Abstract
The introduction of multiple treatments for cancer, including chemotherapeutic agents and radiation therapy, has significantly reduced cancer-related morbidity and mortality. However, these therapies can promote a variety of toxicities, among the most severe being the ones involving the cardiovascular system. Currently, for many surviving cancer patients, cardiovascular (CV) events represent the primary cause of morbidity and mortality. Recent data suggests that CV injury occurs early during cancer treatment, creating a substrate for subsequent cardiovascular events. Researchers have investigated the utility of noninvasive imaging strategies to detect the presence of CV injury during and after completion of cancer treatment because it starts early during cancer therapy, often preceding the development of chemotherapy or cancer therapeutics related cardiac dysfunction. In this state of the art article, we review the utility of current clinical and investigative CV noninvasive modalities for the identification and characterization of cancer treatment-related CV toxicity.
Keywords: Chemotherapy-related cardiotoxicity, noninvasive imaging, cardiovascular imaging
Introduction
Advancements in the treatment of cancer occurring over the past three decades have resulted in decreased cancer-related morbidity and mortality, and increased long-term survivorship. Today, data from billing codes related to cancer patients indicate that cardiovascular disease (CVD) is the leading cause of death among breast cancer survivors – replacing recurrent cancer or development of a new cancer (1). In childhood cancer survivors (2,3), the risk of CV death is now higher than the actual risk of tumor recurrence (with a reported seven-fold increase in cardiac mortality rate relative to siblings without cancer).
In this article, we review the cancer therapies and their associated CV events, existing noninvasive imaging study results highlighting methods to detect early evidence of CV injury upon receipt of treatment for cancer, and emerging noninvasive imaging technologies that may further enhance the detection of CV injury. Results of several studies raise the possibility that noninvasive imaging may be useful for identifying CV injury after receipt of cancer treatment.
Cardiovascular injury from cancer treatment
The type and duration of cancer treatment still plays an important role in determining CV injury or toxicity. Cardiovascular toxicity can be caused by a) direct injury to or death of cardiac myocytes, b) stimulation of myocardial fibrosis, c) provocation of stress induced myocardial ischemia via endothelial dysfunction, d) vascular injury, e) myocardial and/or pericardial inflammation; f) arrhythmogenic or conduction abnormalities; g) autonomic dysfunction; h) valvular disease, or i) exacerbation of known CV risk factors (e.g., hypertension, accelerated atherosclerosis, or Raynaud's syndrome, etc.) (4,5). In addition to traditional cardiotoxic agents, such as anthracyclines or radiation related heart disease, newer therapies including tyrosine kinase inhibitors (6-11) and even therapies that are not necessarily classified as “chemotherapy” may also promote CV disease or events. For example, the administration of hormone deprivation therapies, which have dramatically reduced cancer recurrence and improved survival in women with breast cancer or men with prostate cancer, are now increasingly associated with CV events (12-16). Table 1 in the online appendix presents a summary of the types of cardiac injuries, the agents that commonly cause these injuries, and noninvasive investigations to determine the extent of these injuries.
Current Clinical Noninvasive Imaging Strategies for Screening Cancer Treatment-Related Cardiotoxicity
A literature review from the American Society of Clinical Oncology has recently noted that there are no available systematic evaluations published regarding the role of routine noninvasive testing for cardiac dysfunction in patients treated for cancer. Moreover, the effectiveness of screening techniques for detecting subclinical CV injury in asymptomatic survivors of cancer is not established. Yet, there are recent research initiatives suggesting the possible utility of noninvasive imaging technologies for identifying subclinical CV injury in those receiving treatment for and surviving cancer. In the following sections, we provide an overview of the data accumulated to date that address the utility of noninvasive imaging strategies for assessing cancer therapy related to CV disease.
Nuclear Medicine Imaging
The first studies of cancer therapy-related cardiac toxicity relied on equilibrium radionuclide angiography (ERNA) to measure LV function through determination of LV ejection fraction (LVEF). Established in the 1970s, reductions in LVEF identified those with anthracycline-related cardiotoxicity; in the single largest study using serial ERNA, 19% of patients who dropped their LVEF by >10% from baseline, or to a value < 50% went on to develop heart failure (17). Today, ERNA is used to identify LV dysfunction from other cardiotoxic agents (17,18).
In addition to resting measures of LVEF, investigators have also assessed the utility of ERNA appreciated stress induced changes in LVEF as markers of early anthracycline induced cardiomyopathy. McKillop, et al., found that the sensitivity for detecting patients that may develop heart failure increased from 58% to 100%, but this occurred with a concomitant decrease in specificity from 75% to 41% (19). Thus, to date, stress nuclear assessments of LVEF to identify cardiac injury after receipt of anthracycline are not widely performed.
In addition to systolic dysfunction, LV diastolic function is often assessed with radio-isotope based techniques. Count-time curves, the peak filling rate (PFR), the PFR normalized to stroke volume (PFRSV), and time-to-peak filling rate (TPFR) detected with planar equilibrium radionuclide ventriculography (ERNV) are associated with anthracycline-induced diastolic dysfunction (20,21). Reductions in these ERNV measures of LV diastolic function correlate with the simultaneous decreases in LVEF, suggesting that anthracyclines impair both systolic and diastolic function (21). Moreover, a recent study by Cochet, et al., demonstrates that baseline prolongation of TPFR (which reflects impairment of diastolic function before treatment) is an independent predictor for trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer (22).
It is important to recognize that while ERNA is widely available for identifying LV dysfunction associated with chemotherapy-related cardiotoxicity (17,20,23,24), there are limitations to the procedure. First, the procedure exposes patients to ionizing radiation dose (estimated at 7.8 mSv per examination). This is problematic for childhood cancer patients or those who receive repeated exposures by surveillance protocol guidelines. Second, the procedure produces little information regarding other cardiac parameters such as those related to valvular structure or the pericardial space. Finally, the technique is not well suited for detecting small changes in LVEF or direct measures of myocardial injury that may provide important evidence of early injury that predispose one to future CV events.
In patients with heart failure, the single-photon emission computed tomography (SPECT) and positron emission tomography (PET) techniques using radiolabeled neurotransmitters and receptor ligands have been used to evaluate pre-synaptic reuptake, neurotransmitter storage, and also activity of post-synaptic receptors (25-27). Metaiodobenbenzylguanidine (MIBG) is a quanethidine analog that shares type I adrenergic neuroreceptor uptake storage and release mechanisms throughout the body with norepinephrine (25-27). After being labeled with 123I, uptake of regional 123I-MIBG reflects neuronal integrity and its release reflects adrenergic function (25-27). Calculation of the heart-to-mediastinum count ratio (H/M ratio) of 123I-MIBG uptake and delay in the 4 hour post injection washout rates have been observed in patients with heart failure or those receiving anthracycline-based chemotherapy. Also, a decrease in the H/M ratio correlated with a higher cumulative dose of anthracycline (27-29). Decreases of MIBG uptake may be seen up to 10 years after development of heart failure in patients with a history of severe anthracycline-induced cardiomyopathy, regardless of recovery of LV function. These findings suggest myocardial cell injury and adrenergic dysfunction from destruction of adrenergic nerve tissue and functional alteration or adrenergic nerves by cytotoxic effect of itself, as in animal or human models, may persist for years after the initial exposure to anthracyclines (30,31).
Echocardiography
Its wide availability and absence of non-ionizing radiation render echocardiography a very attractive imaging option for assessing patients with cardiac abnormalities during or after cancer treatment (32-37). In addition to evaluating LV structure, echocardiography provides information on both systolic function (LVEF and fractional shortening in the pediatric population), and diastolic function (E/A ratio, E/e’,e’, isovolumic relaxation time [IVRT] and pulmonary venous flow) (38-44). Also, recent techniques have become available to measure myocardial deformation, including LV strain, strain rate, or twist and torsion that may provide new understanding regarding the early stages of the pathophysiology of cardiac dysfunction upon receipt of cancer treatment (37,45-50). Moreover, echocardiography provides additional information about valvular function and pericardial fluid/physiology that might occur after cancer treatment (32,51,52).
From a standard two-dimensional (2D) echocardiogram, LVEF can be estimated visually, or quantified by M-mode (the fractional shortening method), single or biplane area-length methods, or the modified Simpson summation of disks technique as per ASE chamber quantification guidelines (53). In general, cardiovascular medicine consultations should be considered for those patients experiencing reductions in LVEF of ≥5% to <55% with symptoms of heart failure, or an asymptomatic reduction in the LVEF of ≥10% (54,55). It is important to note, however, that while 2D echocardiography can appreciate relatively large drops in LVEF (e.g., from 60% to 40%), smaller changes such as from 54% to 48% are more difficult to obtain with a high degree of certainty (56,17,23).
To address this limitation, three dimensional (3D) methods are now available to improve the detection of small changes in LVEF (40,42). Recently, Thavendiranathan, et al., demonstrated that 3D echocardiography was more reproducible and had lower inter-observer variability LVEF and volume measurements. This finding correlated well with a previous study by Walker, et al., that found the technique to be more accurate when compared with 2D, and not inferior when compared with MUGA and cardiac MRI (35,57). To date, however, researchers have not accomplished the utilization of 3D echocardiographic strategies in measuring LVEF on a large scale in community hospitals or clinically in large numbers of patients treated for cancer for the purpose of detecting CV injury.
Some studies have shown that left ventricular diastolic properties, such as a decrease in the E/A ratio, or prolongation of isovolumic relaxation time (IVRT) or deceleration time of early diastolic filling (DT) can predict doxorubicin-induced LV systolic dysfunction (58,59). However, other studies have not observed a relationship between changes in diastolic measures and long-term changes in LVEF (60,61). In fact, increases in E/A ratio and shortening of IVRT occurring 1 hour after administration of the first dose of doxorubicin can return to prechemotherapy levels within 3 weeks (60, 61). Given the transient nature of these diastolic findings, they have not been widely utilized to direct cardio-protective strategies to prevent chemotherapy-related cardiotoxicity.
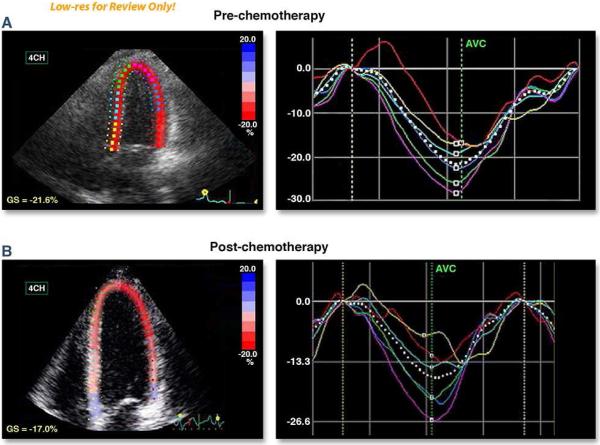
One newer measure that may be helpful to identify cardiac injury includes the assessment of global longitudinal strain (GLS) (Figure 1; 39,41). In general, longitudinal LV mechanics are the most vulnerable and highly reproducible component of LV mechanics that can be assessed with well-performed transthoracic echocardiography (39-41). Stoodley, et al., demonstrated that anthracyline chemotherapy can reduce global and regional longitudinal and radial strain by more than 10% as early as 1 week after receipt of treatment. This corresponds well with results by Sawaya, et al., where reduced global longitudinal and radial strain after 3 months of cancer treatment with an anthracycline and trastuzumab predicted the later development of a reduction of LVEF 6 months after initiation of these therapies. In this same study, reductions in longitudinal strain of > 10% from baseline predicted future declines in LVEF with a sensitivity of 78% and specificity of 79% and a negative predictive value of 93% (37,50). Abnormalities of global measures of longitudinal strain evaluated in combination with determinations of ultrasensitive troponin I measured at the completion of treatment may prognosticate subsequent development of LV dysfunction 12 and 15 months after completion of chemotherapy treatment (p=0.0003 and p=0.04, respectively) (62,63). In a recent systematic review, a 10-15% early reduction in GLS by STE during therapy appears to be the most useful parameter for the prediction of cardiotoxicity defined as a drop in LVEF or heart failure (64).
Figure 1. 2D Speckle tracking Echocardiogram-based strain in patient with invasive ductal carcinoma (ER-, PR-, Her2-neu+), treated with TCH Regimen (Docetaxel, Carboplatinum and trastuzumab). Baseline EF was 65%. EF after 3 months of therapy was 58%.
Panels A and B utilize color to illustrate the global longitudinal strain (GLS) and regional strain values obtained at baseline (pre-chemotherapy) and 3 months after the initiation of trastuzumab-based regimen. The Septal and anteroseptal segments exhibit abnormal regional strain after treatment. (Courtesy of Dr. Juan Carlos Plana, MD, FACC. Co-Director Cardio-Oncology Center. Cleveland Clinic. Cleveland, Ohio)
Myocardial twist, untwist, and torsion of the LV apex have been studied with transthoracic echocardiography. Myofilament disorganization and cardiomyocyte necrosis impact the passive and restoring forces of the ventricle in in-vitro animal model studies (43,65). To this end, Motoki, et al., identified deterioration in LV apical and both torsion, twisting rates and untwisting rates 1 month after chemotherapy that correlated with prolongation of IVRT 3 months after chemotherapy. However, this finding did not forecast future reductions in LVEF nor CV events (48). Cheung, et al., demonstrated that 1 year after treating children with acute lymphoblastic leukemia, LV apical torsion, twisting and untwisting velocities were reduced. Future studies are required to determine the prognostic utility of echocardiographic measures of twist and torsion in those treated for cancer (45).
The role of microbubble contrast in assessing cardiac function after treatment for cancer is not well studied and has produced conflicting results related to its overall utility. In those patients with poor LV endocardial visualization, the American Society of Echocardiography (ASE) suggests the intravenous administration of microbubble contrast may improve assessment of LV wall motion and LVEF post cancer treatment especially in those undergoing mastectomy or breast implants (66). However, recently, Thavendiranathan et al. demonstrated that in breast cancer patients post chemotherapy with stable measures of global longitudinal strain (GLS), non-contrast 3D assessments of LVEF exhibited lower temporal variability in comparison with contrast based methods (57).
Cardiac Computed Tomography (CCT)
The use of cardiovascular computed tomography (CCT) to assess the CV system after treatment for cancer and to forecast future CV events has not been well studied. This technology may be useful in two respects: first, for evaluating the pericardium of patients that received radiation or surgical treatments to identify abnormal thickening and calcification of the pericardium, and second, to measure coronary artery calcium or directly visualize the coronary arteries (67). Although coronary artery calcium scores are elevated when mediastinal radiation is administered at doses >20 Gray (68,69), and anthracycline chemotherapy has been associated with accelerated atherosclerosis (69-72), in the absence of symptomatic CAD, there is currently insufficient data to recommend a routine use of coronary CT angiography or calcium scoring in patients who underwent high-dose radiation therapy. In addition, the presence of coronary artery calcification prior to treatment for cancer has not been shown to predict future CV risk upon receipt of chemotherapy, tyrosine kinase inhibitors or radiation therapy. For these reasons, CCT has not been widely used to screen for adverse subclinical CV disease after cancer treatment or predict CV risk pre-cancer treatment. Whether existing planning or surveillance images acquired as components of clinical exams used to stage cancer could be used for these purposes requires further study (68-73). At present, information related to the CV system is often not reported on these relatively routinely acquired cancer surveillance studies.
Cardiovascular Magnetic Resonance (CMR)
Cardiovascular magnetic resonance (CMR) is a versatile imaging modality in that with a single examination, one can gather information pertaining to cardiac and vascular anatomy, tissue characteristics (presence of fibrosis, inflammation, injury, etc.), left and RV systolic or diastolic function, blood flow, and myocardial perfusion or metabolism (35,42,57,74). These assessments are accurate and reproducible, exhibit high spatial and temporal resolution, and do not expose individuals without exposure to ionizing electromagnetic radiation. For this reason, the ACC/AHA recognizes CMR as a method to identify cardiovascular dysfunction after treatment for cancer and has incorporated it across research studies to define the pathophysiology of cancer treatment-related CV toxicity (75). In addition, CMR can appreciate myocardial masses associated with metastases, or evaluate the pericardium and pericardial space, and when necessary, assess valuable function (76).
Researchers and clinicians have used LV myocardial mass, volume, and systolic and diastolic function assessments measured from cine white blood imaging sequences to identify evidence of cardiomyopathy among adult cancer survivors (77). Neilan et al., demonstrated an inverse correlation between anthracycline dosage and CMR-derived LV mass index (LVMi) (r=0.67; p<0.001), and an association of LVMi with major adverse CV events (HR 0.89, p<0.001). These results indicated a sensitivity of 100% and specificity of 85% to predict major adverse CV events if the LVMi was ≤57 g/m2 after treatment with anthracycline chemotherapy (78).
In addition to reductions in LV mass, an increase in LV cavity end systolic volume is associated with the subsequent reduction in LVEF after treatment with trastuzumab or anthracycline-based chemotherapy (35,79,80). Drafts, et al., followed 51 subjects treated with anthracycline-based chemotherapy and identified early increases in LV end-systolic volume commensurate with deteriorations in LV ejection fraction, myocardial strain, and ability to perform activities of daily living. In addition, these cardiac and of quality of life metrics occurred commensurate with increases in serum troponin levels (35,76,77).
A unique feature of CMR is the ability to characterize myocardial tissue by the use of relaxation times (T1, T2 and T2*) in order to identify myocardial injury and fibrosis. Specifically, T2-weighted images are sensitive to regional or global increases of myocardial water content that accumulates in the setting of myocellular or microvascular injury or inflammation (81). A previous small study by Oberholzer, et al., identified myocardial edema from a T2-weighted study post-anthracycline treatment (82). Further research is ongoing regarding the utility of T2 mapping techniques of the LV myocardium in patients receiving treatment for cancer (83).
In addition to assessing myocardial T2 relaxation, properties related to T1 relaxation may also provide insight regarding myocardial injury and fibrosis related to the administration of chemotherapy. In rodent models, Lightfoot, et al., demonstrated that an increase in gadolinium enhanced signal intensity on T1 weighted images after treatment with doxorubicin was associated with histopathologic evidence of intracellular vacuolization (consistent with doxorubicin-induced cardiotoxicity) and forecasted a subsequent reduction in LVEF (84). In a clinical study by Wassmuth, et al., an increase of Gd-SI on T1 on post-contrast T1-weighted images within 3 days of the receipt of anthracycline infusions predicted a significant decline in LVEF at 28 days (p < 0.05) (85). Tham, et al., demonstrated that changes in myocardial T1 values occurred in children post-exposure to anthracycline without correlation to anthracycline (86). Long term clinical outcome studies are needed to determine if T1/T2 mapping findings are associated the adverse clinical CV outcomes in patients treated for cancer.
Myocardial fibrosis by late gadolinium enhancement is associated with an adverse CV prognosis in patients with CAD, hypertrophic cardiomyopathy or infiltrative diseases such as amyloidosis and sarcoidosis (86). For those treated for cancer, data pertaining to the association of LGE with cancer treatment is mostly anecdotal or observational, and somewhat conflicting in regards to reported results. In a chemo-toxic cardiomyopathy study by Catalano, et al., midmyocardial LGE is shown in the mid-basal septum and anterior, basal anterolateral, and mid-inferior wall after treatment with anthracycline/cyclophosphamide (87), and a study by Fallah-Rad, et al. demonstrates mid-myocardial LGE patterns in the lateral wall after treatment with trastuzumab for 12 months (88,89). In contrast, Neilan, et al. determined that LGE is an infrequent finding occurring in only 6% (5 cases/91 cases) of patients treated with anthracycline-base chemotherapy despite a reduced LVEF (85). In addition, a study by Lawley, et al. demonstrated that LGE occurs only in 8% (2 cases/25 cases) of patients treated with adjuvant trastuzumab without any change in systolic function or routine diastolic filling parameters (90).
In addition to functional and structural abnormalities pertaining to the heart, treatment for cancer with hormonal deprivants, tyrosine kinase inhibitors, or anthracyclines may impact the vasculature and thereby contribute to other CV events such as stroke and myocardial infarction. Recently, Chaosuwannakit, et al., demonstrated that proximal aortic wall stiffness increased 3 months after receipt of anthracycline-based chemotherapy after controlling for factors such as age, gender, diabetes, hyperlipidemia and hypertension (91). The increase in stiffening occurred soon after administration of chemotherapy, was not dose dependent, and was equivalent to that associated with aging the CV system by 10-20 years (Figure 2). In other patient populations such as those with diabetes, hypertension, renal failure, and advanced age, abnormal increases in proximal aortic stiffness have been associated with LV hypertrophy, exercise intolerance, and future CV events (92).
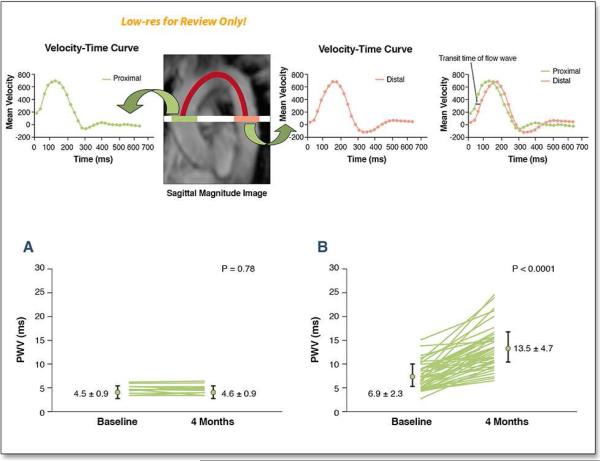
Figure 2. Pulse wave velocity assessments of aortic stiffness after cancer treatment.
Sagittal magnitude image of the thoracic aorta was used to select the axial plane at the level of pulmonary artery and perpendicular to aortic flow (solid white line). The distance between ascending and descending thoracic aorta was obtained by tracing the centerline of the aortic lumen (red line). The two velocity–time curves are shown across the thoracic aorta. The sagittal magnitude image demonstrates the velocity–time curves for the ascending and descending thoracic aorta. Transit time of the flow wave was computed on the basis of the upstroke time difference of the velocity–time curve at two different regions (blue line). The location of the best cross-correlation of two partial upstroke velocity curves was used to estimate the time delay. Pulse wave velocity (PWV) was calculated by dividing the distance between the ascending and descending thoracic aorta by the transit time of the flow wave.
CMR-derived aortic stiffness by the measurement of pulse wave velocity (PWV) between control participants without cancer (Panel A) and participants who are receiving cancer therapy (Panel B) at the baseline and after 4 months of treatment. As shown, the PWV increased in participants receiving anthracycline-based therapy. The magnitude of the increase in PWV is equivalent in other populations to an aortic stiffness age associated increase of 15 years.Reprinted with Permission. Chaosuwannakit N, et al. J Clin Oncol. 2012;28:166-72. (118).
It is important to note that although CMR is accurate and reproducible, it does not expose one to ionizing radiation, and assesses multiple aspects of the CV system in a single exam. Its availability is relatively low and is not well suited for use in those with cardiac pacemakers, cardiac resynchronization therapy devices, internal cardiac defibrillators, or intracranial metal. Moreover, in patients with renal insufficiency, (estimated glomerular filtration rates ≤30 ml/min) precaution is needed when gadolinium contrast is considered due to an increased incidence of nephrogenic systemic fibrosis (93).
Investigative Noninvasive Imaging Strategies for Screening Cancer Treatment-Related Cardiotoxicity
In addition to current clinical applications, there are additional initiatives underway in research venues to image processes involved in cancer therapy related CV injury. These include molecular and metabolism-targeted imaging. These forms of imaging characterize and biological processes at the cellular and molecular level within living organisms, utilizing injectable imaging agents or genetically encoded reporters. Although originating with targeted-nuclear imaging, there are now a variety of imaging agents and modalities evolving as methods to detect cardiotoxicity after treatment for cancer (88,94).
Imaging of Apoptosis and Cell Death
Apoptosis, the physiologic adenosine triphosphate (ATP)-dependent, non-inflammatory process of programmed cell death resulting in fragmentation and shrinkage of nuclear material, or myocyte death culminates in the activation of a variety of proteins that can serve as imaging biomarkers. Phosphatidylserine (PS), one such protein, is expressed on the cell membrane and serves as a noninvasive imaging biomarker of apoptosis (88,94,95).
Annexin V, a high affinity calcium-depending PS-binding protein conjugated to radioisotopes (such as 99mTc) in SPECT imaging, to magnetic iron oxide nanoparticles and Gd-containing liposomes in CMR, to positron emitters in PET, and to fluorescence markers in optical imaging has been used to detect in vivo cell death due to myocardial infarction, heart transplant rejection, end stage LV dysfunction in human subjects, and cancer-related therapy in animal models. In animal studies of acute and chronic doxorubicin cardiac toxicity, a significant increase in 99mTc-Annexin V uptake in the myocardium, with dose-dependent cell death confirmed by histopathology and immunohistochemistry, was related to subsequent ventricular dysfunction confirmed by echocardiography (96-98). Recently, Annexin V-based magnetoflourescent supraparamagnetic iron oxide (SPIO) nanoparticles in combination with T2*-weighted CMR revealed diffuse myocardial T2* signal loss that correlated with increased caspase activity in an animal receiving anthracyclines (99).
Another imaging related biomarker of cellular apoptosis relates to the activation of caspase proteins. Activation of caspases is associated with cellular apoptosis. In animals, a significant increase in caspase-3 activity has been observed within LV myocardium after treatment with doxorubicin (100,101).
Inflammation Imaging
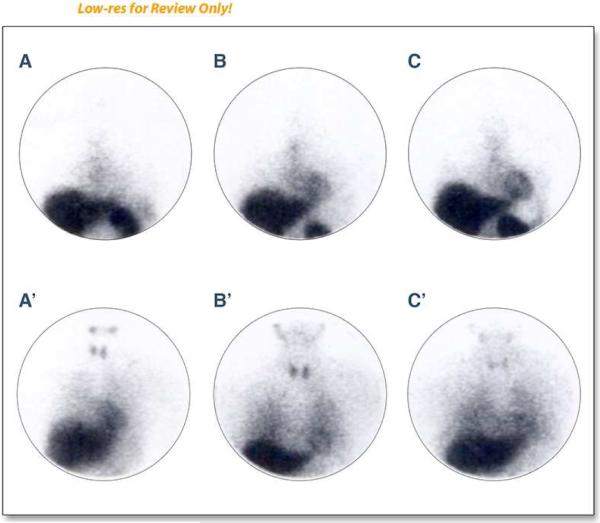
Inflammatory injury to cardiac myocytes disrupts the cellular membrane promoting the release of a myosin heavy chain. Investigators developed monoclonal antibodies, 111In and 99mTc, to identify these heavy chains and thereby assess the degree of myocyte damage in response to inflammation. Studies by Valdes Olmos, et al., and Maini, et al., both show positive correlation between cumulative dose of anthracycline and the uptake of antimyosin in the myocardium, with later deterioration of LVEF (102,103; Figure 3). Importantly, however, the specificity of 111In-antimyosin scintigraphy is low (25-50%) for predicting decrements in LVEF 12 months after receipt of cancer treatment (78).
Figure 3. Sequential antimyosin (A, B, C) and MIBG (A', B', C') studies before chemotherapy (A, A') at 240-300mg/m 2 (B, B') and at 420-600 mg/rn2of doxorubicin.
There is a pattern of increasing myocardial antimyosin uptake with decreasing myocardial MIBG uptake both reflecting ongoing myocellular injury from anthracycline-based chemotherapy. Reprinted with permission. This research was originally published in JNM. Carrió I, Estorch M, Berná L, López-Pousa J, Torres G. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med. 1995;36:2044-2049. © by the Society of Nuclear Medicine and Molecular Imaging, Inc. (28).
Myocardial Metabolism Imaging
Magnetic resonance spectroscopy (MRS) imaging can assess multiple metabolic pathways simultaneously without exposure to ionizing radiation. The principle of MRS is that the chemical shift influences the different resonance frequencies, allowing for the differentiation of nuclei of the same species in different molecules. MRS allows direct measurement of biochemical information about in vivo processes involving phosphorous (31P), hydrogen (1H), carbon (13C), sodium (23Na), nitrogen (15N), and fluorine (19F). Currently, only 31P has been studied in the assessment of doxorubicin related cardiotoxicity. In animals receiving doxorubicin, phosphocreatine (PCr) to adenosine triphosphate ratios (89) and PCr levels (90) differed after stress compared to those not receiving doxorubicin. These pre-clinical data suggest that MRS may be able to detect abnormal mitochondrial ATP production/utilization related anthracycline therapy.
Myocardial fatty acid metabolism assessed with the SPECT radiotracers 15-(piodophenyl)-pentadecanoic acid (IPPA) and 123I-betamethyl-P-iodophenyl pentadecanoic acid (BMIPP) have been measured in subjects receiving anthracycline and other chemotherapeutic agents. A study by Saito, et al., demonstrated early decreased uptake of 123I-BMIPP in patients with preserved LV function after treatment with an anthracycline; similar decreases in 123I-BMIPP uptake were observed in patients experiencing a decline in LVEF after receipt of a taxane and carboplatin (104,105). Recently, Carboni, et al., evaluated mitochondrial metabolism with 99mTc-sestamibi (MIBI) in patients receiving multi-agent chemotherapy. These investigators demonstrated both early and delayed cardiac MIBI uptake with rapid washout rates reflective of mitochondrial membrane dysfunction that were associated with an adverse cardiovascular prognosis (106).
Positron emission tomography with its ability to quantify myocardial blood flow, oxygen extraction (using 15O as a tracer), myocardial glucose metabolism, and fatty acid metabolism has been preliminarily investigated regarding the detection of cardiac toxicity after receipt of cancer treatment. A recent study by Borde, et al., demonstrated enhanced myocardial 18FFluorodeoxyglucose (FDG) uptake in patients treated with anthracyclines (107). In addition, Toubert, et al., demonstrated decreased myocardial uptake of 18F-FDG in patients treated with a combination of tyrosine kinase inhibitors (imatinib-sorafenib) who later developed a cardiac event (108). It is important to note that FDG uptake is nonspecific and its uptake can change after other disease processes such as diabetes, and thus, fasting status and pre-scan diet must be considered when interpreting the study results.
Angiogenesis Imaging
Angiogenesis is generally defined as the development of new capillaries from preexisting microvessels. This complex multi-step process involves a variety of cells responding to both stimulatory and inhibitory factors. Several conditions stimulate the angiogenic process including: ischemia, hypoxia, inflammation, shear stress, and traumatic injury (109). Tumors also modify angiogenesis to enhance their blood supply. For this reason, therapy directed to prevent tumor-associated angiogenesis (“anti-angiogenesis therapy”) has become one of the cornerstones of many modern chemotherapeutic regimens (109). To assess the efficacy of anti-angiogenesis therapy, angiogenesis imaging utilizing a) non-endothelial cell targets (molecules associated with monocytes, macrophages, and stem cells), b) endothelial cell targets (vascular endothelial growth factor [VEGF], integrins, CD13, and syndecan-4), and c) extracellular matrix proteins have been developed (95,109,110).
While anti-angiogenesis therapy has been found useful for treating cancer, it is now recognized that adverse microcirculatory effects (e.g. hypertension, organ dysfunction) of non-tumor related host organ tissues occurs after administration of these agents (110). Currently, studies have relied on clinical endpoints to identify and determine the functional importance of injury related to these agents. In animals, investigators have explored the use of isotopes or paramagnetic traces linked directly to VEGFRs and integrin αvβ3 to directly monitor progression of angiogenesis within CV tissues exposed to anti-angiogenic cancer therapy (95,109,110).
Direct Imaging of Chemotherapeutics
Directly imaging chemotherapeutic agents is also an area of active research. Thus far, however, results have been somewhat contradictory. For example, Behr, et al., observed reduced uptake of 111In-labeled trastuzumab in the myocardium of patients who developed heart failure and arrhythmia; whereas, Perik, et al., noticed no myocardial uptake in patients who developed severe symptomatic LV dysfunction during treatment with trastuzumab (111,112). Directly imaging effects of these agents may also depend on the timing of the image acquisition relative to the administration of the cancer treatment. For example, de Korte, et al., identified that myocardial HER2 over-expression was upregulated by cardiac stress induced by the anthracycline administration, but was not present in patients receiving non-anthracycline based regimens months after receiving their treatment (113).
Current Recommendations in CV Imaging
To date, few, if any, guideline statements exist regarding the implementation of noninvasive imaging techniques for the purpose of monitoring patients receiving treatment for cancer. There have, however, been several suggested management algorithms published over the last 10 years. To date, these algorithms have been accomplished for assessment of those receiving radiation, anthracyclines, or trastuzumab.
In regards to assessments of patients receiving radiation therapy, recently the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) published an expert consensus statement for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults (114). This document encompasses assessments of left ventricular function, pericardial diseases, and valvular heart disease. Also, in the case of breast cancer, the document addresses issues associated with irradiation of the right and left breast.
In regards to receipt of anthracycline-based chemotherapy, there are no evidenced-base guidelines for CV monitoring during therapy. The only established guidelines for CV monitoring in children during treatment were published in 1992, by Steinherz et al. This report of the Cardiology Committee of the Children's Cancer Study Group (CCSG), suggested an algorithm for the use of echocardiograms or nuclear medicine scans for assessing children scheduled to receive anthracycline-based chemotherapy (50). This particular statement, does not address newer methodologies available (biomarkers, advanced imaging with echocardiography or CMR) that may provide evidence of early cardiac or vascular injury prior to a decline in LVEF or fractional shortening. More recently, the Cardiology Committee of the Children's Cancer Study Group Lipsultz et al. (115) indicated that echocardiography with its widespread availability and when not suitable, cardiovascular magnetic resonance could be utilized to identify CV injury upon receipt of chemotherapy in children.
For adults, the European Society for Medical Oncology provided recommendations for assessment of adult patients scheduled to receive anthracycline-based chemotherapy (116). Similar to the recommendations produced in 1992 for children, the current suggestions for assessment of anthracyclines in adults remain primarily based on radioisotope, echocardiographic, or CMR assessments of LVEF. As with assessments in children, these recommendations do not incorporate assessments of subclinical CVD that may portend the future occurrence of advanced CV events. Moreover, these suggestions are developed to prevent the occurrence of marked deteriorations in LVEF. Following either of the children or adult recommendations for monitoring LVEF upon receipt of anthracycline-based chemotherapy does not guarantee the absence of any future CV events should individuals survive their cancer treatment.
Raschi, et al., published algorithms for monitoring LVEF upon receipt of trastuzumab or Herceptin (117). Unlike the recommendations pertaining to the administration of anthracyclines, the suggestions provided by Raschi do incorporate measurements of serum biomarkers such as troponin and B-type natriuretic peptide. In addition, suggestions for restarting trastuzumab in the setting of patients that recover their LVEF are provided. However, as with the suggestions to date regarding the administration of radiation therapy and anthracyclines to children or adults, these current recommendations for trastuzumab do not address the development of subclinical CV injury nor protection against the late occurrence of CV events in cancer survivors. Similar to the suggestions provided for radiation treatment and anthracyclines, the suggestions for assessments of patients receiving trastuzumab are directed primarily to prevent relatively large declines in LVEF.
Summary and Future Directions
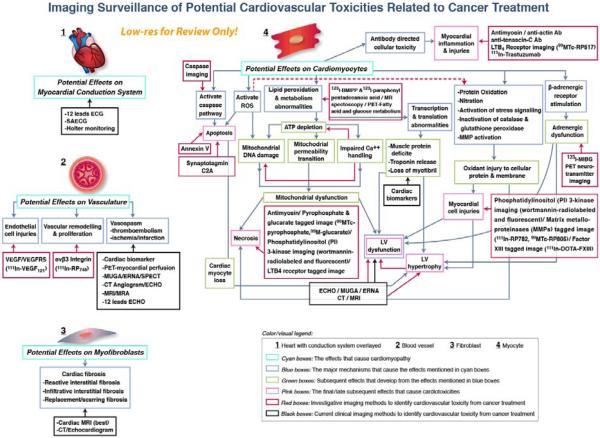
As shown in Figure 4, there are multiple potential sites and pathways that can be affected by the administration of chemotherapy, and multiple possibilities for clinical (radionuclide, transthoracic echocardiographic, and magnetic resonance) and research developmental (targeted molecular imaging) methods that may enable detection of the CV effects of cancer treatment.
Figure 4. Clinical & Developmental Imaging of Cardiovascular Injury after cancer treatment.
Opportunities for utilizing existing (black boxed) and developmental (red boxed) noninvasive imaging technologies for identifying processes associated with myocellular, myofibroblast, myocardial conduction and vascular injuries associated with the administration of cancer therapies that may adversely impact the cardiovascular system. As shown, existing technologies identify mainly clinically evident manifestations of CV injury while developmental technologies may facilitate assessment of biomolecular pathways that precede end organ damage.
What then should be current areas of focus for research in CV imaging as it pertains to the field of cardio oncology? First, to date, the majority of the management algorithms have focused on the primary use of noninvasive imaging to assess LVEF. While LVEF is important, it may not reflect the underlying advancements of subclinical CVD that could portend the development of CV events in patients actively treated for or those that survive cancer. Imaging research is necessary to understand the entirety of CV effects after treatment for cancer. This research needs to address therapies beyond the administration of anthracyclines, as shown in Table 1.
Table 1.
Anticancer Treatment, Mechanisms, Risk factors, Manifestations of Cardiotoxicity and Prevention
| Treatments/Drugs | Max limited dose | Therapeutic Indication | Mechanisms of Cardiotoxicity | Risk Factors | Manifestations of Cardiotoxicity | Prevention & Risk Reduction strategies |
|---|---|---|---|---|---|---|
| Anthracycline and analogues | ||||||
| Doxorubicin Daunorabicin Epirubicin Idarubicin |
450-500 mg/m2 400-550 mg/m2 800-900 mg/m2 60 mg/m2 |
Breast cancer Gastric tumor Leukemias Lymphomas Lung cancer Ovarian tumor Sarcomas |
↑Toxic oxygen free radical ↑Oxidative stress Lipid peroxidation of membrane Replacement fibrous tissue ↓Endogenous antioxidant enzyme Iron accumulations complex Cellular apoptosis, DNA damage |
-Concurrent Chemotherapy -Prior/Concurrent chest irradiation -High dose per cycle (>50mg/m2) -High cumulative dose (≥300mg/m2 of Doxorubicin, ≥600 mg/m2 of Epirubicin) -IV bolus -Extreme age (Elderly >65, children <18) -Woman, Pregnancy -Pre-existing CV disease (CAD,HT,LV dysfunction) -Undergoes for hematopoitic cell transplantation |
Acute (Initial to several week after Tx) -Arrhythmia (AF,SVT,VT,CHB,Mobiz II) -ST-T wave abnormalities -Anginal pain -LV dysfunction/CHF -Pericarditis/Myocarditis -Myocardial Ischemia/Infaction Chronic (early within 1 Yr and late delay beyond 1 Yr after Tx) -Asymptomatic diastolic/systolic dysfunction, overt HF - Dilated cardiomyopathy Stroke |
-Limiting lifetime cumulative dose -Structural modification (Liposome encapsulated molecule) & IV infusion schedule (>6 Hr) -CV risk assessment before start chemotherapy -Intensive monitoring cardiac function during & after Tx -F/U ECG if QRS in limb lead ↓≥30% associate with cardiomyopathy (Daunorubicin) -Adjunctive cardioprotective agent Dexrazoxane (Doxorubicin/Epirubicin) Beta blocker (Carvedilol) ACE-I |
| Anthraquinolones | ||||||
| Mitoxantrone | 140 mg/m2 | Breast cancer NHL, AML Multiple sclerosis |
↑Toxic oxygen free radical ↑Oxidative stress |
-Concurrent Chemotherapy -Prior/Concurrent chest irradiation -High cumulative dose |
Pericarditis-Myocarditis syndrome CHF, Arrhythmia Myocardial Ischemia/Infarction |
-Limiting lifetime cumulative dose -CV risk assessment before start chemotherapy -Intensive monitoring cardiac function during and after treatment |
| Treatments/Drugs | Therapeutic Indication | Mechanisms of Cardiotoxicity | Risk Factors | Manifestations of Cardiotoxicity | Prevention & Risk Reduction strategies |
|---|---|---|---|---|---|
| Non-Anthracycline Chemotherapy | |||||
| Antimetabolite Agents | |||||
| Fluorouracil (5-FU) Capecitabine |
Breast Cancer Colorectal cancer Pancreatic cancer |
Endothelial cytotoxicity Vascular vasospasm (coronary) Exaggerated sympathetic stimulation |
-Underlying CV disease -Infusion both in long term & short term -previously treatment with 5-FU [Capecitabine] -Concomitant chemotherapy |
Chest pain/Angina [5FU] Myocardial Ischemia/Infarction Myocarditis/Pericarditis CHF Stress-induced cardiomyopathy (Tako-tsubo) [5FU] |
-Termination of treatment (reversible), retreatment after symptom improve -IV bolus regimen, Lower dose regimen -Anti-anginal Treatment -Prophylactic coronary vasodilator (limit efficacy) |
| Fludarabine Pentostatin Cladribine Cytarabine Methotrexate (MTX) |
Leukemias Lymphomas Various solid tumors Autoimmune Disease |
Unknown | -Unknown | Pericarditis (Pericardial effusion/tamponade)[cytarabine] Arrhythmia (bradycardia, supra/ventricular arrhythmia) [MTX] Hypotension/Chest pain [Fludarabine] Myocardial Ischemia/Infarction CHF |
-Termination of treatment (reversible), retreatment after symptom improve -Steroid treatment in case of pericarditis from cytarabine -Anti-anginal Treatment -Prophylactic coronary vasodilator (limit efficacy) |
| Microtubule-Targeting Agents | |||||
| Vinca Alkaloids Vinblastine Vincristine Vinorelbine |
Leukemias Lymphomas Nephroblastoma TTP/chronic ITP Brest cancer SCLC |
Possible vasospasm | -Unknown | Hypertension Myocardial Ischemia/Infarction Vaso-occlusive complication Raynaud's phenomenon |
-Unknown |
| Taxane derivative Paclitaxel Docetaxel Eribulin Ixabepilone |
Breast cancer Ovarian cancer NSCLC Kaposi's sarcoma Prostate cancer Gastric Adrenocarcinoma |
Anaphylaxis and Hypersensitivity reaction | -Congenital long QT syndrome -Concomitants chemotherapy -Concomitants with drug that prolonged QTc -Underlying CV disease |
Conduction abnormalities (bradycardia, CHB) Arrhythmia (SVT), Angina Prolong QTc [Eribulin] Myocardial Ischemia/Infarction Ventricular dysfunction Hypotension |
-Pre-treatment with corticosteroid, H1 &2 blocker agents -Dose adjustment and limiting dose of anthracycline -ECG monitoring [Paclitaxel, Eribulin] -Continuous Infusion with Infusion monitoring for hypersensitivity reaction |
| Alkylating Agents | |||||
| Cyclophosphamide | Leukemias Lymphomas Various solid tumors |
Endothelial Capillary damage | -High dose (not relate to cumulative dose) -Elderly -Prior abnormal ejection fraction -Prior irradiation to chest wall -Prior anthracyclines |
Hemorrhagic myocarditis/pericarditis [common 1st week after cyclophosphamide] Pericardial effusion/tamponade LV dysfunction/CHF LVH |
-Corticosteroid & analgesic with termination of treatment -Dose adjustment |
| Ifosfamide | Lymphomas Various solid tumors |
Myocardial fiber fragmentation | -High dose regimen -Use for lymphoma |
Arrhythmias, ST-T wave change CHF |
-Dose adjustment -ECG monitoring |
| Busulfan | CML | Unknown | -Unknown | Endocardial Fibrosis/Pulmonary Fibrosis HT, arrhythmias Pericardial effusion |
|
| Cisplatin | Germ cell tumors Ovarian cancer Lymphomas Head/Neck tumors Lung cancer Sarcomas |
Anaphylaxis and Hypersensitivity reaction Vascular fibrosis E'lyte disturbance [hypokalemia,hypomagnesemia] |
-Elderly -Prior mediastinal irradiation -Use for metastatic testicular cancer -Use with cyclophosphamide |
Early manifestation -Myocardial Ischemia/Infarction -CHF Late manifestation -Myocardial Ischemia/Infarction -HTN, Stroke, LVH -Arrhythmia (SVT, bradycardia,LBBB) -LV dysfunction/CHF -Ischemic cardiomyopathy -Vascular toxicity - Raynaud's phenominon |
-Adequate hydration and Fluid balance - E'lyte check up and correction -Avoid concomitant renal toxic drug -Continuous Infusion with Infusion monitoring for hypersensitivity reaction |
| Anti-tumor Antibiotics | |||||
| Mitomycin C | Gastric cancer Pancreatic cancer Bladder cancer Lung cancer Lymphomas |
Unknown | -High cumulative dose >30 mg/m2 [mytomycin C] -Concomitant with anthracyclines or non-anthracyclines base chemotherapy -Prior coronary artery disease |
CHF Sub-sternal chest pain Pericarditis [Bleomycin] Myocardial Ischemia/Infarction [Bleomycin] |
-Dose adjustment and limiting dose of other chemotherapies -Supportive symptomatic with termination of treatment in case with pericarditis due to Bleomycin |
| Bleomycin | Germ cell tumors Squamous cell CA |
||||
| Monoclonal Antibodies | |||||
| Trastuzumab | Breast cancer Gastric cancer Esophageal cancer |
Direct blockage to HER2 effect to impair embryonic heart development & loss protective effect from cardiotoxin Anaphylaxis and Hypersensitivity reaction |
-Prior or concurrent anthracyclines >300 mg/m2 -Pre-existing poor LV function -Age > 50 Yrs -High BMI -Woman with underlying DM -Antihypertensive treatment |
Asymptomatic LV dysfunction Anaphylactic reaction CHF, non-ischemic cardiomyopathy Arrhythmias Myocardial Ischemia/Infarction |
-Dose adjustment and limiting dose of anthracycline -Termination of treatment (reversible), retreatment after symptom improve - Continuous Infusion with Infusion monitoring for hypersensitivity reaction -Intensive monitoring in case with high risk (baseline, 3, 6, 9 mo then 12, 18 mo after Treatment) -Trop I level can predict later cardiotoxicity and prognosis for Trastuzumab-related cardiotoxicity |
| Rituximab | Lymphomas Leukemias Autoimmune Ds |
Unknown Anaphylaxis and Hypersensitivity reaction |
-Pre-existing CV disease -High number of circulating malignant cells (≥25,000/mm3) with or without evidence of high tumor burden |
Anginal pain Arrhythmia (Ventricular fibrillation) Myocardial Ischemia/Infarction Cardiogenic shock |
-Termination of treatment (reversible) - Continuous Infusion with Infusion monitoring for hypersensitivity reaction |
| Bevacizumab | Colorectal cancer Glioblastoma Breast cancer NSCLC RCC |
Unknown Possible exacerbate of pre-existing coronary and peripheral vessels due to antibody of PIGF Infusion hypersensitivity reaction |
-Pre-existing CV disease -Prior or Concurrent anthracyclines -Age > 65 Yrs -Previous arterial thromboembolic event |
Anginal pain, dsypnea Myocardial Ischemia/Infarction Stroke , arterial thromboembolic event Severe Hypertension CHF |
-Monitoring BP during and after Treatment -Infusion monitoring for hypersensitivity reaction |
| Alemtuzumab | Lymphoma Leukemias Multiple Sclerosis GVHD |
Unknown Anaphylaxis and Hypersensitivity reaction |
-Unknown | Hypotension , cardiogenic shock Arrhythmia (AF, VT) CHF Myocardial Ischemia/Infarction |
-Termination of treatment (reversible) - Continuous Infusion with Infusion monitoring for hypersensitivity reaction -Premedication with corticosteroid and H1&2 blockage agents |
| Cetuximab | Colorectal cancer Head/Neck tumors |
Unknown Anaphylaxis and hypersensitivity reaction E'lyte imbalance [hypomagnesemia] |
-Pre-existing CV disease (previous CAD, HT , LV dysfunction or arrhythmia) | Arrhythmia, QTc prolongation CHF Myocardial Ischemia/Infarction Sudden cardiac arrest |
-Termination of treatment (reversible) -Continuous Infusion with Infusion monitoring for hypersensitivity reaction -Monitor and correction of serum E'lyte and magnesium -Dose modification |
| Topoisomerase Inhibitors | |||||
| Etoposide | Testicular cancer SCLC Hematologic cancer |
Coronary artery vasospasm Direct injury to the myocardial Induction of an immune response |
-Concurrent chemotherapy (esp. bleomycin, cisplatin, ifosfamide) -Pre-existing CV disease |
Myocardial Ischemia/Infarction | -Unknown |
| Biologic Response Modifiers | |||||
| Interferon-alpha | Leukemia Lymphomas Melanoma Various solid tumor |
Unknown Complex of interferon and cardiac tissue stimulating an autoimmune or inflammatory reaction (Unclear mechanism) |
-Pre-existing CV disease -History of CAD disease |
Early manifestation -Arrhythmias (supraventricular/ventricular) and heart block -Sudden Cardiac Death (Case report) -Hypertension -Acute pericarditis (effusion/tamponade)-case report in high dose related case Late manifestation -LV dysfunction/CHF -Dilated Cardiomyopathy |
-Termination of treatment (reversible) |
| Interleukin-2 | Melanoma RCC |
Capillary leakage syndrome with increase vascular permeability (high dose) Direct myocardial toxicity (Unclear mechanism) |
-Unknown |
Early manifestation -Hypotension ↓.SVR,↑HR)-peak at 4 hr after Tx -Arrhythmias (SVT/VT) -Myocarditis -Thrombotic events Late Manifestation -Dilated Cardiomyopathy |
-Termination of treatment (reversible) -Response well with fluid replacement Tx -Vasopressors as indicated |
| Denileukin difitox | Lymphomas Leukemias | Fatal capillary leakage syndrome Hypersensitivity reaction | -Pre-existing CV disease | -Dilated Cardiomyopathy Hypotension, tachycardia Chest pain Myocardial Ischemia/Infarction Arrhythmias CHF |
-Termination of treatment (reversible) -Continuous Infusion with Infusion monitoring for hypersensitivity reaction |
| Differentiation Agents | |||||
| All-trans retinoic acid (AYRA) Arsenic trioxide (ATO) |
Leukemias [AML esp. in acute promyeloblastic leukemia] | Direct myocyte injury and damage ↑oxidative stress and DNA fragmentation ↑Apoptosis of myocardial cells Capillary leakage syndrome |
-WBC ≥ 10,000/cu.mm -Rapidly increasing WBC count -Presence of CD 13 expression on leukemic cells -Hypokalemia, Hypomagnesemia [ATO] |
Differentiation syndrome [Both] -Hypotension -CHF/Pulmonary edema -Pericardial effusion/tamponade -Myocardial Ischemia/Infarction QTc Prolongation/Torsades de points/SCD [ATO] |
-Prompt IV corticosteroid -Continue chemotherapy (not recommend of termination, termination only in case severe differentiation syndrome) -Generalize supportive care (oxygen, diuretic) -Monitoring EKG and correction of serum E'lyte and magnesium [ATO] -Resveratrol Treatment [ATO-case report] |
| Multitargeted kinase inhibitors | |||||
| Tyrosine kinase inhibitors | |||||
| Sorafenib Sunitinib |
RCC/HCC/GIST Pancreatic neuro-endocrine tumors Melanoma |
Inhibition of ribosomal S6 kinase (RSK) Inhibition of RAF1 kinase activity Causes trigger and activation targeting apoptotic pathway result in ↑fapoptosis, myocyte loss and ATP depletion lead to left ventricular (LV) dysfunction. |
-History of HT and CAD | Arrhythmias Prolong of QTc/ST-T changes LV dysfunction/CHF ACS-Myocardial Ischemia/Infarction HT |
-Termination of treatment (reversible) -Monitoring EKG and LV function -Cardiac Troponin and BNP show yield in early detection for cardiotoxicity |
| Regorafenib | Colorectal cancers | Unknown | -Pre-existing CV disease | Myocardial Ischemia/Infarction (rare) | -Unknown |
| Vandetanib | Medullary thyroid cancer Breast/Lung cancers CML/CEL/ALL Mesothelioma |
Unknown | -Congenital long QT syndrome -E'lyte imbalance, hypocalcemia and hypomagnesemia -Concurrent drugs that prolong QTc interval |
Prolong QTc/Torsades de points/SCD LV dysfunction/CHF HT |
-Termination of treatment (reversible) -Monitoring EKG and QTc interval prior and during treatment -Correction of serum E'lye, Ca and Mg before treatment -Avoiding for concurrent drugs which causes of prolong QTc or strong CYP3A4 Inducers |
| Imatinib Nilotinib Dasatinib Bosutinib |
GIST CML |
Significant mitochondrial dysfunction Declines in ATP concentration ABL and/or ARG inhibition result in Unrecognized function of cardiomyocytes |
-Pre-existing CV disease -Congenital long QT syndrome -E'lyte imbalance, hypocalcemia and hypomagnesemia -Concurrent drugs that prolong QTc interval |
Prolong QTc Systolic and Diastolic dysfunction Chest pain, HT Pericardial effusion Fatal myocardial ischemia/infarction LV dysfunction/CHF |
-Termination of treatment (reversible) -Monitoring EKG and LV function -Correction of serum E'lye and Mg before treatment -Avoiding for concurrent drugs which causes of prolong QTc or strong CYP3A4 Inhibitors |
| Lapatinib | Breast cancer Brain cancer |
Unknown (Rare cardiotoxicity, usually occur when combine with other cardiotoxic agents) | -Concurrent systemic cardiotoxic agents | Prinzmetal' angina LV dysfunction/CHF |
-Termination of treatment (reversible) -Dose adjustment and limiting dose of other chemotherapies |
| Crizotinib Vemurafenib |
NSCLC Melanoma |
Unknown | -Congenital long QT syndrome -E'lyte imbalance and hypomagnesemia -Concurrent drugs that prolong QTc interval |
Severe sinus bradycardia (≤45 bpm) [Crizotinib] Arrhythmia, QTc prolongation, Torsades de points CHF, hypotension, syncope [Crizotinib] |
-Termination of treatment (reversible) -QTc > 500ms during treatment advice for termination of Tx -Monitoring EKG & correction of E'lyte and Mg before Tx -Avoiding for concurrent drugs which causes of prolong QTc or strong CYP3A4 Inhibitors |
| Miscellaneous Agents | |||||
| Diethylstilbestrol Estramustine |
Breast cancer Prostate cancer |
Unknown Estrogen related CV side effect |
Pre-existing CV disease | Arterial thrombosis - Myocardial Ischemia/Infarct/stroke Venous thrombosis - DVT,PE HT ↑CV death for overall |
-Use with precaution in patient with CV risk factors -Diethylstilbestrol : now no longer available in US due to teratogenic effect. |
| Proteasome inhibitors -Bortezomib -Carfilzomib |
Multiple myeloma Mantel cell lymphoma |
Decreased ATP synthesis Decreased cardiac myocyte contractility Ubiquinated protein accumulation Proteasomal dysfunction |
Pre-existing CV disease Concurrent systemic cardiotoxic chemotherapies Concomitant with drug associate hypotension |
Orthostatic hypotension, Syncope New onset/Worsening pre-existing HF with ↓ LV function Arrhythmia (Bradycardia, heart block, AF) Myocardial Ischemia/Infarction Pulmonary arterial hypertension |
-Termination of treatment (reversible) -Dose modification based upon cardiac toxicity -Caution in patient with history of syncope -Avoid of dehydration |
| Histone deacetylase inhibitors [HDAC-I] -Vorinostat -Romidepsin |
T-Cell Lymphoma NSCLC |
Unknown | -Pre-existing CV disease -Congenital long QT syndrome -Concurrent drugs that prolong QTc interval or inhibit CYP3A4 -E'lyte disturbance or hypomagnesemia |
Cardiac arrest Prolongation of QTc, ST-T changes [transients] Arrhytmias [supraventricular/ventricular] Hypotension, Syncope DVT |
-No routine EKG monitoring recommendation only in high risk -Monitor and correction for e'lyte and magnesium before start medication -Avoiding for concurrent drugs which causes of prolong QTc or inhibit CYP3A4 |
| Radiation therapies | |||||
| Radiation Therapies [RT] | Breast cancer Lymphomas Malignant involve thoracic regions |
Damage to blood vessels due to -↑ROS that disrupt DNA strands -2nd Inflammatory changes lead to fibrosis Direct damage into involving area of irradiation -Pericardium -Myocardium -Endocardium (valvular) -Coronary arteries & other capillaries -Conduction systems |
-Total radiation dose to the heart > 30Gy -Dose per fraction (Daily dose fraction > 2Gy/d) -Volume of irradiated heart -Specific RT technique (Orthovoltage radiation) -Absence of subcarinal blocking -Field of RT -Concomitant cardiotoxic systemic agents -Younger age at time of treatment (<18 Yrs) -Pre-existing risk factors for CAD -Associate mediastinal irradiation [HF,Valve Ds] |
Chest pain, Angina pectoris Myocardial Ischemia/Infarction - Typical of atherosclerosis Valvular disease - Fibrotic changes with/without calcification in the cusp and/or valvular leaflets [TR,AR and MR] Diastolic dysfunction - Myocardial fibrosis Cardiomyopathy - Myocardial cell death & fibrosis [Restrictive] LV dysfunction/CHF Arrhythmias - Fibrotic cells in the conduction systems [bradycardia, complete and lesser degrees heart block and SSS] Pericarditis - common acute [rare - constrictive pericarditis] |
-↓Dose & Volume of heart in RT field -Omitting RT in early stage disease -Minimum necessary total dose of others concurrent systemic chemotherapy esp. anthracyclines -Screening and reduce for modification able CV risk factors -Radionuclide myocardial perfusion imaging for early assessment of RT-induced cardiac change [largest prospective trial with SPECT-gated scans F/U in 6 yrs] or CT coronary CA score if irradiation exposure to coronary arteries ≥ 35 Gy beginning at 5 years after RT or after age 30-35 years -Concomitant with anthracyclines > 300 mg/m2 should be screening cardiac function and valve status with noninvasive screening by nuclear imaging and/or echocardiography |
| Modified from Monsuez, et al. (26), Curigliano, et al. (22), Bovelli, et al. (21), Pai, et al. (27), Senkus, et al. (28), Floyd, et al. (23,24), and Marks, et al. (25). | |||||
Abbreviations: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), angiotensin-converting-enzyme inhibitor (ACE-1), atrial fibrillation (AF), coronary artery disease (CAD), chronic eosinophilic leukemia (CEL), congestive heart failure (CHF), complete heart block (CHB), chronic myelogenous leukemia (CML), computed tomography (CT), cardiovascular (CV), cytochrome p450 3a4 (CYP3A4), deep vein thrombosis (DVT), electrocardiogram (ECG, EKG), gastrointestinal stroma tumor (GIST), graft versus host disease (GVHD), heart failure (HF), heart rate (HR), hormone therapy (HT), hypertension (HTN), idiopathic thrombocytopenic purpura (ITP), left bundle branch block (LBBB), left ventricular (LV), left ventricular hypertrophy (LVH), non Hodgkin's lymphoma (NHL), non small cell lung carcinoma (NCLC), pulmonary embolism (PE), renal cell carcinoma (RCC), reactive oxygen species (ROS), radiation therapy (RT), sudden cardiac death (SCD), small cell lung carcinoma (SCLC), single photon emission computerized tomography (SPECT), sick sinus syndrome (SSS), supraventricular tachycardia (SVT), stroke volume ratio (SVR), thrombotic thrombocytopenic purpura (TTP), ventricular tachycardia (VT), white blood cells (WBC)
Second, the array of current and future imaging metrics (Figure 4) need to be evaluated in terms of predicting future CV events in patients treated for cancer. These imaging related out care measures need to be evaluated in the context of existing risk factor prediction models for forecasting risk. Are these imaging markers beneficial and reliable? In which patients receiving specific subsets of cancer therapies should the imaging markers be applied? In addition, when should they be utilized and what are the economic consequences of their use?
Third, does CV imaging have a role in guiding therapy to prevent CV injury after treatment for cancer? If so, how and when should this imaging be implemented, to what extent, and by whom? To date, CV imaging studies are interpreted primarily by MD or DO physicians with advanced imaging training. Can surveillance imaging programs be designed that utilize this high physician level of expertise in a supervisory rather than a direct interpretive role?
In summary, new advancements related to the treatment of cancer have improved cancer related survival, but, in many cases, have also increased the risk of CV injury and CV events. To date, noninvasive imaging has been used to assess LVEF prior to initial of cancer treatment and in those who develop symptoms after treatment commences. Ongoing and future investigations will help determine the suitability of noninvasive imaging modalities to identify those at risk of developing CV injury upon receipt of cancer treatment and whether noninvasive imaging can be utilized to guide the administration of additional protective therapies to prevent CV injury and events after treatment for cancer. Preventing CV events in patients treated for cancer provides an opportunity to improve overall cancer survivorship and quality of life.
Supplementary Material
Acknowledgments
Disclosures: Research supported in part by National Institute of Health grants R33 CA121296, BCTR0707769, R01 CA167821, R01 HL118740, R01 HL076438, and P30 CA12197
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med. 2006;57:485–98. doi: 10.1146/annurev.med.57.121304.131240. [DOI] [PubMed] [Google Scholar]
- 4.Mewton N, Liu CY, Croisille P, et al. Assessment of myocardioal fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie HS, McGann CJ, Wilson BD. Noninvasive diagnosis of chemotherapy related cardiotoxicity. Curr Cardiol Rev. 2011;7:234–44. doi: 10.2174/157340311799960672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Maitland ML. Sunitinib, hypertension, and heart failure: a model for kinase inhibitor-mediated cardiotoxicity. Curr Hypertens Rep. 2011;13:430–5. doi: 10.1007/s11906-011-0229-4. [DOI] [PubMed] [Google Scholar]
- 9.Pantaleo MA, Mandrioli A, Saponara M, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer. 2012;12:231. doi: 10.1186/1471-2407-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–97. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 12.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252–60. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer MS, Gluck S. A woman's heart: The impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009;115:1813–26. doi: 10.1002/cncr.24219. [DOI] [PubMed] [Google Scholar]
- 14.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 15.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: Results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–56. doi: 10.1200/JCO.2010.29.1567. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy: Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–18. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 18.de Geus-Oei LF, Mavinkurve-Groothuis AM, Bellersen L, et al. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J Nucl Med. 2011;52:560–71. doi: 10.2967/jnumed.110.082784. [DOI] [PubMed] [Google Scholar]
- 19.McKillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J. 1983;106:1048–56. doi: 10.1016/0002-8703(83)90651-8. [DOI] [PubMed] [Google Scholar]
- 20.Hesse B, Lindhardt TB, Acampa W, et al. EANM/ESC guidelines for radionuclide imaging of cardiac function. Eur J Nucl Med Mol Imaging. 2008;35:851–85. doi: 10.1007/s00259-007-0694-9. [DOI] [PubMed] [Google Scholar]
- 21.Cottin Y, Touzery C, Coudert B, et al. Impairment of diastolic function during short-term anthracycline chemotherapy. Br Heart J. 1995;73:61–4. doi: 10.1136/hrt.73.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochet A, Quilichini G, Dygai-Cochet I, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat. 2011;130:845–54. doi: 10.1007/s10549-011-1714-9. [DOI] [PubMed] [Google Scholar]
- 23.Steinherz LJ, Graham T, Hurwitz R, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: Report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89:942–9. [PubMed] [Google Scholar]
- 24.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation. 2003;108:1404–18. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 25.Merlet P, Valette H, Dubois-Rande JL, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med. 1992;33:471–7. [PubMed] [Google Scholar]
- 26.Nakata T, Miyamoto K, Doi A, et al. Cardiac death prediction and impaired cardiac sympathetic innervation assessed by MIBG in patients with failing and nonfailing hearts. J Nucl Cardiol. 1998;5:579–90. doi: 10.1016/s1071-3581(98)90112-x. [DOI] [PubMed] [Google Scholar]
- 27.Valdes Olmos RA, ten Bokkel Huinink WW, ten Hoeve RF, et al. Assessment of anthracycline-related myocardial adrenergic derangement by [123I] metaiodobenzylguanidine scintigraphy. Eur J Cancer. 1995;31A:26–31. doi: 10.1016/0959-8049(94)00357-b. [DOI] [PubMed] [Google Scholar]
- 28.Carrio I, Estorch M, Berna L, Lopez-Pousa J, Tabernero J, Torres G. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med. 1995;36:2044–9. [PubMed] [Google Scholar]
- 29.Wakasugi S, Fischman AJ, Babich JW, et al. Metaiodobenzylguanidine: Evaluation of its potential as a tracer for monitoring doxorubicin cardiomyopathy. J Nucl Med. 1993;34:1283–6. [PubMed] [Google Scholar]
- 30.Jeon TJ, Lee JD, Ha JW, Yang WI, Cho SH. Evaluation of cardiac adrenergic neuronal damage in rats with doxorubicin-induced cardiomyopathy using iodine-131 MIBG autoradiography and PGP 9.5 immunohistochemistry. Eur J Nucl Med. 2000;27:686–93. doi: 10.1007/s002590050563. [DOI] [PubMed] [Google Scholar]
- 31.Nousiainen T, Vanninen E, Jantunen E, Remes J, Kuikka J, Hartikainen J. Anthracycline-induced cardiomyopathy: long-term effects on myocardial cell integrity, cardiac adrenergic innervation and fatty acid uptake. Clin Physiol. 2001;21:123–8. doi: 10.1046/j.1365-2281.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003;16:1091–110. doi: 10.1016/S0894-7317(03)00685-0. [DOI] [PubMed] [Google Scholar]
- 33.Corapcioglu F, Sarper N, Berk F, Sahin T, Zengin E, Demir H. Evaluation of anthracycline-induced early left ventricular dysfunction in children with cancer: A comparative study with echocardiography and multigated radionuclide angiography. Pediatr Hematol Oncol. 2006;23:71–80. doi: 10.1080/08880010500313603. [DOI] [PubMed] [Google Scholar]
- 34.van Royen N, Jaffe CC, Krumholz HM, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–50. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 35.Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: Comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol. 2010;28:3429–36. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 36.Oreto L, Todaro MC, Umland MM, et al. Use of echocardiography to evaluate the cardiac effects of therapies used in cancer treatment: What do we know? J Am Soc Echocardiogr. 2012;25:1141–52. doi: 10.1016/j.echo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–80. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ammar KA, Paterick TE, Khandheria BK, et al. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography. 2012;29:861–72. doi: 10.1111/j.1540-8175.2012.01712.x. [DOI] [PubMed] [Google Scholar]
- 39.Biswas M, Sudhakar S, Nanda NC, et al. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography. 2013;30:88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 40.Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: A systematic review and meta-analysis. J Am Coll Cardiol. 2012;59:1799–808. doi: 10.1016/j.jacc.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: A comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 43.Maffessanti F, Nesser HJ, Weinert L, et al. Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol. 2009;104:1755–62. doi: 10.1016/j.amjcard.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 44.Urbano-Moral JA, Patel AR, Maron MS, Arias-Godinez JA, Pandian NG. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography. 2012;29:997–1010. doi: 10.1111/j.1540-8175.2012.01773.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheung YF, Li SN, Chan GC, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography. 2011;28:738–45. doi: 10.1111/j.1540-8175.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 46.Erven K, Florian A, Slagmolen P, et al. Subclinical Cardiotoxicity Detected by Strain Rate Imaging up to 14 months After Breast Radiation Therapy. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Ho E, Brown A, Barrett P, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart. 2010;96:701–7. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 48.Motoki H, Koyama J, Nakazawa H, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 49.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Stoodley PW, Richards DA, Hui R, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12:945–52. doi: 10.1093/ejechocard/jer187. [DOI] [PubMed] [Google Scholar]
- 51.Galderisi M, Marra F, Esposito R, Lomoriello VS, Pardo M, de Divitiis O. Cancer therapy and cardiotoxicity: The need of serial Doppler echocardiography. Cardiovasc Ultrasound. 2007;5:4. doi: 10.1186/1476-7120-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raikhelkar JK, Steingart RM, Chen CL. Role of echocardiography in cancer care. Herz. 2011;36:333–9. doi: 10.1007/s00059-011-3443-6. [DOI] [PubMed] [Google Scholar]
- 53.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, and branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Martin M, Esteva FJ, Alba E, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: Review and expert recommendations. Oncologist. 2009;14:1–11. doi: 10.1634/theoncologist.2008-0137. [DOI] [PubMed] [Google Scholar]
- 55.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 56.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 57.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 58.Stoddard MF, Seeger J, Liddell NE, Hadley TJ, Sullivan DM, Kupersmith J. Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J Am Coll Cardiol. 1992;20:62–9. doi: 10.1016/0735-1097(92)90138-d. [DOI] [PubMed] [Google Scholar]
- 59.Tassan-Mangina S, Codorean D, Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: Early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7:141–6. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Marchandise B, Schroeder E, Bosly A, et al. Early detection of doxorubicin cardiotoxicity: interest of Doppler echocardiographic analysis of left ventricular filling dynamics. Am Heart J. 1989;118:92–8. doi: 10.1016/0002-8703(89)90077-x. [DOI] [PubMed] [Google Scholar]
- 61.Lange SA, Ebner B, Wess A, et al. Echocardiography signs of early cardiac impairment in patients with breast cancer and trastuzumab therapy. Clin Res Cardiol. 2012;101:415–26. doi: 10.1007/s00392-011-0406-0. [DOI] [PubMed] [Google Scholar]
- 62.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Negishi K, Negishi T, Hare JL, et al. Independent and incremental value of deformation indices for prediciton of trastuzumab-induced cardiotoxicity. J Am So Echocardiogr. 2013;26:493–98. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Thavendirananthan P, Poulin F, Lim K, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy-A Systematic Review. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.01.073. doi: 10.1016/j.jacc.2014.01.073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Fontana A, Zambon A, Cesana F, Giannattasio C, Trocino G. Tissue Doppler, triplane echocardiography, and speckle tracking echocardiography: Different ways of measuring longitudinal myocardial velocity and deformation parameters. A comparative clinical study. Echocardiography. 2012;29:428–37. doi: 10.1111/j.1540-8175.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 66.Mulvaqh SL, Rakowski H, Vannan MA, et al. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. 2008;11:1179–201. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Little WC, Freeman GL. Pericardial Disease. Circulation. 2006;113:1622–1632. doi: 10.1161/CIRCULATIONAHA.105.561514. [DOI] [PubMed] [Google Scholar]
- 68.Rademaker J, Schoder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin's lymphoma: Coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol. 2008;191:32–7. doi: 10.2214/AJR.07.3112. [DOI] [PubMed] [Google Scholar]
- 69.Kupeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol. 2010;28:1025–30. doi: 10.1200/JCO.2009.25.2627. [DOI] [PubMed] [Google Scholar]
- 70.Apter S, Shemesh J, Raanani P, et al. Cardiovascular calcifications after radiation therapy for Hodgkin lymphoma: computed tomography detection and clinical correlation. Coron Artery Dis. 2006;17:145–51. doi: 10.1097/00019501-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Leeuwen-Segarceanu EM, Bos WJ, Dorresteijn LD, et al. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat Rev. 2011;37:391–403. doi: 10.1016/j.ctrv.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Andersen R, Wethal T, Gunther A, et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin's lymphoma. Am J Cardiol. 2010;105(2):149–52. doi: 10.1016/j.amjcard.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Asferg C, Usinger L, Kristensen TS, Abdulla J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: a systematic review and meta-analysis. Eur J Radiol. 2012;81:e757–62. doi: 10.1016/j.ejrad.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–62. doi: 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: An overview of current uses and future directions of cardiovascular magnetic resonance. JCMR. 2013;15:66. doi: 10.1186/1532-429X-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–86. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 80.Drafts BC, Twomley KM, D'Agostino R, Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasice imaging evidence of subclinical cardiovascular disease CME. J Am Coll Cardiol Img. 2013;6(8):877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–88. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oberholzer K, Kunz RP, Dittrich M, Thelen M. Anthracycline-induced cardiotoxicity: Cardiac MRI after treatment for childhood cancer. Rofo. 2004;176:1245–50. doi: 10.1055/s-2004-813416. [DOI] [PubMed] [Google Scholar]
- 83.Bonner F, Neizel M, Gruenig S, Jacoby C, Kelm M, Sievers B. T2 mapping in different cardiomyopathies: First clinical experience. Journal of Cardiovascular Magnetic Resonance. 2013;15:P53. [Google Scholar]
- 84.Lightfoot JC, D'Agostino RB, Jr., Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–8. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141:1007–13. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 86.Tham E, Chow K, Spavor M, Pagano J, Haykowsky M, Thompson R. Degree of diffuse fibrosis measured by MRI correlates with LV remodelling in childhood cancer survivors after anthracycline chemotherapy. Journal of Cardiovascular Magnetic Resonance. 2011;13:P276. [Google Scholar]
- 87.van der Meer RW, Doornbos J, Kozerke S, et al. Metabolic imaging of myocardial triglyceride content: reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology. 2007;245:251–7. doi: 10.1148/radiol.2451061904. [DOI] [PubMed] [Google Scholar]
- 88.Dobrucki LW, Sinusas AJ. Cardiovascular molecular imaging. Semin Nucl Med. 2005;35:73–81. doi: 10.1053/j.semnuclmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Dekker T, van Echteld CJ, Kirkels JH, et al. Chronic cardiotoxicity of Adriamycin studied in a rat model by 31P NMR. NMR Biomed. 1991:416–24. doi: 10.1002/nbm.1940040104. [DOI] [PubMed] [Google Scholar]
- 90.Bittner V, Reeves RC, Digerness SB, Caulfield JBm Pohost GM. 31P NMR spectroscopy in chronic Adriamycin cardiotoxicity. Magn Reson Med. 1991;17:69–81. doi: 10.1002/mrm.1910170112. [DOI] [PubMed] [Google Scholar]
- 91.Chaosuwannakit N, D'Agostino R, Hamilton CA, Lane KS, Ntim WO, Lawrence J, Melin SA, Ellis LR, Torti FM, Little WC, Hundley WG. Aortic stiffness increases upon receipt of anthracyclin chemotherapy. J Clin Oncol. 2010;28:166–72. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenei Z, Bardi E, Magyar MT, Horvath A, Paragh G, Kiss C. Anthracycline Causes Impaired Vascular Endothelial Function and Aortic Stiffness in Long Term Survivors of Childhood Cancer. Pathol Oncol Res. 2012 doi: 10.1007/s12253-012-9589-6. [DOI] [PubMed] [Google Scholar]
- 93.Wiginton CD, Kelly B, Oto A, et al. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue. AJR Am J Roentgenol. 2008;190:1060–8. doi: 10.2214/AJR.07.2822. [DOI] [PubMed] [Google Scholar]
- 94.Korngold EC, Jaffer FA, Weissleder R, Sosnovik DE. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev. 2008;13:163–73. doi: 10.1007/s10741-007-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrison AR, Sinusas AJ. New molecular imaging targets to characterize myocardial biology. Cardiol Clin. 2009;27:329–44. doi: 10.1016/j.ccl.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bennink RJ, van den Hoff MJ, van Hemert FJ, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45:842–8. [PubMed] [Google Scholar]
- 97.Gabrielson KL, Mok GS, Nimmagadda S, et al. Detection of dose response in chronic doxorubicin-mediated cell death with cardiac technetium 99m annexin V single-photon emission computed tomography. Mol Imaging. 2008;7:132–8. [PMC free article] [PubMed] [Google Scholar]
- 98.Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol. 2007;49:2457–64. doi: 10.1016/j.jacc.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 99.Sosnovik DE, Schellenberger EA, Nahrendorf M, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54:718–24. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 100.Ueno M, Kakinuma Y, Yuhki K, et al. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101:151–8. doi: 10.1254/jphs.fp0050980. [DOI] [PubMed] [Google Scholar]
- 101.Zhou D, Chu W, Rothfuss J, et al. Synthesis, radiolabeling, and in vivo evaluation of an 18F-labeled isatin analog for imaging caspase-3 activation in apoptosis. Bioorg Med Chem Lett. 2006;16:5041–46. doi: 10.1016/j.bmcl.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 102.Maini CL, Sciuto R, Ferraironi A, et al. Clinical relevance of radionuclide angiography and antimyosin immunoscintigraphy for risk assessment in epirubicin cardiotoxicity. J Nucl Cardiol. 1997;4:502–8. doi: 10.1016/s1071-3581(97)90008-8. [DOI] [PubMed] [Google Scholar]
- 103.Valdes Olmos RA, Carrio I, Hoefnagel CA, et al. High sensitivity of radiolabelled antimyosin scintigraphy in assessing anthracycline related early myocyte damage preceding cardiac dysfunction. Nucl Med Commun. 2002;23:871–7. doi: 10.1097/00006231-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 104.Saito K, Takeda K, Imanaka-Yoshida K, Imai H, Sekine T, Kamikura Y. Assessment of fatty acid metabolism in taxan-induced myocardial damage with iodine-123 BMIPP SPECT: Comparative study with myocardial perfusion, left ventricular function, and histopathological findings. Ann Nucl Med. 2003;17:481–8. doi: 10.1007/BF03006439. [DOI] [PubMed] [Google Scholar]
- 105.Saito K, Takeda K, Okamoto S, et al. Detection of doxorubicin cardiotoxicity by using iodine-123 BMIPP early dynamic SPECT: Quantitative evaluation of early abnormality of fatty acid metabolism with the Rutland method. J Nucl Cardiol. 2000;7:553–61. doi: 10.1067/mnc.2000.108351. [DOI] [PubMed] [Google Scholar]
- 106.Carboni GP. A novel clinical indicator using cardiac technetium-99m sestamibi kinetics for evaluating cardiotoxicity in cancer patients treated with multiagent chemotherapy. Am J Cardiovasc Dis. 2012;2:293–300. [PMC free article] [PubMed] [Google Scholar]
- 107.Borde C, Kand P, Basu S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J Radiol. 2012;4:220–3. doi: 10.4329/wjr.v4.i5.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toubert ME, Vercellino L, Faugeron I, Lussato D, Hindie E, Bousquet G. Fatal heart failure after a 26-month combination of tyrosine kinase inhibitors in a papillary thyroid cancer. Thyroid. 2011;21:451–4. doi: 10.1089/thy.2010.0270. [DOI] [PubMed] [Google Scholar]
- 109.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–28S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 110.Stacy MR, Maxfield MW, Sinusas AJ. Targeted molecular imaging of angiogenesis in PET and SPECT: A review. Yale J Biol Med. 2012;85:75–86. [PMC free article] [PubMed] [Google Scholar]
- 111.Behr TM, Behe M, Wormann B. Trastuzumab and breast cancer. N Engl J Med. 2001;345:995–6. doi: 10.1056/NEJM200109273451312. [DOI] [PubMed] [Google Scholar]
- 112.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24:2276–82. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 113.de Korte MA, de Vries EG, Lub-de Hooge MN, et al. 111-Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: A clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur J Cancer. 2007;43:2046–51. doi: 10.1016/j.ejca.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 114.Lancellotti P, Nkomo VT, Badano LP, et al. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 115.Lipshultz SE, Adams MJ, Colan SD. Long-term cardiovascular toxicity in children, adolescents, and young adults who reveice cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement for the American Heart Association. Circulation. Oct 22. 2013;128(17):1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 116.Barrett-Lee PJ, Dixon JM, Farrell C, et al. Expert opinion of the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol. 2009;20(5):816–827. doi: 10.1093/annonc/mdn728. [DOI] [PubMed] [Google Scholar]
- 117.Raschi E, De Ponti F. Cardiovascular toxicity of anticancer-targeted therapy: Emerging issues in the era of cardio-oncology. Intern Emerg Med. 2012;7:113–31. doi: 10.1007/s11739-011-0744-y. [DOI] [PubMed] [Google Scholar]
- 118.Chaosuwannakit N, D'Agostino R, Jr., Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–72. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.