Abstract
Adolescence refers to the transition from childhood to adulthood that begins with the onset of puberty and ends with successful independence from the parent. A paradox for human adolescence is why, during a time when the individual is probably faster, stronger, of higher reasoning capacity and more resistant to disease, there is such an increase in mortality relative to childhood. These untimely deaths are not due to disease, but rather to preventable forms of death (accidental fatalities, suicide and homicide) associated with adolescents putting themselves in harm’s way due, in part, to diminished self control – the ability to suppress inappropriate emotions, desires and actions. This paper highlights how self control varies as a function of age, context and the individual and delineates its neurobiological basis.
Keywords: Adolescence, prefrontal cortex, self control, ventral striatum, reward, salience, development
INTRODUCTION
During adolescence we are probably the quickest that we will ever be; the crushes will never be better; and the thrills will never quite be the same. That’s the good news. The bad news is that during this time your chances of death from putting yourself in harm’s way will increase by 200% relative to your childhood (Dahl, 2001). This paper focuses on the challenges of adolescence in the context of self control – the ability to suppress inappropriate emotions, desires and actions. We highlight the specific contexts in which adolescents’ self control is most likely to falter and its underlying neurobiological basis.
Over the past decade there has been a marked increase in neurobiological research on the behavioral changes that occur during adolescence. Too often, in simplifying the findings for the media or policy makers, this work is reduced to adolescents having no self control and no prefrontal cortex, basically being “all gasoline, no brakes, and no steering wheel” (Bell & McBride, 2010). Such simple claims can have positive and negative consequences for the treatment of adolescents, justifying both diminished responsibility for criminal acts (see Scott and Bonnie in this issue) and limited ability to make life choices (e.g., terminate or continue a pregnancy). Reading popular science magazines that have made such claims led our group to undertake the studies of self control described in this article.
In this paper, we present our work in the context of three common “myths” or overgeneralizations about adolescence to clarify and temper some of these claims. The first is that adolescent behavior is irrational or deviant. Such descriptions may be understandable in light of the peak incidence in criminal activity and of many psychiatric disorders during this developmental period. Yet, this description psychopathologizes an important phase of normal development for the individual to learn how to function relatively independently in society. A second over generalization is that adolescents are incapable of making rational decisions because of their less mature prefrontal cortex (Yurgelun-Todd, 2007), the so-called rational, Vulcanized region of the brain (J. D. Cohen, 2005). Clearly, the prefrontal cortex is not the only part of the brain that is changing during this developmental period and the child’s prefrontal cortex is even less mature than the adolescent’s. Thus, this explanation does not sufficiently explain spikes in risky and emotive behavior during adolescence. We present evidence that underscores the importance of considering brain regions as part of a developing circuit that is fine-tuned with experience during this time. Third is the century old claim that all adolescents experience “sturm and drang” originally proposed by G Stanley Hall (Hall, 1904). Although adolescents show poor self control as group, we provide evidence for when self control is most likely to break down during adolescence and for striking individual differences in this ability across the lifespan that may put some teens at greater risk than others. Each of these overgeneralizations is addressed below in the context of a neurodevelopmental framework.
SELF CONTROL AND THE TEENAGE BRAIN
Over Generalization 1: Adolescents are incapable of making optimal decisions
Adolescence, by definition, involves new demands on the individual as she/he moves from dependence on the family unit to relative independence. This developmental period is not specific to humans as evidenced by increases in novelty seeking, interactions with peers and fighting with parents that is observed in other species (see Spear, Sisk, and Romeo articles in this issue). These behaviors are thought to have evolved to serve adaptive functions (Spear & Varlinskaya, 2010) related to successful mating and obtainment of resources necessary for survival. A heightened sensitivity to socially relevant cues (e.g., peers, monetary gain) would seem to be an ideal mechanism for meeting some of these developmental challenges. However, such a system may appear less than optimal when the pull by these socially relevant cues are at the expense of long-term goals and the overall well being of the adolescent.
To suggest that this period is one of no brakes or steering wheel (Bell & McBride, 2010) greatly oversimplifies this period of development. In a series of recent experiments in our laboratory (Somerville, Hare, & Casey, 2011) we measured self control using a variant of a go/nogo paradigm that contained social cues (e.g., positive, negative or neutral facial expressions). By using socially relevant and emotionally salient stimuli together with neutral ones, we could test how well adolescents regulate their impulses in both emotional and nonemotional contexts (Hare et al 2008; (Somerville et al., 2011).
Self control, in this case, suppressing a compelling action, showed a different developmental pattern in the context of emotional information than in its absence, especially for males (Tottenham et al 2011). As illustrated in Figure 1, (see Figure 1, Hare et al 2008; National Research Council, 2011) when no emotional information is present, not only do many adolescents perform as well as adults, some perform even better. However when decisions are required in the heat of the moment (i.e., in the presence of emotional cues, Figure 2A), then performance falters (Figure 2B). Specifically, adolescents have difficulty suppressing a response to appetitive social cues relative to neutral ones. This diminished ability is not observed in children and adults, who show equal difficulty regardless of emotional content of the nontarget. Thus, the description of teens as “all gasoline, no brakes, and no steering wheel” (Bell & McBride, 2010) more accurately reflects their behavior in heated situations rather than cool, less immediate and less emotional ones. In these cool situations, the teen is quite capable of acting rationally and making optimal decisions.
Figure 1. Performance of a standard Go/Nogo Task by Age.
D-prime, a measure of accuracy that includes both hits and false alarms, is plotted as a function of age illustrating improvements in performance with age, but high variability with some adolescents performing as well or better than some adults as indicated in gray box (Data from Hare et al. 2008, National Research Council, 2011).
Figure 2. Developmental and Individual Differences in Behavior and the Brain.
Teens unlke children and adults, make more false alarms to positive social cues than neutral ones on a go/nogo task (A and B). This behavioral performance is paralleled by enhanced activity of the ventral striatum (D and E), part of the reward circuit) to appetitive cues in teens relative to children and adults (bottom left). Low delayers make more false alarms to positive social cues than high delayers on a go/nogo task (C). This behavioral performance is paralleled by enhanced activity of the ventral striatum in low dealyers realtive to high delayers (F). Adapted from Somerville, Hare & Casey, 2011; Casey et al. 2012.
Overgeneralization 2. Adolescents have no Prefrontal Cortex
To say one studies the adolescent brain, has often been met with comic skepticism and relief that adolescents do indeed have a brain. There is no hole in the head or missing parts to suggest a lesion related impairment during this period. Moreover, the prefrontal cortex, a region important in self control and rational decisions is clearly present even from birth. What is changing during this period of development is the strength of connections within prefrontal circuitry as we learn to adapt to changing environmental demands (Liston et al 2005 Cerebral Cortex). This development reflects a combination of evolutionarily shaped biological constraints and experiential history, which interact to shape the brain and behavior.
Evidence from human imaging and animal studies of regional neurochemical, structural and functional brain changes with development have led to a theoretical account of adolescence referred to as the imbalance model of brain development (Somerville & Casey, 2010). According to this view, reward-related subcortical regions and prefrontal control regions interact differently across development. Specifically, motivational and emotional subcortical connections develop earlier than those supporting prefrontal control. This developmental imbalance results in relatively greater reliance on motivational subcortical regions versus prefrontal regions during adolescence (i.e., imbalance in reliance of systems), compared to adulthood, when this circuitry is fully mature and also compared to childhood, when this circuitry is still developing. With age and experience, the connectivity between these regions is strengthened and provides a mechanism for top down modulation of the subcortically-driven emotional behavior that increases the capacity for self control.
Recently a number of human imaging studies have attempted to evaluate this model and test for unique patterns of brain activity in adolescents during stereotypical risky behavior in the context of incentives (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; J. R. Cohen et al., 2010; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Van Leijenhorst et al., 2010). This work challenges the view that diminished self control in adolescents is due to a less mature prefrontal cortex, thus less successfully exerting regulatory control on behavior (Bell & McBride, 2010). In contrast, these studies show a unique sensitivity to motivational cues during adolescence that appear to challenge the less mature cognitive control systems when called upon simultaneously in tasks that involve inhibiting attention or actions toward potential incentives. Accordingly, developmental differences in self control arise due to maturational constraints of developing brain circuitry and the connectivity between these interacting brain systems with experience (Liston et al., 2006).
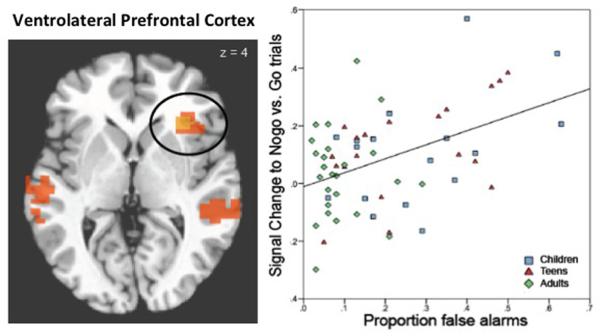
To better understand changes in self control during adolescence, we used functional brain imaging together with our previously described go/nogo task. Specifically we examined the neural correlates of self control in the face of emotional and nonemotional cues. We found that the ability to suppress a habitual response, regardless of emotional content, relied on the ventrolateral prefrontal cortex (Figure 3). Activity in this region, showed a monotonic increase with age for correct trials that was correlated with behavioral performance. In contrast, the ability to suppress a response to emotional cues revealed a different pattern of brain activity. Specifically diminished behavioral performance by adolescence in suppressing responses to positive emotional cues was paralleled by enhanced activity in the ventral striatum (Figure 2D and E), a region critical for detecting and learning about novel and rewarding cues in the environment. These findings suggest an exaggerated ventral striatal representation of appetitive cues in adolescents that may serve to “hijack” a less fully mature prefrontal control response. Thus, adolescent decisions and actions are not solely due a less mature prefrontal cortex, but rather to a tension within neural circuitry involving the ventral striatum, implicated in reward processing, and the prefrontal cortex implicated in control processing.
Figure 3. Ventral Prefrontal Activity correlates with Go/Nogo Task Performance.
The left panel illustrates localization of the ventral prefrontal cortical region that correlates with behavioral performance. The right panel (Adapted from Somerville, Hare & Casey, 2011) illustrates the correlation between BOLD signal in the ventral lateral prefrontal cortex and go/nogo task performance by age group.
Overgeneralization 3. All Adolescents Experience Similar Degrees of Storm and Stress
Most everyone reading this article has survived adolescence reasonably well. Clearly we’re not all doomed during adolescence as suggested by G. Stanley Hall’s theory of adolescence (Hall, 1904). Rather, adolescence falls somewhere between the extreme views of Hall’s storm and stress theory of adolescence and Margaret Mead’s cultural- not biological argument of adolescence (Mead, 1928). Basically, our behavior is a reflection of environmental and genetic factors that impact our brain’s ability to adapt to changing environmental demands. Some environmental demands are universally expected and some are specific to an individual’s experiences. How well we adapt to these changing environmental demands is a function of biological constraints and experiential history. Thus, even as adults we may differ in our ability to face new challenges and adequately regulate our behavior accordingly.
A hallmark of self regulation is the ability to resist temptation of an immediate reward for a later larger reward later, known as delay of gratification. A classic paradigm for assessing this ability was developed by Mischel (Mischel, Shoda, & Rodriguez, 1989) for use with young children. He examined whether children would choose a small reward (one marshmallow) sooner than a larger reward (two marshmallows) later. Children’s behavior fell into two clusters: 1) they would eat the treat almost immediately (low delayers); or 2) they would wait for some time in an attempt to gain two treats (high delayers). These two different patterns of behavior provide an example of individual differences in self control that can be detected and measured in early childhood (Mischel et al., 1989). However, how do these individuals fare in self control ability later in life?
We recently examined self control in a 40-year follow-up of the original cohort of children Mischel tested on the delay of gratification task to address this question. Using both neutral (“cool”) and emotional (“hot”) cues in a go/nogo task we examined the ability of these individuals, now in their mid-forties, to stop habitual responses to emotional or neutral cues. Since marshmallows don’t quite have the same appeal for adults as children, rather than using marshmallows we used social cues (e.g., happy faces relative to neutral and fearful faces), as nontargets in a go/nogo task.
The results indicated that even 40 years later, the same individuals who could not stop themselves from immediately eating the marshmallow and thus not getting two, also had difficulty stopping themselves when a positive social cue was present, even when instructed not to respond (Figure 2C). However, they had no problem stopping a habitual response to neutral cues (Casey et al., 2011). Thus, individuals who, as a group, had more difficulty delaying gratification at 4 years of age continued to show reduced self-control abilities 40 years later. These findings highlight individual differences in self control independent of age that can persist throughout the lifespan. However, a remaining question is whether the neural correlates underlying individual differences in self control, are similar to those observed in adolescents in our previously described study?
To address this question, high- and low- delaying individuals were imaged during performance of the “hot” go/nogo task (Casey et al., 2011). The findings showed that while prefrontal activity was associated with accurately withholding a response, activity in the ventral striatum mapped onto the behavioral finding of poorer performance when specifically suppressing a response to an appetitive social cue (Figure 2F).
These findings underscore the importance of the stimulus qualities a person has to resist in self control. Sensitivity to characteristics of environmental cues (salience, reward value) can significantly influence an individual’s ability to suppress inappropriate actions in favor of appropriate ones. This tension between regulation of behavior and sensitivity to positive environmental cues in many ways parallels observations from our adolescent study (Somerville et al., 2011). Perhaps not surprisingly, delay of gratification ability at 4 years of age predicts parental ratings of adolescent self control too (Mischel, Shoda & Peake, JPSP 1988). Both examples show how the stimulus qualities such as rare positive social cues can compromise an individual’s self control and suggest that both developmental and individual differences affect this ability. Thus, individuals who have diminished self-control may be especially vulnerable during adolescence when a heightened sensitive to emotional environmental cues can further hinder this ability.
DISCUSSION
Our findings suggest that adolescence can show remarkable restraint in controling habitual responses, but tend to fail when controling habitual responses to salient positive cues in the environment. Specifically, we showed that adolescents have comparable or even better impulse control than some adults (Figure 1) in neutral contexts. However, in emotional contexts, adolescents’ impulse control ability is severely taxed relative to children and adults (Figure 3). This behavioral pattern is paralleled by exagerated responses in reward related circuitry teenagers that presummably are difficult to regulate due to less mature prefrontal control regions. This tension between motivational and control processes during adolescence can vary by individual leading to enhanced or diminished self control. To say that the adolescent is “all gasoline, no brakes, and no steering wheel” (Bell & McBride, 2010) is a disservice to this essential phase of typical development. If indeed the objective of adolescence is to gain independence from the family unit, then providing opportunities for adolescents to engage in new responsibilities is essential. Without opportunities and experiences to help shape their brain and behavior optimally, the objectives of this developmental phase will not easily be met.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Mental Health grant nos. P50MH062196 and P50MH079513, the National Institute of Drug Abuse grant nos. R01DA018879 and R01 HD069178, National Science Foundation Grant 06-509, and the MacArthur Foundation Law and Neuroscience Network.
REFERENCES
- Bell CC, McBride DF. Affect regulation and prevention of risky behaviors. JAMA : the journal of the American Medical Association. 2010;304(5):565–566. doi: 10.1001/jama.2010.1058. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental science. 2011;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD. The vulcanization of the human brain: A neural perspective on interactions between cognition and emotion. Journal of Economic Perspectives. 2005;19(4):3–24. [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS spectrums. 2001;6(1):60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. Stanley. Adolescence: In psychology and its relation to physiology, anthropology, sex, crime, religion, and education. Vol. I & II. Englewood Cliffs, NJ; Prentice-Hall: 1904. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Mead Margaret. Coming of age in samoa; a psychological study of primitive youth for western civilisation. W. Morrow & Company; New York: 1928. [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of cognitive neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? Developmental psychobiology. 2010;52(3):236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current opinion in neurobiology. 2007;17(2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
SUGGESTED READINGS
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14998–15003. doi: 10.1073/pnas.1108561108. This study provides empirical evidence of stable individual differences over 40 years in self control that are associated with differences in ventral frontostriatal circuitry.
- Casey BJ, Duhoux S, Cohen MM. Adolescence: What Do Transmission, Transition, and Translation Have to Do with It? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. A review highlighting that adolescence is not special to humans but rather observed across species and is an evolutionarily adaptive and necessary phase of typical development.
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of cognitive neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. This study provides empirical evidence showing that adolescence can show remarkable restraint in controling habitual responses, but tend to fail when controling habitual repsonse to salient positive cues in the environment. This behavioral pattern is paralleled by exagerated responses in reward related circuitry relative to children and adults.
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. Using a passive viewing gambling task, this article reports striatal and prefrontal regions that are more sensitive to the anticipation and receipt of rewards in adolescence.
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. Using an antisaccade task, this article demonstrates how reward motivation dynamically influences recruitment of striatal and prefrontal regions in adolescents.
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental science. 2011;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. Chein and colleagues emphasize the role that peers play in adolescent decision making, and highlight the ventral striatum as a brain region through which the presence of peers influence risky decision making in adolescents.
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. This study provides empirical evidence that adolesents reative to children and adults have a faster response to stimuli associated with large rewards and a greater positive predicition error in the ventral striatum. Cohen and colleagues discuss how adolescent decision making and behavior may be understood using quantitative behavioral decision making approaches.