Abstract
American Indians have a high prevalence of obesity. Evidence exists to support a relationship between increased dietary calcium intake and lower body weight. This study was conducted to investigate the association between dietary calcium intake, BMI, and percentage of body fat (PBF) in American Indians (ages 47–79 y) in the Strong Heart Study (SHS) (2nd exam: 1992–1995). SHS data were compared with data for the general U.S. adult population from the National Health and Nutrition Examination Survey (NHANES III) (1988–1994). BMI was calculated as weight (kg)/height (m2). PBF was estimated by bioelectrical impedance, using an equation based on total body water. The clinical examination included measures of blood chemistry. Dietary data were collected using a 24-h dietary recall. Calcium intake was significantly lower among SHS participants than among age-matched NHANES III participants. Mean calcium intake in the SHS was 680 mg/d (range: 103 – 4574 mg/d) for men and 610 mg/d (range: 71 – 4093 mg/d) for women (P < 0.001). After adjustment for potential confounders, BMI and PBF were lower by 0.80 kg/m2 (95% CI: −1.53 to −0.08, P = 0.046) and 1.28% (95% CI: −2.10 to −0.47, P = 0.011) in SHS participants with higher (≥ 873 mg/d in the 5th quintile) versus lower calcium intake (< 313 mg/d in the 1st quintile). No relation between calcium intake and BMI or PBF was observed in NHANES III participants. Our data may be used to develop nutritional interventions aimed at weight control in culturally appropriate clinical trials.
Keywords: Dietary calcium, BMI, Body fat, American Indians
INTRODUCTION
Although much effort has been devoted to studying the effect of macronutrients on weight control, the role of micronutrients has been less well studied (1). Dietary calcium appears to be related to energy metabolism (2), and there is evidence to support a relationship between increased dietary calcium intake and lower body weight, specifically reduced fat mass (3). Lower dietary calcium intake has been associated with higher body weight and adiposity in the Quebec Family Study (4). This concept has been further evaluated in other epidemiological studies, including the Third National Health and Nutrition Examination Survey (NHANES III)** and the Coronary Artery Risk Development in Young Adults (CARDIA) study (5,6). Some of this work suggested gender differences in the association between calcium intake and body fat (5).
Despite evidence of a relationship between calcium and adiposity, findings on this association have been inconsistent (7). Further, ethnic minorities, such as American Indians, have not been adequately represented in any of the national health and dietary intake surveys (8). Dietary studies in American Indians have been limited to only a few tribes or have small sample sizes (9,10). These limitations are notable because of the high prevalence of obesity in American Indians (11). Studies of American Indian diets have focused on specific groups, such as pregnant women, children, or adolescents (12–18). The current study was designed to address these limitations by examining the role of dietary calcium in development of obesity in a broad sample of American Indians.
SUBJECTS AND METHODS
Study Sample
This analysis is based on the 3638 subjects who participated in the second Strong Heart Study (SHS) examination (1992–1995), which included resident members ages 47 to 80 years from 13 American Indians tribes in Arizona, Oklahoma, and North and South Dakota. Nutritional data (described below) were available for 3450 (95% of Exam 2 participants) participants. Of these, we excluded individuals who were older than 79 (n = 2), to match the ages in NHANES III; were taking dietary supplements (n = 144); had extreme values for total energy intake ≤ 2510 kJ/d (n = 131) or ≥ 33472 kJ/d and/or body mass index (BMI) ≥ 50 kg/m2 (n = 38); had conditions that might affect energy intake, such as dialysis, kidney transplant, and cirrhosis (n = 153); and those missing data for key variables (n = 7). These exclusions yielded an analysis sample of 2975, representing 82% of individuals participating in the second examination.
The Indian Health Service, institutional review boards, participating tribes, and the MedStar Research Institute approved the study. Written informed consent was obtained from each participant.
Measurements
As described elsewhere (19), the second SHS examination included measures of body circumferences and fat, as well as blood chemistry. Height was obtained with the participant standing erect in a Frankfort plane, using a stadiometer fixed to the wall. Weight was measured using a Detecto model 683-p scale, which was calibrated and adjusted daily (20). BMI was calculated as weight (kg)/height (m2). Percentage of body fat (PBF) was estimated by bioelectrical impedance with an RJL impedance meter (model B1410) using an equation based on total body water (21). The examination also included a fasting blood draw, from which determinations including fasting glucose were measured (19).
All participants at the second examination had dietary data collected via a single 24-h dietary recall. Interviewers were centrally trained and certified in data collection and form completion according to standardized methods (22). Use of dietary supplements was assessed as part of the medication inventory. Dietary intake was analyzed using the Minnesota Nutrition Data System (NDS Version 2.1) (23,24).
U.S. Population Data
To compare the relationship between calcium intake and obesity in American Indians in the SHS versus the general U.S. population, we used data for U.S. adults ages 47–79 years from NHANES III (1988–1994) (25,26), which occurred at about the same time as the second SHS examination. Exclusion criteria in NHANES III were similar to those for the SHS sample: reported energy intake ≤ 2510 kJ /d or ≥ 33472 kJ /d; BMI ≥ 50 kg/m2; dietary calcium intake > 5000 mg/day (no one had this extreme calcium intake in the SHS); those missing data on key variables; and those without a fasting blood draw, including all participants assigned to an afternoon or evening examination. These criteria yielded an NHANES III analysis sample of 2755 individuals. Both NHANES III and SHS excluded dietary supplements.
As in the SHS, total energy intake in NHANES III was estimated using a single 24-h dietary recall. However, NHANES III nutrient estimates were obtained using the USDA Survey Nutrient Data Base (26).
Statistical analysis
SHS data were analyzed with the Statistical Analysis System (SAS) Version 9.00 (27). NHANES III data were analyzed with SAS-Callable SUDAAN software version 9.0 (28), which permits appropriate weighting of NHANES III data per published guidelines (29). For both SHS and NHANES III, continuous variables were presented as means and standard errors of means (mean ± SEM). In the current study, the t-test and X2 test were used to compare the means and proportions within genders between the SHS and NHANES III.
Both SHS and NHANES III excluded supplemental calcium intake. Consistent with previous methodology (30), dietary calcium and energy intake were categorized into quintiles, with the first quintile serving as the reference group for the multivariate analyses.
Mean energy, PBF, and BMI were presented according to quintiles of calcium intake. To test linear trend according to ascending quintiles of calcium intake, the statement CONTRAST in the general linear models procedure (PROC GLM) in SAS was used with the SHS data (27); PROC REGRESS in SUDAAN was used with the NHANES III data (28).
The adjusted mean PBF and BMI within quintiles of calcium intake were calculated by using the statement LSMEANS in PROC GLM in SAS (27) for the SHS data and by using PROC REGRESS in SUDAAN for the NHANES III data (28). Variables entered into the model for all participants included gender, age, study center, years of education, income, alcohol consumption, smoking, diabetes status (diabetes, impaired fasting glucose [IFG], or normal fasting glucose), diabetes duration in participants with diabetes, and energy intake. Diabetes was defined according to American Diabetes Association criteria (31). The data were examined for interactions between gender or diabetes and quintiles of calcium. No interactions were observed. We also evaluated the effect of adding fat, protein, potassium, and magnesium intakes on the association between calcium and PBF and BMI by adding these factors to the model as potential confounders.
To examine odds ratios of being overweight (defined as 25 ≤ BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) compared with normal weight (BMI < 25) across calcium quintiles after controlling for the confounders listed above, the generalized logit model was used (SAS PROC CATMOD or SUDAAN PROC MULTILOG), because the parallel lines assumption was violated when using the logistic regression model. Tests for trend were conducted by modeling the median of each quintile of calcium intake as a continuous variable in the generalized logit model.
RESULTS
Mean age did not differ significantly between the two studies (Table 1). BMI was significantly higher in both men and women in the SHS compared with those in NHANES III (P < 0.001), while mean calcium intake was lower (P < 0.001) in SHS participants. Cutoff points for BMI quintiles in the SHS were 25.6, 28.1, 30.7, 33.9 kg/m2 for men and 26.2, 29.7, 32.7, 36.6 for women. Cutoff points for BMI quintiles in NHANES III were 23.5, 25.6, 27.9, 30.6 for men and 22.4, 25.2, 27.8, 32.2 for women. In women, mean energy and fat intake (percentage of energy) were higher but carbohydrate intake (percentage of energy) was lower in the SHS compared with NHANES III. In contrast, among men, energy and carbohydrate intakes were lower while fat intake as percentage of energy was higher in the SHS than in NHANES III. Nearly half of the SHS sample had diabetes (48%) and 61% had either diabetes or IFG. As expected, glucose dysregulation in the SHS was more prevalent than in NHANES III (in which 12% had diabetes and 23% had diabetes or IFG).
Table 1.
Clinical and demographic characteristics of American Indians in the SHS and the general U.S. adult population in NHANES III.1
| Women | Men | |||
|---|---|---|---|---|
| SHS | NHANES III | SHS | NHANES III | |
| (n = 1823) | (n = 1391) | (n = 1152) | (n = 1364) | |
| Age (y) | 60.3 ± 0.2 | 60.8 ± 0.3 | 59.4 ± 0.22 | 60.3 ± 0.3 |
| Body mass index (kg/m2) | 31.7 ± 0.12 | 27.5 ± 0.2 | 29.9 ± 0.22 | 27.2 ± 0.2 |
| Body fat (%) | 41.2 ± 0.22 | 34.6 ± 0.4 | 28.5 ± 0.22 | 22.8± 0.3 |
| Education (y) | 11.3 ± 0.12 | 11.7 ± 0.1 | 11.3 ± 0.12 | 12.1 ± 0.2 |
| Calcium intake (mg/d) | 610 ± 102 | 673 ± 13 | 680 ± 142 | 852 ± 20 |
| Energy intake (kJ)/d) | 7128 ± 652 | 6873 ± 102 | 8478 ± 1102 | 9564 ± 158 |
| Dietary fat (g/d) | 67 ± 12 | 63 ± 2 | 82 ± 12 | 89 ± 2 |
| Dietary fat (% of energy) | 34.7 ± 0.22 | 33.3 ± 0.5 | 35.9 ± 0.32 | 33.4 ± 0.4 |
| Dietary protein (g/d) | 67 ± 12 | 63 ± 1 | 80 ± 12 | 89 ± 2 |
| Dietary protein (% of energy) | 15.9 ± 0.1 | 15.8 ± 0.2 | 16.2 ± 0.13 | 15.9 ± 0.2 |
| Dietary carbohydrates (g/d) | 213 ± 2 2 | 207 ± 3 | 240 ± 42 | 275 ± 6 |
| Dietary carbohydrates (% of energy) | 50.3 ± 0.32 | 51.4 ± 0.5 | 47.8 ± 0.42 | 49.0 ± 0.4 |
| Alcohol use (%) | ||||
| Current | 24.4 | 32.93 | 47.6 | 55.83 |
| Former | 48.8 | 44.3 | 46.8 | 37.83 |
| Never | 26.8 | 22.73 | 5.6 | 6.4 |
| Smoking status (%) | ||||
| Current | 26.3 | 20.8 3 | 37.6 | 26.92 |
| Former | 34.4 | 27.73 | 45.1 | 46.4 |
| Never | 39.3 | 51.43 | 17.3 | 26.72 |
| Diabetes status (%) | ||||
| Diabetes | 50.8 | 8.43 | 42.9 | 12.62 |
| Impaired fasting glucose | 12.4 | 11.0 | 15.1 | 14.2 |
| Normal fasting glucose | 36.8 | 80.6 | 42.0* | 73.2 |
All data are presented as mean ± SEM for SHS and NHANES III.
Different from the same sex in SHS; P < 0.05
Different from men in that study
In the SHS, calcium intake was 610 ± 9.56 mg/d (range: 103 – 4574 mg/d) for women and 680 ± 14.11 mg/d (range: 71 – 4093 mg/d) for men (P < 0.001). There was no significant interaction between calcium intake and study center; thus data from the three study centers were combined. Mean BMI and PBF were significantly higher in the SHS than in NHANES III. As expected, BMI and PBF were highly correlated for both genders in the SHS and NHANES III (r = 0.69 in men and 0.83 in women in the SHS and 0.60 in men and 0.84 in women in NHANES III, P < 0.0001; data not shown).
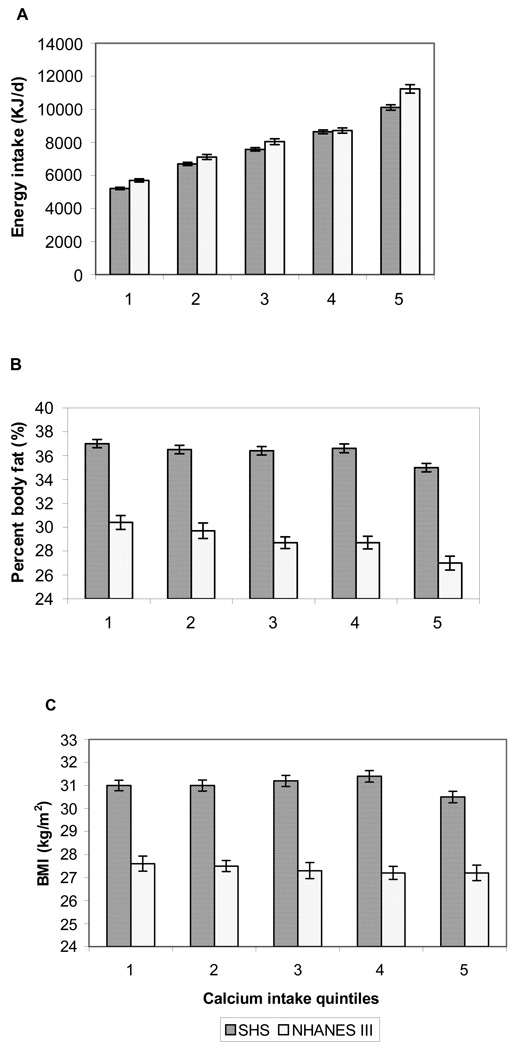
In NHANES III, mean calcium intake was higher overall and in each quintile among both men and women compared with SHS participants (Table 2). However, in women, energy intake was lower in NHANES III compared with SHS. As expected, energy intake was higher with increasing calcium intake in each study (Figure 1a). In both SHS and NHANES III, a significant trend of decreasing PBF was observed with increasing quintile of calcium intake in a univariate analysis (Figure 1b), but this association was not observed for BMI in either study (Figure 1c).
Table 2.
Quintiles of calcium and energy intake by gender, SHS and NHANES III.1
| Women | Men | |||
|---|---|---|---|---|
| SHS | NHANES III | SHS | NHANES III | |
| (n = 1823) | (n = 1391) | (n = 1152) | (n = 1364) | |
| Calcium intake | Mean ± SEM of calcium intake (mg/d) | |||
| Quintile | ||||
| 1 | 222 ± 4 | 233 ± 6 | 242 ± 4 | 289 ± 8 |
| 2 | 370 ± 3 | 418 ± 4 | 407 ± 3 | 511 ± 4 |
| 3 | 514 ± 3 | 581 ± 4 | 557 ± 4 | 733 ± 6 |
| 4 | 700 ± 4 | 803 ± 6 | 788 ± 5 | 997 ± 9 |
| 5 | 1244 ± 29 | 1325 ± 21 | 1411 ± 37 | 1721 ± 41 |
| Energy intake | Mean ± SEM of energy intake (kJ/d) | |||
| Quintile | ||||
| 1 | 3875 ± 33 | 3837 ± 52 | 4175 ± 50 | 4995 ± 69 |
| 2 | 5418 ± 19 | 5384 ± 25 | 6107 ± 35 | 7310 ± 38 |
| 3 | 6746 ± 21 | 6451 ± 27 | 7927 ± 35 | 8931 ± 41 |
| 4 | 8252 ± 27 | 7775 ± 32 | 9915 ± 45 | 10962 ± 71 |
| 5 | 11359 ± 115 | 10869 ± 132 | 14285 ± 188 | 15584 ± 352 |
All data presented as mean ± SEM.
Figure 1.
Mean ± SEM energy intake, percent body fat, and BMI by calcium intake quintiles (n = 2975 in the SHS and 2755 in NHANES III).A: energy intake, P for trend < 0.001 in both samples. B: percent of body fat, P for trend < 0.001 in both samples. C: BMI, P for trend = 0.19 in the SHS and 0.31 in NHANES III.
In the SHS, PBF and BMI (calculated as adjusted mean differences in higher calcium quintiles compared to the lowest quintile) decreased as calcium intake increased after controlling for other confounders (Table 3). In NHANES III, we observed no trend in BMI or PBF across calcium categories. These results were unchanged following adjustment for total fat, protein, magnesium, and potassium intake (data not shown).
Table 3.
Absolute adjusted mean differences (95% CI) in percent body fat (PBF [%]) and BMI per quintile of calcium intake, SHS and NHANES III1,2
| Calcium intake quintiles |
P for trend3 |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (Reference) | ||||||
| Absolute adjusted mean difference compared with reference (95% CI) | ||||||
| SHS | ||||||
| PBF (%)4 | 36.9 | −0.82 (−1.54, −0.10) | −0.59 (−1.33, 0.14) | −0.57 (−1.33, 0.19) | −1.28 (−2.10, −0.47) | 0.011 |
| BMI5 | 31.3 | −0.37 (−1.02, 0.27) | −0.07 (−0.73, 0.59) | −0.09 (−0.77, 0.60) | −0.80 (−1.53, −0.08) | 0.046 |
| NHANES III | ||||||
| PBF (%)6 | 28.5 | 0.50 (−1.03, 2.02) | 0.25 (−1.40, 1.90) | 0.69 (−0.51, 1.88) | 0.46 (−1.20, 2.12) | 0.634 |
| BMI7 | 27.5 | −0.22 (−0.69, 1.13) | −0.21 (−1.19, 0.77) | −0.33 (−1.28, 0.63) | −0.46 (−1.72, 0.80) | 0.252 |
Model adjusted for gender, age, study center, years of education, income, alcohol use, smoking status, diabetes status (diabetes, impaired glucose tolerance, normal fasting glucose tolerance), and reported energy intake.
Value presented is adjusted mean for the reference group.
Tests for linear trend were conducted by the statement CONTRAST in PROC GLM in SAS and PROC REGRESS in SUDAAN.
Multiple R2 = 0.54.
Multiple R2 = 0.14.
Multiple R2 = 0.46
Multiple R2 = 0.13.
Because the prevalence of diabetes is much higher in the SHS as compared to NHANES, diabetes, which may affect dietary intake, was included as a covariate. Analyses repeated in participants without diabetes yielded the same results regarding the associations mentioned above (data not shown). Calcium intake was similar in those with and without diabetes (638 ± 11.8 mg/d and 638 ± 11.0 mg/d; P = 0.98, respectively). BMI and PBF were slightly higher in individuals with diabetes (32.2 ± 0.2 kg/m2, and 30 ± 0.1; P < 0.001 kg/m2, and 37.5% ± 0.2 and 35.2% ± 0.2; P < 0.001, adjusted for sex and age, respectively).
In sex-specific analyses of SHS data, PBF decreased as calcium intake increased in women, but not in men after controlling other confounders. In women, the adjusted mean PBF difference and 95% CI in ascending calcium intake quintiles compared with the 1st quintile (41.7%) were −0.44% (95% CI: −1.41 to 0.53), −0.55% (95% CI: −1.54 to 0.45), −0.45% (95% CI: −1.48 to 0.57), −1.28% (95% CI: −2.36 to −0.20); P for trend = 0.026, while in men the adjusted mean PBF difference and 95% CI in ascending calcium intake quintiles, compared with the 1st quintile (29.0%), were −0.42% (95% CI: −1.48 to 0.65), −0.75% (95% CI: −1.83 to 0.33), −0.61% (95% CI: −1.74 to 0.51), and −0.80% (95% CI: −2.03 to 0.43); P for trend = 0.30. There were similar trends with increasing BMI, but they did not reach significance (P for trend = 0.17 and 0.12 in women and men, respectively).
Calcium intake was not correlated with BMI or PBF in either gender in NHANES III participants (P for trend = 0.33 in women and 0.94 in men and P = 0.92 in women and 0.18 in men, respectively).
In the SHS, physical activity data were collected only at the first examination, and these levels were low (32). Adjustment for baseline physical activity did not change the results.
In the SHS, the odds of being obese vs. normal weight decreased over increasing calcium intake quintiles, applying a generalized logit mode after controlling for cofounders (Table 4). This association was not observed in NHANES III.
Table 4.
Odds ratios (95% CI) for being overweight or obese vs. normal, 1by calcium intake quintiles, SHS and NHANES III2
| Calcium intake quintiles |
P for trend3 |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| SHS | ||||||
| Overweight vs. normal | 1.0 | 0.97 (0.66–1.42) | 0.81 (0.55–1.21) | 1.01 (0.67–1.52) | 0.78 (0.51–1.20) | 0.30 |
| Obese vs. normal | 1.0 | 0.78 (0.54–1.13) | 0.84 (0.58–1.23) | 0.81 (0.54–1.21) | 0.56 (0.37–0.85) | <0.01 |
| NHANES III | ||||||
| Overweight vs. normal | 1.0 | 0.83 (0.49–1.39) | 1.16 (0.72–1.84) | 1.16 (0.67–2.02) | 1.27 (0.72–2.26) | 0.29 |
| Obese vs. normal | 1.0 | 0.79 (0.49–1.29) | 0.95 (0.65–1.37) | 0.88 (0.54–1.42) | 0.84 (0.50–1.41) | 0.25 |
normal defined as BMI < 25 (kg/m2), overweight as BMI ≥ 25 and < 30 (kg/m2) and obese as BMI ≥ 30 (kg/m2).
Odds ratios are estimated from the generalized logits model, controlling for gender, age, study center, years of education, income, alcohol use, smoking status, diabetes status (diabetes, impaired glucose tolerance, normal fasting glucose tolerance), and energy intake.
Tests for trend were conducted by modeling the median of each quintile of calcium intake as a continuous variable in the generalized logits models.
DISCUSSION
In this cross-sectional analysis of American Indian men and women, ages 47–79 years, we observed negative associations between calcium intake and both BMI and PBF, although not all reached statistical significance. Mean calcium intake was low, but mean intakes in the highest quintile exceeded the U.S. Dietary Reference Intake for calcium, which is 1000 mg/day for individuals ages 19–50 and 1200 mg/day for individuals ages > 51 (33). These finding differ from observations on calcium intake and either PBF or BMI for the general U.S. population sampled in NHANES III. Energy intake was higher in American Indian women, while American Indian men's energy intake was lower. These findings may result from lower levels of physical activity among American Indians or from recall bias in older American Indian men as compared with younger American Indians or the more educated population in NHANES III, as well as from other unmeasured factors that differ between the two groups.
A previous report from NHANES III that focused on data for younger adults (mean age 28.7 in women and 43.5 in men) showed that after controlling for energy intake, the odds of being in the highest quartile of percent body fat vs. other quartiles were 1.00, 0.75, 0.40, 0.16 for the lowest to highest quartiles of calcium intake (255 ± 20, 484 ± 13, 773 ± 28, and 1346 ± 113 mg/day), respectively, in women (n = 380, P < 0.0009). A similar inverse association was noted in men (n = 7114, P < 0.0006), although a comparable dose-response relation was not evident from the model (5). When recalculating PBF applying the equation used by Zemel (5,34) in our analysis, the odds of being in the highest quartile of PBF vs. other quartiles for participants in the lowest to highest quartiles of calcium intake also were not evident (data not shown). The differing results across the two analyses may result from differences in the age and gender distributions, as well as fewer adjustments for confounders in the NHANES III analysis.
Analyses in 65 Pima Indian adults failed to find an association between calcium intake and body size or adiposity (35). Differences between this study and our findings may result from the much larger sample size in the SHS.
To the best of our knowledge, this is the first examination of diet- and obesity-related factors that compares American Indians to the general U.S. population. The higher PBF and BMI and the lower calcium intake in American Indian men and women, compared with the general U.S. population in NHANES III, is notable and supported by a suggested biological mechanism, as well as by some longitudinal and small intervention trials (36). Other trials of calcium intake (in both diet and supplements) in other populations have generally shown little effect on weight loss (37, 38).
One possibility is that calcium intake is reflective of a diet lower in energy density. Stang et al. (39) showed that intakes of several vitamins were low in this population, possibly reflecting a diet low in vegetables and fruits. Our analyses show that the SHS diet is lower in potassium and magnesium, as well as calcium (data not shown). This supports a possible association between lower calcium intake and a less energy-dense diet. Another possible explanation for our findings of a lower calcium intake among American Indians may relate to the high prevalence of lactose intolerance among American Indians (40). The range between the lowest and the highest calcium intake quintiles, in the SHS is potentially large enough to produce an association, whereas this range in the NHANES III was not as large.
Other epidemiologic studies have reported associations between dietary intake of dairy products and calcium and obesity. The CARDIA study reported that overweight individuals consumed fewer dairy products than their normal-weight counterparts (6). Dairy consumption also was reported to be inversely associated with prevalence of insulin resistance among individuals with a baseline BMI of ≥ 25 kg/m2. Consistent with this finding is a report from the Women's Health Study that dietary intakes of calcium and dairy products may be associated with lower prevalence of metabolic syndrome, as well as incidence of type 2 diabetes (41). A meta-analysis of 13 randomized controlled trials failed to show an association between increased consumption of either calcium supplements or dairy products and weight loss (37). However, it is possible that higher calcium intake is associated with lower weight in an obese population. This hypothesis is supported by data from at least one study (42). In an additional study, calcium intake of about 600 mg/d was associated with predicted mean gain of 0.5 kg per year. By contrast, at a range of intake of 1000–1500 mg/d, average weight gain was zero (43).
The inverse relationship between adiposity and calcium intake observed in American Indians and the lack of any relation among the general U.S. population may reflect other nutritional or lifestyle differences between these groups. Nonetheless, mechanisms underlying the association between calcium intake and adiposity merit further investigation. Because we do not have data on food sources for the relevant nutrients from these dietary recalls that were performed in the mid 1990s (the NDS database at that time did not allow extraction of food data), we cannot present the results by food groups or food patterns.
This study was limited by several factors. Because this was a cross-sectional analysis, cause and effect cannot be established. Dietary intake alone is unlikely to be the sole factor underlying lower BMI and PBF. The use of a single 24-h recall is limited because of day-to-day individual variability (44). In addition, diet was only measured once during the SHS and may have changed during the follow-up period. The 24-h recall can provide detailed information on specific foods (45); and, therefore, is considered ideal for intercultural comparisons of mean dietary intake levels, because it is an open-ended method which allows detailed reporting of heterogeneous types of food (43). On the other hand, it tends to underestimate energy intake. Our aim was to use the SHS cohort to compare quintiles of calcium intake with PBF and BMI. For this more limited objective, we believe it is sufficient to assume that constant scaling bias is of a relatively constant magnitude, as in NHANES III (44). Finally, although SHS is a population-based sample of 13 communities, the data may not be reflective of all American Indian groups (46).
Our data support an association between calcium intake and obesity in American Indians and may be useful in developing approaches for nutritional interventions aimed at weight control. However, well-designed, culturally appropriate clinical trials are needed to determine whether interventions to increase calcium intake are effective in weight control in American Indians.
ACKNOWLEDGMENTS
The authors acknowledge the assistance and cooperation of the Ak-Chin Tohono O’Odham (Papago)/Pima, Gila River and Salt River Pima/Maricopa in Arizona, Apache, Caddo, Comanche, Delaware, Fort Sill Apache, Kiowa, and Wichita in Oklahoma, and Oglala Sioux, Cheyenne River Sioux, and Spirit Lake communities in North/South Dakota, without whose support this study would not have been possible. The authors also wish to thank the directors of the Strong Heart Study clinics, Dr. Marie Russell, Dr. Tauqeer Ali, Marcia O’Leary, and their staffs. We wish to thank Rachel Schaperow, MedStar Research Institute, Hyattsville, MD, for her assistance in the editing of this manuscript. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
This study was supported by cooperative agreement grants (No. U01HL-41642, U01HL-41652, and U01HL-41654) from the National Heart, Lung and Blood Institute.
Footnotes
Abbreviations used: CARDIA, Coronary Artery Risk Development in Young Adults; IFG, impaired fasting glucose; NHANES III, Third National Health and Nutrition Examination Survey; PBF, percentage of body fat; SAS, Statistical Analysis System; SHS, Strong Heart Study.
LITERATURE CITED
- 1.Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. Journal of Nutritional Biochemistry. 2003;14:492–506. doi: 10.1016/s0955-2863(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 2.Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr. 2003;133:252S–256S. doi: 10.1093/jn/133.1.252S. [DOI] [PubMed] [Google Scholar]
- 3.Teegarden D. Calcium intake and reduction in weight or fat mass. J Nutr. 2003;133:249S–251S. doi: 10.1093/jn/133.1.249S. [DOI] [PubMed] [Google Scholar]
- 4.Jacqmain M, Doucet E, Despres JP, Bouchard C, Tremblay A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 2003;77:1448–1452. doi: 10.1093/ajcn/77.6.1448. [DOI] [PubMed] [Google Scholar]
- 5.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEBJ. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 6.Pereira MA, Jacobs DR, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the Insulin Resistance Syndrome in young adults: the CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 7.Boon N, Koppes LLJ, Saris WHM, Van Mechelen WV. The relation between calcium intake and body composition in a Dutch population. Am J Epidemiol. 2005;162:27–32. doi: 10.1093/aje/kwi161. [DOI] [PubMed] [Google Scholar]
- 8.Interagency Board for Nutrition Monitoring and Related Research. Nutrition monitoring in the United States: the directory of federal and state nutrition monitoring activities. In: Wright J, editor. Hyattsville (MD): Public Health Service; 1992. [Google Scholar]
- 9.Broussard BA, Sugarman JR, Bachman-Carter K, Booth K, Stephenson L, Strauss K, Gohdes D. Toward comprehensive obesity prevention programs in Native American communities. Obes Res. 1995;3:289S–297S. doi: 10.1002/j.1550-8528.1995.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 10.Broussard BA, Johnson A, Himes JA, Story M, Fichtner R, Hauck F, Bachman-Carter K, Hays J, Frohlich K, et al. Prevalence of obesity in American Indians and Alaska Natives. Am J Clin Nutr. 1991;53(suppl):1535S–1542S. doi: 10.1093/ajcn/53.6.1535S. [DOI] [PubMed] [Google Scholar]
- 11.Story M, Marguerite E, Fabitz RR, Clay TE, Rock BH, Broussard B. The epidemic of obesity in American Indian communities and the need for obesity-prevention programs. Am J Clin Nutr. 1999;69:747S–754S. doi: 10.1093/ajcn/69.4.747S. [DOI] [PubMed] [Google Scholar]
- 12.Interdepartment Committee for National Defense and Division of Indian Public Health. Washington: Public Health Service; 1964. Fort Belknap Indian Reservation nutrition survey. [Google Scholar]
- 13.Interdepartment Committee for National Defense and Division of Indian Public Health. Blackfeet Indian Reservation nutrition survey. Washington DC: Public Health Service; 1964. [Google Scholar]
- 14.Bass MA, Wakefield LM. Nutrient intake and food consumption patterns of Indians on Standing Rock Reservation. J Am Diet Assoc. 1974;64:36–41. [PubMed] [Google Scholar]
- 15.Butte NF, Calloway DH, Van Duzen JL. Nutritional assessment of pregnant and lactating Navajo women. Am J Clin Nutr. 1981;34:2216–2228. doi: 10.1093/ajcn/34.10.2216. [DOI] [PubMed] [Google Scholar]
- 16.Teufel NF, Dufour DL. Patterns of food use and nutrient intake of obese and non-obese Hualpai Indian women of Arizona. J Am Diet Assoc. 1990;90:1229–1235. [PubMed] [Google Scholar]
- 17.Owen G, Garry PJ, Seymoure RD, Harrison GG, Acosta PB. Nutrition studies with White Mountain Apache preschool children in 1976 and 1969. Am J Clin Nutr. 1981;34:266–277. doi: 10.1093/ajcn/34.2.266. [DOI] [PubMed] [Google Scholar]
- 18.Story M, Topkins RA, Bass MA, Wakefield LM. Anthropometric measurement and dietary intakes of Cherokee Indian teenagers in North Carolina. J Am Diet Assoc. 1986;86:1555–1560. [PubMed] [Google Scholar]
- 19.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le N-A, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study - A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 20.Sosenko JM, Hu D, Welty T, Howard BV, Lee E, Robbins DC. Albuminuria in recent-onset in type 2 diabetes. The Strong Heart Study. Diabetes Care. 2002;51:1078–1084. doi: 10.2337/diacare.25.6.1078. [DOI] [PubMed] [Google Scholar]
- 21.Collis R, Devereux RB, Roman MJ, Simone GD, Yeh JL, Howard BV, Fabsitz RR, Welty TK. Relation of stroke volume and cardiac output to body composition. The Strong Heart Study. Circulation. 2001;103:820–825. doi: 10.1161/01.cir.103.6.820. [DOI] [PubMed] [Google Scholar]
- 22.The Strong Heart Study Operational Manual Volume V. Dietary and psychosocial studies. Oklahoma City, OK: The Strong Heart Study Coordinating Center, University of Oklahoma Health Sciences Center; 1993. [Google Scholar]
- 23.Schakel SF, Sievert YA, Buzzard LM. Sources of data for developing and maintaining a nutrient data base. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 24.Schakel SF. Procedures for estimating nutrient values for food composition databases. J Food Comp and Anal. 1997;10:102–114. [Google Scholar]
- 25.National Center for Health Statistics. Washington, DC: U.S. Government printing office; 1994. Plan and operation of the third National Health and Nutrition Examination Survey 1988–94. [Google Scholar]
- 26.U.S. Department of Health and Human Services (DHHS) National Center for Health Statistics. [Accessed 8 February 2005];NHANES III data files series 11, No. 1A and 2A. 1998 http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm#Data%20Files%202a.
- 27.SAS. SAS Release 9.00. Cary, NC: SAS Institute; 2002. [Google Scholar]
- 28.Research Triangle Institute. SUDAAN Release 9.0. Research Triangle Park, NC: 2004. [Google Scholar]
- 29. [Accessed February 10, 2006]; http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/analytical_guidelines.htm.
- 30.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 31.Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Yurgalevitch SM, Kriska AM, Welty TK, Go O, Robbins DC, Howard BV. Physical activity and lipids and lipoproteins in American Indians ages 45–74. Med Sci Sports Exerc. 1998;30:543–549. doi: 10.1097/00005768-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes. Am J Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 34.Segal KR, Van Loran M, Fitzgerals PI, Hodgdon JA, Van itallie TB. Lean body mass estimation by bioelectrical inpedance: a four-site cross-validation study. Am J Clin Nutr. 1988;47:7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Venti CA, Tataranni PA, Salbe AD. Lack of relationship between calcium intake and body size in an obesity-prone population. J am Diet Assoc. 2005;105:1401–1407. doi: 10.1016/j.jada.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Parikh SJ, Yanovski J. Calcium intake and adiposity. Am J Clin Nutr. 2003;77:281–287. doi: 10.1093/ajcn/77.2.281. [DOI] [PubMed] [Google Scholar]
- 37.Trowman R, Dumville JC, Hahn S, Torgerson DJ. A systematic review of the effects of calcium supplementation on body weight. Br J Nutr. 2006;95:1033–1038. doi: 10.1079/bjn20051727. [DOI] [PubMed] [Google Scholar]
- 38.Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, Leboff M, Margolis K, Powell L, Uwaifo G, Whitlock E, Wylie-Rosett J, LaCroix A. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain) Arch Intern Med. data in press doi: 10.1001/archinte.167.9.893. [DOI] [PubMed] [Google Scholar]
- 39.Stang J, Zephier EM, Story M, Himes JH, Yeh JL, Welty T, Howard BV. Dietary intakes of nutrients thought to modify cardiovascular risk from three groups of American Indians: The Strong Heart Dietary Study, Phase II. J Am Diet Assoc. 2005;105:1895–1903. doi: 10.1016/j.jada.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Swagerty DL, Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845–1850. [PubMed] [Google Scholar]
- 41.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 42.Heaney RP. Low calcium intake among African Americans: effects on bones and body weight. J Nutr. 2006;136:1095–1098. doi: 10.1093/jn/136.4.1095. [DOI] [PubMed] [Google Scholar]
- 43.Heaney RP. Normalizing calcium intake: projected population effects on body weight. J Nutr. 2003;133:268S–70S. doi: 10.1093/jn/133.1.268S. [DOI] [PubMed] [Google Scholar]
- 44.Witschi JC. Short-term recall and recording methods. In: Willett WC, editor. Nutritional epidemiology. New York: Oxford University Press; 1990. pp. 53–68. [Google Scholar]
- 45.Block G, Hartman AM. Issues in reproducibility and validity of dietary studies. Am J Clin Nutr. 1989;50:1133–1138. doi: 10.1093/ajcn/50.5.1133. [DOI] [PubMed] [Google Scholar]
- 46.Howard BV, Lee ET, Cowan LD, Fabsitz RR, Howard WmJ, Oopik AJ, Robbins DC, Savage PJ, Yhe JL, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142:254–268. doi: 10.1093/oxfordjournals.aje.a117632. [DOI] [PubMed] [Google Scholar]