: superior parietal lobule.
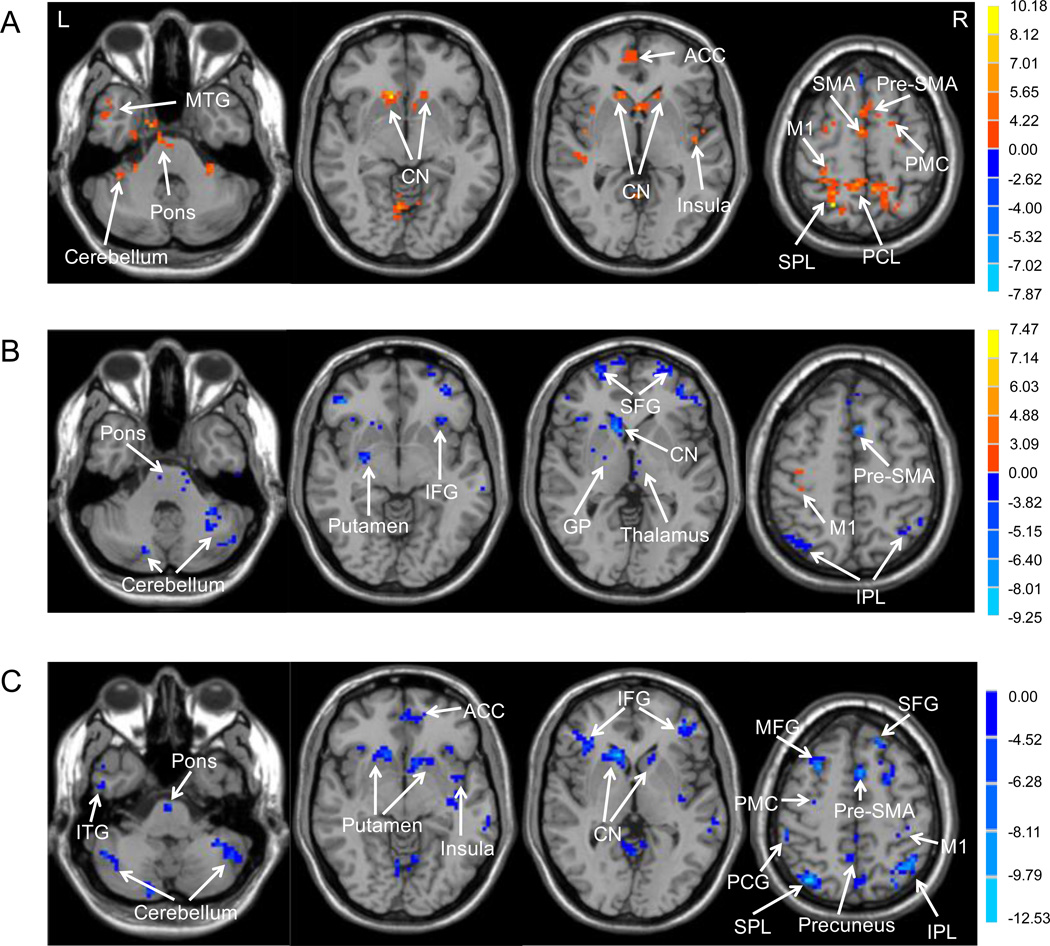
The brain areas that showed connectivity with the pre-SMA in the healthy controls, asymptomatic carriers and patients in the off state are shown in Supplementary Fig. 1. In asymptomatic carriers compared to controls, the pre-SMA had stronger connectivity with extensive regions like the cortical motor areas (e.g., M1, SMA-proper, CMA, and premotor cortex (PMC)), caudate nucleus, pons, and cerebellum (Fig. 3A, Supplementary Table 4). In patients compared to controls, the pre-SMA showed decreased connectivity with the BG, thalamus, prefrontal cortex, parietal cortex, cerebellum, and pons, and only had increased connectivity with the bilateral M1 (Fig. 3B, Supplementary Table 5). In patients compared to asymptomatic carriers, the pre-SMA had less connectivity with the M1, PMC, prefrontal cortex, parietal cortex, striatum, insula, cerebellum, and pons, but without any increased connectivity (Fig. 3C, Supplementary Table 6).
Figure 3. Differences of functional connectivity with the pre-SMA between the groups.
Hot and cold colors indicate increases and decreases of connectivity with the pre-SMA in asymptomatic carriers compared to healthy controls (A), in familiar parkinsonism patients compared to healthy controls (B), and in familiar parkinsonism patients compared to asymptomatic carriers (C), respectively.
Abbreviations: L, left; R, right; ACC: anterior cingulate cortex; CN: caudate nucleus; GP: globus pallidus; IFG: inferior frontal gyrus; IPL: inferior parietal lobule; ITG: inferior temporal gyrus; M1, primary sensorimotor cortex; MFG: middle frontal gyrus; MTG: middle temporal gyrus; PCG: postcentral gyrus; PCL: paracentral lobule; PMC, premotor cortex; pre-SMA, rostral supplementary motor area; SFG: superior frontal gyrus; SMA: caudal supplementary motor area; SPL: superior parietal lobule.
Administration of levodopa increased connectivity of putamen-thalamo-cortical, putamen-cerebellar, and cortical motor circuits in both asymptomatic carriers and patients, and increased putamen-pons connectivity in the patients (Supplementary Fig. 2). In patients in the off state, correlation analysis between connectivity results and UPDRS scores showed that there was a negative correlation between the pre-SMA and putamen, insula, and PMC (Supplementary Fig. 3A), and there was a negative correlation between the posterior putamen and anterior putamen, globus pallidus, caudate nucleus, thalamus, insula, pre-SMA, and prefrontal cortex (Supplementary Fig. 3B).
Discussion
The present study investigated the functional connectivity of brain networks in the preclinical stage of familial parkinsonism, and explored how the network connectivity is modulated in the preclinical stage compared to the clinical stage. The novel finding is that besides weakened BG-cortical connectivity, there are extensively strengthened network connections at the preclinical stage of parkinsonism. These increased functional connectivities, however, are not significant in the clinical stage. This special pattern of neural network changes provides a new insight to explain underlying pathophysiological changes in parkinsonism.
The asymptomatic carriers present a characteristic pattern of network connectivity. First, there are decreased connectivities between the posterior putamen and other BG nuclei (e.g., anterior putamen, caudate nucleus, globus pallidus), and cortical areas (e.g., pre-SMA, insula, and temporal lobe, Fig. 2A). The putamen, globus pallidus, and pre-SMA are a part of the BG-thalamo-cortical motor loop. These weakened connections were normalized after levodopa administration, which demonstrates that even early dopaminergic deficits can disrupt functional connectivity of BG motor and other striatal-cortical circuits. Another feature is that there are increased connectivities in asymptomatic carriers compared to controls, including the connectivity between the posterior putamen and the M1 and pons, areas that are also involved in the BG-thalamo-cortical motor loop (Fig. 2A), the cortico-cortical motor network (e.g., pre-SMA, SMA-proper, M1, PMC, and CMA), the cortico-cerebellar loop, and cortico-pons loop (Fig. 3A).
The dysfunction of the BG and reduced connectivity of BG motor circuit should lead to deterioration in motor performance. However, our asymptomatic carriers did not show any motor symptoms. As these cortico-cortical motor, cortico-cerebellar, or cortico-brainstem circuits are important in various components of movements, such as motor preparation, initiation, or execution [23,25], we speculate that increased connectivity of these networks might to compensate for the dysfunction of the BG motor loop required in order to maintain motor function in the early stage of dopaminergic deficit [2–4, 26,27].
The combination of our findings with previous reports [2–4] demonstrates that both the local brain activity, and the interactions of large-scale brain networks are modulated in the preclinical stage of parkinsonism. Compared to our results, these earlier studies also found additional recruitments of the premotor areas, but did not detect striatal dysfunction or recruitment of other areas, e.g., the cerebellum and pons. These differences may be due to the variation of genotype (SCA2 vs. Parkin, PINK1 mutation), or different experimental paradigms (resting vs. movement), but may also suggest that network connectivity analysis may be a more sensitive method to detect neural changes than local activation amplitude [28].
This pattern of network connectivity in the preclinical stage, however, is significantly different in the clinical stage. The connectivity of the BG-thalamo-cortical motor circuits is further decreased in patients than that in the asymptomatic carriers. The connectivity of BG motor loop is negatively correlated with the UPDRS scores, and can be relatively normalized by levodopa administration in the patients. These findings indicate that as the dopaminergic deficits progress, the functional disconnection of BG motor loop becomes more significant.
In patients, the connectivity of the striato-brainstem, cortico-cortical motor, cortico-brainstem, and cortico-cerebellar circuits is no longer strengthened, but is now weakened compared to asymptomatic carriers, or healthy controls (Figs. 2 and 3). Only the connectivity between the pre-SMA and M1 is still increased in patients compared to controls (Fig. 3). Our findings suggest that although the compensation is still apparent in the clinical stage [5–7,11,12,26,27,29] it is largely broken down compared to that in the preclinical stage. It is logical to speculate that progressive breakdown of compensatory effects in addition to disruption of BG loop is critical in onset of clinical parkinsonism. However, as the current report was not a longitudinal follow-up study, it is unclear when this breakdown of compensation happens. Another limitation of the current study is the relatively small sample size. Thus, a further large sample follow-up study on asymptomatic mutation carriers is necessary to explore the relationship between progressive decreases of BG loop connectivity and compensatory effects and clinical onset of parkinsonism symptoms.
Although the symptoms, severity, and duration are different, the pattern of network connectivity in familial parkinsonism patients with SCA2 mutation is comparable with previous reports on Parkinson’s disease patients in the resting condition [11,12]. For example, in one such study, the putamen had decreased connectivity with the insula, temporal lobe, precentral gyrus, and postcentral gyrus, but without any increased connectivity in the patients compared to controls [12]; in another study, the pre-SMA had increased connectivity with the M1, but had decreased connectivity with the putamen, insula, and parietal cortex [11]. A previous study also showed that patients with Parkin mutation and sporadic Parkinson’s disease had similar movement-related activation patterns [30]. Our results, at least partially, may also reflect preclinical and clinical neural network modulations in Parkinson’s disease. In future, we need to investigate other types of hereditary parkinsonism, like Parkin, or LRRK2, to clarify whether this pattern of preclinical and clinical neural network changes is common in parkinsonism.
In conclusion, our study demonstrates that in the preclinical stage of SCA2 parkinsonism, the connectivity of a part of the BG motor loop is weakened secondary to dopaminergic deficits; meanwhile, the interactions of extensive brain networks are strengthened presumably to compensate for the dysfunctional BG in order to maintain motor function in the early stage of dopaminergic deficit. The combination of progressive functional disconnection of BG motor circuits and loss of compensatory effects as dopaminergic dysfunction progresses may be neural correlates contributing to the onset of clinical parkinsonism.
Supplementary Material
Brain regions showing significantly functional connectivity in the pre-SMA in the resting state in healthy controls (A), asymptomatic SCA2 mutation carriers (B), and familiar parkinsonism patients with SCA2 mutation carriers (C). The results are shown across each group (one-sample t-test, p < 0.05, FDR corrected). T-score bars are shown on the right. Abbreviations: L, left; R, right.
(A) Brain regions show increased connectivity with the pre-SMA after levodopa administration in asymptomatic carriers; (B) Brain regions show increased connectivity with the putamen after levodopa administration in asymptomatic carriers; (C) Brain regions show increased connectivity with the pre-SMA after levodopa administration in parkinsonism patients; (D) Brain regions show increased connectivity with the putamen after levodopa administration in parkinsonism patients.
Brain regions showing a negative correlation between functional connectivity and UPDRS score from the pre-SMA (A), and putamen (B) in patients at off state (correlation analysis, p < 0.05, FDR corrected).
Acknowledgements
This work was supported by grants from the National Science Foundation of China (30570530, 30870693, and 81071012).
References
- 1.Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Buhmann C, Binkofski F, Klein C, Büchel C, van Eimeren T, Erdmann C, et al. Motor reorganization in asymptomatic carriers of a single mutant Parkin allele: a human model for presymptomatic parkinsonism. Brain. 2005;128:2281–2290. doi: 10.1093/brain/awh572. [DOI] [PubMed] [Google Scholar]
- 3.van Nuenen BF, Weiss MM, Bloem BR, Reetz K, van Eimeren T, Lohmann K, et al. Heterozygous carriers of a Parkin or PINK1 mutation share a common functional endophenotype. Neurology. 2009;72:1041–1047. doi: 10.1212/01.wnl.0000338699.56379.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders S, Sack B, Pohl A, Münte T, Pramstaller P, Klein C, Binkofski F. Compensatory premotor activity during affective face processing in subclinical carriers of a single mutant Parkin allele. Brain. 2012;135:1128–1140. doi: 10.1093/brain/aws040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010;133:2394–2409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. NeuroImage. 2010;49:2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett. 2009;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 2011;32:1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb. Cortex. 2010;20:1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- 13.Gwinn-Hardy K, Chen JY, Liu TY, Liu TY, Boss M, Seltzer W, et al. Spinocerebellar ataxia type 2 with parkinsonism in ethnic Chinese. Neurology. 2000;55:800–805. doi: 10.1212/wnl.55.6.800. [DOI] [PubMed] [Google Scholar]
- 14.Shan DE, Soong BW, Sun CM, Lee SJ, Liao KK, Liu RS. Spinocerebellar ataxia type 2 presenting as familial levodopa-responsive parkinsonism. Ann Neurol. 2001;50:812–815. doi: 10.1002/ana.10055. [DOI] [PubMed] [Google Scholar]
- 15.Lu CS, Wu Chou YH, Kuo PC, Chang HC, Weng YH. The parkinsonian phenotype of spinocerebellar ataxia type 2. Arch Neurol. 2004;61:35–38. doi: 10.1001/archneur.61.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Wang JL, Xiao B, Cui XX, et al. Analysis of SCA2 and SCA3/MJD repeats in Parkinson's disease in mainland China: genetic, clinical, and positron emission tomography findings. Mov Disord. 2009;24:2007–2011. doi: 10.1002/mds.22727. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Hong S, Kim GP, Choi YJ, Kim YK, Park SS, et al. Importance of low-range CAG expansion and CAA interruption in SCA2 Parkinsonism. Arch Neurol. 2007;64:1510–1518. doi: 10.1001/archneur.64.10.1510. [DOI] [PubMed] [Google Scholar]
- 18.Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of neurological deficit. Boston: Butterworths; 1989. pp. 285–309. [Google Scholar]
- 19.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 21.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 22.Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–555. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- 23.Jahanshani M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 25.Purzner J, Paradiso GO, Cunic D, Saint-Cyr JA, Hoque T, Lozano AM, et al. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. J Neurosci. 2007;27:6029–6036. doi: 10.1523/JNEUROSCI.5441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120:103–110. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 28.Palmer SJ, Li J, Wang ZJ, McKeown MJ. Joint amplitude and connectivity compensatory mechanisms in Parkinson's disease. Neuroscience. 2010;166:1110–1118. doi: 10.1016/j.neuroscience.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, et al. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain. 2007;130:2898–2914. doi: 10.1093/brain/awm208. [DOI] [PubMed] [Google Scholar]
- 30.van Eimeren T, Binkofski F, Buhmann C, Hagenah J, Strafella AP, Pramstaller PP, et al. Imaging movement-related activity in medicated Parkin-associated and sporadic Parkinson's disease. Parkinsonism Relat Disord. 2010;16:384–387. doi: 10.1016/j.parkreldis.2010.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brain regions showing significantly functional connectivity in the pre-SMA in the resting state in healthy controls (A), asymptomatic SCA2 mutation carriers (B), and familiar parkinsonism patients with SCA2 mutation carriers (C). The results are shown across each group (one-sample t-test, p < 0.05, FDR corrected). T-score bars are shown on the right. Abbreviations: L, left; R, right.
(A) Brain regions show increased connectivity with the pre-SMA after levodopa administration in asymptomatic carriers; (B) Brain regions show increased connectivity with the putamen after levodopa administration in asymptomatic carriers; (C) Brain regions show increased connectivity with the pre-SMA after levodopa administration in parkinsonism patients; (D) Brain regions show increased connectivity with the putamen after levodopa administration in parkinsonism patients.
Brain regions showing a negative correlation between functional connectivity and UPDRS score from the pre-SMA (A), and putamen (B) in patients at off state (correlation analysis, p < 0.05, FDR corrected).