Abstract
Epithelia form the building blocks of many tissue and organ types. Epithelial cells often form a contiguous 2-dimensional sheet that is held together by strong adhesions. The mechanical properties conferred by these adhesions allow the cells to undergo dramatic three-dimensional morphogenetic movements while maintaining cell–cell contacts during embryogenesis and post-embryonic development. The Drosophila Folded gastrulation pathway triggers epithelial cell shape changes that drive gastrulation and tissue folding and is one of the most extensively studied examples of epithelial morphogenesis. This pathway has yielded key insights into the signaling mechanisms and cellular machinery involved in epithelial remodeling. In this review, we discuss principles of morphogenesis and signaling that have been discovered through genetic and cell biological examination of this pathway. We also consider various regulatory mechanisms and the system's relevance to mammalian development. We propose future directions that will continue to broaden our knowledge of morphogenesis across taxa.
Keywords: Apical constriction, Morphogenesis, Cell signaling, Folded gastrulation, Actin, Myosin
Introduction
Epithelial morphogenesis, the process through which simple sheets of cells are rearranged and change shape to form mature structures and organs, is an area of intense focus in the field of developmental biology (Nelson and Gleghorn, 2012; Spear and Erickson, 2012; Suzuki et al., 2012). A key morphogenetic movement, which occurs in almost all multicellular animals, is the folding or bending of flat epithelial sheets to form more complex configurations. These changes are often driven at least in part by actin- and myosin-based apical constriction (Sawyer et al., 2010). One of the best-studied developmental signaling pathways regulating this process is the Drosophila Folded gastrulation (Fog) pathway in which many of the crucial molecular events are known, from initiation by transcription factors (TFs) to the mechanics of cell shape changes. This pathway, which drives apical constriction, therefore allows examination of some of the intricacies of cell signaling during development in vivo.
Many stereotypical signaling mechanisms are exemplified in the Fog pathway, including patterned induction of gene expression by TFs, G-protein coupled receptor (GPCR) to G-protein signaling, and actin rearrangement induced by Rho GTPase signaling. The Fog pathway also reveals some novel insights, such as how multiple signaling pathways can be integrated into a single outcome and that GPCRs, among their many other functions, have morphogenetic roles. While certain aspects of the Fog pathway have been worked out in great detail, many questions still remain. What mechanisms recruit signaling components apically? How are Fog pathway components spatially and temporally patterned in tissues and time and what role does this patterning play in development? Which mechanisms regulate the attenuation of Fog signaling? We will explore these questions in this review.
Pathway overview
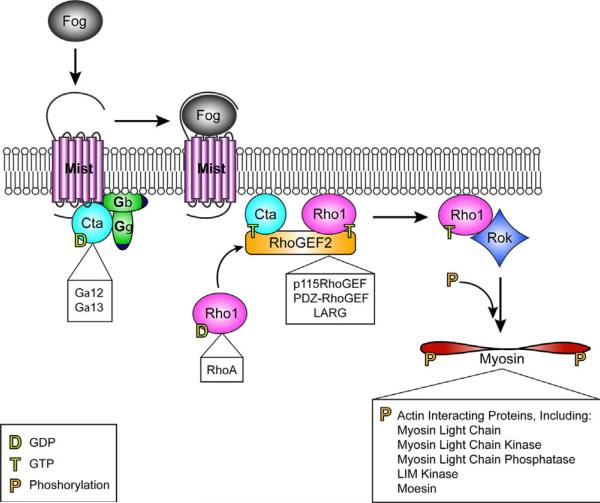
The Fog pathway, diagramed in Fig. 1, begins with the specific expression of Fog in subsets of cells fated for actomyosin-based shape changes. Fog is a large secreted protein that is thought to signal primarily as an autocrine factor (Costa et al., 1994). The Fog signal is transmitted across the plasma membrane by the GPCR Mesoderm invaginating signal transducer (Mist), a member of the secretin family of GPCRs, to a G-protein of the Gα12/13 family, Concertina (Cta; Parks and Wieschaus, 1991; Manning et al., 2013). In turn, RhoGEF2, a Dbl family Rho guanine nucleotide exchange factor (RhoGEF), the small GTPase Rho1, and the Rho effector, Rho Kinase (Rok) are all activated (Barrett et al., 1997; Dawes-Hoang et al., 2007). Rok phosphorylates the regulatory light chain of non-muscle myosin II to induce contraction of the apical actomyosin network in the cells that receive the Fog signal. While the ligand, Fog, is not conserved outside of Drosophila and the receptor, Mist, is not conserved outside of insects, the axis of signaling from Gα12/13 proteins through Rho to affect actin rearrangement is highly conserved and is important in human development and disease (Fig. 1; Waterhouse et al., 2011). For example, lysophosphatidic acid and sphingosine 1-phosphate are membrane lipid derivatives known to signal through GPCRs, the Gα12/13 family, RhoGEFs, RhoA, and various downstream effectors in mammals (Suzuki et al., 2009; Xiang et al., 2013). These pathways modulate cytoskeletal and cell shape changes such as neurite outgrowth and retraction, tumor cell invasion, or angiogenesis.
Fig. 1.
The Fog Signaling Pathway. Fog is a large secreted protein which acts as a ligand for Mist, a seven pass transmembrane GPCR. In its ligand-free state Mist is predicted to interact with inactive, GDP-bound Cta. Once Fog binds Mist, it likely stimulates Cta's exchange of GTP for GDP, which allows Cta to dissociate from its trimer partners, Gβ and Gγ. Cta-GTP binds to RhoGEF2 which can then act as a GEF for Rho1. In its GTP-bound form Rho1 then activates Rok. Finally, the regulatory light chain of non-muscle myosin II, Spaghetti squash, is phosphorylated by active Rok to induce apical actomyosin network contraction in the cells which receive the Fog signal. Boxed are vertebrate components of Rho axis signaling which act in a similar manner to induce actomyosin cytoskeleton rearrangements. In vertebrates, Rok is known to phosphorylate many proteins which interact with actin, activating some and inactivating others.
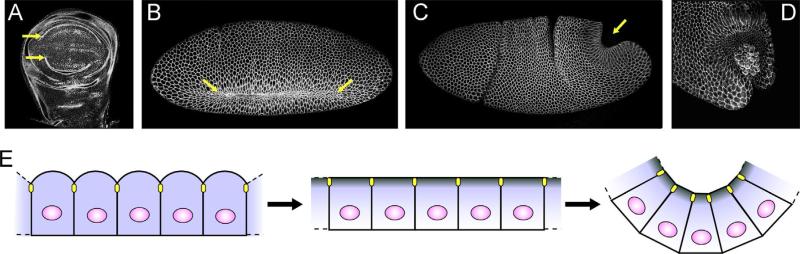
The Fog pathway is active in several morphogenetic events in Drosophila development, with known roles in ventral mesoderm and posterior midgut (PMG) invagination during gastrulation, salivary gland internalization in mid-embryogenesis, and imaginal disc folding during larval development (Fig. 2A–D; Costa et al., 1994; Nikolaidou and Barrett, 2004). It has also been proposed that Fog is involved in morphogenesis of the central nervous system during late embryogenesis (Ratnaparkhi and Zinn, 2007). In most of these cases Fog induces apical constriction, although in the CNS the cellular results of Fog's action are not known.
Fig. 2.
Morphogenetic changes induced by the Fog pathway: (A) Third instar imaginal wing disc. Actin staining highlights epithelial folds. (B) Ventral furrow invagination. (C) Posterior midgut invagination. (A–C) yellow arrows denote cell groups undergoing Fog pathway induced apical constriction. (D) Closer view of posterior midgut cells undergoing apical constriction. Germ cells are carried in with this invagination. (B–D) embryos are stained for Neurotactin to outline cells. (E) Cartoon of cell shape changes induced by the Fog pathway. When cellularization is complete, adherens junctions (yellow ovals) are sub-apical and apical cell surfaces are rounded. Fog pathway members become apically concentrated (denoted by shading of cells) and apical cell surfaces flatten. When the Fog pathway is activated cell apices constrict and cells elongate apicobasally.
Before cells begin apical constriction proper, they generally have domed apical surfaces which become flat before constriction begins (Fig. 2E; Dawes-Hoang et al., 2007). During apical constriction the myosin in the actin network along the apical membrane of the contracting cells is activated, reducing the size of the network, pulling on apical junctions, and reducing the apical area of the cell (Sweeton et al., 1991). Because of the junctional connections bound to the actin, each cell pulls its neighbors inward during this process. At the same time as their apices are shrinking cells elongate in the apical–basal direction which aids in internalization. After apical constriction is complete, cells shorten apicobasally to become fully internalized (Pouille and Farge, 2008). Apical constriction, along with other concomitant shape changes, in cells of the ventral mesoderm, PMG, and salivary gland eventually results in complete internalization of these cell groups. The cells of imaginal discs only invaginate as far as to form U-shaped folds within the plane of the tissue.
During ventral furrow (VF) formation there are two phases of apical constriction: a stochastic, nonproductive phase, when individual cells contract and relax without any overall reduction in apical area, and a concerted, coordinated phase, when individual cells undergo cyclical ratchet-like rounds of reductions in apical area which are much more stable (Sweeton et al., 1991; Martin et al., 2009). During both phases, actin and myosin periodically coalesce and these concentrations tend to move toward the center of a cell (Martin et al., 2009). Via these contractions, the plasma membrane is pulled inward. During random constriction the membrane relaxes to its original position when actomyosin coalescences are disassembled. Once the concerted phase of constriction begins, membrane deformations are stabilized to reduce apical cell area. This pulsatile mode of actomyosin constriction has also been observed in other contracting groups of cells in the Drosophila embryo, as well as in C. elegans and Xenopus (Munro et al., 2004; Skoglund et al., 2008; Solon et al., 2009; Roh-Johnson et al., 2012).
In addition to the conserved nature of the signaling components, the cell shape changes elicited by Fog signaling are similar to morphogenetic processes in mammals (Schoenwolf and Franks, 1984; Sweeton et al., 1991). Internalization of the mesoderm during Drosophila gastrulation closely resembles neural tube formation in vertebrates. In both cases, a subset of epithelial cells within a flat sheet undergoes apical constriction to invaginate and form a tube sealed off from the surrounding epithelium (Copp and Greene, 2010). When these processes are disrupted Drosophila embryos die at the end of embryogenesis; in humans debilitating congenital defects such as spina bifida or anencephaly can occur, sometimes leading to death. Working out the intricacies of the Fog signaling pathway and its resulting cell and tissue movements will ultimately lead to a more profound understanding of our own development and greater potential for medical interventions in disease states.
Ligand and receptor
Any discussion of the core components of the Fog signaling pathway must begin with Fog itself. Although it has not been studied biochemically, Costa et al. originally predicted that Fog is a secreted protein based on the presence of a putative amino-terminal secretion signal sequence and multiple sites for N- and O-linked glycosylation (Costa et al., 1994; Morize et al., 1998). This prediction was later confirmed when Fog was localized by immunofluorescence to secretory vesicles in presumptive mesodermal cells (Dawes-Hoang et al., 2007). Embryos lacking Fog exhibit disorganized VF cell apical constriction, although most mesodermal cells are eventually internalized (Costa et al., 1994). Major problems arise in the next steps of development, however, since PMG cells do not invaginate and improper germ band extension (GBE), the elongation of the anterior-posterior body axis, leads to twisting of that body axis. All embryos mutant for fog die before hatching. Embryos lacking fog in subsets of cells that cross the VF have a distinct division between apically constricting cells (wild-type) and non-constricting cells (fog mutant). This result suggested that the Fog signal does not diffuse farther than a couple of cell widths and acts primarily cell autonomously, consistent with it being a large, secreted protein.
The most recent addition to our knowledge of Fog signaling was the discovery of a receptor, Mist, that can function downstream of Fog (Manning et al., 2013). Mist is a GPCR with a large extracellular domain, appropriate for interacting with a large ligand such as Fog. This receptor, which had eluded conventional genetic approaches, was identified in an RNAi screen for GPCRs using a cell culture model that reproduced Rho1 pathway activation by Fog. Conditioned media was collected from a stable Drosophila S2 cell line inducibly expressing Fog protein and then applied to Drosophila S2R+ cells. These cells respond to exogenously added Fog by changing their shape from a flat profile to a conical shape due to actomyosin constriction and, thus, provide a visual readout for pathway activation. Mist is both necessary and sufficient for Fog-induced contractility of cultured cells. This system has the exciting potential to be used to interrogate other aspects of Fog signaling, as well.
In gastrulating embryos, Mist was found to be a zygotic gene specifically expressed in the VF and PMG primordia and mist mutants exhibit gastrulation defects similar to fog and cta (Parks and Wieschaus, 1991; Manning et al., 2013). fog and mist transcription are both precisely regulated in space, but seem to be under independent control, with overlapping but not completely coincident expression patterns (Dawes-Hoang et al., 2007; Manning et al., 2013). This redundancy helps explain how the formation of Fog-induced epithelial invaginations is so regular within the complex developmental dynamics of wild-type animals.
Ubiquitous overexpression of Fog in the early embryo results in a normal VF and no precocious apical constriction (Dawes-Hoang et al., 2007). This can now be explained by mist's restriction to ventral and posterior cells and its upregulation at the end of cellularization when VF invagination normally begins (Manning et al., 2013). The opposite is also true–ubiquitous expression of Mist does not significantly disrupt gastrulation, presumably due to spatial restriction of Fog expression. Adding complexity to the situation, however, is that ubiquitous Fog overexpression results in apical flattening without apical constriction in cells outside the VF (Morize et al., 1998; Dawes-Hoang et al., 2007). This observation may be explained by a low level of Mist in dorso-lateral cells that allows flattening, but does not reach the threshold for full apical constriction. There is also the possibility of additional Fog receptors working either redundantly with, in concert with, or differently from Mist in the same or different tissues. For example, there may be a second receptor in cells outside the VF and PMG invaginations in the early embryo that responds to Fog by inducing apical flattening specifically. Another possibility is a redundant receptor in other tissues, though it is not likely this plays an essential role in the VF and PMG given the similarities of mist and fog zygotic phenotypes (Manning et al., 2013). Mist may also have an obligate coreceptor, in which case missing either one of the pair would phenocopy a complete lack of receptor. Overexpressing Fog in the mist mutant will help to answer some of these questions. A candidate receptor that could work with or in parallel to Mist is another GPCR, CG31660, which was suggested by genetic screening to play a role during the morphogenetic movements of gastrulation (Mathew et al., 2009). The precise actions of this receptor and its possible interactions with Mist or Fog have not yet been determined.
The recent discovery of a receptor connecting Fog and Cta activation across the plasma membrane in the well-studied Fog signaling pathway establishes an experimentally tractable system to examine GPCR activity in vivo. Complementary approaches using Drosophila cell lines will add to our understanding of GPCR signaling. As Mist is a primary example of G-protein signaling in morphogenesis, it will be extremely interesting to learn all that we can from this system.
Heterotrimeric G-protein signaling
Among all of the known Fog pathway components, Cta was discovered first and yet comparatively little is known about it (Schüpbach and Wieschaus, 1989; Parks and Wieschaus, 1991). Embryos lacking maternal Cta exhibit similar gastrulation pheno-types to fog or mist zygotic mutants. Cta is required to organize myosin apically in the contractile VF cells, though it is not essential for apical actin accumulation (Dawes-Hoang et al., 2007; Fox and Peifer, 2007). Cta is expressed much more broadly throughout embryogenesis than are Fog and Mist, and likely has roles outside the VF and PMG. One possible Fog-independent role of Cta is in maintenance of cortical cytoskeletal stability throughout the blastoderm (Kanesaki et al., 2013).
In the early embryo, ubiquitous expression of constitutively active Cta or injection of cholera toxin, which activates Cta, phenocopies ubiquitous expression of Fog, including apical flattening but not apical constriction of all cells (Morize et al., 1998). This result suggests that Fog-dependent apical flattening works through Cta, though as mentioned above it may not work through Mist. Receptor-specific Cta activation and subcellular localization in certain cells may help restrict which effectors are activated downstream of Cta and therefore which cellular pathways are triggered. Unfortunately, no method for visualizing endogenous Cta has been developed, making it difficult to learn about this protein in more detail. A reliable antibody to Cta or replacement of the endogenous gene with a tagged version would be highly beneficial to the field and open up a wealth of new information about how G-proteins function during development in vivo.
Gα proteins function with Gβs and Gγs in obligate heterotrimers. Gβ13f and Gγ1 have been suggested as partners for Cta during gastrulation as embryos lacking either have gastrulation and cuticle phenotypes similar to those lacking Cta, consistent with a role for these proteins in Fog signaling (Fig. 3; Schaefer et al., 2001; Izumi et al., 2004; Wang et al., 2005). They are also important for Fog-induced cellular constriction in culture (Peters and Rogers, 2013). Gα proteins are generally thought to be the primary signal transducing members of heterotrimeric G-proteins, but it is now well established that β and γ subunits can signal independently of Gαs (Clapham and Neer, 1997; Dupré et al., 2009). However, their precise signaling role in the Fog pathway remains unknown. Additionally, some Gαs have been reported to require the chaperone-like cofactor, Ric-8, for proper localization (Wang et al., 2005). In the Fog-cell culture model, Ric-8 regulates Cta localization and is required for it to signal downstream of Fog (Peters and Rogers, 2013). Embryos lacking Ric-8 have disrupted VF apical constriction resulting in similar cuticle phenotypes to embryos from cta mutant mothers (Wang et al., 2005; Kanesaki et al., 2013). Ric-8 is also necessary for apical myosin accumulation and cortical tension during VF formation (Kanesaki et al., 2013). It will be interesting to further investigate the roles of these three essential co-factors in epithelial morphogenesis.
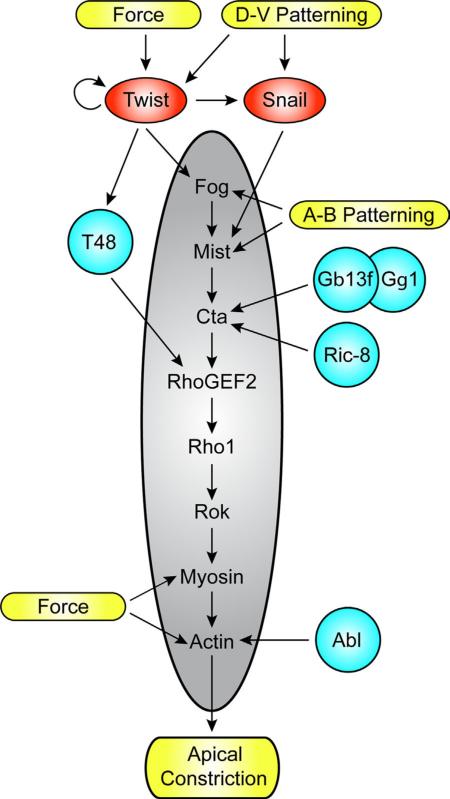
Fig. 3.
Known Inputs into the Fog Signaling Pathway. The core Fog signaling pathway components are shown in the central gray oval. Transcription factors are in red ovals. Accessory proteins are in aqua circles. Yellow bars denote physical changes. Physical forces act on Twist, myosin, and actin to change their abundance and localization, though the mechanisms of these functions and whether they are direct are not entirely clear. Dorsal–ventral patterning sets up Twist and Snail expression. Twist induces transcription of fog and T48 in VF cells. Similarly, Snail is necessary for mist transcription in the VF. Apical–basal patterning organizes Fog and Mist subcellular organization. T48, a single pass transmembrane protein, helps to localize RhoGEF2 apically in the VF. Gβ13f, Gγ1, and Ric8 are all required for Cta protein stability and function. Abl helps organize actin apically in contracting cells. All of these inputs, and likely more, help organize and activate Fog signaling in developmental time and space.
The Rho signaling axis
The intracellular signaling components of the Fog pathway fit into the well-established Rho signaling axis that leads from activation of a Gα12/13 family member to actin cytoskeletal rearrangement, (e.g. Somlyo and Somlyo, 2000). Some vertebrate members of this pathway are listed in boxes in Fig. 1. Cta, RhoGEF2, Rho1, Rok, myosin, and actin are present in all cells in Drosophila early embryos and imaginal discs (Warn and Magrath, 1983; Kiehart et al., 1990; Parks and Wieschaus, 1991; Barrett et al., 1997; Hacker and Perrimon, 1998; Mizuno et al., 1999). They are all supplied maternally to embryos, suggesting they have broad importance during the early stages of development. However, these proteins are apically localized specifically in cells undergoing apical constriction (Fig. 2E). The presence and activity of their upstream activators and their limited subcellular localization help give developmental control to their downstream effects. This section aims to highlight some of the important points we have learned about how this pathway enacts cell shape changes from studying Fog signaling and what we can potentially learn from further examining the Rho axis signaling in Drosophila.
RhoGEFs act to transduce upstream signals to Rho and specify the subcellular location where Rho will be activated. Maternal RhoGEF2 mutant gastrulation phenotypes are much more severe than either zygotic fog or maternal cta mutants, with no mesoderm or endoderm (PMG) internalization at all (Barrett et al., 1997; Hacker and Perrimon, 1998). Additionally, unlike fog and cta mutants, RhoGEF2 mutants have defects in both actin and myosin accumulation at the apical sides of VF cells (Fox and Peifer, 2007). These data suggest that there is another pathway feeding into the activation of RhoGEF2 in the VF that is somewhat additive with the input from Fog–Mist–Cta. (Some possibilities will be discussed in the “Other inputs into Fog-induced cell shape change” section below.)
Rho1 acts in early embryos and cell culture to organize both the actin and myosin networks, with Cta upstream of its action on myosin (Halsell et al., 2000; Fox and Peifer, 2007). Disruption of Rho1 function in early embryos by exogenous expression of a dominant negative version mimics the genetic loss of RhoGEF2 (Barrett et al., 1997; Hacker and Perrimon, 1998). Embryos with disruptions in RhoGEF2 or Rho1 do exhibit sporadic apical constriction but not in a coordinated or concerted fashion. However, Rho1 and RhoGEF2 maternal mutants have noticeably different phenotypes, with Rho1 mutants having more and varied cell shape defects throughout embryogenesis (Barrett et al., 1997; Magie et al., 1999). The interpretation of these results is complicated by the requirements for Rho1 during egg formation and cellularization, but does suggest that Rho1 can be activated by other RhoGEFs in addition to RhoGEF2 or by other mechanisms during embryogenesis (Crawford et al., 1998; Magie et al., 1999; Simões et al., 2006). Overall, RhoGEF2 does not seem to be absolutely necessary for actin and myosin rearrangement but acts to organize and maintain actomyosin structures and contractions.
In addition to their roles in embryogenesis, Rho1, RhoGEF2, and zipper (encoding the heavy chain of myosin II) all interact genetically in leg and wing morphogenesis, during imaginal disc folding and/or limb eversion (Halsell et al., 2000; Nikolaidou and Barrett, 2004). Fog, Mist, and Cta have all been implicated in these processes, as well (Nikolaidou and Barrett, 2004; Manning et al., 2013). Improper expression levels or patterns of Fog pathway components in wing imaginal discs leads to stochastic rather than patterned folding of the epithelium. Proliferation, specification, and polarity of discs do not seem to be altered when the Fog pathway is disrupted, but normal growth of the tissue forces once flat epithelial sheets to adopt random folds within the confines of the disc in the absence of proper patterning information (Nikolaidou and Barrett, 2004). These data again confirm that patterning and specificity of Rho activation is crucial during morphogenesis. Imaginal disc development will continue to provide a powerful tool to study the signaling pathways involved in tissue morphogenesis. The ability to visualize a living flat epithelium undergoing morphogenetic movements while visualizing patterns of small GTPase activation using recently developed bioprobes represents an exciting area for future work (Kamiyama and Chiba, 2009; Aldaz et al., 2010).
Transcription factors
There are several factors that contribute to the expression pattern of Fog pathway components, as well as initiation and organization of the pathway itself. First, transcriptional control of certain Fog pathway members can influence pathway activation within developmental space and time. We know the most detail about this topic relative to VF formation. During egg production, a nuclear gradient of the Dorsal TF is maternally set, with the highest levels on the ventral side of the egg (Roth et al., 1989). The cells with the highest nuclear levels of Dorsal then zygotically transcribe the TFs Twist, a member of the basic helix–loop–helix family, and Snail, a zinc finger TF (Leptin and Grunewald, 1990). Twist in the ventral mesoderm reinforces both its own expression and that of Snail (Ip et al., 1992). Twist and Snail are each independently required for both mesoderm specification and the morphogenetic movements of gastrulation, though they have slightly different phenotypes (Fig. 3; Leptin, 1991). twist single mutants retain some ability to accumulate myosin and constrict VF cells, though they are never able to transition to the coordinated, productive phase of apical constriction (Martin et al., 2009). Twist is required to stabilize actomyosin-based constrictions, perhaps due in part to mechanosensation (see “Mechanical inputs” below). snail mutants do not undergo visible apical myosin coalescence, though some mesodermal cells are eventually internalized, suggesting that Snail is required for the initial stages and coordination of apical constriction (Martin et al., 2009). In snail twist double mutants VF cells do not accumulate myosin apically, contract, or form an invagination suggesting that these two TFs together are necessary to transcribe key molecules involved in all steps of VF cell shape change (Leptin, 1991; Martin et al., 2009).
Some of the transcriptional targets of these two TFs are known. Twist activates the transcription of both fog and T48, a single pass transmembrane protein that acts to apically localize RhoGEF2 during VF formation (see “Other inputs into Fog-induced cell shape change” below; Fig. 3; Morize et al., 1998; Kolsch et al., 2007). Snail's only known target necessary for gastrulation is mist (Fig. 3; Manning et al., 2013). fog mRNA and mist mRNA have similar expression patterns in wild type embryos, with enrichments along the ventral side and the posterior end of the embryo. One marked difference between them is that mist RNA is present in a continuous stripe while fog RNA exhibits a gap between its mesodermal (VF) and endodermal (PMG) patches (Costa et al., 1994; Manning et al., 2013). fog RNA in twist mutant embryos and mist RNA in snail mutant embryos both lose expression in the ventral mesoderm while retaining it in the PMG, suggesting that an independent set of TFs is probably required in the PMG (Seher et al., 2006; Manning et al., 2013). These somewhat independent and overlapping patterns of receptor and ligand expression help provide robust spatial control of apical constriction is important during this morphogenetic event.
Identification of Mist as a transcriptional target of Snail clarified several previously unexplained results. First, ectopic Fog expression in wild-type or fog mutant embryos induces a VF to form in its normal location (Morize et al., 1998). Twist is not required for this to occur. In snail mutants, however, ectopic Fog expression fails to induce flattening of VF cell apices (Morize et al., 1998; Dawes-Hoang et al., 2007). Mist is likely the Snail target required for apical flattening, at least in VF cells. Second, the stochastic phase of VF apical constriction occurs in twist but not snail mutants (Martin et al., 2009). Twist, T48, and, importantly, Fog are not required for random cellular constrictions, but a Snail target is. This could be explained by spontaneous agonist-free excitation of Mist, which is a property of many GPCRs (reviewed in Smit et al. (2007)). Overlapping expression patterns of Mist and Fog by means of Snail and Twist provide a novel mechanism for robustly controlling the location and timing of a developmental signaling pathway.
Outside of the VF we do not know the transcriptional regulators controlling Fog pathway members. The Fork head TF is necessary for salivary gland primordium apical constriction and invagination (Myat and Andrew, 2000). As Fork head is also expressed at the extreme ends of the early embryo, it may also be involved in PMG invagination, though it has not been specifically implicated in controlling Fog signaling in either of these processes (Weigel et al., 1989). Fog and Mist expression patterns in the wing imaginal disc are complex and do not simply follow any known TF patterns (Manning et al., 2013). They are thus likely under combinatorial control of many TFs in this tissue. The downstream players in the Fog pathway are maternally deposited in embryos and are widely expressed in other tissues, thus their localized activity rather than expression is likely to determine their site of action.
Mechanical inputs
Another mode of control feeding into Fog signaling is mechanical force (Fig. 3). As a flat sheet of cells folds the apically constricting cells produce force that pulls on neighboring cells. Therefore, even cells within a folding sheet that are not actively contributing to the deformation can experience significant mechanical strain. We do not know all of the implications of these forces yet, but some interesting concepts have been advanced in the literature. For instance, cell volume is conserved throughout these complex shape changes and coordinates cell lengthening with apical constriction (Gelbart et al., 2012). Also, stresses across the apical surfaces of cells undergoing Fog signaling could increase the membrane tension enough to reduce endocytosis, leaving more activated Mist at the membrane for signaling (Driquez et al., 2011). Conversely, apical–basal shortening toward the end of furrow invagination could result in a reduction in cell surface area and membrane tension leading to an increase in endocytosis and termination of signaling.
As mentioned previously, VF cell contraction occurs in two phases: a random unproductive period of contraction and then a coordinated period that forms an epithelial fold (Sweeton et al., 1991; Martin et al., 2009). The trigger that allows for the change from the stochastic phase to the collective phase is not yet known. It has been suggested that this transition occurs when a threshold of strain is reached which has built up across the tissue during the stochastic contractions (Martin et al., 2010). This mechanical strain may feed directly into the actomyosin network through its connections to cell–cell contacts. Another mechanism whereby force can directly affect morphogenesis is through the anisotropy of the embryo. The Drosophila embryo is football shaped with the long axis corresponding to both the anterior-posterior axis and the VF axis. When VF cells begin to contract they attempt to do so isotropically, or equally in all directions, but the lower tension along the lateral axes due to the embryo shape encourages more constriction in that direction (Martin et al., 2010). In contrast to the potential roles of apical membrane strain, however, basolateral membranes present no barrier and move with the fluid flow of cytoplasm in embryos undergoing the VF formation (He et al., 2014). In other words, division of the cytoplasm by basolateral membranes is dispensable for apical constriction. It will be interesting to further study the interactions between signaling and mechanics during apical contraction and to investigate their roles in other organisms.
Force may also feed less directly into Twist, Fog, and T48 expression, as Twist protein expression has been positively correlated with the mechanical deformation of cells during GBE (Farge, 2003). Just after gastrulation, large-scale tissue rearrangements comprising GBE produce compressive forces on the dorsal side of the embryo and stretching forces on the ventral side. Physically disrupting GBE movements reduces Twist expression, but artificial force on these disturbed embryos can rescue Twist levels (Desprat et al., 2008). However, Twist expression remains in embryos with disrupted cell– cell adhesion, suggesting that force only plays a modulatory role (Harris and Peifer, 2005). (After VF formation Twist is no longer required for Fog signaling, but it is still necessary for proper mesoderm differentiation Leptin, 1991.) Similarly, Snail is required for apical myosin localization in the VF, but experimental indentation of snail mutant embryos can rescue myosin localization and promote complete mesoderm invagination (Pouille et al., 2009).
There is evidence for mechanical strain influencing RNA transcription, cytoskeletal dynamics, and tissue movements in many systems. For instance, formation of the head fold in the chick embryo, an epithelial folding event, exerts significant forces on the surrounding tissues (Varner et al., 2010). Application of ectopic forces to embryo explants undergoing this process alters their morphogenetic movements. Force has been hypothesized to be a conserved mechanism for initiation of gastrulation and/or mesoderm induction, being required in both Drosophila and zebrafish early embryogenesis for these processes (Brunet et al., 2013). We do not yet know how forces are involved in most tissues where Fog signaling is active, but this pathway and its resulting epithelial invaginations can be used to investigate the problem in a very detailed manner. The early Drosophila embryo and imaginal discs can be mechanically manipulated and methods have already been developed to do so, (e.g. Farge, 2003). The early embryo is a relatively simple, yet 3-dimensional in vivo system in which we can simultaneously modulate gene activity and mechanical stress. Insights about the interaction between these two inputs into the Fog signaling pathway will likely be broadly applicable to many developmental processes.
Subcellular localization
We know that much of the signal transduction within the Fog pathway must occur at or near the apical surface of contractile cells in order to restrict actomyosin contraction to cell apices, but we know very little about how this is achieved (Fig. 2E). We do know that fog mRNA is localized apically in invaginating PMG and imaginal disc cells, and mist mRNA is apical in imaginal discs (Fig. 3; Dawes-Hoang et al., 2007; Manning et al., 2013). Fog protein localizes to punctate vesicles in the apical portion of PMG cells during invagination, suggesting that it may be specifically apically secreted (Dawes-Hoang et al., 2007). Mist protein is also present in discrete punctae on the apical surface of VF cells during invagination (Manning et al., 2013). Localized translation and directional trafficking likely contribute to the apical localization of these proteins. Specific association of Cta with apical Mist or apical trafficking of Cta by Ric-8 in cells undergoing Fog signaling may act to restrict Cta to the apical domain, but these mechanisms have yet to be studied.
Before gastrulation, RhoGEF2 localizes to the basal ends of cellularization furrows but is redistributed throughout the cytoplasm once cellularization is complete (Barmchi et al., 2005; Grosshans et al., 2005; Fox and Peifer, 2007). RhoGEF2 then moves to the apical surface of VF cells just before constriction occurs. This striking relocalization may be promoted by RhoGEF2's association with the plus-ends of microtubules (MTs; Rogers et al., 2004). After activation of Cta, RhoGEF2 dissociates from MTs, possibly allowing for RhoGEF2 to associate with Cta itself and/or interact with lipids in the plasma membrane. Most MTs in the blastoderm epithelium are generally thought to be oriented with their plus-ends basally, the reverse orientation to that which would bring RhoGEF2 to the apical surface (Harris and Peifer, 2005). The MT arrays in many interphase Drosophila cells are acentrosomal, however, so there may be mixed polarity MT arrays or short MTs along apical cell surfaces which may contribute to localization of RhoGEF2 or other Fog signaling components (Rogers and Rogers, 2008). Alternatively, RhoGEF2's association with MT plus-ends could be a mechanism for keeping it basally localized and inactive before Fog pathway activation. This model is consistent with a recent study suggesting that dynamic microtubules are able to inhibit RhoGEF2 in epidermal cells (Bulgakova et al., 2013). The orientation and dynamics of MTs in contractile cells in vivo should be examined in greater detail in order to determine whether and how they play a role in localizing Fog signaling components.
Myosin localizes apically in cells undergoing VF formation, PMG invagination, salivary gland invagination, and imaginal disc folding (Fig. 2E; Nikolaidou and Barrett, 2004; Zhang and Ward, 2011). It is concentrated basally in all cells during cellularization, and then lost from the basal surface and enriched apically only in VF cells. This accumulation during VF formation is reduced in embryos lacking Fog, Mist, Cta, RhoGEF2, or Rok, suggesting that a complete Fog pathway is required for establishment or maintenance of the apical myosin network (Nikolaidou and Barrett, 2004; Dawes-Hoang et al., 2007; Manning et al., 2013). Myosin polarization seems to be important for organization of actin and coordination between cells during apical constriction events.
Recently, a novel kind of cellular polarity has come to light. Rho1 and Rok display radial polarity within the apical planes of VF cells (Mason et al., 2013). They both exhibit specific localization to the center of cell apices during apical constriction, with Rho1 also present at cell margins. Myosin colocalizes with Rok in medial apical accumulations, which may aid in its stabilization. How this organization is achieved is still unknown, but it likely aids in coordinating the ratchet-like mechanism of constriction.
A major determinant of epithelial apical behavior in most organisms is the apical PAR complex, traditionally thought to include Par-6, Par-3/Bazooka, and aPKC, which must be in place for apically restricted events to occur properly (reviewed in Goldstein and Macara (2007)). These apical proteins likely have direct as well as indirect roles in organizing Fog. In the early Drosophila embryo cellular polarity is established during cellularization, immediately preceding VF invagination, and Bazooka is a key player in this process (Cox et al., 1996; Müller and Wieschaus, 1996; Harris and Peifer, 2004). Bazooka, through recruitment of several partner proteins, localizes Gα proteins apically in Drosophila neuroblast cells (Siegrist and Doe, 2005). A similar mechanism may help localize Cta. The PAR complex also interacts with the proteins that set up subapical adherens junctions, the physical connections between cells, in the early embryo (Fig. 2E). These cell–cell contacts are necessary for tissue cohesion during gastrulation (Cox et al., 1996; Müller and Wieschaus, 1996; Dawes-Hoang et al., 2007). Adherens junction proteins move from their normal subapical localization to a more extreme apical localization in the VF cells just before apical constriction (Fig. 2E; Dawes-Hoang et al., 2007). We do not know how much influence their location along the apical-basal axis has on the ability of cells to invaginate in the VF, although adherens junction migration is known to be a driving force in Drosophila dorsal epithelial folding (Wang et al., 2012).
The transmembrane protein Crumbs is also a major player in apical membrane identity and recruitment of proteins to the apical region of cells during later stages (Assémat et al., 2008). During salivary gland invagination, Rho1 activity in the invaginating cells positively regulates crumbs transcription and aids in crumbs mRNA and protein apical localization (Xu et al., 2008). Crumbs, in turn, helps to organize the apical domain of these cells, leading to proper actomyosin constriction downstream of Rho1. Crumbs does not play a role in gastrulation but may be important in later Fog-induced events (Tepass and Knust, 1993). How Crumbs- and PAR complex-induced polarity interacts with other signaling complexes is a convoluted matter and will likely take years more work to figure out. The strict localization and restricted timing of Fog signaling offer a good system with which to study these interactions.
Negative regulation of Fog signaling
One thoroughly unknown aspect of the Fog pathway is how the contractile signal is terminated. The mRNAs or proteins of pathway members may be degraded to terminate signaling. mist RNA persists in the presumptive mesodermal cells well after they have been internalized (Manning et al., 2013). However, fog RNA is lost from mesodermal cells shortly after the VF has invaginated (Costa et al., 1994). If there is no activating ligand there should be no pathway activation, whether other pathway components are competent for signaling or not. Translational or transcriptional regulation may not be rapid enough for termination of the signal in VF formation, as mesoderm internalization only lasts about ten minutes. Other Fog pathway-dependent morphogenetic processes probably occur on a longer time scale, however.
GPCR signaling is canonically terminated by phosphorylation of the C-terminal tail of ligand-bound GPCRs by G-protein coupled receptor kinases (GRKs; Premont and Gainetdinov, 2007). Once phosphorylated, GPCRs are bound by β-Arrestins, which can induce receptor internalization, cause receptor degradation, compete for GPCR binding with Gαs, and potentially activate independent signaling cascades. Vertebrate genomes encode many GRKs and β-Arrestins, some of which are visual system specific and some of which are utilized more generally across tissues. Drosophila only has one non-visual GRK and one non-visual β-Arrestin, GPRK2 and Kurtz (Krz), respectively (Cassill et al., 1991; Roman et al., 2000). GPRK2 is required maternally for egg production (Schneider and Spradling, 1997). However, of the few eggs laid by GPRK2 mutant mothers some do display disrupted gastrulation phenotypes. GPRK2 also interacts zygotically with fog and cta suggesting a role in regulating VF invagination (Fuse et al., 2013). Eggs lacking Krz also display cuticle phenotypes suggestive of gastrulation defects (Tipping et al., 2010). Alteration of levels of either protein in wings also causes morphological defects (Molnar et al., 2011). These data raise the possibility that Krz could play a role with GPRK2 in termination of Fog signaling. Further investigation of the roles of GPRK2 and Krz in this pathway could allow us to more precisely determine how and when signal termination is achieved during other morphogenetic signaling events.
There are a few canonical molecules that terminate Rho axis signaling in many contexts: Rho GTPase activating proteins (GAPs) and myosin phosphatase. RhoGAPs accelerate the inherent GTPase activity of Rho proteins, increasing the ratio of inactive to active Rho. During Drosophila posterior spiracle invagination, an apical constriction event not connected to Fog signaling, Rho1 activity is restricted to the apical sides of cells (Simões et al., 2006). In these cells RhoGEFs remain apical while RhoGAPs are baso-lateral. The complementary localization of these regulatory proteins organizes Rho1 activation and also allows for its deactivation promptly after termination of an activating signal. However, we do not yet know whether or which GAPs are acting in Fog signaling or how they may contribute to signaling dynamics.
Myosin phosphatase removes the activating phosphates from regulatory myosin subunits. Rok can phosphorylate both myosin light chain to activate it and phosphorylate myosin phosphatase to inactivate it, a twofold way of maintaining myosin activity (Amano et al., 2010). When negative regulation is not exerted on myosin phosphatase, it can act to down-regulate myosin activity. The role of this deactivation mechanism in Fog signaling is not yet known.
There may be other contributing factors to the termination of Fog signaling. For instance, Par-6 has been found to negatively regulate Rho in several contexts, thus Rho activation within an apical PAR domain must overcome this localized down-regulation (Goldstein and Macara, 2007). Changes in membrane trafficking could also influence certain aspects of signaling such as Mist presentation on the apical plasma membrane and secretion of Fog. Alteration of membrane tension during cell shape change may also influence the ability of the actomyosin cytoskeleton to pull against the plasma membrane. These questions may be difficult to approach in vivo, but are ideal problems to solve using a cell culture model of apical constriction.
Other inputs into Fog-induced cell shape change
There are several other accessory proteins that have been shown, genetically or mechanistically, to influence Fog signaling but do not fit into a well-defined category. First, the single pass transmembrane protein T48, a Twist transcriptional target, is expressed along the ventral side of early embryos and is restricted to their apical membranes (Fig. 3; Gould et al., 1990; Leptin, 1991). Interestingly, it is required for organized VF invagination by apically recruiting RhoGEF2, but it is not expressed in the PMG. T48 also helps to organize the transition of adherens junctions from subapical to apical localization in VF cells as constriction begins. Just as Fog and Cta are not absolutely required for mesoderm internalization, neither is T48, but embryos lacking both Cta and T48 fail to form a VF (Kolsch et al., 2007). T48 may act as an accessory protein in Fog signaling or in a parallel pathway, though the mechanism of its influence is not yet known.
MTs have been implicated in working with the actin cytoskeleton in order to enact cell shape changes during morphogenesis, potentially in nuclear positioning or membrane trafficking (e.g. Suzuki et al., 2012). Within the cytoplasm actin regulatory proteins could also influence the organization or formation of the apical contractile array during Fog-induced cell shape changes. For instance, the formin Diaphanous (Dia) is an actin filament elongation factor that is also a Rho effector in several systems (reviewed in Young and Copeland (2010)). It is localized to cell margins in the early embryo (Afshar et al., 2000; Mason et al., 2013). Embryos with reduced maternal dia have defects in coordinating apical constriction in the VF so that only a subset of cells constrict (Homem and Peifer, 2008). Disruption of Dia's radial polarity decreases coherence of the actin network in VF cells and therefore decreases the ability of those cells to constrict in an organized fashion (Mason et al., 2013).
One actin regulator with a well-defined role in VF formation is Abelson kinase (Abl), a non-receptor tyrosine kinase that interacts directly with the actin cytoskeleton (Fig. 3; Van Etten et al., 1994). Abl is present apically in all cells during early embryogenesis and is enriched and activated in VF and PMG invaginations (Fox and Peifer, 2007). Embryos lacking Abl maternally and zygotically have similar gastrulation defects to those lacking Cta maternally. Abl mutants have uncoordinated VF cell contraction with disorganized apical networks of actin, but do internalize most, if not all, mesodermal cells. The double mutant phenotype of abl and cta is much stronger than either alone, and resembles RhoGEF2 mutants. Abl likely acts parallel to Cta, with Abl regulating actin assembly and Cta affecting the myosin network to coordinate apical constriction. Loss of Abl and Abl-related gene in mice leads to strong neural tube closure defects, implicating a similar molecular mechanism of cell shape change in mammalian development (Koleske et al., 1998). The interaction between G-protein signaling and actin regulatory proteins in Rho activation and cell shape change should be more deeply studied, with VF formation being a great model.
Conclusions
Drosophila morphogenesis and VF invagination has long been used as a simplified model for vertebrate morphogenesis and signaling. Many wide-reaching paradigms have been discovered and investigated in depth using this model, not the least of which is the complement of physical cell shape changes which occur during apical constriction. Additionally, quantification of different aspects of VF cellular contraction in wild-type and perturbed embryos has allowed us to analyze how physical forces are coupled to cellular contractions and ultimately to tissue-scale movements (Martin et al., 2010; Driquez et al., 2011). The intimate integration of multiple signaling pathways to trigger a single outcome has become clearer in recent years as well, with the study of how cell polarity affects cell shape and Rho signaling (Xu et al., 2008). Fog signaling is also a pioneer model for GPCR-G-protein signaling in morphogenesis (Manning et al., 2013).
The mechanistic interactions between known players in Fog-activated morphogenetic events require additional study in the coming years. There is much to learn from this system in terms of spatial and temporal regulation of cellular morphogenesis. The complementary patterns of Fog and Mist expression throughout Drosophila development in combination with all of the accessory proteins required for normal tissue invagination give us a hint as to the level of robust control required by evolution for development. Looking forward, one of the main questions will be how the timing of Fog signaling is regulated, which will likely lead to the discovery of more auxiliary players. Our current and future knowledge of Fog-induced cell shape changes in Drosophila has contributed to the understanding of signaling and morphogenesis in our own development and will continue to do so.
Abbreviations
- Fog
Folded gastrulation
- TF
transcription factor
- GPCR
G-protein coupled receptor
- Mist
mesoderm invagination signal transducer
- Cta
Concertina
- RhoGEF
Rho guanine nucleotide exchange factor
- Rok
Rho kinase
- PMG
posterior midgut
- GBE
germ band extension
- VF
ventral furrow
- MT
microtubule
- GRK
G-protein coupled receptor kinase
- Krz
Kurtz
- GAP
GTPase activating protein
- Dia
Diaphanous
- Abl
Abelson kinase
References
- Afshar K, Stuart B, Wasserman S. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127(9):1887–1897. doi: 10.1242/dev.127.9.1887. [DOI] [PubMed] [Google Scholar]
- Aldaz S, Escudero L, Freeman M. Live imaging of Drosophila imaginal disc development. Proc. Natl Acad. Sci. USA. 2010;107(32):14217–14222. doi: 10.1073/pnas.1008623107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67(9):545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assémat E, et al. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778(3):614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Barmchi M, Rogers S, Häcker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 2005;168(4):575–585. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91(7):905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Brunet T, et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. 2013;4:2821. doi: 10.1038/ncomms3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova N, et al. Dynamic microtubules produce an asymmetric E-cadherin–Bazooka complex to maintain segment boundaries. J. Cell Biol. 2013;201(6):887–901. doi: 10.1083/jcb.201211159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassill J, et al. Isolation of Drosophila genes encoding G protein-coupled receptor kinases. Proc. Natl. Acad. Sci. USA. 1991;88:11067–11070. doi: 10.1073/pnas.88.24.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D, Neer E. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Copp A, Greene N. Genetics and development of neural tube defects. J. Pathol. 2010;220(2):217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Wilson E, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76(6):1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Cox R, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 1996;134(1):133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, et al. Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function. Dev. Biol. 1998;204(1):151–164. doi: 10.1006/dbio.1998.9061. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang R, et al. folded gastrulation, cell shape change and the control of myosin localization. Development. 2007;132(18):4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Desprat N, et al. Tissue deformation modulates Twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Driquez B, Bouclet A, Farge E. Mechanotransduction in mechanically coupled pulsating cells: transition to collective constriction and mesoderm invagination simulation. Phys. Biol. 2011;8(6):066007. doi: 10.1088/1478-3975/8/6/066007. [DOI] [PubMed] [Google Scholar]
- Dupré D, Robitaille M, Rebois R, Hébert T. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. 49, 31–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 2003;13(16):1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Fox D, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Fuse N, Yu F, Hirose S. Gprk2 adjusts Fog signaling to organize cell movements. Development. 2013;140:4246–4255. doi: 10.1242/dev.093625. [DOI] [PubMed] [Google Scholar]
- Gelbart M, et al. Volume conservation principle involved in cell lengthening and nucleus movement during tissue morphogenesis. Proc. Natl. Acad. Sci. USA. 2012;109(47):19298–19303. doi: 10.1073/pnas.1205258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara I. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 2007;13(5):609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A, Brookman J, Strutt D, White R. Targets of homeotic gene control in Drosophila. Nature. 1990;348(6299):308–312. doi: 10.1038/348308a0. [DOI] [PubMed] [Google Scholar]
- Grosshans J, et al. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132(5):1009–1020. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsell S, Chu B, Kiehart D. Genetic analysis demonstrates a direct link between Rho signaling and nonmuscle myosin function during Drosophila morphogenesis. Genetics. 2000;155(3):1253–1265. doi: 10.1093/genetics/155.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 2004;167(1):135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 2005;170(5):813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Doubrovinski K, Polyakov O, Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature. 2014;(508):392–396. doi: 10.1038/nature13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem C, Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 2008;135:1005–1018. doi: 10.1242/dev.016337. [DOI] [PubMed] [Google Scholar]
- Ip Y, et al. dorsal–twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992;6(8):1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- Izumi Y, et al. Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division. J. Cell Biol. 2004;164(5):729–738. doi: 10.1083/jcb.200309162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama D, Chiba A. Endogenous activation patterns of Cdc42 GTPase within Drosophila embryos. Science. 2009;324(5932):1338–1340. doi: 10.1126/science.1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanesaki T, Hirose S, Grosshans J, Fuse N. Heterotrimeric G protein signaling governs the cortical stability during apical constriction in Drosophila gastrulation. Mech. Dev. 2013;130(2–3):132–142. doi: 10.1016/j.mod.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Kiehart D, et al. Contractile proteins in Drosophila development. Ann. N. Y. Acad. Sci. 1990;582(1):233–251. doi: 10.1111/j.1749-6632.1990.tb21683.x. [DOI] [PubMed] [Google Scholar]
- Koleske A, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21(6):1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Kolsch V, et al. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110(1):73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- Magie C, Meyer M, Gorsuch M, Parkhurst S. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126(23):5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Manning A, Peters K, Peifer M, Rogers S. Regulation of epithelial morphogenesis by a G-protein coupled receptor, Mist, and its ligand, Fog. Sci. Signal. 2013;12(6):301. doi: 10.1126/scisignal.2004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, et al. Integration of contractile forces during tisue invagination. J. Cell Biol. 2010;188(5):735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Kaschube M, Wieschaus E. Pulsed constrictions of an actinmosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F, Tworoger M, Martin A. Apical domain polarization localizes actiin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 2013;15(8):926–936. doi: 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Kerridge S, Leptin M. A small genomic region containing several loci required for gastrulation in Drosophila. PLos One. 2009;4(10):e7437. doi: 10.1371/journal.pone.0007437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Amano M, Kaibuchi K, Nishida Y. Identification and characterization of Drosophila homolog of Rho-kinase. Gene. 1999;238(2):437–444. doi: 10.1016/s0378-1119(99)00351-0. [DOI] [PubMed] [Google Scholar]
- Molnar C, et al. Role of the Drosophila non-visual β-arrestin kurtz in hedgehog signalling. PLoS Genet. 2011;7(3):e1001335. doi: 10.1371/journal.pgen.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morize P, et al. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development. 1998;125(4):589–597. doi: 10.1242/dev.125.4.589. [DOI] [PubMed] [Google Scholar]
- Müller H, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 1996;134(1):149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess J. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Myat M, Andrew D. Fork head prevents apoptosis and promotes cell shape change during formation of the Drosophila salivary glands. Development. 2000;127(19):4217–4226. doi: 10.1242/dev.127.19.4217. [DOI] [PubMed] [Google Scholar]
- Nelson C, Gleghorn J. Sculpting organs: mechanical regulation of tissue development. Annu. Rev. Biomed. Eng. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
- Nikolaidou K, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr. Biol. 2004;14(20):1822–1826. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64(2):447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Peters K, Rogers S. Drosophila Ric-8 interacts with the Gα12/13 subunit, Concertina, during activation of the Folded gastrulation pathway. Mol. Biol. Cell. 2013;24:3460–3471. doi: 10.1091/mbc.E12-11-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille P-A, Ahmadi P, Brunet A-C, Farge E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2009;2(66):ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- Pouille P, Farge E. Hydrodynamic simulation of multicellular embryo invagination. Phys. Biol. 2008;5:015005. doi: 10.1088/1478-3975/5/1/015005. [DOI] [PubMed] [Google Scholar]
- Premont R, Gainetdinov R. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Ratnaparkhi A, Zinn K. The secreted cell signal Folded Gastrulation regulates glial morphogenesis and axon guidance in Drosophila. Dev. Biol. 2007;308158(1):6. doi: 10.1016/j.ydbio.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Rogers G. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat. Protoc. 2008;3:606–611. doi: 10.1038/nprot.2008.18. [DOI] [PubMed] [Google Scholar]
- Rogers S, et al. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 2004;14(20):1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M, et al. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335(6073):1232–1235. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, He J, Davis R. kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics. 2000;155:1281–1295. doi: 10.1093/genetics/155.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59(6):1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Sawyer J, et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 2010;341(1):5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, et al. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107(2):183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- Schneider L, Spradling A. The Drosophila G-protein-coupled receptor kinase homologue Gprk2 is required for egg morphogenesis. Development. 1997;124:2591–2602. doi: 10.1242/dev.124.13.2591. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G, Franks M. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev. Biol. 1984;105(2):257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121(1):101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seher T, Natasimha M, Vogelsang E, Leptin M. Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev. 2006;124:167–179. doi: 10.1016/j.mod.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Siegrist S, Doe C. Microtubule-induced pins/gai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Simões S, et al. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development. 2006;133(21):4257–4267. doi: 10.1242/dev.02588. [DOI] [PubMed] [Google Scholar]
- Skoglund P, et al. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, et al. Pharmacogenomic and structural analysis of constitutive g protein-coupled receptor activity. Annu. Rev. Pharmacol. Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137(7):1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Somlyo A, Somlyo A. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522(2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P, Erickson C. Interkinetic nuclear migration: a mysterious process in search of a function. Dev. Growth Differ. 2012;54(3):306–316. doi: 10.1111/j.1440-169X.2012.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Morita H, Ueno N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev. Growth Differ. 2012;54(3):266–276. doi: 10.1111/j.1440-169X.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hajicek N, Kozasa T. Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals. 2009;17(1):55–70. doi: 10.1159/000186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeton D, Parks S, Costa M, Wieschaus E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development. 1991;112(3):775–789. doi: 10.1242/dev.112.3.775. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 1993;159(1):311–326. doi: 10.1006/dbio.1993.1243. [DOI] [PubMed] [Google Scholar]
- Tipping M, et al. β-arrestin Kurtz inhibits MAPK and Toll signalling in Drosophila development. EMBO J. 2010;29(19):3222–3235. doi: 10.1038/emboj.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R, et al. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J. Cell Biol. 1994;124(3):325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner V, Voronov D, Taber L. Mechanics of head fold formation: investigating tissue-level forces during early development. Development. 2010;137(22):3801–3811. doi: 10.1242/dev.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat. Cell Biol. 2005;7(11):1091–1098. doi: 10.1038/ncb1317. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kha Z, Kaschube M, Wieschaus E. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature. 2012;484(7394):390–393. doi: 10.1038/nature10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn R, Magrath R. F-actin distribution during the cellularization of the Drosophila embryo visualized with FL-phalloidin. Exp. Cell Res. 1983;143(1):103–114. doi: 10.1016/0014-4827(83)90113-1. [DOI] [PubMed] [Google Scholar]
- Waterhouse R, et al. OrthoDB: the hierarchical catalog of eukaryotic orthologs in 2011. Nucleic Acids Res. 2011;39:D283–D288. doi: 10.1093/nar/gkq930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, et al. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Xiang S, Dusaban S, Brown J. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim. Biophys. Acta. 2013;1831(1):213–222. doi: 10.1016/j.bbalip.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Keung B, Myat M. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Dev. Biol. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Young K, Copeland J. Formins in cell signaling. Biochim. Biophys. Acta. 2010;1803(2):183–190. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ward R. Distinct tissue distributions and subcellular localizations of differently phosphorylated forms of the myosin regulatory light chain in Drosophila. Gene Expr. Patterns. 2011;11(1–2):93–104. doi: 10.1016/j.gep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]