Abstract
Posttraumatic stress disorder (PTSD) is a moderately heritable anxiety disorder that may develop after exposure to trauma. However, only few genetic variants that relate to PTSD have been studied. This study examined the relationship between 12 single nucleotide polymorphisms (SNPs) in the corticotropin-releasing hormone receptor 1 gene (CRHR1) and post-disaster PTSD symptoms and diagnosis in adults exposed to 2004 Florida hurricanes. CRHR1 regulates the hypothalamic-pituitary-adrenal (HPA) axis; dysregulation of the HPA axis is characteristic of stress phenotypes. Final analyses were conducted in the European-American (EA) subsample (n=564) due to population stratification. After correction for multiple testing, rs12938031 and rs4792887 remained associated with post-hurricane PTSD symptoms. Additionally, rs4792887 was associated with post-hurricane diagnosis of PTSD. This study is the first to examine CRHR1 in relation to PTSD in adults, and provides evidence for the importance of CRHR1 variation in the etiology of PTSD. Although results are preliminary and require replication, they justify follow-up efforts to characterize how this gene relates to PTSD.
Keywords: Posttraumatic stress disorder, CRHR1, HPA axis, disasters, trauma
1. Introduction
The majority of the population (about 60-80% in the United States) will be exposed to at least one traumatic event at some point during their lifetimes (Bonanno, 2004; Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993). Across all traumatic events, only about 8% of those exposed will develop PTSD (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). This difference between the high prevalence of exposure to a potentially traumatic event (PTE) and the comparatively low prevalence of people who develop PTSD has led to research efforts aimed at explaining this variation. Although most research in the field has focused on how psychosocial factors increase risk for PTSD (Brewin et al., 2002), data from both civilian and non-civilian twin studies also suggest that PTSD is moderately heritable (Lyons et al., 1993; Sartor et al., 2012; M.B. Stein, Jang, Taylor, Vernon, & Livesley, 2002; True et al., 1993). As for most complex traits, the genetic underpinnings of this phenotype have not been thoroughly elucidated.
Two of the primary methods employed to identify molecular variants that influence the latent genetic heritability estimates yielded from twin studies include genome-wide association studies (GWAS) and candidate gene studies. GWAS assesses polymorphisms across the entire genome for association with the phenotype, but does not embody an underlying biological hypothesis. While GWAS would greatly advance the PTSD genetic literature, existing published PTSD studies have small sample sizes (Cornelis, Nugent, Amstadter, & Koenen, 2010).
Unlike GWAS, the candidate gene approach nominates regions of interest based on their hypothesized biological relationship with neurobiological pathways involved with the development or maintenance of the phenotype (or other information, such as genetic linkage). Serotonergic and dopaminergic systems have been the focus of most genetic studies of PTSD (Cornelis, et al., 2010), although there are other neurobiological pathways of interest. The hypothalamic-pituitary-adrenal (HPA) axis, activated by corticotrophin-releasing hormone (CRH), moderates the release of stress hormones such as cortisol (Claes, 2004). In most individuals, the normal stress response is followed by a return to baseline once the stressor has passed. However, the initial stress response is altered in some individuals, becoming chronic. Dysregulation of the HPA axis is characteristic of stress-related phenotypes, including PTSD (Yehuda, 2009). Numerous findings suggest a sensitized HPA axis among individuals with PTSD, such as increased corticotrophin-releasing factor levels in cerebrospinal fluid, higher cortisol levels, and elevated glucocorticoid receptor number and responsiveness (Yehuda, 2009). Thus, studying the relationship between regulatory genes of the HPA axis and PTSD may be useful.
There are many genes that influence HPA axis reactivity (A. B. Amstadter, Nugent, & Koenen, 2009; Koenen, 2007); however, CRH genes are seemingly particularly consequential (Baker et al., 2005; Smoller et al., 2005; Tyrka et al., 2009; van Gaalen, Stenzel-Poore, Holsboer, & Steckler, 2002). Of candidate gene studies of PTSD to date, few have examined genes influencing the HPA axis (Bachmann et al., 2005; Binder et al., 2008), with one prior study examining a CRH gene (A. B Amstadter et al., 2011). In this small study of pediatric injury patients, variants of CRHR1 were significantly associated with acute and longitudinal PTSD symptoms, with rs12944712 significantly predictive.
Numerous studies have investigated CRHR1 variation and depression in the context of a traumatic event. For example, Heim et al. (Heim et al., 2009) showed that rs110402 was associated with cortisol levels and developing depression in adulthood in males who experienced childhood trauma. Bradley et al. (Bradley et al., 2008) found that after correction for multiple testing, rs110402 and rs7209436 moderated the relationship between child abuse and adult depression, with the effect of rs110402 consistent with that reported by Heim et al. (Heim, et al., 2009). Bradley et al. (Bradley, et al., 2008) also found a protective effect of the CRHR1 TAT haplotype (rs7209436, rs110402, rs242924), which was later replicated in a study by Polanczyk et al. (Polanczyk et al., 2009) examining adult depression following childhood maltreatment.
Overall, evidence suggests that CRHR1 variation may be important in post-trauma psychopathology. The primary aim of the current study was to test for an association between variation in CRHR1 and PTSD symptoms in an epidemiologic sample of adults exposed to one of the 2004 Florida hurricanes (R. Acierno, Ruggiero, Kilpatrick, Resnick, & Galea, 2006; A. B. Amstadter et al., 2009). This disaster-exposed sample is very suitable for genetic PTSD studies, as it controls for possible gene-environment correlations. We hypothesized that variants in CRHR1 would be associated with post-hurricane PTSD symptoms.
2. Methods
2.1. Data Collection
This study examined data from 626 individuals residing in Florida in 2004 who were exposed to one of four hurricanes (Charley, Francis, Ivan, and Jeanne). A probability sample of adults was randomly selected from telephone households in counties that were declared “disaster zones” following the 2004 Florida hurricanes through a Random Digit Dial (RDD) procedure. Interested individuals completed a structured telephone interview and provided a saliva sample for genotyping. Methodological information for this Florida hurricanes study is described elsewhere (R. Acierno et al., 2007; R. Acierno, et al., 2006; Galea, Acierno, Ruggiero, Resnick, & Kilpatrick, 2006; Kilpatrick et al., 2007).
Participants gave verbal consent and were mailed detailed information regarding the components of the study and consent. Participants received $20 for completion of the interview and saliva sample for DNA extraction.
2.2. Assessment Procedure
Self-report data collection occurred 6-9 months after the hurricanes via telephone interview. Interviews were conducted using computer-assisted telephone interview (CATI) methodology, which allows for maximum quality control. From the phone interview, we evaluated: 1) demographic characteristics, 2) social support, 3) exposure to hurricane(s), and 4) PTSD symptoms.
Demographic Characteristics
Information about the participant regarding sex, marital status, age, employment status, ethnicity, race, and household income were collected.
Social support
Participants were evaluated for social support during the six months before the hurricanes using a modified version of the Medical Outcomes Study module (Sherbourne & Stewart, 1991). This assesses emotional, instrumental, and appraisal social support with five items (range=0–20). A score of 15 or less was considered “low social support” based on the cutoff score derived from prior work (Galea et al., 2002). This scale had excellent reliability (Chronbach’s alpha= .86).
Exposure to hurricane(s)
Hurricane exposure was evaluated using behaviorally specific indicators identified in previous research (Freedy, Saladin, Kilpatrick, Resnick, & Saunders, 1994): 1) being personally present during the hurricane and exposed to winds or flooding; 2) at least one week of inadequate access to food, water, electricity, telephone service, or clothing; 3) losses or significant damage in at least two of five categories of hurricane-related loss (i.e., furniture, appliances, or other household contents; sentimental possessions, such as photographs; automobiles; pets; and crops, trees, or garden); 4) one week or longer of home displacement; and 5) un-reimbursed losses of $1,000 or more. As used in previous research (A. B. Amstadter, Koenen, et al., 2009), high hurricane exposure was defined as having experienced two or more of these indicators.
PTSD
The National Women’s Study (NWS) PTSD module, which is used often in population-based research, was used to evaluate PTSD symptoms. This module has concurrent validity and reliability (e.g., temporal stability, internal consistency, diagnostic reliability) (Ruggiero, Rheingold, Resnick, Kilpatrick, & Galea, 2006). The NWS PTSD module was validated in the DSM-IV PTSD field trial against the Structured Clinical Interview for DSM (SCID), yielding an inter-rater kappa coefficient of 0.85 for the diagnosis of PTSD. Comparisons between scores on the National Women’s Study PTSD module and the SCID yielded a kappa coefficient of 0.71 for current PTSD (Kilpatrick et al., 1998). Research demonstrates considerable correspondence between telephone versus in-person administration of this module (R Acierno, Resnick, Kilpatrick, & Stark-Riemer, 2003). For our main analyses we examined post-hurricane PTSD symptom count, which had excellent internal reliability in this sample (α=.86) (R. Acierno, et al., 2007). In a follow-up analysis we examined post-hurricane PTSD at the diagnostic level; we defined PTSD based on DSM-IV symptom requirements (i.e., three avoidance, one intrusion, and two arousal symptoms), including functional impairment.
2.3. Saliva Collection and DNA Extraction
Individuals who participated in the phone interview were given the option of providing a saliva sample using a mouthwash protocol. A total of 637 individuals mailed their saliva samples back to the Yale University School of Medicine laboratory for DNA extraction and analysis, and 626 samples gave valid genotype data for CRHR1 markers (see below). Participants who did versus did not return a saliva sample did not differ with regard to main study variables. Details on response rate and associations of participation are described elsewhere (Galea et al., 2006). DNA was extracted from these samples using PURGENE kits (Gentra Systems, Minneapolis).
2.4. Bioinformatics
The SNPs that were selected include previously studied SNPs and tagging SNPs with an r2 value greater than 0.8 using Haploview’s Tagger (Barrett, Fry, Maller, & Daly, 2005). Tag SNPs are representative SNPs in each haplotype block at the gene locus (Zhang et al., 2004). The following SNPs were genotyped: rs12938031, rs7209436, rs171441, rs242924, rs4792887, rs2664008, rs242936, rs110402, rs17689966, rs242939, rs16940686, and rs173365.
2.5. Genotyping Procedures
Ancestry Proportion Scores
A total of 36 markers were genotyped to yield ancestry information (M. B. Stein, Schork, & Gelernter, 2004; Yang, Zhao, Kranzler, & Gelernter, 2005a, 2005b). One additional highly informative SNP marker, SLC24A5(Lamason et al., 2005), was added to the panel. Ancestry proportion scores were created to control for spurious associations that can occur from variation in allele frequency and prevalence of trait by population. Bayesian cluster analysis was used to estimate participants’ ancestries with the marker panel described above on the procedures and STRUCTURE software developed by Falush, Stephens, and Pritchard (Falush, Stephens, & Pritchard, 2003) and Pritchard and Rosenberg (Pritchard & Rosenberg, 1999). For the STRUCTURE analysis, the “admixture” and “allele frequencies correlated” models were specified and we used 100,000 burn-in and 100,000 Markov chain Monte Carlo iterations.
TaqMan probe-based chemistry (Lee, Connell, & Bloch, 1993) was used to genotype the samples at the Virginia Commonwealth University laboratory. The Taqman SNP genotyping assays were designed and ordered from Life Technologies.
2.6. Statistical Analyses
Descriptive statistics were compiled to examine the study sample and for SNP quality evaluation. Analyses were conducted in the full sample and the EA subsample to control for population stratification. To test for possible gene-environment correlations, Chi-square analyses were conducted for all stressor variables and all genotypes. The prevalence of post-hurricane PTSD diagnosis in the full sample was 3.2%, while the prevalence of post-hurricane PTSD diagnosis in the EA subsample was 2.8%. In order to maximize power, symptom counts were used as the primary outcome variable. A linear regression model was conducted to determine if there was an association between CRHR1 SNPs and post-hurricane PTSD symptoms after adjusting for sex, age, ancestral proportion scores, social support, and hurricane exposure. A follow-up logistic regression analysis was conducted with the SNPs that were significantly associated with PTSD symptoms in the linear regression analysis to examine their relation to post-hurricane PTSD diagnosis in the EA subsample, again, controlling for sex, age, ancestral proportion scores, social support, and hurricane exposure.
3. Results
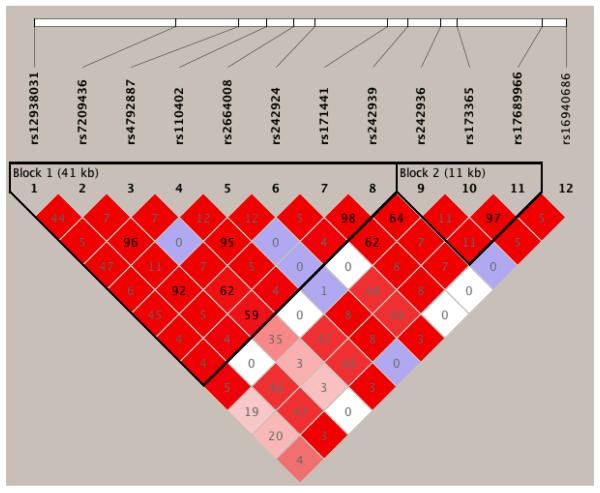
SNPs and their chromosomal position, minor/major alleles, minor allele frequency, Hardy-Weinberg Equilibrium (HWE) p-values, and call rates are summarized in Table 1 for the full sample. Call rates ranged from 90.4% to 98.6%. As shown in Table 1, 4 of 12 SNPs nominally deviated from HWE expectations in the full sample. Given the non-homogenous composition of the study sample, we selected the 564 participants who were EA and calculated the HWE tests for this subsample to determine if population stratification influenced the results in the full sample. Indeed, three of four SNPs that violated HWE in the full sample were in HWE in the EA subsample. The fourth SNP (rs2664008) was not in nominal HWE (p= 0.02), but with Bonferroni correction this is not considered significant. Following, we performed the subsequent analyses in the EA participants only. Haploview (Barrett, et al., 2005) was used to estimate the LD structure of the SNPs (Figure 1). Table 2 summarizes the LD relationship between previously studied SNPs and tag-SNPs.
Table 1.
List of CRHR1 SNPs and their chromosomal position, minor/major alleles, minor allele frequency, Hardy-Weinberg p-values, and call rates for the full sample.
| SNP | Chromosomal Position |
Minor/major alleles |
Minor allele frequency |
Hardy- Weinberg p- value |
Call rate % |
|---|---|---|---|---|---|
| rs12938031 | 41210268 | G/A | 0.35 | 0.462 | 90.4 |
| rs7209436 | 41225913 | T/C | 0.43 | 0.018 | 92.7 |
| rs171441 | 41249125 | A/G | 0.07 | 0.343 | 92.0 |
| rs242924 | 41241147 | T/G | 0.44 | 0.047 | 95.4 |
| rs4792887 | 41232791 | T/C | 0.09 | 0.114 | 97.3 |
| rs2664008 | 41238883 | A/G | 0.08 | 0.016 | 94.4 |
| rs242936 | 41254990 | A/G | 0.09 | 0.814 | 96.6 |
| rs110402 | 41235818 | A/G | 0.44 | 0.059 | 97.1 |
| rs17689966 | 41266236 | G/A | 0.47 | 0.566 | 96.2 |
| rs242939 | 41251360 | C/T | 0.07 | 0.028 | 98.6 |
| rs16940686 | 41268811 | T/G | 0.04 | 1.000 | 97.1 |
| rs173365 | 41256855 | A/G | 0.47 | 0.681 | 95.2 |
Table 4.
Linear regression results for post-hurricane PTSD symptoms in the EA subsample.
| Variable | B | t | p-value |
|---|---|---|---|
| Sex | 0.03 | 0.61 | 0.54 |
| Age | 0.11 | 2.30 | 0.02 |
| Ancestry | 0.01 | 0.16 | 0.87 |
| Hurricane Exposure | 0.14 | 3.09 | 0.002 |
| Social Support | 0.23 | 4.93 | <0.001 |
| rs12938031 | −0.33 | −4.06 | <0.001* |
| rs7209436 | 0.05 | 0.22 | 0.83 |
| rs171441 | 0.33 | 0.96 | 0.34 |
| rs242924 | −0.40 | −1.62 | 0.11 |
| rs4792887 | −0.31 | −3.22 | 0.001* |
| rs2664008 | 0.06 | 1.13 | 0.26 |
| rs242936 | −0.05 | −0.51 | 0.61 |
| rs110402 | 0.03 | 0.09 | 0.93 |
| rs17689966 | 0.76 | 1.98 | 0.049 |
| rs242939 | −0.19 | −0.57 | 0.57 |
| rs16940686 | 0.04 | 0.81 | 0.42 |
| rs173365 | −0.77 | −1.99 | 0.047 |
Note: SNPs significant after Bonferroni correction.
Figure 1.
LD plot of all tested SNPs in the EA subsample using r2 as the measure of LD. Higher numbers indicate a greater amount of LD.
Table 2.
LD relationship (r2) between previously studied SNPs (x-axis) and tag-SNPs (y-axis).
| rs7209436 | rs242924 | rs4792887 | rs110402 | rs173365 | |
|---|---|---|---|---|---|
| rs12938031 | 0.448 | 0.455 | 0.055 | 0.474 | 0.190 |
| rs171441 | 0.055 | 0.056 | 0.622 | 0.057 | 0.081 |
| rs2664008 | 0.116 | 0.125 | 0.009 | 0.120 | 0.088 |
| rs242936 | 0.003 | 0.003 | 0.355 | 0.004 | 0.117 |
| rs17689966 | 0.472 | 0.486 | 0.031 | 0.480 | 0.977 |
| rs242939 | 0.047 | 0.048 | 0.597 | 0.049 | 0.073 |
| rs16940686 | 0.037 | 0.038 | 0 | 0.038 | 0.054 |
Demographic characteristics are presented in Table 3 for the full sample and the EA subsample. As shown, the EA subsample consisted of 19.3% adults younger than 60 years old, 64.0% were female, 43.8% experienced high hurricane exposure, and 35.1% had low social support. Among EA participants with valid genotype data, the average number of post-hurricane PTSD symptoms reported was 1.61 (SD= 2.65).
Table 3.
Sample characteristics.
| Full Sample n= 626 |
EA Subsample n= 564 |
|||
|---|---|---|---|---|
| Variable | n | % | n | % |
| Age | ||||
| <60 years | 140 | 22.6 | 108 | 19.3 |
| ≥60 years | 479 | 77.4 | 451 | 80.7 |
| Sex | ||||
| Male | 224 | 35.9 | 203 | 36.0 |
| Female | 400 | 64.1 | 361 | 64.0 |
| Race/Ethnicity | ||||
| European American | 564 | 90.8 | 564 | 100 |
| African American | 23 | 3.7 | — | — |
| Hispanic | 23 | 3.7 | — | — |
| Other | 11 | 1.8 | — | — |
| Hurricane Exposure | ||||
| Low | 343 | 55.0 | 317 | 56.2 |
| High | 281 | 45.0 | 247 | 43.8 |
| Social Support | ||||
| Low | 227 | 36.5 | 197 | 35.1 |
| High | 395 | 63.5 | 365 | 64.9 |
Two SNPs were nominally significantly related to social support (rs2664008 [χ2= 5.81, p= 0.02] and rs110402 [χ2= 6.01, p= 0.049]) in individual tests, but not significant after correction for multiple testing. No SNPs were related to hurricane exposure. The following results between SNPs and PTSD symptoms are not likely due to a gene-environment correlation.
As shown in Table 4, psychosocial characteristics were related to post-hurricane PTSD symptom count. The major allele (A) of rs12938031 (β= −0.33, t= −4.06, p< 0.001), the major allele (C) of rs4792887 (β= −0.31, t= −3.22, p= 0.001), the minor allele (G) of rs17689966 (β= 0.76, t= 1.98, p= 0.049), and the major allele (G) of rs173365 (β= −0.77, t= −1.99, p= 0.047) were nominally associated with higher post-hurricane PTSD symptoms (Table 4). After a Bonferroni adjustment for multiple testing was employed (α=.004), rs12938031 and rs4792887 remained significant.1 To determine if rs12938031 and rs4792887 were related to post-hurricane PTSD diagnosis a logistic regression was conducted. Results indicated that rs12938031 was associated with PTSD diagnosis (OR=.18, p=.006) and that rs4792887 was not significantly related to diagnostic status (OR=.58, ns).
4. Discussion
This is the first study to examine the relationship between CRHR1 SNPs and PTSD in adults. The results show that four SNPs (rs12938031, rs4792887, rs17689966, and rs173365) were nominally significantly related to post-hurricane PTSD symptoms. After an adjustment for multiple testing, rs12938031 and rs4792887 remained significant. This implies that the major alleles of rs12938031 and rs4792887 may increase risk for symptoms of PTSD. Further, the logistic regression results indicated that rs12938031 was significantly associated with PTSD diagnosis.
A strength of this study is its use of a disaster-exposed epidemiologic sample, which reduces the possible effects of a gene-environment correlation (rGE). Exposure to certain types of PTEs (e.g., assaultive traumas) is influenced by heritable personality characteristics such as neuroticism (Jang, Livesley, & Vernon, 1996). However, exposure to a non-assaultive trauma (e.g., natural disaster) has been shown not to be heritable (M.B. Stein, et al., 2002). Of note, no SNPs were related to hurricane exposure, and no control for rGE is needed for these models.
Our results are consistent with the limited literature on CRHR1 variation and stress-relevant phenotypes. One study that investigated SNPs in relation to PTSD symptoms following an acute injury showed that rs12944712 was related to PTSD symptoms (A. B Amstadter, et al., 2011). The SNP found in Amstadter et al’s (2011) investigation is in high LD (r2= 0.76) with rs12938031, a SNP found in the current study to be related to post-hurricane PTSD symptoms. Therefore, future studies of this gene may consider fine mapping of this region to identify functional variants. Similar to the results in this study, Bogdan et al. (Bogdan, Santesso, Fagerness, Perlis, & Pizzagalli, 2011) found that under conditions of stress, individuals with the AA genotype for rs12938031 showed a reduced ability to develop a response bias toward a more frequently rewarded stimulus, resulting in reward learning abnormalities.
Although functional variants of CRHR1 and their downstream effects are yet to be determined, the biologic function of this gene is relevant to PTSD and other stress-related phenotypes due to the key role of CRH on activating the HPA axis. Demonstrations that PTSD patients hypersuppress cortisol in response to low dose dexamethasone treatment (Yehuda, Boisoneau, Lowry, & Giller, 1995) led to the expansion of PTSD development models to incorporate altered post-trauma cortisol response. Exaggerated catecholamine increases during a traumatic event without the regulatory influence of cortisol may lead to abnormal memory formation and result in the intrusion symptoms that characterize PTSD (Yehuda, Shalev, & McFarlane, 1998). Variation in CRHR1 may play a role in this process, although this specific question has not yet been explored. Nevertheless, preclinical and clinical studies support the importance of CRHR1 in stress-related pathology (Koenen, 2007; van Gaalen, et al., 2002).
Our preliminary results suggest that polymorphisms in the CRHR1 gene are related to post-hurricane PTSD symptoms and for rs12938031 PTSD diagnosis in adults. The current study is not without limitations. Recall bias may exist, as these data were collected 6-9 months following hurricane exposure. In addition, DNA samples were returned for less than one half of the sample. However, there were no significant differences between returners and non-returners of saliva samples on a number of key variables (Galea, Acierno, Ruggiero, Resnick, & Kilpatrick, 2006; Kilpatrick, et al., 2007). Results from this study are not definitive and additional replication is needed, especially in a more diverse sample with ample power to analyze subsamples other than EAs. The major limitation is the small sample size we obtained, only a fraction of which is informative with respect to the phenotype of interest; therefore we were underpowered. Although a disaster-exposed sample has advantages, the generalizability of the results may be limited. Future studies should examine a wide range of traumatic events to determine if the results in the current study can be applied to other traumas. Future studies of this gene would also be strengthened by additional mapping and sequencing to identify functional variants, as well as gene expression studies. Overall, these results along with other literature on CRHR1 and stress-related health suggest that this gene is implicated in the development of anxiety disorders.
Footnotes
Analyses were also run in the full sample, and the pattern of results was similar.
6. References
- Acierno R, Resnick H, Kilpatrick DG, Stark-Riemer W. Assessing elder victimization: demonstration of a methodology. Social Psychiatry and Psychiatric Epidemiology. 2003;38:644–653. doi: 10.1007/s00127-003-0686-4. [DOI] [PubMed] [Google Scholar]
- Acierno R, Ruggiero KJ, Galea S, Resnick HS, Koenen KC, Rotizsch J, Kilpatrick DG. Psychological sequelae of the 2004 Florida hurricanes: Implications for post-disaster intervention. American Journal of Public Health. 2007;97(Supplement 1):S103–S108. doi: 10.2105/AJPH.2006.087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acierno R, Ruggiero KJ, Kilpatrick D, Resnick H, Galea S. Risk and protective factors for psychopathology among older versus younger adults after the 2004 florida hurricanes. American Journal of Geriatric Psychiatry. 2006;14:1051–1059. doi: 10.1097/01.JGP.0000221327.97904.b0. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders. 2009;23(3):369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. 2009;39(6):358–367. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Koenen KC. Corticotrophin-releasing Hormone Type 1 Receptor Gene (CRHR1) Variants Predict Posttraumatic Stress Disorder Onset and Course in Pediatric Injury Patients. Disease Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology. 2005;30(3):297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD., Jr Higher Levels of Basal Serial CSF Cortisol in Combat Veterans With Posttraumatic Stress Disorder. American Journal of Psychiatry. 2005;162(5):992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-Releasing Hormone Receptor Type 1 (CRHR1) Genetic Variation and Stress Interact to Influence Reward Learning. The Journal of Neuroscience. 2011;31(37):13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience. Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Ressler KJ. Influence of child abuse on adult depression moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Rose S, Andrews B, Green J, Tata P, McEvedy C, Foa EB. Brief screening instrument for post-traumatic stress disorder. The British Journal of Psychiatry. 2002;181:158–162. doi: 10.1017/s0007125000161896. [DOI] [PubMed] [Google Scholar]
- Claes SJ. Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Annals of Medicine. 2004;36(1):50–61. doi: 10.1080/07853890310017044. [DOI] [PubMed] [Google Scholar]
- Cornelis M, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Current Psychiatry Reports. 2010;12(4):313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of Population Structure Using Multilocus Genotype Data: Linked Loci and Correlated Allele Frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedy JR, Saladin ME, Kilpatrick DG, Resnick HS, Saunders BE. Understanding acute psychological distress following natural disaster. Journal of Traumatic Stress. 1994;7(2):257–273. doi: 10.1007/BF02102947. [DOI] [PubMed] [Google Scholar]
- Galea S, Acierno R, Ruggiero K, Resnick H, Tracy M, Kilpatrick D. Social context and the psychobiology of posttraumatic stress. Annals of the New York Academy of Sciences. 2006;1071:231–241. doi: 10.1196/annals.1364.018. [DOI] [PubMed] [Google Scholar]
- Galea S, Acierno R, Ruggiero KJ, Resnick HS, Kilpatrick DG. Social context and the psychobiology of trauma. Annals of the New York Academy of Sciences. 2006;1071:231–241. doi: 10.1196/annals.1364.018. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Vlahov D. Psychological sequelae of the September 11 terrorist attacks in New York City. New England Journal of Medicine. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Binder EB. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Frontiers in Behavioral Neuroscience. 2009;41(3):1–10. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: a twin study. Journal of Personality and Social Psychology. 1996;64(3):577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Gelernter J. Serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164(11):1–7. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Freedy JR, Pelcovitz D, Resick PA, Roth S, van der Kolk B. The posttraumatic stress disorder field trial: evaluation of the PTSD construct—criteria A through E. In: Widiger T, Frances A, Pincus H, Ross R, First M, Davis W, Kline M, editors. DSM-IV sourcebook. Vol. 4. American Psychiatric Press; Washington, DC: 1998. pp. 803–844. [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. Journal of Traumatic Stress. 2007;20(5):737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, Cheng KC. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310(5755):1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Research. 1993;21(16):3761–3766. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM. Do genes influence exposure to trauma? A twin study of combat. American Journal of Medical Genetics. 1993;48(1):22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment. Replication and extension. Archives of General Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. The American Journal of Human Genetics. 1999;65(1):220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Ruggiero KJ, Rheingold AA, Resnick HS, Kilpatrick DG, Galea S. Comparison of two widely used PTSD-screening instruments: implications for public mental health planning. Journal of Traumatic Stress. 2006;19:699–707. doi: 10.1002/jts.20141. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Nelson EC. Common Heritable Contributions to Low-Risk Trauma, High-Risk Trauma, Posttraumatic Stress Disorder, and Major Depression. Archives of General Psychiatry. 2012;69(3):293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social Science and Medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Sklar P. The corticotropin releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biological Psychiatry. 2005;57(12):1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. American Journal of Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. A polymorphism of the beta1-adrenergic receptor is associated with low extraversion. Biological Psychiatry. 2004;56(4):217–224. doi: 10.1016/j.biopsych.2004.05.020. [DOI] [PubMed] [Google Scholar]
- True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry. 2009;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. European Journal of Neuroscience. 2002;15(12):2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Characterization of a Likelihood Based Method and Effects of Markers Informativeness in Evaluation of Admixture and Population Group Assignment. BMC Genetics. 2005a;6(1):50. doi: 10.1186/1471-2156-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: Characteristics and properties of Bayesian clustering via STRUCTURE. Genetic Epidemiology. 2005b;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of Glucocorticoid Alterations in Post-traumatic Stress Disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowry MT, Giller EL. Dose response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without PTSD. Archives of General Psychiatry. 1995;52(7):583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Shalev AY, McFarlane AC. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Zhang K, Qin ZS, Liu JS, Chen T, Waterman MS, Sun F. Haplotype Block Partitioning and Tag SNP Selection Using Genotype Data and Their Applications to Association Studies. Genome Research. 2004;14(5):908–916. doi: 10.1101/gr.1837404. [DOI] [PMC free article] [PubMed] [Google Scholar]