Abstract
The Stop Atherosclerosis in Native Diabetics Study (SANDS) was a randomized open‐label clinical trial in type 2 diabetics designed to examine the effects of intensive reduction of blood pressure, aggressive vs standard goals (≤115/75 mm Hg vs ≤130/80 mm Hg), and low‐density lipoprotein (LDL) cholesterol on the composite outcome of change in carotid intimal‐medial thickness and cardiovascular events. The study demonstrated that in conjunction with a lower LDL cholesterol target of 70 mg/dL, aggressive systolic blood pressure–lowering resulted in a reduction in carotid intimal‐medial thickness and left ventricular mass without measurable differences in cardiovascular events. The blood pressure treatment algorithm included renin‐angiotensin system blockade, with other agents added if necessary. The authors conclude that both standard and more aggressive systolic blood pressure reduction can be achieved with excellent safety and good tolerability in patients with type 2 diabetes mellitus.
Persons with type 2 diabetes are particularly prone to develop cardiovascular (CV) disease. Since many diabetic individuals develop CV events at blood pressure (BP) and cholesterol levels that meet recommended treatment goals, lower goals might improve CV risk reduction. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommended systolic BP (SBP) and diastolic BP (DBP) goals of 130/80 mm Hg for people with diabetes or chronic kidney disease (glomerular filtration rate <60 mL/minute/1.73 m2). 1 However, it is not clear whether lower targets may be more effective in preventing CV disease. While several studies have shown little increase in side effects when low‐density lipoprotein (LDL) cholesterol levels are reduced below current goals, 2 , 3 few randomized trial data exist on the success or tolerability of reducing SBP to levels appreciably below 130 mm Hg.
The Stop Atherosclerosis in Native Diabetics Study (SANDS) was a randomized controlled trial that tested the hypothesis that lowering BP and lipids to goals below current targets may be beneficial in adults with type 2 diabetes. Participants randomized to lower target SBP of 115 mm Hg and LDL cholesterol of 70 mg/dL had reduced carotid intimal‐medial thickness and reduced left ventricular mass after 3 years of follow‐up compared with those randomized to standard (STD) targets of 130 mm Hg and 100 mg/dL, respectively, without a significant difference in CV events. 4 An important consideration in the clinical applicability of these findings is the safety and tolerability of achieving the lower SBP goal of 115 mm Hg in diabetic patients who frequently require drugs for multiple conditions and who may be at greater risk for orthostatic hypotension due to autonomic neuropathy. Accordingly, the present report analyzes the 3‐year experience regarding the efficacy, tolerability and safety of treatment to a more intensive BP goal of 115/75 mm Hg compared with the STD goal of 130/80 mm Hg in the SANDS trial.
Methods
Population
The target population consisted of American Indians in 4 geographic areas in the United States: Southwest Oklahoma, areas near Phoenix and Chinle, Arizona, and the area in and around Rapid City, South Dakota. Patients were 40 years and older with documented type 2 diabetes mellitus by 1997 American Diabetes Association (ADA) criteria and/or diagnosed previously by ADA or World Health Organization criteria. 5 Patients were required to have had an SBP >130 mm Hg within the previous 12 months. Patients with an SBP >180 mm Hg or with known reversible causes of hypertension were excluded. Patients with known secondary causes of hypertension, renal dysfunction (serum creatinine >2.0 mg/dL for women, >2.4 mg/dL for men), and orthostatic hypotension (defined as a >20‐mm Hg change in SBP from sitting to standing and with symptoms lasting longer than 1 minute) were excluded. All participants provided written informed consent before enrollment. The protocol was approved by participating tribes and institutional review boards of area Indian Health Service and participating institutions.
Study Design
The protocol incorporated a 3‐year, 2‐arm, open‐label, randomized, blinded to end point trial design comparing aggressive (AGG) with STD treatment for both BP (115/75 vs 130/80 mm Hg) and LDL cholesterol (70 vs 100 mg/dL) goals, respectively. The schedules of procedures performed at each visit were published previously. 4 , 6
After initial screening and randomization, patients were seen at monthly intervals until they achieved BP goal and then every 3 months during follow‐up, with additional visits as needed for side effects or adjustments in BP medication.
Each center had a physician investigator who was familiar with Indian Health Service care practices and a nurse or nurse practitioner who implemented the algorithm in consultation with the study physician. All centers employed community members when possible to carry out the study protocol; they also served as translators in the case of language barriers. A nephrologist (MRW) served as BP consultant and worked with each study site to review treatment plans and address individual patient problems or barriers to achieving randomized BP goals using a combination of regularly scheduled and ad hoc telephone consultations.
All medication was dispensed through local Indian Health Service pharmacies. Use of the Indian Health Service pharmacists provided access to additional professional adherence counseling as well as assistance in medication reconciliation, in avoiding drug interactions, and in avoiding duplication. In addition, this system provided an opportunity to document when a prescription was filled or dispensed and whether the accompanying education was provided.
Hypertension Management
An algorithm to achieve STD and AGG goals was developed to standardize the care throughout the course of the study (Table I). The algorithm was based on the Sixth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI). Medications were chosen that might be effective not only in lowering BP in people with type 2 diabetes, but also in reducing CV morbidity and mortality. 1 The step‐1 drug was a renin‐angiotensin system (RAS) blocker: either an angiotensin‐converting enzyme (ACE) inhibitor, in most cases lisinopril, titrated to a dosage of 40 mg/d, or an angiotensin receptor blocker, in most cases losartan, titrated to 100 mg/d. The latter choice was incorporated as an alternate to the ACE inhibitor if there was a history of ACE inhibitor intolerance. The step‐2 drug was hydrochlorothiazide, to be used in a dosage of 12.5 to 25 mg/d. It could also be used as a fixed‐dose combination with losartan. A thiazide diuretic was chosen because of its efficacy in lowering BP with RAS inhibitors and its demonstrated advantages in reducing CV morbidity and mortality in both diabetic and nondiabetic patients. 7 , 8 The step‐3 drug was either a β‐blocker or a calcium channel blocker. Atenolol could be titrated to 100 mg/d or amlodipine to 10 mg/d. The step‐4 drug was the alternative step‐3 medications. The step‐5 drug was an α‐blocker, doxazosin, which could be titrated to 8 mg daily. Step‐6 drugs were direct‐acting vasodilators, either hydralazine up to 100 mg twice a day or minoxidil up to 10 mg twice a day. A loop diuretic could be substituted for hydrochlorothiazide if needed to achieve more volume reduction. Reserpine up to 0.3 mg could also be added if BP was not controlled with the previously described medications.
Table I.
Algorithm for Hypertension Management
| Step | Medication | Minimal Starting Dose | Usual Starting Dose | Titration Sequence |
|---|---|---|---|---|
| 1 | Lisinopril or | 10 mg po QD | 10 mg po QD | 10–20–40 mg po QD |
| Losartan | 50 mg po QD | 50 mg po QD | 50 mg po QD | |
| 2 | Lisinopril/HCTZ or | 10/12.5 mg po QD | 20/12.5 mg po QD | 10/12.5–40/25 mg po QD |
| Losartan/HCTZ | 50/12.5 mg po QD | 50/12.5 mg po QD | 50/12.5–100/25 mg po QD | |
| 3 | Atenolol or | 25 mg po QD | 50 mg po QD | 25–50–100 mg po BID |
| Nifedipine | 30 mg po QD | 30 mg po QD | 30–60–90 mg po QD | |
| 4 | Add alternate #3 agent at specified doses: if no atenolol, can add nifedipine and vice versa. | |||
| 5 | Doxazosin | 1 mg po QD | 1 mg po QD | 1–2–3–4–5–6–7–8 mg po QD |
| 6 | Hydralazine or | 25 mg po BID | 25 mg po BID | 25–50–100 mg po BID |
| Minoxidil or | 2.5 mg po BID | 2.5 mg po BID | 2.5–5–10–20 mg po BID | |
| Reserpine | 0.1 mg po QD | 0.1 mg po QD | 0.1–0.2–0.3 mg po QD | |
Abbreviations: BID, twice a day; po, orally; HCTZ, hydrochlorothiazide; QD, every day.
Definitions
The SBP goals were 115 mm Hg and 130 mm Hg in AGG and STD groups, respectively. Achievement of goals was defined as an SBP <117 mm Hg for the AGG group and 124 to 136 mm Hg for the STD group, in view of the known variability of BP measurements. Left ventricular hypertrophy was defined as a left ventricular mass index >49.2 g/m2.7 for men and 46.7 g/m2.7 for women. 9
Adverse Event Assessment
At every visit after randomization, information was collected on potential side effects by direct questioning of patients by the nurse or physician investigators. Seated‐to‐standing BP was measured at every visit to assess for orthostatic hypotension, a concern in this group due to potential autonomic neuropathy. Adverse events (AEs) were recorded as being possibly, probably, or definitely related to pharmacotherapy. All AEs and serious AEs defined by the investigators were reviewed by the Morbidity and Mortality Committee and an independent Data Safety and Monitoring Board (DSMB) appointed by the study sponsor, the National Heart, Lung, and Blood Institute. The side effects by treatment group were blinded to the Morbidity and Mortality Committee, but unblinded to the DSMB.
BP Measurement
BP was taken by staff members who were certified in performance of BP measurement using the OMRON 907 device (Omron Healthcare, Inc, Bannockburn, IL). This device was chosen for its accuracy in obtaining BP. 10 Use of this automated device eliminated problems in BP measurements such as digit preference and personnel errors in calculating means. Appropriate arm size cuffs were used for each patient. Seated BP in the brachial artery was measured 3 times consecutively after 5 minutes of rest and the average of the second and third SBP and DBP measures was used in determining need for drug titration and in analyses.
Data Analysis
Descriptive statistics are presented as means and standard deviations for continuous variables and number of observations and percentages for categoric variables. Comparisons to detect differences between the STD and AGG groups used 2‐sample t tests or chi‐square tests. For all analyses, 2‐tailed P<.05 defined statistical significance. The null hypothesis was that lower SBP (AGG) goals could be achieved with similar success as STD goals.
Results
Baseline Characteristics of Participants
A total of 548 patients were randomized, of whom 96.6% completed 36‐month visits. Although 49 participants with a history of CV disease at baseline were excluded from analyses of the primary end point, 4 all SANDS participants are included in the present BP‐related analyses. A total of 272 patients were randomized to the STD group and 276 to the AGG group (Table II). More women than men participated in the trial. Baseline characteristics including sex, body mass index, and durations of diabetes and hypertension did not differ between treatment groups. Baseline SBP in the AGG group was 3.9 mm Hg lower than the STD group (P=.003); however, there was no difference between groups in target organ consequences such as renal function or left ventricular hypertrophy. Renal function was well preserved in both groups, and only about 10% of patients had microalbuminuria.
Table II.
Baseline Characteristics of Study Population
| Aggressive Group | Standard Group | P Value a | |
|---|---|---|---|
| No. randomized | 276 | 272 | |
| Arizona (Phoenix) | 70 | 68 | |
| Arizona (Chinle) | 63 | 73 | |
| Oklahoma | 71 | 66 | |
| Dakota | 72 | 65 | |
| Sex | |||
| Male | 101 | 101 | |
| Female | 171 | 175 | .90 |
| Age, y | 55.8 (9.3)b | 57.4 (9.3) | .04 |
| Body mass index, kg/m2 | 33.5 (6.5) | 33.0 (6.1) | .41 |
| Diabetes duration, y | 10.3 (8.4) | 9.8 (8.4) | .52 |
| Hypertension duration, y | 10.1 (8.6) | 10.6 (8.7) | .61 |
| SBP, mm Hg | 128.7 (14.7) | 132.6 (16.4) | .003 |
| DBP, mm Hg | 74.1 (10.4) | 75.9 (10.4) | .04 |
| Pulse pressure, mm Hgc | 54.6 (13.4) | 56.7 (14.2) | .08 |
| Mean arterial pressure, mm Hgd | 92.2 (10.3) | 94.8 (10.8) | .01 |
| Blood urea nitrogen, mg/dL | 15.5 (5.6) | 15.5 (5.7) | .93 |
| Serum creatinine, mg/dL | 0.85 (0.25) | 0.86 (0.23) | .44 |
| Estimated GFR (MDRD), mL/min/1.73 m2 | 89.7 (24.3) | 87.1 (23.3) | .20 |
| Albuminuria, % | |||
| Normal | 91.1 (87.0–95.1) | 87.7 (82.9–92.5) | |
| Microalbuminuria | 8.4 (4.5–12.4) | 11.7 (7.0–16.4) | |
| Macroalbuminuria | 0.5 (−0.5 to 1.6) | 0.6 (−0.5 to 1.7) | .65 |
| Left ventricular hypertrophy, % | 24.2 (19.0–29.3) | 19.4 (14.6–24.2) | .18 |
Abbreviations: DBP, diastolic blood pressure; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease study group; SBP, systolic blood pressure. a T test/Chi‐square test/Fisher exact test was used to calculate the P value. bNumbers in parentheses are one standard deviation for continuous variables and 95% confidence interval for percentages. cPulse pressure = SBP – DBP. dMean arterial pressure=[(2*DBP) + SBP]/3.
Targets
During the last 24 months of the study, the achieved SBP was significantly lower in the AGG group (116±13 mm Hg) than in the STD group (128±13.2 mm Hg; P<.0001). Mean change in SBP from baseline to 36 months was −3.8±17.2 mm Hg in the STD group and −11.1±17.1 mm Hg in the AGG group (P<.0001) (Table III and Figure 1), with a parallel significant difference in DBP reduction (Table III and Figure 1). Achievement of BP goals was similar in all 4 field sites. As shown in Figure 1, despite statistically lower pretreatment BP in the AGG group, there was a further separation in SBP within 1 month and in DBP within 9 months. Differences reached a maximum at 18 months and were maintained throughout the remaining 18 months of follow‐up. For SBP in the AGG and STD groups, 67% vs 46%, respectively, met the SBP goals for at least half of the visits, 32% vs 4% of patients met the SBP goals for at least 80% of the visits, and 17% vs 2% met the goals for all visits. For DBP in the AGG compared with the STD group, 88% vs 82% of patients met the DBP goals for at least half of the visits, 64% vs 62% of patients met the DBP goals for at least 80% of the visits, and 52% vs 44% of the patients met the DBP goals at all visits. At the request of primary care providers, some patients in the STD group were maintained on a low dose of an RAS‐blocking drug, despite being below the recommended SBP treatment goal of 130 mm Hg at times. This explains the somewhat lower percentage of patients who reached goal SBP in the STD group compared with the AGG group. There was steady improvement throughout the study in the percentage of patients who reached SBP goals in both groups.
Table III.
SBP and DBP for Each Group Averaged During the 12‐ Through 36‐Month Period
| Aggressive Group | Standard Group | P Value a | |
|---|---|---|---|
| Mean SBP, mm Hgb | 116.0 (13.0)c | 128.2 (13.2) | <.0001 |
| Mean SBP change from randomization visits, mm Hg | −11.1 (17.1) | −3.8 (17.2) | <.0001 |
| Mean % of visits BP goal metd | 61.2 (54.0–68.4)c | 43.6 (38.1–49.1) | <.0001 |
| Mean DBP, mm Hgb | 67.3 (9.5)c | 72.6 (9.8) | <.0001 |
| Mean DBP change from randomization visits, mm Hg | −6.4 (9.7) | −2.9 (9.8) | <.0001 |
| Mean % of visits BP goal metd | 80.7 (74.3–87.2)c | 77.0 (70.2–83.7) | .12 |
a T test was used to calculate P value. bN for systolic blood pressure/diastolic blood pressure (SBP/DBP) is 528, and the means are the average blood pressures (BPs) during the 12‐ through 36‐month visits. cNumbers in parentheses are one standard deviation for continuous variables and 95% confidence interval for percentages. dGoal: aggressive group (SBP ≤117/DBP ≤75 mm Hg); standard group (SBP 124–136/DBP ≤80 mm Hg).
Figure 1.
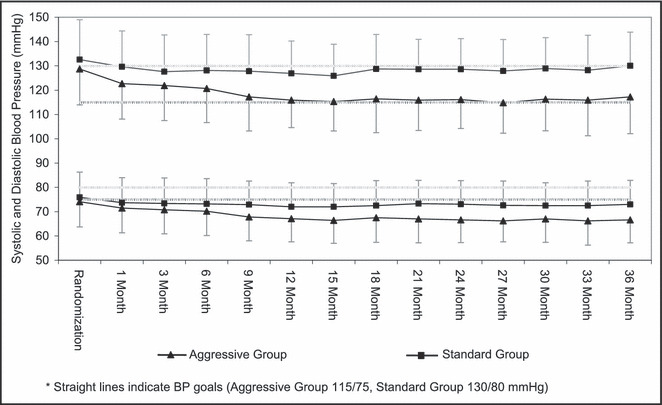
Mean (standard deviation) blood pressure (BP) by treatment group.
Algorithm Adherence
The overall adherence to the algorithm was good. Most patients (81% in the AGG group, and 73% in the STD group) were receiving one or both of the first‐ and second‐step drugs at study end. At that time, 92% of AGG group patients were taking an RAS blocker compared with 67% in the STD group (Figure 2). The majority of patients in the AGG group received a thiazide diuretic (55%), compared with 32% in the STD group. In the AGG group, 19% of patients received ≥4 antihypertensive medications compared with 8% in the STD group. In the STD group, 22% of patients did not receive any medication compared with only 5% of the AGG group. The mean number of drugs used was 2.3±1.3 per patient in the AGG group, and 1.5±1.3 in the STD group. Figure 2 illustrates the time course of medication use during the study. There was a greater incremental change in use of drugs over time in the AGG group.
Figure 2.
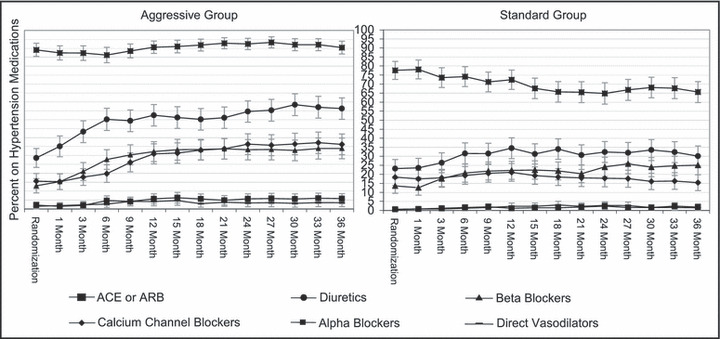
Percent (95% confidence interval) of participants in each group using the various hypertension medications at each visit. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker.
Adverse Events
Twenty‐five percent of patients in the AGG group (n=69) and 14% of patients in the STD group (n=39) had AEs that were possibly, probably, or definitely related to drug therapy (P=.002). The most common AEs were dizziness, cough, and fatigue (Table IV). Orthostasis was reported in 5 patients (2 were reported as AEs with no recorded BP change and 3 were reported with a BP decrease >20 mm Hg). The most commonly reported AEs were with atenolol, lisinopril, and hydrochlorothiazide. No patients dropped out of the study because of side effects considered to be related to therapy. AEs due to antihypertensive medication led to dose reduction (n=32 for AGG, n=10 for STD) or drug discontinuation more commonly in the AGG group (n=69 for AGG, n=33 for STD). Serious AEs are shown in Table V. The 2 cases of hyperkalemia occurred at 10 months (6.9 meq/L) and at 36 months (5.8 meq/L). Both resolved with drug cessation. Few of these events were drug‐related with 4 in the AGG group and 2 in the STD group. All 6 cases were believed by the investigators to be possibly related to lisinopril, the ACE inhibitor, and other BP‐ (5 cases) or lipid‐lowering (2 cases) drugs. In 2 cases, no action was taken, in 2 cases drug therapy was interrupted, and in 2 cases drug therapy was discontinued.
Table IV.
Summary of AEs Relateda to Hypertension Medications
| Aggressive Group | Standard Group | P Value b | |
|---|---|---|---|
| Participants with ≥1 AE, % | 25.0 (19.9–30.1)c | 14.3 (10.2–18.5) | .002 |
| No. of AEs | 118 | 54 | <.001 |
| Most common AEs | |||
| Dizziness (lisinopril, atenolol, amlodipine, HCTZ, valsartan)d | 41 (34.8%)e | 9 (16.7%)e | |
| Cough or nonproductive cough (lisinopril, enalapril)d | 13 (11.0%) | 15 (27.8%) | |
| Fatigue (lisinopril, atenolol, HCTZ, doxazosin)d | 13 (11.0%) | 3 (5.6%) | |
| Occurrences of orthostatic hypotension, No. | 2 | 3 | |
| Drugs reported most often | |||
| Lisinopril (cough, dizziness)f | 40 (33.9%)e | 19 (35.2%)e | |
| Atenolol (dizziness, bradycardia, fatigue)f | 21 (17.8%) | 7 (13.0%) | |
| HCTZ (dizziness, nausea, fatigue)f | 17 (14.4%) | 10 (18.5%) | |
| Amlodipine (dizziness, edema)f | 10 (8.5%) | 5 (9.3%) | |
| Drugs with highest incidence of AEs (AEs/100 person‐years) | |||
| Atenolol | 8.86 (5.24–12.48) | 4.52 (1.25–7.79) | .1 |
| Lisinopril | 6.15 (4.30–8.00) | 4.44 (2.49–6.39) | .2 |
| Amlodipine | 5.13 (2.03–8.23) | 4.57 (0.66–8.48) | .8 |
| HCTZ | 4.12 (2.20–6.04) | 4.30 (1.69–6.91) | .9 |
Abbreviations: AEs, adverse events; HCTZ, hydrochlorothiazide. aPossibly, probably, or definitely related. bChi‐square test was used to calculate the P value. cNumbers in parentheses are 95% confidence interval for percentages. dMost frequently reported drugs. eNumber in parentheses is percentage of total AEs for group. fMost frequently reported AEs.
Table V.
Summary of SAEs Relateda to Hypertension Medications
| Aggressive Group | Standard Group | P Value b | |
|---|---|---|---|
| Participants with ≥1 SAEs, % | 1.45 (0.04–2.86)c | 0.74 (−0.28–1.76) | .69 |
| Number of SAEs | 4 | 2 | .41 |
| Outcome of SAEs | |||
| Death | 0 | 0 | |
| Life‐threatening event | 0 | 0 | |
| Prolongation of hospital stay | 0 | 0 | |
| Event resulting in permanent disability | 0 | 0 | |
| Hospitalization | 4 | 2 | |
| Symptoms of SAEs | |||
| Syncope (pre‐syncope) | 2 | 1 | |
| Hypotension | 0 | 2 | |
| Acute renal failure | 1 | 0 | |
| Hyperkalemia | 2 | 0 | |
Abbreviation: SAEs, serious adverse events. aPossibly, probably, or definitely related. bChi‐square test was used to calculate the P value. cNumbers in parentheses are 95% confidence interval for percentages.
Discussion
The description of optimal BP goals in diabetic adults has focused largely on DBP, 11 with little information on the relationship of lower SBP with better CV outcomes. Consequently, SANDS provides some of the first available information on the feasibility, safety, and efficacy of more intensive SBP reduction in diabetes. In conjunction with a lower LDL cholesterol goal of <70 mg/dL, the lower SBP goal of 115 mm Hg in this study was associated with a reduction in carotid intimal‐medial thickness and a greater reduction in left ventricular mass when compared with standard treatment goals of 100 mg/dL and 130 mm Hg, but no measurable difference in the small number of CV events. 4
The current results provide objective evidence that lower BP goals can be safely achieved in middle‐aged, obese, type 2 diabetic adults. The regimen was effective and well tolerated with limited side effects, particularly with regard to dizziness and fatigue, and with little documented orthostasis. Although there were more reported AEs in the AGG than in the STD arm, these were generally mild, and most patients remained on the clinical trial algorithm for BP treatment.
SANDS goes beyond previous studies that have evaluated the safety of reducing BP by achieving substantially lower in‐trial pressures. In comparison to the Hypertension Optimal Treatment (HOT) trial, 11 in which the most intensively treated group achieved an average DBP <83 mm Hg (range, 81–85 mm Hg in diabetic patients), mean on‐treatment DBPs in the AGG and STD treatment arms of SANDS were 67 and 73 mm Hg, respectively. The SBP levels achieved in diabetics in the HOT study participants were only 140 to 144 mm Hg. Among the large group of diabetic participants in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), 12 mean DBP after 4 years in the 3 treatment arms was 75 to 76 mm Hg, approaching that in SANDS participants on STD therapy, whereas average SBPs of 135 to 137 mm Hg remained substantially higher than the average in‐treatment levels of 116 and 128 mm Hg in aggressive and STD treatment arms of SANDS. Similarly, mean attained BPs in SANDS were lower than the average level of 132/74 mm Hg attained after initial up‐titration of combination antihypertensive therapy in the Avoiding Cardiovascular Events Through Combination in Patients Living With Systolic Hypertension (ACCOMPLISH) study, 13 in which 60% of participants had diabetes.
Study Strengths and Limitations
This study has a number of strengths. It is the first to directly compare 2 specific targets for SBP in diabetic patients. Multiple epidemiologic studies have documented a stronger effect of SBP than DBP on CV events, particularly stroke and heart failure. 14 The study evaluated a large number of well‐characterized participants, BP was measured by a standardized protocol, and retention and adherence were high. A study limitation is that it was conducted in a single ethnic group. American Indians with diabetes often experience CV events despite BP levels below those traditionally recommended as a goal of treatment (<130/80 mm Hg), 1 , 15 , 16 making it important to evaluate lower BP targets in this group. The current observations should be applicable to type 2 diabetics in other racial/ethnic groups. However, future trials are necessary to verify this supposition.
Another important concern is whether the present results, achieved in a controlled clinical trial, can be achieved in a primary care setting. In this study, physician investigators and coordinators at each site supervised the care of approximately 125 to 130 patients each. They also worked with tribal community members when possible to assist with translation and encouragement; a BP consultant was readily available. Also important is that all medications were provided through Indian Health Service pharmacy providers. This organizational structure is probably beyond that which would be typical for clinical practice. However, many other aspects of the study were similar to, if not more challenging than, those encountered in a primary care setting. Not all patients had easy access to the study clinic, and they often had to drive several hours to be seen. Many had limited financial means. At times, referring physicians and cultural barriers interfered with opportunities to achieve appropriate BP treatment goals. Moreover, as in clinical practice, obesity (mean body mass index of 33) and alcohol use (28% current users, 46% former users) were often barriers to achieving better BP control. Despite these potential obstacles, the BP goals were achieved with excellent tolerability and safety.
Conclusions
Lower BP goals than those currently recommended in JNC 7 can be safely achieved and maintained in men and women with type 2 diabetes. Lower BP and cholesterol goals can have a measurable impact on surrogate measures of CV disease such as left ventricular mass and carotid intimal‐medial thickness. SANDS also shows that a systematic approach to antihypertensive therapy with a predefined algorithm can be helpful to facilitate effective and safe treatment in this high‐risk population.
Disclosures: Dr Weir had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Indian Health Service, the Office of Public Health and Science, or the National Institutes of Health. Medications were donated by First Horizon Pharmacy, Merck and Co, and Pfizer, Inc. Dr Howard has served on the advisory boards of Merck, Shering Plough, the Egg Nutrition Council, and General Mills and has received research support from Merck and Pfizer. Dr Weir has served on the speakers’ bureau and as an ad hoc consultant to Merck, Novartis, Boehringer‐Ingelheim, and Sanofi/Bristol Myers Squibb. Dr Devereux serves on advisory boards or as a consultant to Merck and Novartis and has received research support from Merck. The other authors have nothing to declare. Funding was provided by the National Heart, Lung, and Blood Institute, National Institutes of Health, NHLBI grant #1U01 HL67031‐01A1. The National Heart, Lung, and Blood Institute has representation on the SANDS Steering Committee governed the design and conduct of the study, interpretation of the data, and preparation and approval of the manuscript. The National Heart, Lung, and Blood Institute Project Office reviewed the manuscript. All statistical analyses for the study were performed by statisticians at The University of Oklahoma Health Sciences Center, under the direction of the principal investigator of the Coordinating Center, Dr Elisa T. Lee.
Acknowledgments
Acknowledgments: We thank the Indian Health Services facilities, SANDS participants, and participating tribal communities for extraordinary cooperation and involvement without which this study would not have been possible. We also acknowledge the valuable contributions of the following SANDS study staff: Tauqeer Ali, PhD; Collen Begay; Stephanie Big Crow; Verna Cable; Damon Davis, RN; Joanne Detwiler, PA‐C; Lynne Dobrovolny, PA; Verdell Kanuho; Tanya Molina; Missy Oines; Maria Ramos, PA‐C; Corinne Wills, CNP; and Jackie Yotter, RN, for coordination of study centers and Tia A. Paul, University of Maryland School of Medicine, Baltimore, MD, for editing the manuscript. We also gratefully acknowledge donations of pharmacologic agents by First Horizon Pharmacy (Triglide); Merck and Co (Cozaar/Hyzaar); and Pfizer, Inc (Lipitor), and we thank Dr John Lachin for providing advice on statistical methods.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin survival study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 4. Howard BV, Roman M, Devereux R, et al. Effects of lower target for blood pressure and ldl cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 6. Russell M, Fleg JL, Galloway WJ, et al. Examination of lower targets for low‐density lipoprotein cholesterol and blood pressure in diabetes–the Stop Atherosclerosis in Native Diabetics Study (SANDS). Am Heart J. 2006;152:867–875. [DOI] [PubMed] [Google Scholar]
- 7. Black HR. The evolution of low‐dose diuretic therapy: the lessons from clinical trials. Am J Med. 1996;101:47S–52S. [DOI] [PubMed] [Google Scholar]
- 8. Neutel JM, Black HR, Weber MA. Combination therapy with diuretics: an evolution of understanding. Am J Med. 1996;101:61S–70S. [DOI] [PubMed] [Google Scholar]
- 9. Palmieri V, Tracy RP, Roman MJ, et al. Relation of left ventricular hypertrophy to inflammation and albuminuria in adults with type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:2764–2769. [DOI] [PubMed] [Google Scholar]
- 10. El Assaad MA, Topouchian JA, Darne BM, et al. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 11. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. HOT study group. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Barzilay J, Cushman WC, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2005;165:1401–1409. [DOI] [PubMed] [Google Scholar]
- 13. Jamerson K, Bakris GL, Dahlof B, et al. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Press. 2007;16:80–86. [DOI] [PubMed] [Google Scholar]
- 14. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham heart study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 15. Howard BV, Lee ET, Cowan LD, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians. The strong heart study. Am J Epidemiol. 1995;142:254–268. [DOI] [PubMed] [Google Scholar]
- 16. Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The strong heart study. Circulation. 1999;99:2389–2395. [DOI] [PubMed] [Google Scholar]


