Abstract
Xenotransplantation of genetically modified pig organs offers great potential to address the shortage of human organs for allotransplantation. Persistence of hyperacute rejection in Gal KO pigs due to elicited non Gal antibody response required further genetic modifications of donor pigs and better control of the B cell response to xeno antigens. We report significant prolongation of graft survival of 8 months when peri-transplant B-cell depletion was added to an established anti CD154 and MMF based immunosuppressive regimen. Specifically Galactosyl transferase “knock-out” and human CD46 transgenic (GTKO.CD46Tg) pig cardiac xenografts were heterotopically transplanted into specific pathogen free (SPF) baboons. The B cell numbers and non Gal antibody production remained suppressed for over 2 months after only 4 doses of induction treatment with an anti CD20 antibody. Continuous evaluation of the transplanted hearts and recipients by telemetry provided accurate assessment of graft function and aided post operative care, allowing prevention of several major complications. The significant difference in graft survival with the addition of anti CD20 antibody identifies a critical role for B cells in the mechanisms of delayed xenograft rejection and represents a significant advance toward clinical application.
Keywords: Xenograft, Gal Knockout pigs, CD46 transgenic, Anti CD20
INTRODUCTION
Thousands of patients with end stage kidney, heart, liver, and lung disease succumb while waiting for a transplant due to the critical shortage of donor organs(1). Even the best available mechanical alternatives to transplantation such as dialysis, mechanical circulatory support, and extracorporeal membrane oxygenation, are constrained by cost and technical limitations, have generally unfavorable impacts on quality of life, and do not adequately address the organ shortage(2). Despite significant progress, tissue engineering remains far from clinical application.
Xenotransplantation, the use of organ transplants from other species, could potentially address the unmet clinical need(3–5). Infectious and ethical concerns associated with xenotransplantation are considered manageable(6;7) “Hyperacute rejection (HAR)” of pig organs usually occurs in non-human primate models, as in man, (8;9) but can be significantly attenuated by genetic modifications to the donor pig, including removal of the Galactose α-1,3Galactose carbohydrate epitope (α-Gal) by galactosyl transferase gene knockout (GTKO)(4;10–12) or expression of human complement regulatory proteins(13–15). Transgenic expression of human CD46 on GTKO organs (GTKO.hCD46Tg) addresses the problem of complement injury driven by pre existing or elicited anti-pig antibodies directed at targets other than α-Gal.(16) However evidence is accumulating that “anti-non–Gal” antibodies play a major role in the post transplant thrombotic microangiopathy (TM) and delayed xenograft rejection (DXR) (17).
In this study, we hypothesized that B-cells, as antigen presenting cells and the dominant source of elicited anti-pig antibodies, are the principle trigger for graft injury in baboon heart xenograft recipients treated even with an established, effective anti-CD154- and mycophenolate mofetil (MMF)-based immunosuppression regimen. We report that efficient depletion of circulating and secondary lymphoid B cells by anti CD20 antibody at the time of transplant prevents elicited anti-pig immune response and resulting graft injury, and significantly delays or prevents systemic coagulation pathway dysregulation and TM.
Material & Methods
Animal
Specific pathogen free (SPF) baboons weighing 7–15Kg from University of Oklahoma (Norman, OK) were housed in a clean pathogen free facility. Gal knockout hCD46 Transgenic (GTKO.hCD46Tg) pigs (Revivicor Inc., Blacksburg, VA) were used at 3–8 weeks old with a maximum weight of 12Kg. All animals were used under the strict guidance of guidelines provided by the National Heart, Lung and Blood Institute (NHLBI) Animal Care and Use Committee (ACUC).
Flow Cytometric Analyses
For α-Gal and CD46 expression
Porcine PBMC were exposed to 100mg/ml Isolectin B4 (Vector labs FL-1201) and anti-Human CD46 antibody (AbD Serotec- 5ml of MCA 2113PE). 5000 events were recorded by flow cytometry (FACSAria-BD).
For B and T cells in peripheral blood
Lymphocytes were labeled with primate anti CD3 (Becton Dickinson, San Diego, CA) and anti CD19 (Becton Dickinson, San Diego, CA) and were analyzed on FACS Calibur, (Becton Dickinson, CA).
Transplant Procedure
The heterotopic transplant procedure has previously been described(18). Briefly, the donor pig’ undergoes a standard median sternotomy. After heparin administration (400 IU/kg), a cardioplegia catheter is placed in the ascending aorta, both superior and inferior vena cavae are ligated, and cross clamp is applied on the aorta. Cold hyperkalemic crystalloid cardioplegia solution (500 ml) is infused at 100 mmHg into the ascending aorta, resulting in prompt diastolic arrest; the IVC and left atria are incised to vent the cardioplegia, and iced saline is used for topical cooling. After cardioplegia administration the heart is excised, and the ascending aorta and main pulmonary artery prepared for subsequent anastomoses. The Pulmonary vein and cavae are oversewn.
The recipient baboon’s infrarenal aorta and inferior vena cava are exposed through a midline abdominal incision. Side-biting clamps are applied, an aortotomy and venotomy made, and the end-to-side anastomoses are performed between donor and recipient aorta and donor pulmonary artery with recipient inferior vena cava.
Telemetry Implantation and Methods of Graft Function
A telemetry device (Konigsberg Instruments, Inc, Pasadena, CA) was implanted into the recipient to monitor graft left ventricular pressure and electrocardiogram, as well as, the recipient’s temperature. The telemetry device data is transmitted wirelessly to a receiver attached to the animal’s cage (RMISS, Wilmington, DE). The parameters recorded included Left Ventricular Pressure (LVP), Heart rate based on LVP (LVPHR), Peak Systolic Pressure (PEAK) and End Diastolic Pressure (END), EKG, Heart rate based on EKG (EKGHR) and recipient’s body temperature (TEMP). Heart function was evaluated daily by telemetry and, when the recipient was sedated, by palpation and/or ultrasound.
Immunosuppressive regimen
Table 1: The study was divided into three groups: Group A: (n=2) Recipient baboons received no immunosuppression. Group B: (n=8) a standard clinically accepted immunosuppressive regimen was employed which included antithymoglobulin (ATG) (Genzyme, Cambridge MA), Mycophenolate Mofetil (MMF) (Genzyme, San Francisco, CA), Cobra Venom Factor (CVF) (Quidel, San Diego, CA), Gancyclovir (Roche, Nutley NJ) and anti CD154 antibody (Beth Israel Medical Center, Boston, MA) and Group C: (n=15) the same standard immunosuppression as Group B along with anti CD20 antibody (Rituxan)(Genetech, South San Francisco, CA).
Table 1.
Immunosuppression regimen
| Drugs | Dose | Timing |
|---|---|---|
|
| ||
| Induction: | ||
| Anti CD20 | 19 mg/Kg | Pre op days −7, 0, 7 & 14 |
| ATG | 40–50 mg/Kg | Pre op days −2 & −1 |
| Anti CD154 | 25 mg/Kg | Pre op days −1 & 0 |
| CVF | 50–100 U/Kg | Pre op days −1, 0 & 1 |
|
| ||
| Maintenance: | ||
| Anti CD 154 | 25 mg/Kg | Post op days 3, 7, 10, 14, 19, q weekly |
| MMF | 20 mg/Kg/2 hr IV infusion | BID daily |
| Steroids | 2 mg/Kg | BID, tapered off in 7 weeks |
| Aspirin | 81 mg | Daily |
| Heparin | Maintain ACT 2x baseline | Continuous infusion |
|
| ||
| Supportive: | ||
| Ganciclovir | 5 mg/Kg/day | daily |
| Cefazolin | 250 mg | BID for 7 days |
| Epogen | 200 U/Kg | Daily from −7 to 7 then weekly |
| Ketorolac | 15 mg | Just before Anti CD154 |
Histology and Immunohistochemistry
Paraffin sections from biopsies and explanted xenografts were stained with hematoxylin and eosin for light microscopy. Sections were analyzed semi quantitatively for the presence of hemorrhage, necrosis, thrombosis, and cellular infiltrates. Frozen tissue sections from explanted xenografts were assessed by immunohistochemistry staining using monoclonal antibodies against human IgG (Pharmigen, Becton Dickinson Co.) at 1 : 100 and IgM at 1 : 50, (Immunotech, Marseille, France). The reactivity of the monoclonal antibodies was revealed by a Cy3-labeled goat-anti-mouse IgG (Jackson Laboratories, West Grove, PA, USA). A double fluorescence staining with a rabbit anti-von Willebrand factor antibody (Dako), revealed by a Cy2-labeled goat-anti-rabbit IgG antibody (Jackson Laboratories, West Grove, PA, USA), was used to confirm the location of deposits on the endothelium. Negative controls included staining of normal heart tissue, and omission of the primary antibody. Sections from hyperacutely rejected heart xenografts, acutely rejected allografts, or baboon lymphoid organs were used as a positive control. Staining for antibody deposition was only scored as positive where it occurred in vWF+ vessels. Staining intensity was assigned “− to +++”, taking into account the extent and intensity of staining from a standard control section.
Detection on Non Gal Antibodies
Porcine Aortic Endothelial Cells (PAEC) were isolated and cultured from GTKO pigs. Cells were cultured in RPMI1640 with 20% heat-inactivated fetal calf serum before being harvested and washed in RPMI1640 only. The cells were adjusted to a concentration of 1×106 cells/ml in FACS buffer, and 100 μl of this suspension was dispensed into each FACS tube. 100 μl cell suspension containing 0.5×106 lymphocytes was mixed with 100 μl of 1:10 baboon sera (heat inactivated at 56°C for 30 min in advance) and incubated for 30 min at 4°C. As a negative control, cells were incubated with media. After incubation, the cells were washed twice with phosphate-buffered saline (PBS) and then incubated for 30 min at 4°C with either 10 μl of 1:10 PE-goat antihuman IgG (Jackson Immuno Research Laboratories, Inc.) or 20 μl of 1:10 FITC-antihuman IgM (BD PharMingen). After incubation, the cells were washed twice with FACS buffer (PBS containing 0.01% sodium azide and 1% bovine serum albumin) and then fixed with 300 μl of 4% paraformaldehyde. Cells were then analyzed on a Becton Dickinson FACScan Flow Cytometer with CellQuest software. The mean fluorescence intensity of the cells for each test serum was compared with that produced by the controls.
Treatment of Rejection
Rejection was diagnosed by decrease in LVP pressure and EKG changes. At this point rescue therapy was initiated with IV methyl prednisolone (10–15mg/Kg) for 6 days. Also Heparin dose was increased to prevent thrombosis. Both measures were unsuccessful in preventing graft rejection.
RESULTS
Low levels of non Gal antibody levels were detected in SPF baboons
Limited experience with GTKO pig organs that did not express hCD46 revealed that xenograft rejection still occurred acutely due to the pre-existing Gal antibody response(19). Therefore GTKO.hCD46Tg hearts that are presumably protected from “early graft failure” were used. Recipient baboons were bred in a Specific Pathogen Free (SPF) facility and had low serum levels of anti-non-Gal antibodies (both IgM and IgG) relative to conventional non-SPF baboons (Fig 1A).
Fig 1.
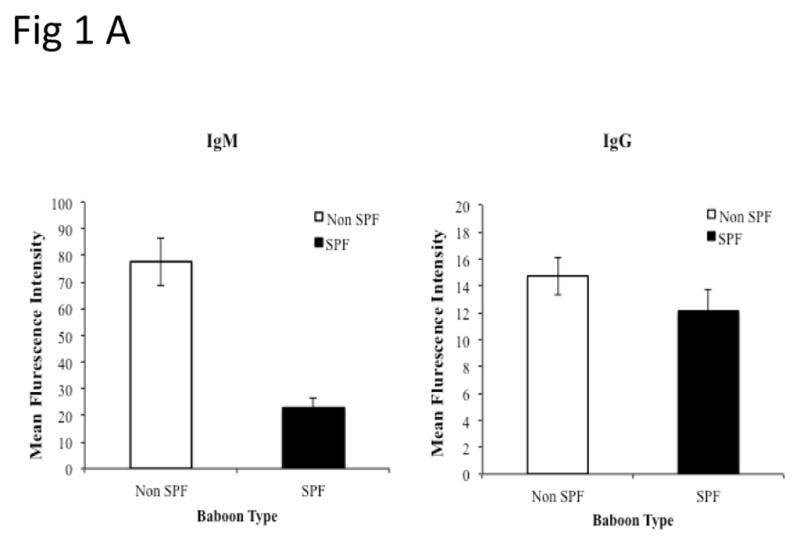
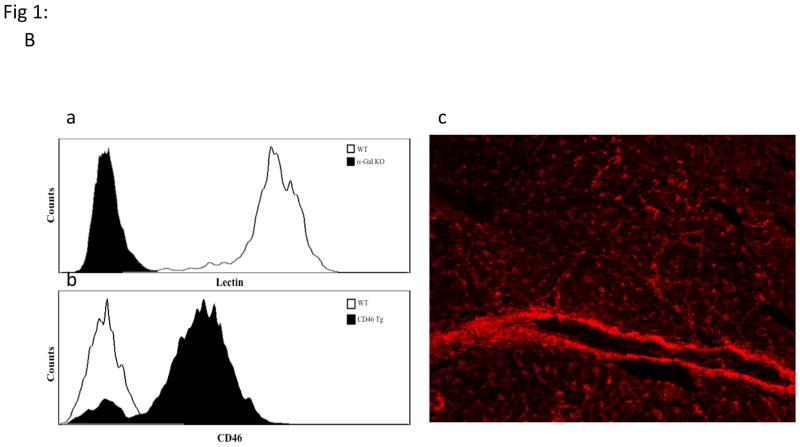
Non Gal antibody levels in SPF baboons and verification of pig genetics: PAEC from GTKO pigs were isolated and mixed with baboon sera (1:10 ratio). After incubation, the cells were further stained with PE-goat anti human IgG/IgM or 20 micro L of 1:10 FITC/PE. The mean fluorescence intensity of the cells for each test and control serum was measured for the estimation of non-Gal antibodies. (B) Expression of Gal epitope and hCD46:Peripheral blood lymphocytes of pigs with modified genetics were tested by flow cytometry for the absence of Gal epitope using IB4 lectin (Ba) and transgenic expression of human CD46 (Bb). Expression of hCD46 was confirmed by immunohistochemical staining of heart vessels (Bc).
Absence of Gal epitope and transgenic expression of hCD46 in donor pigs was confirmed
Cloned or bred GTKO.hCD46Tg donor piglets provided by Revivicor, Inc. lacked the Gal epitope on the cells’ surface as measured by the flow cytometry using IB4 Lectin (Fig 1Ba). The cells and tissue from these genetically modified pigs did express high levels of hCD46 by FACS and immunohistochemistry (IHC) (Fig 1B b&c).
Achievement of longest reported cardiac xenograft survival in group receiving anti CD20 antibody along with the conventional immunosuppression
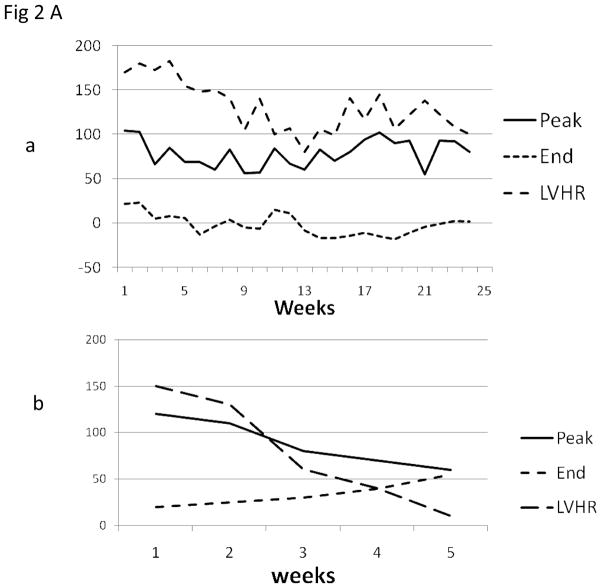
The telemetry provided continuous accurate evaluation of graft function by continuous measurement of LVP, EKG and recipients temperature. Representative tracing of LVP heart rate, PEAK systolic pressure and END diastolic pressure are shown in Fig 2Aa (long term surviving graft) and Fig 2Ab (graft rejecting within one month). A decrease of LVP below 40 mmHg was indicative of the start of the rejection process and pressure below 20 mmHg was consistent with the findings of rejection on ultrasound and confirmatory explants histology. At any significant spike in temperature the recipient baboon was immediately evaluated for leuckocytosis, cultured and treated with antibiotics and in most cases infections were effectively controlled
Fig 2. Cardiac xenograft survival and telemetry interpretations.
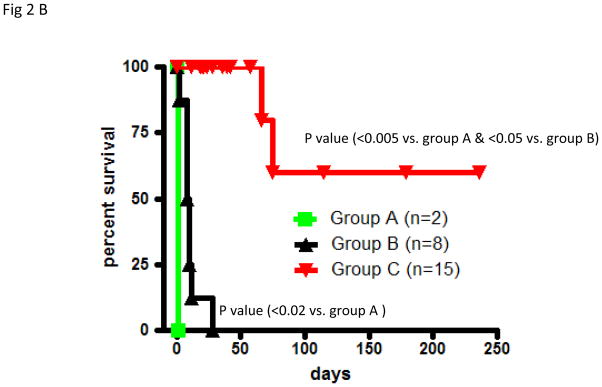
(A): A typical measurement of telemetry readings from one representative baboon is shown in fig 2Aa (Long term survivor) and 2Ab (Rejection within a month) (B): The cardiac xenograft survival in all three groups. In group A and B, grafts were explanted at the time of rejection and data represents the graft survival time while in group C 85% of the recipients died with a functioning xenograft therefore the data points represent the time at which data was censored due to recipient’s death.
Untreated baboons (Group A; n=2) rejected GTKO.hCD46Tg heart xenografts within 24 hours (Fig 2B).Graft injury by preformed antibodies would also account for the unexpectedly short survival of GTKO.hCD46Tg hearts in this group. Group B (n=8) regimen included induction by Anti Thymocyte Globulin (ATG ) and Cobra Venom Factor (CVF), and maintenance by Anti CD154 (5C8) antibody, (MMF) and tapering dose of methyl prednisolone. This group had a 10 day median survival in eight baboons (p=0.02 versus untreated). No Group B grafts exhibited normal function when the recipient died or was euthanized. We suspect ineffective control of elicited antibody response in this group led to shorter graft survival.
Addition of depleting humanized anti-CD20 antibody (Rituxan) to the Group B regimen (Group C; n=15) was associated with graft prolongation up to 236 days, reduced incidence of graft failure (2/15), median graft survival time (censored for baboon death with a functioning graft) of over 100 days and mean recipient survival of 60 days (p<0.05 versus Group B) (Fig 2B). The majority of recipients (12/15) in this group died with a functional graft due to complications related to the surgical procedure and immunosuppression(20). Some of the major complications included bowel obstruction and intra abdominal bleeding>.
Prevalent protection of GTKO.hCD46Tg heart xenografts from functional deterioration for up to 236 days represents a substantial advance. Since many of the complications encountered in this model would be treatable if they occurred in patients, and all the reagents except anti-CD154 are used safely in clinical transplantation, this regimen is approaching clinical feasibility if residual graft injury can be reproducibly prevented.
Histological and Immunohistochemistry (IHC) comparison of three experimental groups
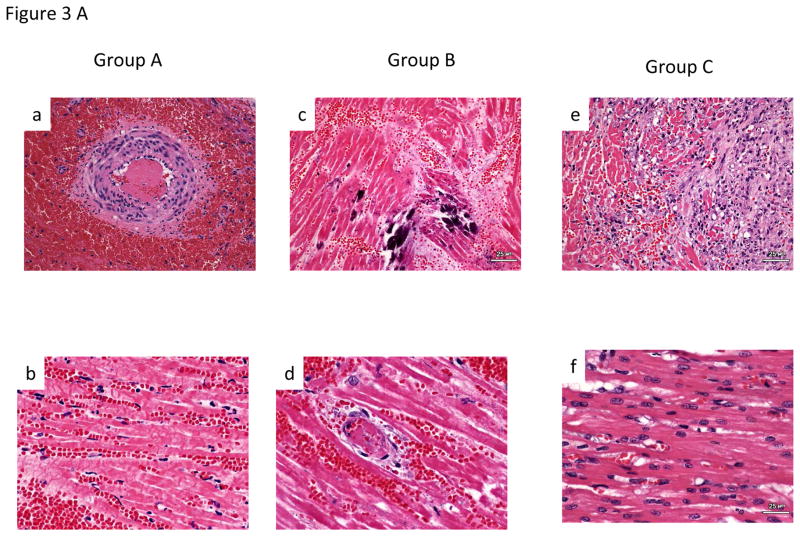
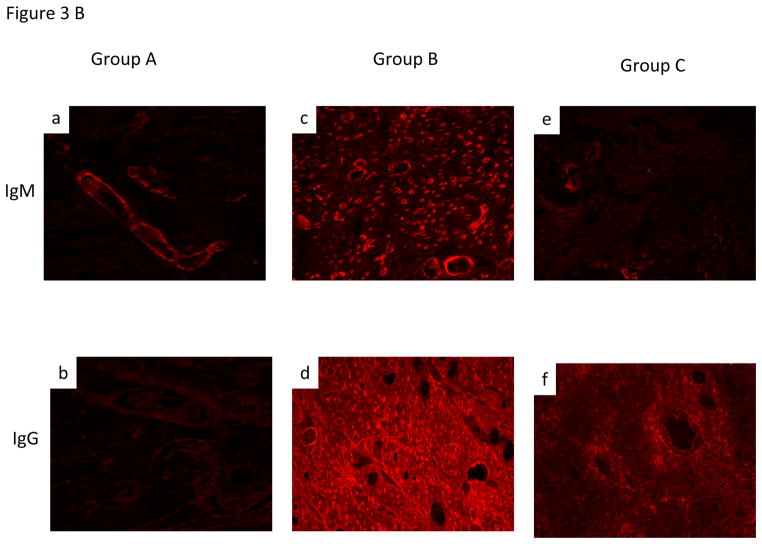
Group A hearts demonstrated histology resembling HAR, including acute interstitial hemorrhage, diffuse coagulative myocytes necrosis and intravascular thrombosis (Fig 3A, a&b) and IHC demonstrating antibody deposition (Fig 3B, a&b). Group B graft histology showed signs of Delayed Xenograft Rejection (DXR), including TM with microvascular thrombosis, interstitial hemorrhage and ischemic myocyte necrosis (Fig 3A, c&d). Antibody deposition similar to Group A was seen on IHC in this group (Fig 3B, c&d). In group C, although some patchy interstitial fibrosis was observed in some areas of explanted Group C grafts (Fig 3Ae) that survived for more than 100 days, myocardial morphology was essentially normal in other regions (Fig 3Af). Antibody deposition on IHC was similar to group B & C (Fig 3B, e&f).
Fig 3. Histological and IHC evaluation of the cardiac xenograft.
A.Histology: Group A-Section of right ventricle with extensive acute interstitial hemorrhage and arterial thrombus (200X) (A). Section of interventricular septum with diffuse coagulative necrosis and acute interstitial hemorrhage (400X)(B). Group B- (C). Section of left ventricle with focal dystrophic mineralization of myofibers (200X) (C). Section of left ventricular with venular thrombus, coagulative necrosis of adjacent cardiac myocytes and acute interstitial hemorrhage (400X) (D). Group C- Section of left atrium with coagulative necrosis of myocytes, loss of myocytes and replacement of myocytes with moderate interstitial fibrosis with increased numbers of fibroblasts and adjacent collagen (200X)(E). Section of interventricular septum demonstrating an area of relatively normal cardiac myocytes (400X)(F).
B. Immunohistochemical staining of graft for non-Gal antibody deposition for both IgM and IgG antibody was consistently observed in all three groups. The stronger intensity of staining in group B likely reflects presence of induced antibodies.
Efficient B and T cell depletion achieved by the immunosuppression regimen
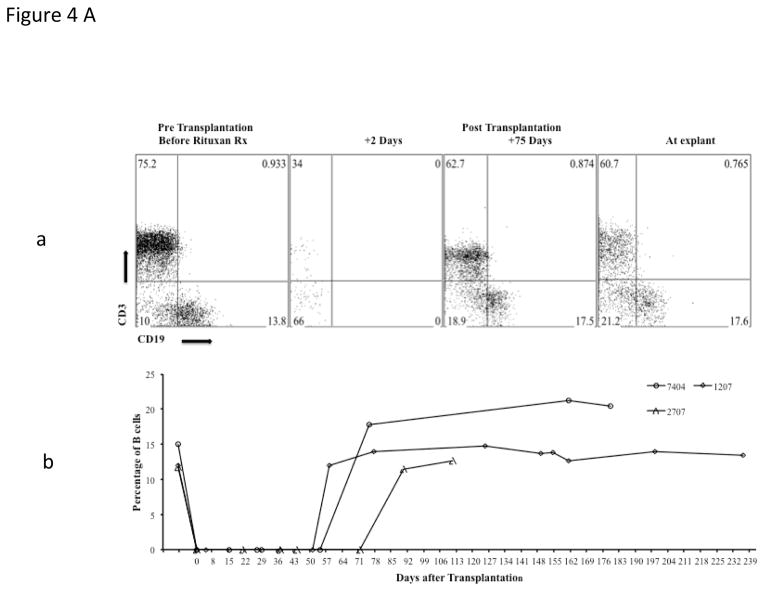
B cell depletion by the anti CD20 antibody was very effective and sustained, with essentially no B cells detected in treated baboons, for at least 60 days post transplant (Fig 4A, a&b). Absence of cellular infiltration in explanted grafts (Fig 3A, e&f) and adequate suppression of circulating T cells in peripheral blood (Figure 4Aa) indicates that the T cell response to xenoantigens was also effectively suppressed by this regimen.
Fig 4. Suppression of B and T cells by the immunosuppression regimen.
Analyses of B cell suppression in representative baboons after four doses of anti CD20, at different time points, by flow cytometery is shown (CD19)(4A, a&b) along with suppression of T cells (CD3) by other agents in the immunosuppressive regimen (4Aa). Complete B cell depletion was observed for 60 days with the last dose of anti CD20 given on day 14 post transplantation.
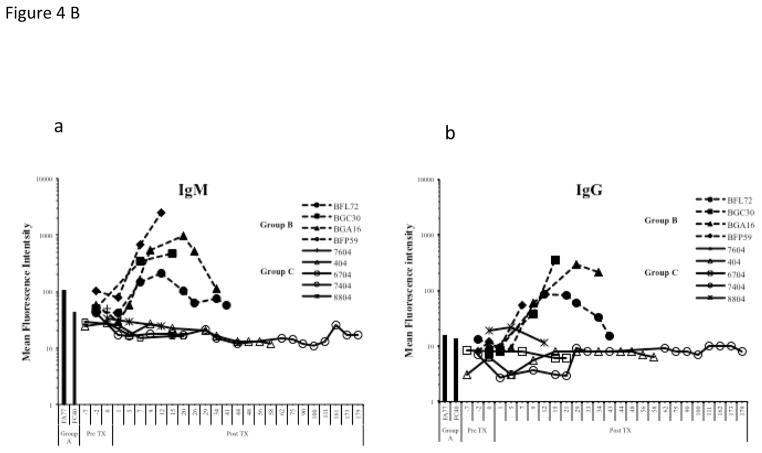
Comparison of non Gal (IgM (4Ba) & IgG (4Bb)) antibody mean fluorescent intensity (MFI) of representative baboons rejecting pig hearts; without immunosuppression (Group A)(shown as bars as there was only one time point), immunosuppression without anti CD20 (Group B), and with full immunosuppression including anti CD20 (Group C).
Low anti non Gal antibodies were detected after anti CD20 treatment but similar antibody deposition in xenograft was observed in all three groups
Recipients in group C had no detectable increase in the level of circulating non Gal antibodies (both IgM and IgG) compared to group A & B (Fig 4B). The marked increased of antibody production in group B may have resulted in DXR. This striking result demonstrates efficient control of elicited xenoimmunity in Group C. As demonstrated, induction B-cell depletion represents a major advance toward safe clinical application. Similar levels of antibody deposition were seen in the transplanted cardiac xenograft in all three experimental groups (Fig 3B).
Discussion
Overcoming xenograft rejection completely in a large animal model has been unsuccessful to date but a tremendous amount of research done on this topic over the past 30 years has provided detailed information about the mechanism of xenograft rejection. As predicted the availability of GTKO pigs has provided significant improvement in graft survival beyond HAR but fail to overcome the antibody mediated rejection barrier completely. The rejection of GalKO pig cardiac xenograft in baboons is still predominately mediated by elicited antibodies to numerous non Gal antigens. The level of conventional immunosuppression required to overcome this rejection is lethal and therefore not clinically applicable. In this study we used a clinically employed immunosuppressive regimen and supplemented it with a potent clinically tested anti B cell antibody to deplete the source of non Gal antigens.
The striking result described in this study demonstrates efficient control of elicited xenoimmunity in the group receiving the anti CD20 antibody. Whether removing a pivotal APC population or incidentally depleting the anti-pig precursor B-cell population, induction B-cell depletion represents a major advance toward safe clinical application of pig xenografts in man. Just four doses of anti CD20 were enough to suppress the B cell response for over a 6 month period. Whether the use of SPF baboons with low levels of anti non Gal antibodies played any significant role in graft prolongation is unclear but inability by others to get similar graft survival strongly indicates the beneficial role of using these “clean” baboons. Along with the B cell response, the T cell response to xeno antigens was also subdued efficiently by induction treatment with Thymoglobulin and maintenance with MMF and anti CD154. The T cells were not eliminated completely and the surviving T cells mostly demonstrated T regulatory cell phenotype (CD4+ CD25hi FoxP3 +) (personal observation). Perhaps these cells contributed to the extended survival of the xenografts.
The use of telemetry in this experiment provided accurate monitoring of graft function and helped in managing complications like infections. In most cases the infections were adequately controlled immediately after their onset.
Detection of anti-pig non Gal antibody in grafts from all groups is not surprising, since CD20 is not expressed on plasma cells and therefore anti-CD20 treatment was not expected to significantly alter elaboration of pre-existing anti-non-Gal by plasma cells. Whether targeting this population, either selectively through an antigen specific immune targeting approach or by addition of a proteasome inhibitor to the Group C regimen, can reduced the incidence of dysregulated coagulation and will reveal the contribution of pre-formed antibody to the pathogenesis of this phenomenon is unknown. Alternatively, expression of coagulation pathway regulatory molecules on the endothelium of GTKO.hCD46Tg pig organs will reveal whether TM and CC can be safely and reliably be prevented without further inhibiting recipient immunity.
The major hurdle for consistent success in this experimental model was the variety of complications which caused recipient’s mortality before graft rejection. Most of the complications occurred only once except for bleeding complications, possibly due to consumption of platelets and coagulation factors. Anti CD154 is known to have thrombotic complications and perhaps resulting thrombocytopenia resulted in intensified bleeding. We are currently testing anti CD40, which doesn’t affect the function of platelets, to avoid this complication in this xenotransplantation model.
This study demonstrates for the first time that xenograft rejection can be avoided, using GTKO.hCD46Tg pigs with recipient’s peri transplant B cell depletion, for over 230 days and the limiting factors are various non rejection related complications. This experiment paves the way for testing orthotopic transplantation in this model and assessing the ability of the transplanted heart to sustain life.
Acknowledgments
We acknowledge staff of Laboratory of Animal Medicine and Surgery (LAMS) and Division of Veterinary Resources (DVR) for their support in surgical procedures and care of animals. Flow cytometry core of NHLBI for their help in FACS analyses. Patricia Jackson for administrative help.
Footnotes
Disclosure:
Two authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
David Ayares is the CEO &president of Revivicor, Inc
Richard Pierson serves on the scientific advisory board of Revivicor, Inc
Reference List
- 1.Mulligan MS, Shearon TH, Weill D, Pagani FD, Moore J, Murray S. Heart and lung transplantation in the United States, 1997–2006. Am J Transplant. 2008 Apr;8(4 Pt 2):977–87. doi: 10.1111/j.1600-6143.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 2.Badiwala MV, Rao V. Left ventricular device as destination therapy: are we there yet? Curr Opin Cardiol. 2009 Mar;24(2):184–9. doi: 10.1097/HCO.0b013e328323f58f. [DOI] [PubMed] [Google Scholar]
- 3.Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. Xenotransplantation-current status and future perspectives. Br Med Bull. 2005;75–76:99–114. doi: 10.1093/bmb/ldh061. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin MM. Clinical xenotransplantation of organs: why aren’t we there yet? PLoS Med. 2007 Mar 27;4(3):e75. doi: 10.1371/journal.pmed.0040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuurman HJ, Pierson RN., III Progress towards clinical xenotransplantation. Front Biosci. 2008;13:204–20. doi: 10.2741/2671. [DOI] [PubMed] [Google Scholar]
- 6.Patience C, Takeuchi Y, Weiss RA. Zoonosis in xenotransplantation. Curr Opin Immunol. 1998 Oct;10(5):539–42. doi: 10.1016/s0952-7915(98)80220-3. [DOI] [PubMed] [Google Scholar]
- 7.Smetanka C, Cooper DK. The ethics debate in relation to xenotransplantation. Rev Sci Tech. 2005 Apr;24(1):335–42. [PubMed] [Google Scholar]
- 8.Cozzi E, Severo M, Bosio E, Besenzon F, Ancona E. Antibody mediated rejection in pig-tononhuman primate xenotransplantation models. Curr Drug Targets Cardiovasc Haematol Disord. 2005 Jun;5(3):233–53. doi: 10.2174/1568006054064799. [DOI] [PubMed] [Google Scholar]
- 9.Daniels LJ, Platt JL. Hyperacute xenograft rejection as an immunologic barrier to xenotransplantation. Kidney Int Suppl. 1997 Mar;58:S28–S35. [PubMed] [Google Scholar]
- 10.Adams DH, Kadner A, Chen RH, Farivar RS. Human membrane cofactor protein (MCP, CD 46) protects transgenic pig hearts from hyperacute rejection in primates. Xenotransplantation. 2001 Feb;8(1):36–40. doi: 10.1046/j.0908-665x.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DK, Ezzelarab M, Hara H, Ayares D. Recent advances in pig-to-human organ and cell transplantation. Expert Opin Biol Ther. 2008 Jan;8(1):1–4. doi: 10.1517/14712598.8.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Sandrin MS, Loveland BE, McKenzie IF. Genetic engineering for xenotransplantation. J Card Surg. 2001 Nov;16(6):448–57. doi: 10.1111/j.1540-8191.2001.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 13.Lam TT, Hausen B, Hook L, Lau M, Higgins J, Christians U, et al. The effect of soluble complement receptor type 1 on acute humoral xenograft rejection in hDAF-transgenic pig-to-primate lifesupporting kidney xenografts. Xenotransplantation. 2005 Jan;12(1):20–9. doi: 10.1111/j.1399-3089.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 14.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005 Sep;130(3):844–51. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Pfeiffer S, Schroder C, Zhang T, Nguyen BN, Lea W, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005 May;12(3):197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhong R. Gal knockout and beyond. Am J Transplant. 2007 Jan;7(1):5–11. doi: 10.1111/j.1600-6143.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 17.Tseng YL, Moran K, Dor FJ, Sanderson TM, Li W, Lancos CJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006 Apr 15;81(7):1058–62. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 18.Adams DH, Chen RH, Kadner A, Naficy S. Technique for heterotopic pig heart xenotransplantation in primates. Ann Thorac Surg. 1999 Jul;68(1):265–8. doi: 10.1016/s0003-4975(99)00488-9. [DOI] [PubMed] [Google Scholar]
- 19.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran PC, Horvath KA, Singh AK, Hoyt RF, Jr, Thomas ML, III, Eckhaus MA, et al. Surgical and nonsurgical complications of a pig to baboon heterotopic heart transplantation model. Transplant Proc. 2010 Jul;42(6):2149–51. doi: 10.1016/j.transproceed.2010.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]