Abstract
Epithelial (E)-cadherin is a homophilic adhesion molecule which is responsible for maintenance of baso-lateral cell adhesion and polarity. E-cadherin can be lost from the cell surface by proteolytic cleavage, resulting in the generation of an 80kDa fragment referred to a soluble E-cadherin (sE-cad). Although originally discovered in the conditioned media of breast cancer cells and later verified in the fluids of cancer patients, today sE-cad has been reported in patients with viral and bacterial infections, organ failure, and benign disease. The proteases implicated in this cleavage event include members of the disintegrin family (ADAM10 and 15), bacterial proteases (gingipains and BFT), cathepsins (B, L, S), matrix metalloproteases (MMP-2, 3, 7, 9, and 14), Kallikrein-7 (KLK7), and plasmin. Stimulus that induces sE-cad generation by ADAMs, MMPs, KLK7, and plasmin in vitro ranges from serum withdrawal to pro-inflammatory cytokines to growth factors. The cellular or physiologic consequences of sE-cad accumulation include the disruption of adherens junctions, cellular migration and invasion, induction of MMPs, as well as cell signaling, suggesting that sE-cad may contribute to disease progression.
Keywords: Soluble E-cadherin, ADAM, MMP, cancer, disease, biomarker
2. INTRODUCTION
2.1. Epithelial cadherin
E-cadherin is a homophilic, calcium-dependent, adhesion protein, which is expressed at adherens junctions between epithelial cells. E-cadherin has an extracellular region comprised of 5 domain repeats, each domain containing a set of seven beta-sheets arranged in an immunoglobulin fold (1). Adhesion is achieved by lateral dimerization between E-cadherin molecules on the same cell, creating a homodimer which can then interact with an adjacent E-cadherin homodimer on a neighboring cell via cadherin repeat 1 (EC1) (2).
Although E-cadherin is predominantly a homophilic adhesion molecule, it also exhibits heterophilic interactions. Of particular interest, E-cadherin can associate with CD103 and Killer cell lectin receptor G1 (KLRG1) (3–5). The E-cadherin receptor, CD103 or alphaEbeta7, is located on T lymphocytes and helps to target T cells to epithelial cells, where it binds extra cellular domains EC1 and EC2 of E-cadherin (3). Another receptor for E-cadherin, KLRG1, is expressed on T cells and natural killer (NK) cells, where the binding of KLRG1 to E-cadherin-expressing cells prevents the lysis of epithelial targets (5). The interaction between KLRG1 and E-cadherin is mediated by the homodimeric interface on EC1, suggesting that monomeric E-cadherin at epithelial cell surfaces is responsible for controlling the activation threshold of NK and T cells and thereby suppressing immune response (6). E-cadherin, therefore, can serve as an adhesion molecule or a targeting molecule, depending on binding partner.
Within the cell, E-cadherin interactions support adhesion established through homodimerization. The cytoplasmic tail of E-cadherin associates directly with beta-catenin, p120, and indirectly with alpha-catenin. Beta-catenin binding to E-cadherin provides structural support and aids in transport of E-cadherin to the baso-lateral plasma membrane (7). The formation of this ordered structure allows for binding of alpha-catenin, which can bind actin, stabilizing and coordinating actin dynamics at the adherens junction (8, 9). p120 binding to E-cadherin also stabilizes the complex and maintains high E-cadherin levels (10). From the cytoplasmic side, regulation of E-cadherin adhesion can occur by altering the composition of the cadherin-catenin complex, the presence growth factors, tyrosine phosphorylation of the cadherin-catenin complex, p120 binding to E-cadherin, and the activity of small GTPases and proteins which aid in cell polarity determination (11). Additionally, E-cadherin adhesion can be disrupted by E-cadherin cleavage in the extracellular or cytosolic domains.
2.2. E-cadherin cleavage
The disruption of E-cadherin function by proteolytic cleavage can occur in the extracellular domain or cytosolic tail of E-cadherin. The initial observation of E-cadherin cleavage was reported by Wheelock et al who observed an 80 kDa soluble E-cadherin in the conditioned media of MCF-7 breast cancer cells (12). This fragment, containing the five extracellular domains of E-cadherin and referred to as sE-cad, could disrupt adherens junctions of mouse mammary tumor cells (12). To date, members of the A Disintegrin and Metalloprotease (ADAM) family (ADAM10 and 15), bacterial proteases (gingipains and BFT/fragilysin), cathepsins (B, L, S), matrix metalloprotease (MMP) family (MMP-2, 3, 7, 9, and 14), KLK7, and plasmin have all been implicated in the generation of sE-cad (13–31).
The extracellular cleavage of E-cadherin can also have implications for the intracellular domains. The generation of sE-cad allows the remaining membrane-bound fragment to undergo further processing by presenilin-1/ gamma-secretase complex at the membrane/cytosol interface, which results in the disassembly of the adherens junction (32). Additionally, p120, which is critical for the association between E-cadherin and gamma-secretase (33), can aid the C-terminal fragment of E-cadherin in entering the nucleus and binding DNA to promote gene transcription (34). This tightly regulated sequential degradation of E-cadherin and resulting disassembly of adherens junctions and nuclear translocation of the C-terminal fragment, however, is not required for all E-cadherin cytoplasmic domain processing.
Apoptosis-induced cleavage of the intracellular domain of E-cadherin by calpain and caspase-3 occurs independently of extracellular processing. Calpain cleavage of E-cadherin results in a 100kDa E-cadherin fragment which can no longer bind beta-catenin and diminishes the survival of prostate cancer cells (35). Conversely, caspase-3 can generate a 24kDa cytosolic fragment of E-cadherin, but requires other metalloproteases to generate the 29kDa fragment and sE-cad (36). The generation of sE-cad, however, is not limited to apoptosis, and there are a variety of stimuli which can induce E-cadherin cleavage.
3. GENERATION OF SE-CAD
3.1. Membrane sheddases
The proteases capable of extracellular E-cadherin cleavage, referred to as sheddases, are a diverse group. While many of these sheddases are misregulated or overexpressed in disease, the authors have limited their descriptions to cell systems and diseases where sE-cad is present in conditioned media of cells or patient fluids. To date, no sheddase unique to E-cadherin has been identified, and the sheddases described below are also responsible for cleavage and shedding events beyond sE-cad generation (Table 1).
Table 1.
E-cadherin sheddases
| Sheddase | Stimulus | System | Studies | Ref |
|---|---|---|---|---|
|
| ||||
| ADAM10 | IL-1 beta, TNF-alpha, IFN-gamma, TGF-beta, Lipopolysaccharide (LPS) | Normal keratinocytes | In, si | (14)A |
| None (growing cultures) | Melanoma cell line | Exp. | (15) | |
| Helicobacter pylori infection | Gastric carcinoma cell line | si, In | (13) | |
| EGF | Benign prostatic hyperplasia cell line | In, sh | B | |
|
| ||||
| ADAM15 | Serum withdrawal | Breast cancer cell line | OE, sh, | (16) |
| Prostate cancer cell line | OE, sh, KO | C | ||
| Bladder cancer cell line | OE, sh | C | ||
|
| ||||
| BFT fragilysin | B. fragilis infection | Colorectal cancer cell line | PP | (18)A |
|
| ||||
| Cathepsins (B, L, S) | Ms pancreatic cancer model | RP | (19) | |
|
| ||||
| Gingipains | P. gingivalis infection | Canine kidney cell line | PP | (17) |
|
| ||||
| KLK7 | ? | Pancreatic cancer cell line | RP | (20) |
|
| ||||
| MMP-2 | Protein Kinase D1 (PKD1) | Prostate cancer cell line | In | (21) |
|
| ||||
| MMP-3 | ? | Breast cancer cell line | RP | (22) |
|
| ||||
| Stromelysin | ? (Activated mutant) | Ms mammary cell line | aOE, In | (23) |
|
| ||||
| MMP-7 | HGF | Gastric cancer cell line | Ind, sh | (24) |
| Matrilysin | HGF | Prostate cancer cell line | RP, si | (25) |
| Lung injury (bleomycin) | Lung cancer cell line, mouse lung injury | aOE, In, KO | (26) | |
| ? | Breast cancer cell line | RP | (22) | |
|
| ||||
| MMP-9 | Collagen binding integrins (alpha2beta1, alpha3beta1) interaction | Ovarian carcinoma cell line | Ind, In, FBA | (27) |
| EGF | Head and neck cancer cell line | Ind, si | (28) | |
| PKD1 | Prostate cancer cell line | In | (21) | |
|
| ||||
| MT1-MMP MMP-14 | Ischemia (mineral oil overlay) | Normal rat kidney cell line | In, FAB, sh | (29) |
|
| ||||
| Plasmin | Lysophosphatidic acid (LPA) | Ovarian carcinoma cell line | In | (30) |
| None (growing cultures) | Canine kidney cell line | RP, In | ||
|
| ||||
| ? | Calcium influx, ionomycin | Lung tumor cell line | In | (65) |
|
| ||||
| ? | Ischemia (lung transplant) | Rat lung transplantation | (68) | |
|
| ||||
| TIMP-2 sensitive | Phorbol ester (PMA) | Breast cancer cell line | In | (22) |
| ? | Serum withdrawal | Breast cancer cell line | In | (12)D |
| TAPIE sensitive | Apoptosis (staurosporine, camptothecin) | Canine kidney cell line | In | (36) |
Read-out is the C-terminal fragment, not sE-cad.
Manuscript submitted
Unpublished observations
Original report of sE-cad
ADAM17 inhibitor which can block other metalloproteases.
In: inhibitor. si: siRNA. sh: shRNA. Exp: expression. OE: Over-expression. RP: recombinant protein. PP: purified protein. Ind: Induction of metalloprotease. aOE: auto-activating mutant metalloprotease. KO: knockout mouse. FBA: function blocking antibody
3.1.1. ADAM family
The human ADAM family consists of 25 members whose expression varies across tissues. Functionally, the ADAMs play roles in adhesion and substrate cleavage. To that end, ADAMs are made up of an inhibitory prodomain, a zinc-dependent metalloprotease domain, a disintegrin domain, a cysteine-rich domain, an EGF-like domain, a transmembrane domain and a cytoplasmic tail. Within the ADAM family, ADAM10 and 15 have been implicated in E-cadherin shedding (13–16).
ADAM10 can be found in mesenchymal stem cells, placenta, blood, myeloid cells, bladder, and bone marrow myeloid cells, where it is predominantly a sheddase with a substrate list featuring 27 proteins, including E-cadherin (37). In terms of E-cadherin cleavage in the skin, ADAM10 has been implicated in generating sE-cad in normal keratinocytes as well as melanoma cell lines. In keratinocytes, Maretzky et al examined the soluble 80kDa form and 37kDa C-terminal fragment (CTF) associated with the membrane and determined that ADAM10 constitutively sheds sE-cad (14). ADAM10-dependet shedding of E-cadherin could be induced by the pro-inflammatory cytokines IL-1 beta, TNF-alpha, IFN-gamma, TGF-beta, and lipopolysaccharide (LPS). Additionally, biopsies from eczema patient lesions revealed elevated levels of ADAM10 and CTF (14). ADAM10 also exists in its active form in melanoma cell lines (15), but to date, no inhibitor or loss of function studies of ADAM10 in melanoma cell lines in terms of sE-cad generation have been undertaken. However, ADAM10 is up-regulated in metastatic melanoma compared to primary melanoma (38), and it is possible that it is the sheddase responsible for E-cadherin cleavage.
ADAM10 has also been implicated in shedding E-cadherin in response to Helicobacter (H.) pylori infection of gastric cancer cell lines (13). Previously, H. pylori infection was found to correlate with increased ADAM10 expression in gastric cancer patient samples and to induce ADAM10 expression in gastric cancer cell lines (39). Indeed, chemical inhibition or shRNA mediated knockdown of ADAM10 resulted in decreased sE-cad generation in response to H. pylori infection, suggesting that induction of ADAM10 by H. pylori in the gut promotes E-cadherin cleavage (13).
Recent work in our lab has determined that in benign prostatic hyperplasia (BPH) cells, epidermal growth factor (EGF) can induce sE-cad generation in an ADAM10-dependent manner (manuscript submitted to Cellular Signaling). In a paper by Arima et al, the authors demonstrated that ADAM10 is highly expressed on the cell surface of BPH patient samples versus cancer samples where ADAM10 resided predominantly within the nucleus (40). This suggests that based on location alone, ADAM10 is likely a major sheddase of E-cadherin in BPH, but not in prostate cancer.
Unlike ADAM10, ADAM15 has only four reported substrates, but it also has three integrin binding partners (37). The expression of ADAM15 is widespread in the human tissues, but it is the highest in mesenchymal stem cells and the urogenital system (37). It is also significantly over-expressed in breast, prostate, and lung cancer (16). Najy et al demonstrated that serum withdrawal from breast cancer cells induced E-cadherin cleavage by ADAM15, and the generation of sE-cad could be abrogated by shRNA knockdown of ADAM15 or increased by ADAM15 over-expression (16). Our unpublished observations also suggest that prostate cancer and bladder cancer cells shed sE-cad in response to serum withdrawal in an ADAM15-dependent manner as well. Additionally, in untransformed mouse cells, ADAM15 knockout prostate cell lines fail to shed appreciable amounts of sE-cad as compared to wild-type control cell lines, suggesting ADAM15 can also cleave E-cadherin in mouse prostate epithelial cells (unpublished observations).
3.1.2. Bacterial proteases (Gingipains, BFT/fragilysin)
Gingipains (HRgpA, RgpB, and Kgp) are secreted cysteine proteases which are encoded in the genome of Porphyromonas gingivalis (P. gingivalis). P. gingivalis has been reported to contribute to adult periodontitis in two ways. First, by infecting epithelial cells, P. gingivalis can influence signal transduction and innate immune response (41). Independent of epithelial cell infection, P. gingivalis can disrupt adherens junctions, allowing for infection of underlying tissues (42). The disruption of the adherens junction is believed to be mediated by Kgp cleavage of E-cadherin (17). Although HRgpA and RgpB can also cleave immunoprecipitated E-cadherin, they are unable to process E-cadherin from the cell surface of Madin-Darby canine kidney (MDCK) cells (17).
Another bacterial protease which has been implicated in E-cadherin cleavage is Bacteroides fragilis (B. fragilis). B. fragilis produces an enterotoxin referred to as B. fragilis toxin (BFT) or fragilysin. Treatment of HT29/C1 colorectal adenocarcinoma cells with BFT results in the generation of the 33kDa and 28kDa cytoplasmic E-cadherin fragments (18). Although the authors demonstrated that BFT did not enter the cells and hence could not generate the cytoplasmic fragments, they were unable to observe BFT-mediated cleavage of their recombinant E-cadherin and generation of sE-cad (18). These studies suggest that E-cadherin cleavage may be an important step in bacterial infection.
3.1.3. Cathepsins
Cysteine cathepsins are intracellular proteases which are responsible for protein degradation in the lysosome and play critical roles in apoptosis, autophagy, and necrosis (43). Although located within the lysosome under normal conditions, an emerging body of evidence suggests cathepsins can be miss-localized or released from the cell. For example, release of active cathepsin B has been demonstrated in mechanically injured mouse gut (44), and pro-cathepsin B can interact and localize with annexin II tetramer on the extracellular surface of human breast cancer and glioblastoma cells (45). These studies suggest that in the context of disease, cathepsins may be a viable candidate for extracellular cleavage of E-cadherin.
Interest in cathepsin cleavage of E-cadherin originated with the observation that pancreatic tumors from cathepsin B, L, S knockout mice on the background of the RT2 pancreatic cancer mouse model retained expression of E-cadherin, suggesting E-cadherin processing was deficient (19). Indeed, when Gocheva et al combined recombinant E-cadherin with active cathepsins B, L, or S, E-cadherin was cleaved to a 64kDa extracellular fragment (19). Because cathepsins B and L are upregulated during pancreatic cancer progression (19) and high cathepsin B expression is an independent prognostic marker for pancreatic cancer recurrence (46), it is likely that the miss-localized cathepsins play a role in E-cadherin cleavage in pancreatic cancer.
3.1.4. Kallikrein-7
Kallikerein-7 (KLK7) is serine proteases which is normally expressed in the salivary gland, nervous system, kidney, mammary gland and skin and to a lesser extent in the uterus, thymus, thyroid, placenta, and trachea (47). It is not expressed in the normal pancreas, but it is dramatically over-expressed in pancreatic cancer (20). In pancreatic cancer cell line cultures, recombinant KLK7 was capable of cleaving E-cadherin in vitro and from the cell surface of pancreatic cancer cell lines (20). Because of its dramatic up-regulation in pancreatic cancer, it is likely that KLK7 is a responsible protease for E-cadherin cleavage in pancreatic cancer patients.
3.1.5. MMP family
Like the ADAM disintegrins, the MMPs are zinc dependent proteases (37, 48). While the majority of the MMP family are secreted, a subset of the MMP family, the membrane type (MT) MMPs remain associated with the cell membrane (48). MMP activity is tightly controlled by MMP gene transcription, pro-enzyme activation, and MMP inhibition (48). In E-cadherin cleavage, five MMPs have been implicated: MMP-2, MMP-3, MMP-7, MMP-9 and MT1-MMP (MMP-14).
In prostate cancer, MMP-2 is an independent predictor of patient survival. Early immunohistochemical studies of MMP-2 in prostate cancer patients demonstrated that epithelial expression of MMP-2 in prostate tumors correlated with a decrease in patient survival (49). In vitro, MMP-2 has been implicated in sE-cad generation in prostate cancer cells which have been transfected with protein kinase D1 (PKD1) (21). The in vitro data and the over-expression of MMP-2 in prostate cancer suggest MMP-2 is a possible candidate for E-cadherin shedding in prostate cancer.
MMP-3 shedding of E-cadherin has been reported in mouse and human mammary cells. Early studies by Lochter et al revealed that an auto-activating MMP-3 mutant transfected into mouse mammary cells resulted in the shedding of sE-cad from their cell surface (22, 23). Later analysis of MMP-3 and 7 by Noe et al demonstrated that E-cadherin can be cleaved in vitro by these metalloproteases in breast cancer cells as well (22). In patients, MMP-3 is over-expressed and activated in breast cancer samples versus normal tissue (50, 51), suggesting MMP-3 could be an E-cadherin sheddase in breast cancer.
MMP-7 generation of sE-cad has been reported in prostate, gastric, and breast cancer cells, as well as in a mouse model of lung injury (22, 24–26). In prostate cancer and gastric cancer cell lines, treatment of cells with hepatocyte growth factor/scatter factor (HGF/SF), results in the release of MMP-7 and cleavage of E-cadherin (24, 25). When MMP-7 levels are decreased by short hairpin (sh) RNA against MMP-7, sE-cad generation is lost (24, 25). Prostate cancer patients with advanced disease have more active MMP-7 in their serum (52), while for gastric patients, expression of MMP-7 correlates with a decrease in patient survival and more advanced stage (53). In breast cancer cell lines, E-cadherin can be cleaved in vitro by MMP-7 (22), and in patients MMP-7 positive tumors by immunohistochemistry correlate with a worse prognosis (54). Because MMP-7 is over-expressed in breast, prostate, and gastric cancer, it is possible that MMP-7 is major sheddase of E-cadherin in these cancers.
MMP-7 over-expression is not unique to cancer and can occur in response to injury. In a mouse lung injury model using bleomycin, MMP-7 is dramatically upregulated in injured lung epithelium (26, 55). McGuire et al also demonstrated that MMP-7 knockout mice did not generate sE-cad from wounded trachea explants, unlike their wild-type controls, implicating that the up-regulation of MMP-7 in response to wounding is responsible for sE-cad generation in this model (55). MMP-7 up-regulation occurs in pulmonary fibrosis patients (56), so MMP-7 generation of sE-cad may play a role in disease progression. Interestingly, in these studies only the lung cancer cells that were transfected with an auto-activating MMP-7 produced the active form of the enzyme; native full length cDNA for MMP-7 did not produce active enzyme (26), suggesting that there is a missing mediator required for MMP-7 activation in these epithelial lung cancer cells.
MMP-9 shedding of E-cadherin appears in ovarian, head and neck, and prostate cancer cell lines. MMP-9 expression is a negative predictor for survival in ovarian cancer, head and neck cancer, as well as prostate cancer (57–59). In ovarian cancer cell lines, aggregation of collagen binding integrins alpha2beta1 and alpha3beta1 induces MMP-9 expression which promotes E-cadherin shedding (27). Early studies of MMP-9 in head and neck cancer patients demonstrated that serum levels of MMP-9 were highest in patients with more advanced disease (59) and in vitro, stimulation of head and neck cell lines with EGF demonstrated increased expression of MMP-9 and increased sE-cad (28). Finally, the re-expression of PKD1 in prostate cancer cells also resulted in increased MMP-9 expression, which correlates with increased sE-cad generation, and could be abrogated by the addition of MMP-9 inhibitors (21). Based on the high expression of MMP-9 in ovarian, head and neck, and prostate cancer patients, it is likely that MMP-9 contributes to sE-cad levels in these patients.
MT1-MMP (MMP-14) has been implicated in sE-cad generation in a model of kidney ischemia. Normal rat kidney cells under ischemic conditions generated sE-cad which, by inhibitor studies, was not mediated by MMP -1, -3, -8, or -9 (29). Covington et al did, however, observe an increase in MT1-MMP expression in response to ischemia, and determined that loss of MT1-MMP by shRNA decreased sE-cad accumulation, confirming that sE-cad can be generated by MT1-MMP under these conditions (29). In a mouse model of hind-limb ischemia, active MT1-MMP was up-regulated in the ischemic limb as compared to the control, sham operated limb (60), suggesting that MT1-MMP may be the sheddase of E-cadherin under ischemic conditions.
3.1.6. Plasmin
Plasmin is a serine protease with limited specificity, which can act on fibrin, fibrinogen, extracellular matrix components, and pro-forms of growth factors either directly or by activating metalloproteases (61). It is also a downstream component of the urokinase-type plasminogen activator (uPA) system, which can be activated in ovarian cancer cells by Lysophosphatidic acid (LPA) (62). LPA is found in high concentration in ovarian cancer ascites and promotes growth in ovarian cancer cell lines in vitro and in vivo (63). In ovarian cancer cell lines, LPA activates the urokinase-type plasminogen activator (uPA) which activates plasmin, resulting in E-cadherin cleavage (30). Although the authors could not rule out metalloproteases downstream of plasmin, they do note that in their studies, LPA only increased pro-MMP-9 slightly, suggesting that plasmin may be directly acting upon E-cadherin (30). Other work in MDCK cells demonstrated that treating cells with plasmin can generate sE-cad and this process can be inhibited by the addition of aprotinin, a serine protease inhibitor (31). Since uPA system disregulation correlates with worse outcome in ovarian cancer patients (64), it is likely that an elevated level of plasmin in these patients generates sE-cad.
3.1.7. Unattributed sheddase activity
In addition to the studies which successfully define sE-cad sheddases, other studies demonstrate the existence of sE-cad as a consequence of stimuli, but do not identify the responsible protease. In these studies, the protease could be one of the aforementioned sheddases or it could be a novel sheddase. Ito et al demonstrated that calcium influx by serum withdrawal or ionomycin treatment allowed for sE-cad to accumulate in the conditioned media of cancer cells (65).
Although they never identified a responsible sheddase, they did report only the membrane fractions and not cell supernatants were capable of cleaving E-cadherin in vitro, suggesting a membrane bound metalloprotease was required and ruled out the classical MMPs in direct cleavage (65). In the breast cancer MCF-7/AZ cell line, the phorbol ester PMA can induce the shedding of E-cadherin by a metalloprotease that is sensitive to tissue inhibitor of metalloprotease-2 (TIMP-2) inhibition (22). In the same study, immunopurified MMP-3 and MMP-7 are shown to cleave E-cadherin directly and from the cell surface of MDCK cells (22), but a direct sheddase from PMA induction was not demonstrated. Other studies of apoptotic MDCK cells also implicated a metalloprotease which was sensitive to TAPI, an ADAM17 inhibitor which can inhibit other metalloproteases (36).
Several studies have determined that the accumulation of sE-cad may be a biomarker for tissue damage and predict surgical outcome (66, 67). Goto et al applied this to a model of lung transplantation in rats and observed that rats with transplanted lungs had a higher level of sE-cad than the sham operated rats (68). Again, no direct evidence implicates a sheddase, but other studies have implicated MMP-7 in rodent lung damage studies (26, 55).
3.2. Proteolytic cascades
The study of sE-cad shedding is greatly complicated by the existence of proteolytic cascades, particularly those involving the MMPs and the uPA system. Synthesized as zymogens, MMPs require proteolytic processing to become active, and this process can be mediated by other MMP family members. As summarized in reviews by Egeblad and McCawley: MMP-2 can generate active MMP-1, 2, 9, and 13; MMP-3 can generate active MMP 1, 3, 7, 8, 9, and 13; MMP-7 can generate active MMP-1, 2, 7 and 9; MMP-9 can generate active MMP-2; finally, MMP-14 can generate active MMP-2 and 13 (69, 70). Based on the ability of MMPs to activate other family members, studies examining upstream MMPs may have difficulty distinguishing effects due to catalytic activity on a substrate by the upstream metalloprotease versus a catalytic activation of a downstream mediator and subsequent substrate cleavage. In the uPA system, the proteolytic cascade is more manageable. Here, uPA converts plasminogen to plasmin, which can then cleave proMMP-2 and 9 to active MMP-2 and 9 (71). Because plasmin is a serine protease which can activate zinc-dependent metalloproteases, these different enzyme classes allow for specific inhibitors and easier determination of the responsible sheddase. The existence of proteolytic cascades coupled with the redundancy observed in E-cadherin cleavage may explain why sE-cad is observed in multiple patient conditions.
4. SE-CAD IS PRESENT IN PATIENT FLUIDS IN A VARIETY OF CONDITIONS
sE-cad was first observed in the conditioned media of MCF-7 cells by Wheelock et al (12), and since then, many studies have been conducted on patient fluids to determine whether sE-cad could serve as a biomarker for disease. The initial report by Katayama et al determined that levels of sE-cad do not vary significantly between men and women or different age groups (72). Although the initial focus on sE-cad was as a cancer biomarker for disease, progression, or recurrence, today there are several studies which describe the presence of sE-cad in other disease states, such as HIV infection and benign prostatic disease (73, 74). In order to be included, studies must have reported on the presence of sE-cad in more than one patient. For example, the initial report of sE-cad in patient samples by Katayama et al included a report for one pancreatic and one ovarian cancer patient (72), which was not sufficient for inclusion in this review (Table 2).
Table 2.
sE-cad can be found in the fluids of patients with multiple conditions
| Patient diagnosis | Type | sE-cad correlates with: | Source | sE-cad Levels (ng/mL) | Ref # |
|---|---|---|---|---|---|
|
| |||||
| Cancer | Bladder | Cancer, grade, number, recurrence | Serum | N: 1013 C: 3955 |
(75) |
| Cancer | Urine | N: .516 mg/mol C: 1.536 mg/mol |
(76) | ||
| Cancer, grade | Urine | N: 1.306 +/− 1.249 mg/mol C: 3.724 +/− 1.892 mg/mol |
(77) | ||
| Recurrence | R: 10.497 +/− 7.47.1 mg/mol | ||||
|
| |||||
| Colorectal | Cancer, Progression | Serum | N1: 3467 B1: 5248 C1: 5495 |
(79) | |
| Not significant | Serum | N: 3.53 C: 3.17 |
(78) | ||
|
| |||||
| Esophageal squamous cell carcinoma | Survival (surgery only) | Serum | PreOp: 5108.96 PreCRT: 3688.932 PostCRT: 3981.029 |
(80) | |
|
| |||||
| Gastric | Cancer | Serum | N: 2515 +/− 744 C: 4735 +/− 2310 |
(81) | |
| Cancer | Serum | N: 5616 C: 9344 |
(82) | ||
| Recurrence | Serum | sE-cad above 10000 | (83) | ||
| Survival | Serum | sE-cad above 10000 | (84) | ||
| Cancer | Serum | N: 2000 C: 3510 +/− 1790 |
(72) | ||
|
| |||||
| Liver | Cancer | Serum | N: 2000 C: 5550 +/− 3110 |
(72) | |
| Cancer | Serum | N: 5798 C: 10759 |
(87) | ||
| Recurrence (early) | sE-cad above 8000 | (87) | |||
|
| |||||
| Non-epithelial | |||||
| Leimyosarcoma | Cancer | Serum | N: 2000 C: 3280 +/− 720 |
(72) | |
| Leukemia | Cancer | Serum | C: 2520 +/− 1000 | (72) | |
| Multiple Myeloma | Cancer | Serum | N: 622.9 C: 3291.4 |
(88) | |
| Survival | Serum | s-Ecad above 3000 | (88) | ||
|
| |||||
| Non-small cell lung | Cancer | Serum | N: 1015 C: 3455 |
(89) | |
| Metastasis | Serum | L2: 2487.8 M2: 4422.2 |
(89) | ||
| Cancer | Serum | N: 1015 +/− 125 NSCL: 3455 +/− 1082.4 SCLC: 3428.3 +/− 1198.8 |
(90) | ||
| Metastasis | Serum | L NSCL: 2460 +/− 388.2 M NSCL: 4579.5 +/− 279.3 L SCLC: 3035 +/− 586.9 M SCLC: 3871 +/− 77.7 |
(90) | ||
|
| |||||
| Ovarian | Not significant | Serum | Luteal cyst: 3677 Dermoid tumor: 2325 Cystadenoma: 2200 C: 2250 |
(92) | |
| Cancer | Cyst | Luteal cyst: 2035 Dermoid tumor: ND Cystadenoma: 2000 C: 14500 |
(92) | ||
| Malignant ascites | Ascites | N: 2061 +/−1968 C: 12241 +/−5314 |
(27) | ||
| Present in ascites | Ascites | C: 89.96 (ug/ul)/ug total protein | (30) | ||
|
| |||||
| Prostate | Cancer, metastasis | Serum | N: 6.270 ug/l L: 9.460 ug/l M: 27.490 ug/l |
(74) | |
|
| |||||
| Skin | |||||
| Basal cell | Not significant | Serum | N: 808 +/− 272 C: 879 +/−485 |
(94) | |
| Melanoma | Cancer, rising S100 | Serum | N: 3198 C: 4975 |
(15) | |
| Cancer, metastasis | Serum | N: 808 +/− 272 M: Values not reported |
(94) | ||
| Paget’s disease | Invasion | Serum | C: Not reported | (94) | |
| Squamous cell | Not significant | Serum | C: 838 +/− 374 | (94) | |
|
| |||||
| Non-cancer | Acute pancreatitis | Severe cases | Serum | N: 5181 +/− 1350 D: 17780 +/− 7853 |
(95) |
|
| |||||
| Benign prostatic hyperplasia (BPH) | BPH | Serum | N: 6.27 ug/l B: 7.26 ug/l |
(74) | |
|
| |||||
| Diabetes | Not significant | Serum | N: 2000 D: 2330 +/− 1580 |
(72) | |
| Not significant | Urine | N: 652.7 +/−87 Diabetic: 721.9 +/−93 |
(96) | ||
|
| |||||
| Diabetic nephropathy (DN) | Nephropathy | Urine | N: 652.7 +/−87 DN0: 721.9 +/−93 DN1: 2751.5 +/− 164 DN2: 5839.6 +/− 428 |
(96) | |
|
| |||||
| Inflammatory skin diseases | (94) | ||||
| Psoriasis | Severe cases | Serum | Values not reported | ||
| Dermatitis | Severe cases | Serum | Values not reported | ||
|
| |||||
| Infection | HIV | Viral load | Plasma | Values not reported | (73) |
|
| |||||
| Hepatitis | Not significant | Serum | N: 2000 D: 2340 +/− 520 |
(72) | |
|
| |||||
| Organ dysfunction | Multi-organ | Sepsis, organ dysfunction | Serum | N: 3280 D: 6000 |
(66) |
|
| |||||
| Cholesystectomy | Inflammation | Serum | Lap: 1850 +/− 250 Open: 3110 +/− 330 |
(67) | |
N: normal, C: cancer, M: metastatic D: disease, R: recurrence, NR: No recurrence, PreOp: Preoperative. CRT: neoadjuvant chemoradiation therapy. L: localized, M: metastatic, ND: not detected, NSCL: non-small cell lung cancer. SCLC: small cell lung cancer. DN0: diabetic, no nephropathy, DN1: diabetic nephropathy, microalbuminuria, DN2: diabetic nephropathy, macroalbuminuria, Lap: laparoscopic
Value conversion from log (sE-cad ng/ml) to sE-cad ng/ml by Grabowska and Day.
Observation reported by study’s author, but numbers generated by Grabowska and Day.
4.1. Cancer
4.1.1. Bladder
Sera from newly diagnosed bladder cancer patients have significantly (P = 0.017) higher levels of sE-cad than normal controls (1,013 ng/ml v. 3,955 ng/ml) (75). Additionally, high levels of sE-cad correlate with higher grade, number of tumors, and recurrence but not tumor bulk (75). In the urine, healthy controls exhibited 582 ng/ml of sE-cad in the urine, while bladder cancer patients averaged 1,272 ng/ml across all stages and grades (P < 0.001) (76). The authors suggested, however, that using total protein in urine is equally effective at this determination (76). In a later study, Shi et al found that urine levels of sE-cad normalized to creatinine were significantly (P <0.01) lower in normal (1.306 mg/mol) versus cancer samples (3.724 mg/mol), and that samples from recurrent patients (10.497 mg/mol) had significantly (P < 0.01) higher levels of sE-cad than primary tumors (77). Additionally, they found that sE-cad correlated well with tumor grade, but not with stage, size, and the number of tumors (77). Based on these data, both serum and urine concentrations of sE-cad can be used to stratify bladder cancer patients.
4.1.2. Colorectal
In colorectal cancer, the initial report determined that there was no statistically significant difference between sE-cad levels from healthy controls and colorectal cancer patients (78). Later, Willmanns et al found that sE-cad levels were statistically different between healthy controls versus benign disease (P = 0.005) versus cancer (P = 0.009) (3,476 ng/ml; 5,248 ng/ml; 5,495 ng/ml) and that the highest levels were found in metastatic patients (79). They also observed that in this cancer cohort, patients with renal or hepatic failure had high levels of sE-cad and that patients who had been treated with chemotherapy had lower sE-cad levels compared to untreated patients (79). From these data, it is apparent that while sE-cad serum levels may be useful in determining cancer spread in untreated patients, it would be important to rule out organ failure or dysfunction.
4.1.3. Esophageal squamous cell carcinoma
The recent study in squamous cell carcinoma compared the pre-operative levels of sE-cad for patients who had surgery alone to those patients who had chemoradiation therapy (CRT) before surgery. Patients in the surgery alone arm had significantly (P = 0.032) higher (5,108 ng/ml) levels of sE-cad than patients who had already received chemoradiation therapy (3,688 ng/ml) (80). This decrease of sE-cad levels after chemotherapy agrees with data from colorectal cancer patients (79), and suggests sE-cad could be used to monitor patient response to therapy. For the patients who received surgery, levels of sE-cad higher than the median pre-surgery sE-cad concentration correlated with a decrease in survival; however, there was no prognostic significance for patients who had undergone neoadjuvant CRT (80). Therefore, sE-cad as prognostic marker for esophageal squamous cell carcinoma would be limited to patients who have yet to undergo any treatment (80).
4.1.4. Gastric
Initials reports of elevated sE-cad in gastric cancer came from Katayama et al (2,000 ng/ml vs. 3,515 ng/ml; P < 0.0001) (72), which were confirmed by Gofuku et al (81). Later studies by Chan et al demonstrated that not only was sE-cad elevated in gastric cancer patients (5,616 ng/ml vs. 9,344 ng/ml; P = 0.001) but also that this correlated with tumor size and poor outcome markers (82). When Chan et al determined the optimal sensitivity and specificity of sE-cad following tumor resection by ROC analysis, they determined 10,000 ng/ml as a point of elevated sE-cad. Once a patient’s serum concentration of sE-cad exceeded 10,000 ng/ml, the patient would eventually recur (83). On average, elevated sE-cad levels predated the recurrence by 13 months (83). Most importantly, the sensitivity of this test was similar in patients with more and less advanced disease (83). In another study, Chan et al determined that a pre-therapeutic 10,000 ng/ml sE-cad concentration was also a predictor of survival, where 90% of patients whose sE-cad levels were above the cutoff had a survival time of less than three years (P = 0.009) (84). Chan et al acknowledged the substantial differences between normal sE-cad levels in the Katayama study (72) and theirs (82) by suggesting that the differences may be attributed to the different populations used in the study. Later studies by Pedrazzani et al determined that while sE-cad is indeed elevated in gastric cancer patients as previously reported, there was a linear increase with sE-cad levels in normal controls and gastric tumor patients over time (85), which is inconsistent with previous observations (72).
sE-cad levels in gastric cancer patients can be decreased by resection or therapy. Resection alone can significantly (P < 0.0001) reduce sE-cad levels (81). Later studies by Zhou et al demonstrated that if surgery was coupled with neoadjuvant Celecoxib therapy, patients showed a significant (P < 0.01) decrease in sE-cad levels during therapy and a significant (P < 0.01) decrease in sE-cad post surgery (86). Based on the data, the use of sE-cad in gastric cancer would be quite informative for patient survival and recurrence, particularly since there is a significant amount of time between elevated sE-cad levels and actual recurrence, which would allow for appropriate therapeutic intervention (83). Additionally, future studies of sE-cad in gastric patient response could provide an early indication of failed therapy and appropriate therapeutic intervention.
4.1.5. Liver
Patients with liver cancer have an increased level of sE-cad (P <0.0001; P < 0.05) (72, 87). The levels reported, however, are quite disparate with normal controls being reported as 2000 ng/ml or 5,798 ng/ml and cancer levels at 5550 ng/ml versus 10,759 ng/ml (72, 87). Soyama et al also demonstrated that levels of sE-cad in the serum of patients did not correlate with levels of E-cadherin in hepatic lesions, tumor markers, size, number, vascular invasion, stage, gender, age, or viral status (87). However, patients with levels higher than 8,000ng/ml were more likely to recur and metastasize (P < 0.05) (87). It appears that sE-cad could be a useful biomarker for disease spread and recurrence in liver cancer.
4.1.6. Non-epithelial
sE-cad levels can also be found in patients with non-epithelial tumors such as leukemia, multiple myeloma, and leiomyosarcoma. Patients with leukemia (myelogenous, monocytic, lymphatic) have increased levels of sE-cad (P < 0.01), as do patients with leiomyosarcoma (P < 0.05) (68). Multiple myeloma patients have five times higher levels of serum sE-cad than control samples (P < 0.0001) (88). sE-cad is also a survival predictor, where patients with levels of sE-cad below 3,000 ng/ml lived longer (P = 0.0015), and an increase in sE-cad of 100ng/ml increased their risk of death from multiple myeloma by 6% (P = 0.013) (88). In a non-epithelial setting, the source of sE-cad cannot come from the tumor cells themselves; instead it is more likely due to the tumors invasion into epithelial tissue. For example, leiomyosarcomas can occur in the smooth muscles cells of the stomach and grow into the stomach proper, resulting in epithelial tissue damage and shed sE-cad. In new multiple myeloma patients, sE-cad levels could be used as a prognostic marker because high levels correlate with poor disease outcome (88).
4.1.7. Non-small cell lung (NSCLC)
In NSCLC patients, sE-cad serum levels are much higher than in healthy volunteers (3,455 ng/ml vs. 1,015 ng/ml; P < 0.001) and the highest levels of sE-cad also correlate with metastasis (P < 0.001) (89). There was, however, no statistically significant difference between the levels of sE-cad and histological type of cancer (adenocarcinoma, squamous cell carcinoma, or large cell carcinoma), sex, or smoking habit (89). Later studies confirmed these observations in NSCL, but also demonstrated elevated levels of sE-cad in small cell lung cancer (SCLC) and that increasing levels of sE-cad correlated with metastasis in SCLC (90). This suggests that while sE-cad might be useful in determining whether a patient has metastatic disease, it would not be useful in disease classification.
As in gastric cancer, sE-cad levels in NSCLC can decrease following therapy. Reckamp et al evaluated serum sE-cad levels in NSCLC patients before and after Celecoxib and Erlotinib treatment (91). Although there was no difference between sE-cad levels between patients with partial response, stable disease, and progressive disease initially, after 8 weeks of therapy, patients who achieved a partial response had significantly lower levels of sE-cad than those with stable or progressive disease (P = 0.021) (91), suggesting sE-cad may be a useful marker for therapeutic response.
4.1.8. Ovarian
In an early report of sE-cad levels in ovarian cancer, Darai et al determined that the levels of serum sE-cad between luteal cyst, dermoid tumor, cystadenoma and malignant tumors did not vary significantly (92). Conversely, when the cyst fluid was examined, the levels of sE-cad were significantly (P = 0.001) higher in patients with malignant versus benign disease (92). Other researchers examined the ascites of benign ovarian disease or ovarian cancer patients and determined that patients with ovarian cancer had very high levels of sE-cad (P < 0.000005) (27). Gil et al later confirmed the presence of sE-cad in malignant ascites of women with advanced ovarian cancer (30). Because serum sE-cad levels fail to distinguish between benign and malignant disease, the use of sE-cad as a biomarker in ovarian cancer would have to be limited to cyst fluid or ascites.
4.1.9. Prostate
The initial report of sE-cad in prostate cancer by Kuefer et al demonstrated the presence of sE-cad in prostate cancer tissues, with increased expression in metastatic deposits and significantly elevated serum levels in patients with metastatic disease (P < 0.001) (93). Later studies comparing BPH, localized and metastatic prostate cancer sE-cad concentrations to healthy controls demonstrated significant differences (normal v. BPH P = 0.023; BPH v. localized prostate cancer P =0.011; localized v. metastatic P < 0.001) (74). In this study, Kuefer et al also evaluated sE-cad as a biomarker to predict outcome. Surprisingly, sE-cad at the time of diagnosis could predict biochemical failure, mainly that localized disease with high levels of sE-cad (above 7.9ug/l) would likely result in late biochemical failure (P < 0.05) (74). In prostate cancer, therefore, sE-cad may be useful in stratifying patients, but the greatest use might be in categorizing high-risk for recurrence patients.
4.1.10. Skin
Levels of sE-cad in different types of skin cancer vary according to type. In basal or squamous cell carcinoma, the levels of serum sE-cad did not vary significantly from the healthy controls (94). In Paget’s disease, the levels of sE-cad were significantly elevated above control samples, but only once the disease became invasive (94). In melanoma, early reports suggested that levels of sE-cad were elevated once patients had metastatic disease (94). Later studies confirmed that levels of sE-cad in melanoma patients were higher than in normal controls (3,198 ng/ml v. 4,975 ug/ml; P < 0.05), and the expression of sE-cad correlated with rising S100 values, indicating melanoma progression (P < 0.05) (15). Interestingly, high sE-cad levels were observed in some patients with low levels of S100, which the authors suggest, may be an indication that generation of sE-cad may serve as an early marker of progression for a subgroup of patients (15).
4.2. Non-cancer
Although sE-cad has been extensively studied as a biomarker in cancer, it has also been observed and evaluated as a biomarker for non-cancer conditions such as BPH, dermatitis, psoriasis, acute pancreatitis, diabetes, and diabetic nephropathy. In BPH patients, the levels of sE-cad were significantly higher than in normal control patients (P = 0.023), but not as high as those patients with prostate cancer (74). Similarly, patients suffering from acute psoriasis and dermatitis had elevated levels of sE-cad in their serum, but unlike skin cancers where sE-cad levels correlated with invasion (15, 94), in the non-cancer setting, sE-cad correlated with the severity of the disease (94).
Serum concentrations of sE-cad are also predictive of acute pancreatitis. As of 2009, when the study was conducted, the standard tests for acute pancreatitis were poor predictors of severity (95), so Sewpaul et al hypothesized that because patients with systemic inflammatory response shed sE-cad (66), sE-cad could be a used as a marker for acute pancreatitis. Indeed, sE-cad levels were elevated in patients with mild acute pancreatitis (7,358 ng/ml) versus acute severe pancreatitis (17,789 ng/ml) versus healthy controls (5,181 ng/ml) (P = 0.0166; P = 0.0039) (95). The levels of sE-cad in severe acute pancreatitis were also significantly higher (P = 0.0073) than other abdominal inflammatory pathologies (acute diverticulitis, perforated duodenal ulcer, cholangitis, acute appendicitis, and acute cholecystitis), suggesting that sE-cad levels could be a specific predictor for acute severe pancreatitis (95). Most importantly, this study demonstrated that sE-cad levels at 12 hours from onset of pain could predict the severity of pancreatitis, allowing for appropriate intervention (95).
In diabetes, levels of sE-cad in the sera or urine of healthy controls versus diabetic patients do not show significant difference (72, 96). However, sE-cad levels in the urine may be useful in determining which diabetic patients are suffering from diabetic nephropathy (96). The urine levels of diabetic patients with normoalbuminuria, microalbuminuria, and macroalbuminuria vary significantly (P < 0.001), suggesting that sE-cad might be a biomarker for diabetic nephropathy (96).
4.3. Infection
For HIV infection, the levels of sE-cad correlate with viral load in patients. Interest in sE-cad in HIV infection arose from the observation that the intestine is a site of increased permeability in infected patients, suggesting a disruption in E-cadherin function (73). Indeed, high HIV viral titers significantly (P = 0.004) correlate with high levels of serum sE-cad, suggesting that sE-cad is a marker for severity of infection (73). Conversely, acute hepatitis does not elevate the levels of sE-cad above controls (72).
4.4. Organ dysfunction
The levels of sE-cad are significantly (P 0.0019) higher in patients with sepsis and organ dysfunction as compared to normal controls, and tend to increase with the amount of organ dysfunction and sepsis in the patient (66). In surgery, the levels of sE-cad can be used as a biomarker of tissue injury and inflammation. For example, a comparison of open to laparoscopic cholecystectomy demonstrated that the less invasive laparoscopic procedure resulted in less sE-cad generated (P = 0.04) (67). These studies suggest that sE-cad levels in patient serum can be used to determine the extent of tissue damage and systemic inflammatory response.
5. CONSEQUENCES OF SE-CAD PRESENCE
5.1. Disruption of cell-cell interactions
The initial report by Wheelock et al demonstrated that sE-cad purified from MCF-7 cells was capable of disrupting cell-cell adhesion between mouse mammary tumor cells which were already growing in clusters (12) (Table 3). Later work demonstrated that treatment of ovarian cancer cell lines with a recombinant human ectodomain of E-cadherin fused to Fc, resulted in disruption of established cell junctions (27). In re-aggregation assays, pancreatic cancer cells and MDCK cells treated with sE-cad immunodepleted media, were more efficient at re-aggregating than the cells which were re-aggregating in the presence of conditioned media with sE-cad present (20, 31). The presence of sE-cad can, therefore, not only disrupt established adherens junctions, but can also interfere with establishing adherence junctions in cell re-aggregation assays.
Table 3.
Consequences of sE-cad presence
| Result | System | sE-cad source | Mechanism (if known) | Ref. |
|---|---|---|---|---|
|
| ||||
| Disruption of adherens junctions | Ovarian cancer cell line | Recombinant, Fc fusion | (27) | |
| Mouse mammary tumor cells | Antibody affinity chromatography | (12) | ||
|
| ||||
| Disruption of anti-viral function | Peripheral blood mononuclear cells from HIV patients | Recombinant sE-cad | Abrogation of IFN-gamma response | (73) |
|
| ||||
| Disruption of cell aggregation | Pancreatic cancer cell line | Immunodepletion | (20) | |
| Canine kidney cell line | Immunodepletion | (31) | ||
|
| ||||
| Invasion | Ovarian cancer cell line | Recombinant, Fc fusion | (30) | |
| Pancreatic cancer cell line | Immunodepletion | (20) | ||
| Lung cancer cells | Immunodepletion; HAV peptide | Induction of MMP-2, 9, 14 | (98) | |
| Canine kidney cells | Immunodepletion | (31) | ||
|
| ||||
| Proliferation | Breast cancer cells | Recombinant, Fc fusion | HER2/HER3 phosphorylation | (16) |
|
| ||||
| Signaling | Breast cancer cells | Endogenous; Recombinant, Fc fusion | Binding to HER2/HER3 ERK signaling | (16) |
| Canine kidney cells | Recombinant, myc tag | pERK/pAKT via EGFR | (99) | |
| Peripheral blood mononuclear cell from HIV patients | Recombinant sE-cad | KLRG1 ligation | (73) | |
|
| ||||
| Survival | Canine kidney cells | Recombinant, myc tag | EGFR signaling | (99) |
sE-cad can also interfere with immune cell interactions by serving as a dummy ligand for KLRG1 and interfering with anti-viral functions. In HIV-infected peripheral blood mononuclear cells, the presence of a recombinant sE-cad interfered with the ability of T cells to secrete IFN-gamma in response to HIV-1 Gag stimulation (73). Because KLRG1 on the CD8+ T cells bound sE-cad, the HIV infected CD4+ T cells were not targeted, resulting in an increase in survival of infected cells (73). These data suggest that sE-cad is sufficient to disrupt cell-cell interactions which has implications for epithelial tissue stability and immune response.
5.2. Migration and invasion
The presence of sE-cad can also induce cells to invade. Ovarian cancer cells exposed to Fc-E-cadherin invade through a modified Boyden chamber (30). Similarly, pancreatic cancer and MDCK cells exposed to conditioned media containing sE-cad versus conditioned media immunodepleted of sE-cad, show much greater inclination toward migration in the presence of sE-cad (20, 97). In the case of lung cancer, the presence of sE-cad in the conditioned media or in the form of an activating HAV peptide based on EC1 of E-cadherin, can induce MMP-2, 9, and 14 transcription and activity as evaluated by zymography and increased invasion (98). sE-cad, therefore, can promote migration and invasion, which may be due to its ability to induce additional metalloprotease activity to aid in these processes.
5.3. Signaling, proliferation, and survival
There have been several studies that have examined the specific effects of sE-cad presence on cells. In breast cancer cells, endogenous sE-cad can be observed bound to HER2 and HER3 by immunoprecipitation (16). Stimulation with exogenous Fc-E-cadherin results in phosphorylation of HER2 and HER3, as well as downstream ERK signaling (16). Work by Najy et al also demonstrated that using a recombinant sE-cad resulted in a proliferative response in breast cancer cells, which was not mediated by full length E-cadherin since the line has a homozygous deletion for CDH1 (16). In our studies, sE-cad can also bind EGFR and signal downstream through ERK (manuscript submitted to Cellular Signaling).
Conversely, in MDCK cells under serum free conditions, the anti-apoptotic signals provided by a myc-tagged sE-cad required E-cadherin expression (99). Treatment of these MDCK cells with a myc-tagged sE-cad resulted in signaling through EGFR and ERK (99). These studies suggest that sE-cad signaling is mediated by EGFR family members and, depending on the cellular context, may or may not be dependent on full length E-cadherin. Moreover, these studies suggest that sE-cad can stimulate proliferation and survival in non-transformed and transformed cells.
6. DISCUSSION
Although the accumulation of sE-cad was initially believed to be solely related to tumorigenesis, cell culture studies have revealed that the generation of sE-cad can be mediated by several mechanisms in a variety of pathological states. E-cadherin cleavage can be induced by pro-inflammatory cytokines, bacterial infection, serum withdrawal, apoptosis, and growth factors (13–31). To date, ADAMs (10 and 15), bacterial proteases (gingipains and BFT), cathepsins (B, L, S), MMPs (2, 3, 7, 9, and 14), KLK7, and plasmin have all been implicated in the generation of sE-cad (13–31), but the study of E-cadherin processing is complicated by redundancy and the presence of proteolytic cascades.
Proteolytic cascades, much like signal transduction cascades, allow for the amplification of a stimulus. Mainly, when protease A is activated, it can activate protease B or C. The problem lies in determining whether it is protease A which is acting on the substrate or protease B or C, particularly when both protease belong to the same family, for example MMP-2, 9, and 13. One definitive measure of protease and substrate specificity is to use recombinant and purified proteins or their catalytic domains in an in vitro cleavage assay. This method requires the protease to be able to act upon the substrate without activating another mediator. The downside to this approach, however, is that it removes the protease and substrate from physiologically relevant situations, such as activation of the protease, presence of protease inhibitors, cell membrane interactions, as well as proteins associated with the substrate. Therefore, while an in vitro cleavage assay will demonstrate cleavage in a best case scenario, it does not prove that the protease can act on the substrate in the context of a cell or biological system. The most thoroughly researched proteases, therefore, have extensive studies into their in vitro cleavage capabilities as well as complementary studies utilizing genetic knockout strategies, protease targeting shRNAs, as well as other specific targeting agents.
Assuming all reports of E-cadherin sheddases are accurate, there is a significant redundancy in mechanisms that generate sE-cad. Because cell culture evidence suggests that sE-cad can disrupt cell adhesion, immune response, as well as induce signaling and invasion (Table 3) and is associated with disease severity in patients (Table 2), sE-cad may be more than a symptom of protease disregulation and may actually be contributing to the progression or severity of disease. Inhibiting E-cadherin cleavage, particularly in cancer, could be beneficial to patients and accomplished either by specific targeting of proteases implicated in a patient’s disease or using broad-spectrum inhibitors. Due to the poorly understood complex role of protease families such as the MMPs in early clinical trials, unintentional targeting of the entire zinc metalloprotease family (MMPs and ADAMs), showed little efficacy in cancer patients with advanced disease (48), and has prompted the development of more specific inhibitors for specific proteases and families, such as inhibitors for ADAM10 and 17 and the MMPs (48, 100, 101). Although these specific inhibitors have not been designed for inhibiting E-cadherin cleavage per se, preventing the generation of sE-cad could provided an additional, albeit unintended, benefit to patients undergoing cancer therapy.
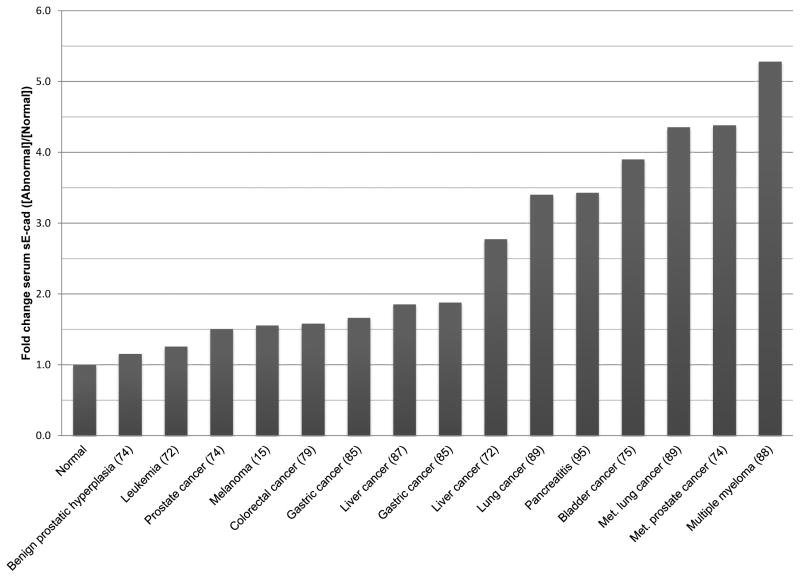
Regardless of the source of sE-cad, based on the published data, there are several observations that come to mind. First, sE-cad is present in a myriad of conditions from cancer to infection to organ failure, which suggests sE-cad will not be a singular biomarker for any specific disease or type. Instead, the use of sE-cad in a clinical setting would, most likely, be a prognostic marker and be used in conjunction with other biomarkers. Another issue with sE-cad as a biomarker is the ranges of sE-cad reported vary greatly for normal controls, and whether this has to do with race (82), healthy volunteers with unknown medical problems, or technical differences between laboratories executing the ELISA remains unclear. Should sE-cad be used as a biomarker for severity of disease, then the ELISA would have to be standardized on a national or international level, and part of that would have to entail analyzing different populations for serum sE-cad concentrations. Another approach would be to divide the sE-cad values by the normal control values, generating fold change values, but even this method produces considerable by overlap between various disease states (Figure 1).
Figure 1. Comparison of serum sE-cad levels among various malignancies.
Malignancy concentrations of sE-cad were divided by the average normal concentrations within each study to provide fold change values.
There are, however, several appealing aspects to using sE-cad in a clinical setting. For one, the majority of conditions use serum or urine samples, which are easy collection procedures versus needle biopsies, etc. Additionally, since sE-cad can be used to screen for many health issues, the ELISA could be run often in a diagnostic laboratory and contribute insights into disease severity, progression, recurrence, therapeutic response, and prognosis. In disease severity, sE-cad can predict cases of severe acute pancreatitis after 12 hours of pain (95) as well as the levels of kidney dysfunction in diabetes patients (96). sE-cad levels can also predict disease progression and recurrence. On average, elevated sE-cad predicts gastric cancer recurrence an average 13 months before the recurrence (83), and higher levels of sE-cad in localized prostate cancer predict early recurrence (74). In melanoma, elevated levels of sE-cad in patients with low S100 values may also indicate patients likely to progress rapidly (15). Bladder, gastric, and liver cancer patients with high sE-cad levels are also more likely recur than patients with lower levels of sE-cad (75, 77, 83, 87). In terms of therapeutic response, NSCLC patients who have a partial response to Celecoxib and Erlotinib treatment show a decrease in sE-cad levels at 8 weeks (91). sE-cad is also an indicator of patient survival: esophageal squamous cell carcinoma, gastric cancer, and multiple myeloma patients with higher levels of sE-cad have a much shorter survival time than lower sE-cad patients (80, 84, 88). These studies provide convincing evidence that sE-cad may provide additional information on disease severity, progression, recurrence, and therapeutic response which could aid in determining appropriate therapeutic intervention.
ABBREVIATIONS
- E-cadherin
epithelial cadherin
- sE-cad
soluble E-cadherin
- ADAM
A Disintegrin and Metalloprotease
- MMP
matrix metalloprotease
- KLRG1
Killer lectin receptor G1
- EC
extracellular domain
- NK
natural killer
- BPH
benign prostatic hyperplasia
- MDCK
Madin-Darby canine kidney
- Il-1 beta
Interleukin-1 beta
- TNF-alpha
tumor necrosis factor alpha
- IFN-gamma
interferon gamma
- LPS
lipopolysaccharide
- CTF
C-terminal fragment
- H. pylori
Helicobacter pylori
- EGF
epidermal growth factor
- PKD1
protein kinase D1
- shRNA
short hairpin RNA
- uPA
urokinase-type plasminogen activator
- LPA
Lysophosphatidic acid
- TIMP
tissue inhibitor of metalloprotease
- CRT
chemoradiation therapy
DOI LINKED REFERENCES
- 1.Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995;267(5196):386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- 2.Boggon Titus J, Murray John, Chappuis-Flament Sophie, Wong Ellen, Gumbiner Barry M, Shapiro Lawrence. C-Cadherin Ectodomain Structure and Implications for Cell Adhesion Mechanisms. Science. 2002;296(5571):1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 3.Kilshaw PJ. Alpha E beta 7. Mol Pathol. 1999;52(4):203–207. doi: 10.1136/mp.52.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundemann Carsten, Bauer Monika, Schweier Oliver, von Oppen Nanette, Lassing Ute, Saudan Philippe, Becker Karl-Friedrich, Karp Klaus, Hanke Thomas, Bachmann Martin F. Hanspeter Pircher: Cutting Edge: Identification of E-Cadherin as a Ligand for the Murine Killer Cell Lectin-Like Receptor G1. The Journal of Immunology. 2006;176(3):1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 5.Ito Masayuki, Maruyama Takuma, Saito Naotoshi, Koganei Satoru, Yamamoto Kazuo, Matsumoto Naoki. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. The Journal of Experimental Medicine. 2006;203(2):289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Seiko, Kuroki Kimiko, Ohki Izuru, Sasaki Kaori, Kajikawa Mizuho, Maruyama Takuma, Ito Masayuki, Kameda Yosuke, Ikura Mitsuhiko, Yamamoto Kazuo, Matsumoto Naoki, Maenaka Katsumi. Molecular Basis for E-cadherin Recognition by Killer Cell Lectin-like Receptor G1 (KLRG1) Journal of Biological Chemistry. 2009;284(40):27327–27335. doi: 10.1074/jbc.M109.038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Yih-Tai, Stewart Daniel B, James Nelson W. Coupling Assembly of the E-Cadherin/Beta-Catenin Complex to Efficient Endoplasmic Reticulum Exit and Basal-lateral Membrane Targeting of E-Cadherin in Polarized MDCK Cells. The Journal of Cell Biology. 1999;144(4):687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasioukhin Valeri, Bauer Christoph, Yin Mei, Fuchs Elaine. Directed Actin Polymerization Is the Driving Force for Epithelial Cell Cell Adhesion. Cell. 2000;100(2):209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 9.Drees Frauke, Pokutta Sabine, Yamada Soichiro, James Nelson W, Weis William I. Alpha-Catenin Is a Molecular Switch that Binds E-Cadherin-Beta-Catenin and Regulates Actin-Filament Assembly. Cell. 2005;123(5):903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ireton Renee C, Davis Michael A, van Hengel Jolanda, Mariner Deborah J, Barnes Kirk, Thoreson Molly A, Anastasiadis Panos Z, Matrisian Linsey, Bundy Linda M, Sealy Linda, Gilbert Barbara, van Roy Frans, Reynolds Albert B. A novel role for p120 catenin in E-cadherin function. The Journal of Cell Biology. 2002;159(3):465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbiner Barry M. Regulation of Cadherin Adhesive Activity. The Journal of Cell Biology. 2000;148(3):399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. Journal of Cellular Biochemistry. 1987;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- 13.Schirrmeister Wiebke, Gnad Thorsten, Wex Thomas, Higashiyama Shigeki, Wolke Carmen, Naumann Michael, Lendeckel Uwe. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Experimental Cell Research. 2009;315(20):3500–3508. doi: 10.1016/j.yexcr.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Maretzky Thorsten, Scholz Felix, Koten Bente, Proksch Ehrhardt, Saftig Paul, Reiss Karina. ADAM10-Mediated E-Cadherin Release Is Regulated by Proinflammatory Cytokines and Modulates Keratinocyte Cohesion in Eczematous Dermatitis. J Invest Dermatol. 2008;128(7):1737–1746. doi: 10.1038/sj.jid.5701242. [DOI] [PubMed] [Google Scholar]
- 15.Billion K, Ibrahim H, Mauch C, Niessen CM. Increased Soluble E-Cadherin in Melanoma Patients. Skin Pharmacology and Physiology. 2006;19(2):65–70. doi: 10.1159/000091972. [DOI] [PubMed] [Google Scholar]
- 16.Najy Abdo J, Day Kathleen C, Day Mark L. The Ectodomain Shedding of E-cadherin by ADAM15 Supports ErbB Receptor Activation. Journal of Biological Chemistry. 2008;283(26):18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz Jannet, Yang Qiu-Bo, Zhang Ping, Potempa Jan, Travis James, Michalek Suzanne M, Balkovetz Daniel F. Hydrolysis of Epithelial Junctional Proteins by Porphyromonas gingivalis Gingipains. Infection and Immunity. 2002;70:2512–2518. doi: 10.1128/IAI.70.5.2512-2518.2002. http://dx.doi.org/10.1128/IAI.70.5.2512-2518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Shaoguang, Lim Kuei-cheng, Huan Julie, Saidi Roxan F, Sears Cynthia. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14979–14984. doi: 10.1073/pnas.95.25.14979. http://dx.doi.org/10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gocheva Vasilena, Zeng Wei, Ke Danxia, Klimstra David, Reinheckel Thomas, Peters Christoph, Hanahan Douglas, Joyce Johanna A. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes & Development. 2006;20:543–556. doi: 10.1101/gad.1407406. http://dx.doi.org/10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson Sarah K, Ramani Vishnu C, Hennings Leah, Haun Randy S. Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer. 2007;109:1811–1820. doi: 10.1002/cncr.22606. http://dx.doi.org/10.1002/cncr.22606. [DOI] [PubMed] [Google Scholar]
- 21.Helal Uddin Biswas M, Du Cheng, Zhang Chuanyou, Straubhaar Juerg, Languino Lucia R, Balaji KC. Protein Kinase D1 Inhibits Cell Proliferation through Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Secretion in Prostate Cancer. Cancer Research. 2010;70:2095–2104. doi: 10.1158/0008-5472.can-09-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. Journal of Cell Science. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Lochter Andre, Galosy Sybille, Muschler John, Freedman Neal, Werb Zena, Bissell Mina J. Matrix Metalloproteinase Stromelysin-1 Triggers a Cascade of Molecular Alterations That Leads to Stable Epithelial-to-Mesenchymal Conversion and a Premalignant Phenotype in Mammary Epithelial Cells. Journal of Cell Biology. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. http://dx.doi.org/10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim J-R. Association of Extracellular Cleavage of E-Cadherin Mediated by MMP-7 with HGF-Induced in vitro Invasion in Human Stomach Cancer Cells. European Surgical Research. 2007;39:208–215. doi: 10.1159/000101452. http://dx.doi.org/10.1159/000101452. [DOI] [PubMed] [Google Scholar]
- 25.Davies Gaynor, Jiang Wen G, Mason Malcolm D. Matrilysin Mediates Extracellular Cleavage of E-Cadherin from Prostate Cancer Cells. Clinical Cancer Research. 2001;7:3289–3297. [PubMed] [Google Scholar]
- 26.McGuire John K, Li Qinglang, Parks William C. Matrilysin (Matrix Metalloproteinase-7) Mediates E-Cadherin Ectodomain Shedding in Injured Lung Epithelium. American Journal of Pathology. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. http://dx.doi.org/10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symowicz Jaime, Adley Brian P, Gleason Kara J, Johnson Jeffrey J, Ghosh Supurna, Fishman David A, Hudson Laurie G, Sharon Stack M. Engagement of Collagen-Binding Integrins Promotes Matrix Metalloproteinase-9-Dependent E-Cadherin Ectodomain Shedding in Ovarian Carcinoma Cells. Cancer Research. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. http://dx.doi.org/10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 28.Zuo Jian-Hong, Zhu Wei, Li Mao-Yu, Li Xin-Hui, Yi Hong, Zeng Gu-Qiang, Wan Xun-Xun, He Qiu-Yan, Li Jian-Huang, Qu Jia-Quan, Chen Yu, Xiao Zhi-Qiang. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. Journal of Cellular Biochemistry. 2011;112:2508–17. doi: 10.1002/jcb.23175. http://dx.doi.org/10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 29.Covington Marisa D, Burghardt Robert C, Parrish Alan R. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14) American Journal of Physiology - Renal Physiology. 2006;290:F43–F51. doi: 10.1152/ajprenal.00179.2005. http://dx.doi.org/10.1152/ajprenal.00179.2005. [DOI] [PubMed] [Google Scholar]
- 30.Gil Orlando D, Lee Catherine, Ariztia Edgardo V, Wang Feng-Qiang, Smith Phillip J, Hope Joanie Mayer, Fishman David A. Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner. Gynecologic Oncology. 2008;108:361–369. doi: 10.1016/j.ygyno.2007.10.027. http://dx.doi.org/10.1016/j.ygyno.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Ryniers Filip, Stove Christophe, Goethals Marc, Brackenier Liesbeth, Noe Veerle, Bracke Marc, Vandekerckhove Joel, Mareel Marc, Bruyneel Erik. Plasmin Produces an E-Cadherin Fragment That Stimulates Cancer Cell Invasion. Biological Chemistry. 2002;383:159–165. doi: 10.1515/BC.2002.016. http://dx.doi.org/10.1515/BC.2002.016. [DOI] [PubMed] [Google Scholar]
- 32.Marambaud Philippe, Shioi Junichi, Serban Geo, Georgakopoulos Anastasios, Sarner Shula, Nagy Vanja, Baki Lia, Wen Paul, Efthimiopoulos Spiros, Shao Zhiping, Wisniewski Thomas, Robakis Nikolaos K. A presenilin-1/[gamma]-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO Journal. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. http://dx.doi.org/10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiss Alexi, Troyanovsky Regina B, Troyanovsky Sergey M. p120-Catenin Is a Key Component of the Cadherin-gamma-Secretase Supercomplex. Molecular Biology of the Cell. 2008;19:4042–4050. doi: 10.1091/mbc.E08-04-0394. http://dx.doi.org/10.1091/mbc.E08-04-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferber Emma C, Kajita Mihoko, Wadlow Anthony, Tobiansky Lara, Niessen Carien, Ariga Hiroyoshi, Daniel Juliet, Fujita Yasuyuki. A Role for the Cleaved Cytoplasmic Domain of E-cadherin in the Nucleus. Journal of Biological Chemistry. 2008;283:12691–12700. doi: 10.1074/jbc.M708887200. http://dx.doi.org/10.1074/jbc.M708887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rios-Doria Jonathan, Day Mark L. Truncated E-cadherin potentiates cell death in prostate epithelial cells. Prostate. 2005;63:259–268. doi: 10.1002/pros.20179. http://dx.doi.org/10.1002/pros.20179. [DOI] [PubMed] [Google Scholar]
- 36.Steinhusen Ulrike, Weiske Jorg, Badock Volker, Tauber Rudolf, Bommert Kurt, Huber Otmar. Cleavage and Shedding of E-cadherin after Induction of Apoptosis. Journal of Biological Chemistry. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. http://dx.doi.org/10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- 37.Edwards Dylan R, Handsley Madeleine M, Pennington Caroline J. The ADAM metalloproteinases. Molecular Aspects of Medicine. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. http://dx.doi.org/10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Sophia B, Schramme Anja, Doberstein Kai, Dummer Reinhard, Abdel-Bakky Mohamed S, Keller Sascha, Altevogt Peter, Oh Shin T, Reichrath Jorg, Oxmann Daniel, Pfeilschifter Josef, Mihic-Probst Daniela, Gutwein Paul. ADAM10 Is Upregulated in Melanoma Metastasis Compared with Primary Melanoma. Journal of Investigative Dermatology. 2009;130:763–773. doi: 10.1038/jid.2009.335. http://dx.doi.org/10.1038/jid.2009.335. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura Tetsuro, Tomita Toshihiko, Dixon Michael F, Axon Anthony TR, Robinson Philip A, Crabtree Jean E. ADAMs (A Disintegrin and Metalloproteinase) Messenger RNA Expression in Helicobacter pylori- Infected, Normal, and Neoplastic Gastric Mucosa. Journal of Infectious Diseases. 2002;185:332–340. doi: 10.1086/338191. http://dx.doi.org/10.1086/338191. [DOI] [PubMed] [Google Scholar]
- 40.Arima Takashi, Enokida Hideki, Kubo Hiroyuki, Kagara Ichiro, Matsuda Ryouichirou, Toki Kazuki, Nishimura Hiroaki, Chiyomaru Takeshi, Tatarano Shuichi, Idesako Toshihiko, Nishiyama Kenryu, Nakagawa Masayuki. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Science. 2007;98:1720–1726. doi: 10.1111/j.1349-7006.2007.00601.x. http://dx.doi.org/10.1111/j.1349-7006.2007.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamont Richard J, Jenkinson Howard F. Life Below the Gum Line: Pathogenic Mechanisms of Porphyromonas gingivalis. Microbiology and Molecular Biology Reviews. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz Jannet, Sambandam Vijaya, Wu John H, Michalek Suzanne M, Balkovetz Daniel F. Characterization of Porphyromonas gingivalis-Induced Degradation of Epithelial Cell Junctional Complexes. Infection and Immunity. 2000;68:1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. http://dx.doi.org/10.1128/IAI.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turk Boris, Turk Vito. Lysosomes as “Suicide Bags” in Cell Death: Myth or Reality? Journal of Biological Chemistry. 2009;284:21783–21787. doi: 10.1074/jbc.R109.023820. http://dx.doi.org/10.1074/jbc.R109.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vreemann Anna, Qu Hong, Mayer Kristina, Andersen Louise Bjorkholt, Irina Stefana M, Wehner Sven, Lysson Mariola, Farcas Anca M, Peters Christoph, Reinheckel Thomas, Kalff Jorg, Brix Klaudia. Cathepsin B release from rodent intestine mucosa due to mechanical injury results in extracellular matrix damage in early post-traumatic phases. Biological Chemistry. 2009;390:481–492. doi: 10.1515/BC.2009.055. http://dx.doi.org/10.1515/BC.2009.055. [DOI] [PubMed] [Google Scholar]
- 45.Mai Jianxin, Finley Russell L, Waisman David M, Sloane Bonnie F. Human Procathepsin B Interacts with the Annexin II Tetramer on the Surface of Tumor Cells. Journal of Biological Chemistry. 2000;275:12806–12812. doi: 10.1074/jbc.275.17.12806. http://dx.doi.org/10.1074/jbc.275.17.12806. [DOI] [PubMed] [Google Scholar]
- 46.Niedergethmann Marco, Wostbrock Birgit, Sturm Jorg W, Willeke Frank, Post Stefan, Hildenbrand Ralf. Prognostic Impact of Cysteine Proteases Cathepsin B and Cathepsin L in Pancreatic Adenocarcinoma. Pancreas. 2004;29:204–211. doi: 10.1097/00006676-200410000-00005. http://dx.doi.org/10.1097/00006676-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Yousef George M, Scorilas Andreas, Magklara Angeliki, Soosaipillai Antoninus, Diamandis Eleftherios P. The KLK7 (PRSS6) gene, encoding for the stratum corneum chymotryptic enzyme is a new member of the human kallikrein gene family -- genomic characterization, mapping, tissue expression and hormonal regulation. Gene. 2000;254:119–128. doi: 10.1016/s0378-1119(00)00280-8. http://dx.doi.org/10.1016/S0378-1119(00)00280-8. [DOI] [PubMed] [Google Scholar]
- 48.Overall Christopher Mark, Lopez-Otin Carlos. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. http://dx.doi.org/10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 49.Trudel Dominique, Fradet Yves, Meyer Francois, Harel Francois, Tetu Bernard. Significance of MMP-2 Expression in Prostate Cancer. Cancer Research. 2003;63:8511–8515. [PubMed] [Google Scholar]
- 50.Garbett EA, Reed MWR, Brown NJ. Proteolysis in human breast and colorectal cancer. British Journal of Cancer. 1999;81:287–293. doi: 10.1038/sj.bjc.6690689. http://dx.doi.org/10.1038/sj.bjc.6690689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiomi Takayuki, Okada Yasunori. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer and Metastasis Reviews. 2003;22:145–152. doi: 10.1023/A:1023039230052. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto Kunihiro, Kihira Yasunori, Matuo Yuhsi, Usui Tsuguru. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. Journal of Urology. 1998;160:1872–1876. doi: 10.1016/S0022-5347(01)62435-2. [DOI] [PubMed] [Google Scholar]
- 53.Lee Kyung Hee, Shin Sang Joon, Kim Kyeong Ok, Kim Min Kyoung, Hyun Myung Soo, Kim Tae Nyeun, Jang Byung Ik, Kim Sang Woon, Song Sun Kyo, Kim Hee Sun, Bae Sung Hwa, Ryoo Hun Mo. Relationship between E-cadherin, matrix metalloproteinase-7 gene expression and clinicopathological features in gastric carcinoma. Oncology Reports. 2006;16:823–830. [PubMed] [Google Scholar]
- 54.Jiang Wen G, Davies Gaynor, Martin Tracey A, Parr Christian, Watkins Gareth, Mason Malcolm D, Mokbel Kefah, Mansel Robert E. Targeting Matrilysin and Its Impact on Tumor Growth In vivo: The Potential Implications in Breast Cancer Therapy. Clinical Cancer Research. 2005;11:6012–6019. doi: 10.1158/1078-0432.ccr-05-0275. [DOI] [PubMed] [Google Scholar]
- 55.Manicone Anne M, Huizar Isham, McGuire John K. Matrilysin (Matrix Metalloproteinase-7) Regulates Anti-Inflammatory and Antifibrotic Pulmonary Dendritic Cells That Express CD103 (AlphaEBeta7-Integrin) American Journal of Pathology. 2009;175:2319–2331. doi: 10.2353/ajpath.2009.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. Journal of Clinical Investigation. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Shi J, Feng J, Klocker H, Lee C, Zhang J. Type IV collagenase (matrix metalloproteinase-2 and -9) in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:327–332. doi: 10.1038/sj.pcan.4500750. [DOI] [PubMed] [Google Scholar]
- 58.Alshenawy Hanan AlSaeid. Immunohistochemical expression of epidermal growth factor receptor, E-cadherin, and matrix metalloproteinase-9 in ovarian epithelial cancer and relation to patient deaths. Annals of Diagnostic Pathology. 2010;14:387–395. doi: 10.1016/j.anndiagpath.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Riedel F, Gotte K, Schwalb J, Hormann K. Serum levels of matrix metalloproteinase-2 and -9 in patients with head and neck squamous cell carcinoma. Anticancer Research. 2000;20:3045–3049. [PubMed] [Google Scholar]
- 60.Muhs Bart E, Plitas George, Delgado Yara, Ianus Ioana, Shaw Jason P, Adelman Mark A, Lamparello Patrick, Shamamian Peter, Gagne Paul. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. Journal of Surgical Research. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 61.McMahon B, Kwaan HC. The Plasminogen Activator System and Cancer. Pathophysiology of Haemostasis and Thrombosis. 2007;36:184–194. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- 62.Pustilnik Terri B, Estrella Veronica, Wiener Jon R, Mao Muling, Eder Astrid, Watt Mary-Anne V, Bast Robert C, Mills Gordon B. Lysophosphatidic Acid Induces Urokinase Secretion by Ovarian Cancer Cells. Clinical Cancer Research. 1999;5:3704–3710. [PubMed] [Google Scholar]
- 63.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical Cancer Research. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 64.Kuhn W, Schmalfeldt B, Reuning U, Pache L, Berger U, Ulm K, Harbeck N, Spathe K, Dettmar P, Hofler H, Janicke F, Schmitt M, Graeff H. Prognostic significance of urokinase (uPA) and its inhibitor PAI-1 for survival in advanced ovarian carcinoma stage FIGO IIIc. British Journal of Cancer. 1999;79:1746–1751. doi: 10.1038/sj.bjc.6690278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito Kiyoharu, Okamoto Isamu, Araki Norie, Kawano Yoshiaki, Nakao Mitsuyoshi, Fujiyama Shigetoshi, Tomita Kimio, Mimori Tatsuyuki, Saya Hideyuki. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- 66.Pittard AJ, Banks RE, Galley HF, Webster NR. Soluble E-cadherin concentrations in patients with systemic inflammatory response syndrome and multiorgan dysfunction syndrome. British Journal of Anaesthesia. 1996;76:629–631. doi: 10.1093/bja/76.5.629. [DOI] [PubMed] [Google Scholar]
- 67.Karayiannakis AJ, Syrigos KN, Savva A, Polychronidis A, Karatzas G, Simopoulos C. Serum E-cadherin concentrations and their response during laparoscopic and open cholecystectomy. Surgical Endoscopy. 2002;16:1551–1554. doi: 10.1007/s00464-001-9221-4. [DOI] [PubMed] [Google Scholar]
- 68.Goto Taichiro, Ishizaka Akitoshi, Katayama Masahiko, Kohno Mitsutomo, Tasaka Sadatomo, Fujishima Seitaro, Kobayashi Koichi, Nomori Hiroaki. Involvement of E-cadherin cleavage in reperfusion injury. European Journal of Cardio-Thoracic Surgery. 2010;37:426–431. doi: 10.1016/j.ejcts.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 69.McCawley Lisa J, Matrisian Lynn M. Matrix metalloproteinases: they’re not just for matrix anymore! Current Opinion in Cell Biology. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 70.Egeblad Mikala, Werb Zena. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 71.Mazzieri Roberta, Masiero Laura, Zanetta Lucia, Monea Sara, Onisto Maurizio, Garbisa Spiridione, Mignatti Paolo. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO Journal. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katayama M, Hirai S, Kamihagi K, Nakagawa K, Yasumoto M, Kato I. Soluble E-cadherin fragments increased in circulation of cancer patients. British Journal of Cancer. 1994;69:580–585. doi: 10.1038/bjc.1994.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streeck Hendrik, Kwon Douglas S, Pyo Augustine, Flanders Michael, Chevalier Mathieu F, Law Kenneth, Julg Boris, Trocha Kasper, Jolin Jonathan S, Anahtar Melis N, Lian Jeff, Toth Ildiko, Brumme Zabrina, Judy Chang J, Caron Tyler, Rodig Scott J, Milner Danny A, Piechoka-Trocha Alicja, Kaufmann Daniel E, Walker Bruce D, Altfeld Marcus. Epithelial adhesion molecules can inhibit HIV-1-specific CD8+ T-cell functions. Blood. 2011;117:5112–5122. doi: 10.1182/blood-2010-12-321588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuefer R, Hofer MD, Zorn CSM, Engel O, Volkmer BG, Juarez-Brito MA, Eggel M, Gschwend JE, Rubin MA, Day ML. Assessment of a fragment of e-cadherin as a serum biomarker with predictive value for prostate cancer. British Journal of Cancer. 2005;92:2018–2023. doi: 10.1038/sj.bjc.6602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffiths TR, Brotherick I, Bishop RI, White MD, McKenna DM, Horne CH, Shenton BK, Neal DE, Mellon JK. Cell adhesion molecules in bladder cancer: soluble serum E-cadherin correlates with predictors of recurrence. British Journal of Cancer. 1996;74:579–584. doi: 10.1038/bjc.1996.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Protheroe AS, Banks RE, Mzimba M, Porter WH, Southgate J, Singh PN, Bosomworth M, Harnden P, Smith PH, Whelan P, Selby PJ. Urinary concentrations of the soluble adhesion molecule E-cadherin and total protein in patients with bladder cance. British Journal of Cancer. 1999;80:273–278. doi: 10.1038/sj.bjc.6690351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi B, Laudon V, Yu S, Dong D, Zhu Y, Xu Z. E-cadherin tissue expression and urinary soluble forms of E-cadherin in patients with bladder transitional cell carcinoma. Urologia Internationalis. 2008;81:320–4. doi: 10.1159/000151412. [DOI] [PubMed] [Google Scholar]
- 78.Velikova G, Banks RE, Gearing A, Hemingway I, Forbes MA, Preston SR, Hall NR, Jones M, Wyatt J, Miller K, Ward U, Al-Maskatti J, Singh SM, Finan PJ, Ambrose NS, Primrose JN, Selby PJ. Serum concentrations of soluble adhesion molecules in patients with colorectal cancer. British Journal of Cancer. 1996;77:1857–1863. doi: 10.1038/bjc.1998.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilmanns C, Grossmann J, Steinhauer S, Manthey G, Weinhold B, Schmitt-Gräff A, von Specht BU. Soluble serum E-cadherin as a marker of tumour progression in colorectal cancer patients. Clinical and Experimental Metastasis. 2004;21:75–78. doi: 10.1023/B:CLIN.0000017204.38807.22. [DOI] [PubMed] [Google Scholar]
- 80.Chung Y, Law S, Kwong DLW, Luk JM. Serum soluble E-cadherin is a potential prognostic marker in esophageal squamous cell carcinoma. Diseases of the Esophagus. 2011;24:49–55. doi: 10.1111/j.1442-2050.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 81.Gofuku J, Shiozaki H, Doki Y, Inoue M, Hirao M, Fukuchi N, Monden M. Characterization of soluble E-cadherin as a disease marker in gastric cancer patients. British Journal of Cancer. 1998;78:1095–1101. doi: 10.1038/bjc.1998.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan A, Lam S, Chu K, Lam C, Kwok E, Leung S, Yuen S, Law S, Hui W, Lai K, Wong C, Hu H, Lai C, Wong J. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808–811. doi: 10.1136/gut.48.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan Annie On On, Chu Kent-Man, Lam Shiu Kum, Cheung Kwan Lok, Law Simon, Kwok Ka-Fai, Wong Wai Man, Yuen Man Fung, Wong Benjamin Chun-Yu. Early prediction of tumor recurrence after curative resection of gastric carcinoma by measuring soluble E-cadherin. Cancer. 2005;104:740–746. doi: 10.1002/cncr.21260. [DOI] [PubMed] [Google Scholar]
- 84.Chan Annie On-On, Chu Kent-Man, Lam Shiu-Kum, Wong Benjamin Chun-Yu, Kwok Ka-Fai, Law Simon, Ko Samuel, Hui Wai-Mo, Yueng Yui-Hung, Won John. Soluble E-cadherin is an independent pretherapeutic factor for long-term survival in gastric cancer. Journal of Clinical Oncology. 2003;15:2288–93. doi: 10.1200/JCO.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 85.Pedrazzani Corrado, Caruso Stefano, Corso Giovanni, Marrelli Daniele, Neri Alessandro, Berardi Anna, Roviello Franco. Influence of age on soluble E-cadherin serum levels prevents its utility as a disease marker in gastric cancer patients. Scandinavian Journal of Gastroenterology. 2008;43:765–766. doi: 10.1080/00365520802037370. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Yongning, Ran Juntao, Tang Chenwei, Wu Jing, Honghua Li, Xingwen Li, Chen Ning, Qiao Liang. Effect of celecoxib on E-cadherin, VEGF, microvessel density and apoptosis in gastric cancer. Cancer Biology and Therapy. 2007;6:269–275. doi: 10.4161/cbt.6.2.3629. [DOI] [PubMed] [Google Scholar]
- 87.Soyama A, Eguchi S, Takatsuki M, Kawashita Y, Hidaka M, Tokai H, Nagayoshi S, Mochizuki S, Matsumoto S, Hamasaki K, Tajima Y, Kanematsu T. Significance of the serum level of soluble E-cadherin in patients with HCC. Hepato- Gastroenterology. 2008;55:1390–1393. [PubMed] [Google Scholar]
- 88.Syrigos KN, Harrington KJ, Karayiannakis AJ, Baibas N, Katirtzoglou N, Roussou P. Circulating Soluble E-Cadherin Levels are of Prognostic Significance in Patients with Multiple Myeloma. Anticancer Research. 2004;24:2027–2031. [PubMed] [Google Scholar]
- 89.Charalabopoulos K, Gogali A, Dalavaga Y, Daskalopoulos G, Vassiliou M, Bablekos G, Karakosta A, Constantopoulos S. The clinical significance of soluble E-cadherin in nonsmall cell lung cancer. Experimental Oncology. 2006;28:83–85. [PubMed] [Google Scholar]
- 90.Gogali Athena, Charalabopoulos Konstantinos, Zampira Iris, Konstantinidis Athanasios K, Tachmazoglou Fanny, Daskalopoulos George, Constantopoulos Stavros H, Dalavanga Yotanna. Soluble Adhesion Molecules E-Cadherin, Intercellular Adhesion Molecule-1, and E-Selectin as Lung Cancer Biomarkers. Chest. 2010;138:1173–1179. doi: 10.1378/chest.10-0157. [DOI] [PubMed] [Google Scholar]
- 91.Reckamp Karen L, Gardner Brian K, Figlin Robert A, Elashoff David, Krysan Kostyantyn, Dohadwala Mariam, Mao Jenny, Sharma Sherven, Inge Landon, Rajasekaran Ayyappan, Dubinett Steven M. Tumor Response to Combination Celecoxib and Erlotinib Therapy in Non-small Cell Lung Cancer Is Associated with a Low Baseline Matrix Metalloproteinase-9 and a Decline in Serum-Soluble E-Cadherin. Journal of Thoracic Oncology. 2008;3:117–124. doi: 10.1097/JTO.0b013e3181622bef. [DOI] [PubMed] [Google Scholar]
- 92.Darai E, Bringuier AF, Walker-Combrouze F, Feldmann G, Madelenat P, Scoazec JY. Soluble adhesion molecules in serum and cyst fluid from patients with cystic tumours of the ovary. Human Reproduction. 1998;13:2831–2835. doi: 10.1093/humrep/13.10.2831. [DOI] [PubMed] [Google Scholar]
- 93.Kuefer Rainer, Hofer Matthias D, Gschwend Juergen E, Pienta Kenneth J, Sanda Martin G, Chinnaiyan Arul M, Rubin Mark A, Day Mark L. The Role of an 80 kDa Fragment of E-cadherin in the Metastatic Progression of Prostate Cancer. Clinical Cancer Research. 2003;9:6447–6452. [PubMed] [Google Scholar]
- 94.Shirahama Shigeho, Furukawa Fukumi, Wakita Hisashi, Takigawa Masahiro. E- and P-cadherin expression in tumor tissues and soluble E-cadherin levels in sera of patients with skin cancer. Journal of Dermatological Science. 1996;13:30–36. doi: 10.1016/0923-1811(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 95.Sewpaul A, French JJ, Khoo TK, Kernohan M, Kirby JA, Charnley RM. Soluble E-Cadherin. An Early Marker of Severity in Acute Pancreatitis. HPB Surgery. 2009;6(2009) doi: 10.1155/2009/397375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang Hongjuan, Guan Guangju, Zhang Rui, Liu Gang, Cheng Jing, Hou Xianghua, Cui Yazhou. Identification of urinary soluble E-cadherin as a novel biomarker for diabetic nephropathy. Diabetes/Metabolism Research and Reviews. 2009;25:232–241. doi: 10.1002/dmrr.940. [DOI] [PubMed] [Google Scholar]
- 97.Ryniers Filip, Stove Christophe, Goethals Marc, Brackenier Liesbeth, Noë Veerle, Bracke Marc, Vandekerckhove Joël, Mareel Marc, Bruyneel Erik. Plasmin Produces an E-Cadherin Fragment That Stimulates Cancer Cell Invasion. Biological Chemistry. 2002;383:159–165. doi: 10.1515/BC.2002.016. [DOI] [PubMed] [Google Scholar]
- 98.Nawrocki-Raby Béatrice, Gilles Christine, Polette Myriam, Bruyneel Erik, Laronze Jean-Yves, Bonnet Noël, Foidart Jean-Michel, Mareel Marc, Birembaut Philippe. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. International Journal of Cancer. 2003;105:790–795. doi: 10.1002/ijc.11168. [DOI] [PubMed] [Google Scholar]
- 99.Inge Landon J, Barwe Sonali P, D’Ambrosio Julia, Gopal Jegan, Lu Kan, Ryazantsev Sergey, Rajasekaran Sigrid A, Rajasekaran Ayyappan K. Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Experimental Cell Research. 2011;317:838–848. doi: 10.1016/j.yexcr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 100.Liu Xiangdong, Fridman Jordan S, Wang Qian, Caulder Eian, Yang Gengjie, Covington Maryanne, Liu Changnian, Marando Cindy, Zhuo Jincong, Li Yanlong, Yao Wenqing, Vaddi Kris, Newton Robert C, Scherle Peggy A, Friedma Steven M. Selective inhibition of ADAM metalloproteases blocks HER-2 extracellular domain (ECD) cleavage and potentiates the anti-tumor effects of trastuzumab. Cancer Biology and Therapy. 2006;5:648–656. doi: 10.4161/cbt.5.6.2707. [DOI] [PubMed] [Google Scholar]
- 101.Moss Marcia L, Bomar Martha, Liu Qian, Sage Harvey, Dempsey Peter, Lenhart Patricia M, Gillispie Patricia A, Stoeck Alexander, Wildeboer Dirk, Bartsch Jorg W, Palmisano Ralf, Zhou Pei. The ADAM10 Prodomain Is a Specific Inhibitor of ADAM10 Proteolytic Activity and Inhibits Cellular Shedding Events. Journal of Biological Chemistry. 2007;282:35712–35721. doi: 10.1074/jbc.M703231200. [DOI] [PubMed] [Google Scholar]