Abstract
Prognostic relevance of blood counts at complete remission (CR) in acute myeloid leukemia (AML) is not clear. To address this issue, we analyzed 891 AML patients in first CR. From the data of randomly selected 446 patients (training set), we first established optimal cutoffs for neutrophil and platelet counts and hemoglobin level at CR in terms of relapse-free survival (RFS). Patients whose counts were higher than each optimal cutoff were shown to have significantly better RFS (p < 0.01 for neutrophil and platelets, and p = 0.02 for hemoglobin). Then we tested whether these cutoffs were, after accounting for better known prognostic covariates, also predictive of RFS in the remaining 445 patients (validation set). Our data revealed that higher neutrophil count was independently predictive of longer RFS in the validation set (hazard ratio 1.38, p = 0.02), as was higher platelet count (hazard ratio 1.35, p = 0.04). These findings suggest that blood counts at CR, information readily available, are useful in prognostication in AML.
Keywords: Acute myeloid leukemia, Blood counts, Complete remission, Relapse-free survival, Prognostication
1. Introduction
Although chemotherapy produces complete remission (CR) in the majority of patients with newly diagnosed acute myeloid leukemia (AML), disease will recur in most. Current research places less emphasis on ascertaining results in the average patient and more on assessing risk in individual patients. Several characteristics serve to distinguish patients at different risks of relapse [1,2]. Generally, as in the case of cytogenetics and various genetic abnormalities, these are measured prior to beginning therapy. Nevertheless, it is intuitive that data gathered after treatment begins can potentially be more useful than pre-treatment data. For example, Keating et al. [3] reported that the number of courses needed to achieve CR was inversely related to remission duration and indeed was more predictive of this outcome than pre-treatment covariates. Most attempts to use post-treatment findings to assess risk of relapse have focused on laboratory parameters such as the percentage of bone marrow blasts [4,5], cytogenetics [6,7], leukemia-associated immunophenotypes [8,9], or genetic abnormalities [10–12], measured during the induction course or at time of CR. Here however we examine whether a much simpler test, namely the complete blood count at time of CR, might also predict relapse-free survival (RFS).
2. Patients and methods
2.1. Patients
923 adults treated for AML (≥20% blasts, acute promyelocytic leukemia excepted) at M. D. Anderson (MDA) from 1991 to 2003 achieved CR with 1 course of cytarabine-containing induction therapy; these 923 represented 95% of those entering CR. Excluding the 32 of the 923 who received allogeneic hematopoietic stem cell transplantation during first CR left 891 patients. Induction therapy contained idarubicin without fludarabine or topotecan in 336 patients (38%), fludarabine with or without idarubicin in 303 (34%), and topotecan, without idarubicin, in 153 (17%); 99 patients (11%) received other cytarabine-containing therapies [13]. Generally patients continued their induction regimen at reduced doses for 3–6 courses (median, 4 courses) in CR. This study was approved by the MDA Institutional Review Board, and patients were treated in accordance with the Declaration of Helsinki.
2.2. Statistical analysis
RFS (dated from achievement of CR, event = relapse or death in first CR) was assessed using Kaplan-Meier methodology, with differences between groups qualified with the log-rank test. Relapse was considered to have occurred when the marrow contained ≥5% blasts unrelated to recovery from prior chemotherapy. We first randomly divided our 891 patients into two groups of 446 and 445 patients. In the first group we divided neutrophil and platelet counts and hemoglobin level at time of CR into quartiles and then assessed whether the smallest log-rank p-value obtained comparing (a) the lower 25% with the upper 75%, (b) the lower 50% with the upper 50%, or (c) the lower 75% with the upper 25%. The cutoff that gave the smallest log-rank p-value was considered the cutoff of interest. Because of the dangers inherent in such optimal cutoff selection we used the cutoffs determined in the first 446 patients to develop a Cox model for RFS both in the first 446 patients and in the second 445 patients. Since we have previously noted that the shorter the time to CR the longer RFS even considering only patients who reach CR in 1 course [14], the inverse relation between time to CR and counts at CR made it mandatory to include both in our multivariate analyses. Other covariates examined in these analyses for a relation with RFS were age (considered numerically), cytogenetics (better/intermediate/worse as defined below), antecedent hematologic disorder (AHD, yes/no), prior chemotherapy (yes/no), sex, performance status at CR (0–2/3–4) and treatment (as noted above) recalling the close correspondence between induction and post-remission therapy.
3. Results
3.1. Patient characteristics
The median age of the entire cohort was 56 years (range, 16–86), with 497 males and 394 females. Cytogenetics was determined pre-treatment in 842 (95%): 117 (14%) had [inv(16)/t(16;16) or t(8;21) with or without other abnormalities] (“better”), 131 (16%) had abnormalities of chromosomes 5 and/or 7 or complex karyotype defined as ≥3 aberrations (“worse”), while the remaining 594 (70%) had other findings (“intermediate”), primarily a normal karyotype. 296 had AHD (documented abnormality in blood count for more than one month before MDA presentation), and 109 (12%) had received chemo/radiotherapy for another condition. For the median follow-up of 5.1 years (range, 0.1–14.3), relapse and death in first CR occurred in 599 and 104 patients, respectively. The probability of RFS for all patients was 21 ± 1% at 5 years.
3.2. Training set
For 446 patients in the training set, the median neutrophil count at CR was 4.0 × 109/L, while the 25th percentile was 2.2 × 109/L and the 75th percentile was 8.2 × 109/L. Among these 3 values, the 75th percentile gave the lowest p-value (p < 0.01). The optimal cutoff for platelet count at CR was also the 75th percentile (= 320 × 09/L, p < 0.01, median 194 × 109/L and 25th percentile 133 × 109/L), while for hemoglobin the 25th percentile (=9.4 g/dL) produced the optimal cutoff (p = 0.02, median 10.3 g/dL and 75th percentile 11.0 g/dL). The 5-year RFS rates for patients with higher and lower counts than each optimal cutoff were 29 ± 4% vs. 18 ± 2% for neutrophils, 22 ± 2% vs. 18 ± 4% for hemoglobin, and 30 ± 5% vs. 18 ± 2% for platelets. The Cox model indicated some relationship between higher neutrophil count at CR (p = 0.03), higher platelet count at CR (p = 0.07) and higher hemoglobin level at CR (p = 0.12) and RFS after accounting for the other covariates named, particularly time to CR (Table 1).
Table 1.
Multivariate analysis of risk factors for relapse-free survival
| Training set (n = 446) |
Validation set (n = 445) |
|||
|---|---|---|---|---|
| HR (95% CI) a | p-Value | HR (95% CI)a | p-Value | |
| Cytogenetic risk | ||||
| Better | 0.59 (0.40–0.89) | 0.01 | 0.54 (0.35–0.85) | <0.01 |
| Intermediate | 1.00 | – | 1.00 | – |
| Worse | 2.10 (1.58–2.80) | <0.01 | 1.85 (1.43–2.39) | <0.01 |
| Age (years) | ||||
| As a numerical variable (10 years older) | 1.09 (1.01–1.17) | 0.03 | 1.04 (0.96–1.13) | 0.36 |
| Sex | ||||
| Male | 1.04 (0.82–1.31) | 0.76 | 1.07 (0.84–1.36) | 0.58 |
| Female | 1.00 | – | 1.00 | – |
| Antecedent hematologic disorder | ||||
| Yes | 0.94 (0.72–1.22) | 0.64 | 1.07 (0.82–1.39) | 0.62 |
| No | 1.00 | – | 1.00 | |
| Prior chemo/radiotherapy | ||||
| Yes | 1.05 (0.72–1.53) | 0.80 | 1.18 (0.84–1.65) | 0.33 |
| No | 1.00 | – | 1.00 | |
| Treatment | ||||
| Idarubicin + cytarabine without fludarabine or topotecan | 1.00 | – | 1.00 | – |
| Flludarabine + cytarabine with or without idarubicin | 1.17 (0.87–1.55) | 0.30 | 1.00 (0.75–1.32) | 0.98 |
| Topotecan + cytarabine without idarubicin | 1.20 (0.88–1.65) | 0.25 | 1.18 (0.85–1.64) | 0.31 |
| Others | 1.08 (0.73–1.58) | 0.71 | 0.84 (0.55–1.29) | 0.43 |
| Neutrophil count at CR (× 109/L) | ||||
| <8.2 | 1.38 (1.03–1.86) | 0.03 | 1.38 (1.05–1.83) | 0.02 |
| >8.1 | 1.00 | – | 1.00 | – |
| Hemoglobin level at CR (g/dL) | ||||
| <9.5 | 1.25 (0.94–1.65) | 0.12 | 0.98 (0.74–1.30) | 0.91 |
| >9.4 | 1.00 | – | 1.00 | – |
| Platelet count at CR (× 109/L) | ||||
| <320 | 1.29 (0.98–1.70) | 0.07 | 1.35 (1.01–1.80) | 0.04 |
| >319 | 1.00 | – | 1.00 | – |
| Performance status at CR | ||||
| 0–2 | 1.00 | – | 1.00 | – |
| 3–4 | 1.50 (0.91–2.47) | 0.12 | 2.34 (1.41–3.87) | <0.01 |
| Time to CR (days) | ||||
| As a numerical variable (7 days longer) | 1.11 (1.05–1.17) | <0.01 | 1.06 (1.00–1.13) | 0.05 |
HR, hazard ratio; CI, confidence interval; CR, complete remission.
A value beyond 1.0 indicates higher risk for failure.
3.3. Validation set
Next, we examined whether the cutoffs established in the training set stratify 445 patients in the validation set in terms of RFS. As shown in Fig. 1, patients with higher neutrophil count had significantly better RFS than those with lower count (26 ± 4% vs. 20 ± 2%, p = 0.02). Analogously, higher platelet count was significantly associated with better RFS (32 ± 5% vs. 19 ± 2%, p < 0.01, Fig. 2). RFS appeared better in patients with higher hemoglobin level than in those with lower level, although we failed to detect statistical significance (23 ± 2% vs. 15 ± 4%, p = 0.22, Fig. 3). Multivariate analysis identified neutrophil and platelet counts at CR as factors independently associated with RFS (p = 0.02 and p = 0.04, Table 1).
Fig. 1.
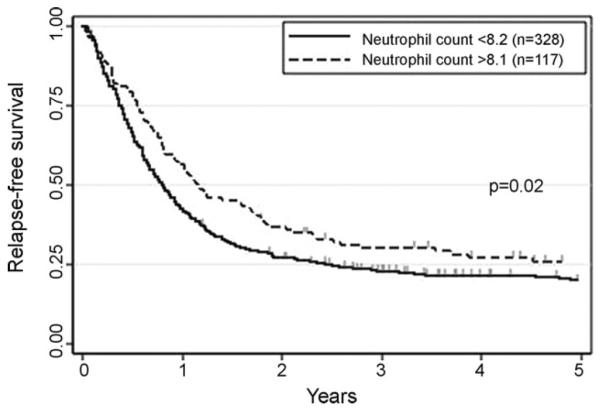
Relapse-free survival according to neutrophil count at time of complete remission in the second group. The cutoff established in the first group (75th percentile, 8.2 × 109/L) were applied.
Fig. 2.
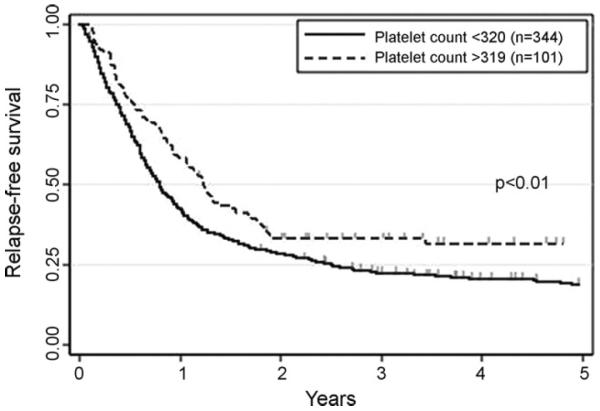
Relapse-free survival according to platelet count at time of complete remission in the second group. The cutoff established in the first group (75th percentile, 320 × 109/L) were applied.
Fig. 3.
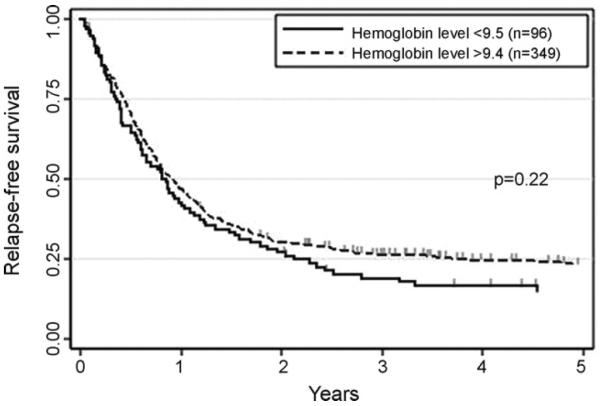
Relapse-free survival according to hemoglobin level at time of complete remission in the second group. The cutoff established in the first group (25th percentile, 9.5 g/dL) were applied.
3.4. Patients with FLT3 data
Information on the fms-like tyrosine kinase (FLT3) status was known in 273 of the 891 patients (31%). By using the methods described previously [15], internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutation of the FLT3 gene were detected in 51 and 19 patients, respectively. 4 patients were positive for both mutations. Findings that FLT-ITD correlated with inferior RFS (p < 0.01) while FLT3-TKD mutation did not (p = 0.20) in the univariate analysis, led us to see the effect of counts at CR after adjusting for FLT3-ITD in these 273 patients. The Cox model including FLT3-ITD in addition to above-mentioned covariates showed that neutrophil and platelet counts at CR remained significantly associated with RFS (p = 0.04 and 0.01, respectively). FLT3-ITD also constituted an independent adverse predictor for RFS (hazard ratio 2.24, 95% confidence interval 1.52–3.28, p < 0.01).
4. Discussion
With the aim of investigating the prognostic relevance of blood counts at time of CR, we randomly divided our 891 patients into 2 groups, and in the first group, patients whose counts were higher than each optimal cutoff were shown to have significantly better RFS than those with lower counts. However, more important issue was the observation that in the second group, using the cutoffs established in the first group, neutrophil count and platelet count at CR had independent value for predicting RFS.
It was possible that our results were confounded by use of granulocyte-colony stimulating factor (G-CSF), which might have been responsible for high neutrophil counts in some patients. Specifically, we looked at the relationship between neutrophil count at CR and RFS considering only regimens that did not call for G-CSF administration. While it is not possible to be sure that some of these patients did not receive G-CSF at physicians' discretion, the findings considering patients treated on protocols not calling for G-CSF administration were analogous to those in all patients (hazard ratio, 1.27, p = 0.03). Furthermore, the finding that platelet count at CR was relevant made it less likely our results reflected use of G-CSF. We also considered the possibility that counts at CR were influenced by when CR was documented. Thus, 2 patients might actually have entered CR on the same day, but if the first patient was seen in clinic, and CR documented, on that day while the second patient was not seen, and CR documented only 1 week later, the second might be found, spuriously, to have higher counts at CR. However, arguing against this possibility was the inverse relationship between time to documented CR and counts at CR (p < 0.01 for neutrophils and p = 0.04 for platelets, Spearman rank-order correlations).
Rather we believe our findings are due to the ability of neutrophil and platelet counts at CR to reflect minimal residual AML. The ability of AML blasts to suppress normal hematopoiesis is responsible for the cytopenias typically present at diagnosis. Hence it is reasonable that although level of blood counts at CR may reflect the degree of recovery of normal progenitors from chemotherapy-induced damage, these levels may also reflect the amount of minimal residual AML present at CR. A similar phenomenon seems to underlie our previous observation of an inverse relation between time to CR and RFS [14]. In the present study, however, platelet and neutrophil counts at CR and time to CR were each independent predictors of RFS. Likewise clinical experience suggests that patients whose counts recover only incompletely and slowly after post-remission therapy are prone to relapse.
The effect of neutrophil and platelet counts at CR is still important even after accounting for FLT3-ITD. Although data on FLT3 were available in only 31% of the patients, those in whom FLT3 status was determined had similar RFS as patients in whom FLT3 status was not determined (p = 0.24), thus suggesting that our results are generally applicable, as does the fact that FLT3-ITD was, as expected, an important independent prognostic factor in our dataset. Given that results of any multivariate analysis are dependent on what covariates are examined, it is certainly possible that the prognostic influence of neutrophil and platelet counts at CR might have disappeared had we examined other factors such as nucleophosmin1 (NPM1) [16] and CEBPA mutations [17]. However, while we had such information in only a minority of patients, we knew blood counts at CR in all patients, reflecting the simplicity, availability, and inexpensive nature of the test. Further exploration of blood counts at CR as a potential predictor of RFS is warranted.
Acknowledgments
M.Y. designed the study, collected and analyzed data, and wrote the paper. G.B., G.G.-M., F.R., S.F., and H.K. provided and analyzed data, and reviewed the paper. S.P. collected data and reviewed the paper. E.E. designed the study, provided and analyzed data, and wrote the paper.
Footnotes
Conflict of interest The authors reported no potential conflict of interest.
References
- [1].Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- [2].Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- [3].Keating MJ, Smith TL, Gehan EA, McCredie KB, Bodey GP, Spitzer G, et al. Factors related to length of complete remission in adult acute leukemia. Cancer. 1980;45:2017–29. doi: 10.1002/1097-0142(19800415)45:8<2017::aid-cncr2820450806>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [4].Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- [5].Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- [6].Konopleva M, Cheng SC, Cortes JE, Hayes KJ, Pierce SA, Andreeff M, et al. Independent prognostic significance of day 21 cytogenetic findings in newly-diagnosed acute myeloid leukemia or refractory anemia with excess blasts. Haematologica. 2003;88:733–6. [PubMed] [Google Scholar]
- [7].Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22:2410–8. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- [8].San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98:1746–51. doi: 10.1182/blood.v98.6.1746. [DOI] [PubMed] [Google Scholar]
- [9].Kern W, Voskova D, Schoch C, Hiddemann W, Schnittger S, Haferlach T. Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood. 2004;104:3078–85. doi: 10.1182/blood-2004-03-1036. [DOI] [PubMed] [Google Scholar]
- [10].Krauter J, Gorlich K, Ottmann O, Lubbert M, Dohner H, Heit W, et al. Prognostic value of minimal residual disease quantification by real-time reverse transcriptase polymerase chain reaction in patients with core binding factor leukemias. J Clin Oncol. 2003;21:4413–22. doi: 10.1200/JCO.2003.03.166. [DOI] [PubMed] [Google Scholar]
- [11].Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20:1103–8. doi: 10.1038/sj.leu.2404149. [DOI] [PubMed] [Google Scholar]
- [12].Weisser M, Haferlach C, Hiddemann W, Schnittger S. The quality of molecular response to chemotherapy is predictive for the outcome of AML1-ETO-positive AML and is independent of pretreatment risk factors. Leukemia. 2007;21:1177–82. doi: 10.1038/sj.leu.2404659. [DOI] [PubMed] [Google Scholar]
- [13].Estey EH, Thall PF, Cortes JE, Giles FJ, O'Brien S, Pierce SA, et al. Comparison of idarubicin+ara-C-, fludarabine + ara-C-, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98:3575–83. doi: 10.1182/blood.v98.13.3575. [DOI] [PubMed] [Google Scholar]
- [14].Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000;95:72–7. [PubMed] [Google Scholar]
- [15].Beran M, Luthra R, Kantarjian H, Estey E. FLT3 mutation and response to intensive chemotherapy in young adult and elderly patients with normal karyotype. Leuk Res. 2004;28:547–50. doi: 10.1016/j.leukres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- [16].Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- [17].Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–23. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]


