Abstract
BACKGROUND
The Primary Immune Deficiency Treatment Consortium was formed to analyze the results of hematopoietic-cell transplantation in children with severe combined immunodeficiency (SCID) and other primary immunodeficiencies. Factors associated with a good transplantation outcome need to be identified in order to design safer and more effective curative therapy, particularly for children with SCID diagnosed at birth.
METHODS
We collected data retrospectively from 240 infants with SCID who had received transplants at 25 centers during a 10-year period (2000 through 2009).
RESULTS
Survival at 5 years, freedom from immunoglobulin substitution, and CD3+ T-cell and IgA recovery were more likely among recipients of grafts from matched sibling donors than among recipients of grafts from alternative donors. However, the survival rate was high regardless of donor type among infants who received transplants at 3.5 months of age or younger (94%) and among older infants without prior infection (90%) or with infection that had resolved (82%). Among actively infected infants without a matched sibling donor, survival was best among recipients of haploidentical T-cell–depleted transplants in the absence of any pretransplantation conditioning. Among survivors, reduced-intensity or myeloablative pre-transplantation conditioning was associated with an increased likelihood of a CD3+ T-cell count of more than 1000 per cubic millimeter, freedom from immunoglobulin substitution, and IgA recovery but did not significantly affect CD4+ T-cell recovery or recovery of phytohemagglutinin-induced T-cell proliferation. The genetic subtype of SCID affected the quality of CD3+ T-cell recovery but not survival.
CONCLUSIONS
Transplants from donors other than matched siblings were associated with excellent survival among infants with SCID identified before the onset of infection. All available graft sources are expected to lead to excellent survival among asymptomatic infants. (Funded by the National Institute of Allergy and Infectious Diseases and others.)
Severe combined immunodeficiency (SCID) is a genetically heterogeneous and lethal disorder of infancy. It is characterized by severe T-cell lymphocytopenia and a lack of antigen-specific T-cell and B-cell immune responses.1
Allogeneic hematopoietic-cell transplantation with the use of bone marrow from an HLA-identical sibling2 or an unrelated donor,3 T-cell–depleted marrow or peripheral-blood stem cells from a haploidentical, related donor,4–7 or umbilical-cord blood8–10 can fully correct the T-cell deficiency and, less consistently, the B-cell deficiency in patients with SCID.2–12 Expanded donor availability and advances in supportive care and treatment of infections have improved long-term outcomes after hematopoietic-cell transplantation.10,13–17 However, owing to the rarity and genetic heterogeneity of SCID, questions remain regarding the contributions of patient characteristics, type of donor and transplant, and conditioning regimen, if used, to survival, immune reconstitution, and the long-term outcome. The development of widespread screening of newborns for SCID18–21 and reports indicating a survival advantage for children with SCID who receive transplants in the first few months of life15,22–24 have sharpened questions regarding immediate treatment.
The Primary Immune Deficiency Treatment Consortium (PIDTC), a collaborative network of institutions in North America, was formed to conduct rigorous multicenter studies addressing critical questions in the treatment of SCID.25 Here we report a retrospective analysis of data from 240 infants with classic SCID who received hematopoietic-cell transplants at 25 PIDTC institutions during a 10-year period.
METHODS
STUDY PARTICIPANTS
Data on all infants who had received a transplant for SCID at each of 25 centers between January 1, 2000, and December 31, 2009, were reviewed centrally. Of the 285 infants who met the criteria for SCID, the 240 infants with classic SCID (on the basis of an absolute T-cell count of <300 per cubic millimeter and an absence of T-cell responses to mitogens) who had undergone allogeneic hematopoietic-cell transplantation were included in the study (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org; and Shearer et al.26). Coded data (age at diagnosis, family history, lymphocyte phenotype, T-cell and B-cell function, genetic subtype, and infection history) were entered into an electronic database with institutional-review-board approval at all centers. Lymphocyte phenotypes were categorized as B+ (B-cell count, >400 per cubic millimeter), Blow (50 to 400 per cubic millimeter), or B− (<50 per cubic millimeter) and NK+ (natural killer [NK] cell count, >100 per cubic millimeter), NKlow (40 to 100 per cubic millimeter), or NK− (<40 per cubic millimeter).27,28
TRANSPLANTS
We recorded age at transplantation, infection status, conditioning regimen, donor type, degree of HLA match, cell source, method of T-cell depletion, and graft-versus-host disease (GVHD) pro-phylaxis. Infection status was categorized as no infection before transplantation, infection resolved before transplantation, and active infection at the time of transplantation. The categories of conditioning regimen (Table S1 in the Supplementary Appendix) were none, immunosuppression (regimens containing one or more of the following: fludarabine, cyclophosphamide, anti-thymocyte globulin, or alemtuzumab), reduced-intensity conditioning (regimens containing melphalan, anti-CD45 antibodies, 200 to 400 cGy of total-body irradiation, or busulfan administered at a total dose of <12 mg per kilogram of body weight), and myeloablative conditioning (regimens containing busulfan at a total dose =12 mg per kilogram). A boost was defined as an additional transplant from the same donor without conditioning. A second transplant was defined as an additional transplant from a different donor (with or without conditioning) or from the same donor with conditioning.
IMMUNE RECONSTITUTION
Data collected at 100 days, at 6 months, and at 1, 2 to 5, and 6 to 10 years after transplantation included absolute numbers of CD3+ T cells, CD19+ or CD20+ B cells, and CD3-CD56+ or CD16+CD56+ NK cells; proliferative response to phytohemagglutinin; serum concentrations of IgG, IgA, and IgM; treatment with intravenous immune globulin (IVIG); and whole-blood and lineage-specific chimerism. T-cell immune reconstitution was defined as a CD3+ count of more than 1000 per cubic millimeter,16 a CD4+ count of more than 500 per cubic millimeter,16 a phytohemagglutinin response that was more than 30% of the lower limit of the normal range, or a stimulation index (i.e., a response to phytohemagglutinin in counts per minute of radiolabeled thymidine incorporated minus counts per minute of background incorporation, divided by counts per minute of background incorporation) of more than than 50. B-cell reconstitution was defined as IgA recovery and independence from IVIG treatment.
STATISTICAL ANALYSIS
Demographic, disease-related, and transplant-related variables were described with the use of frequencies for categorical variables and the median and range for quantitative variables. The association between variables was assessed with the use of the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. Probabilities of survival after transplantation were calculated with the use of the Kaplan–Meier estimator; data from children who were alive at the last follow-up were censored on that date. Probabilities of a second transplantation, acute GVHD, and chronic GVHD were summarized with the use of a cumulative incidence method, with death considered to be a competing event. Confidence intervals were calculated with the use of a log transformation. Multivariate Cox regression models examining risk factors for transplantation outcomes were built with the use of stepwise forward selection, with a P value of 0.05 or less considered to indicate statistical significance. Variables considered were age at transplantation, sex, race or ethnic group, maternal engraftment, genotype, B-cell and NK-cell phenotypes, family history, infection status, failure to thrive, donor type, use of conditioning, graft type, type of T-cell depletion, and GVHD prophylaxis. All variables met the proportional-hazards assumption.
The prevalence of immune recovery at 2 to 5 years after transplantation was analyzed in the group of children who were alive at 2 years and had not received a second transplant. Frequencies of recovery of CD3+ T cells, CD4+ T cells, phytohemagglutinin responsiveness, donor B cells, and IgA and of the need for IVIG therapy were described, and associations with demographic, disease-related, or transplant-related factors were assessed in a univariate analysis with the use of the chi-square test or Fisher’s exact test. Stepwise multivariate logistic-regression models were built to examine risk factors for each immune-recovery outcome.
RESULTS
CHARACTERISTICS OF THE INFANTS AT DIAGNOSIS
Table 1 summarizes the characteristics of the infants. A genetic cause of SCID was identified in 69% of infants, most frequently X-linked mutations in IL2RG (Table S2 in the Supplementary Appendix). Certain genotypes had a wider phenotypic variation than heretofore reported (Fig. S1 in the Supplementary Appendix).1 Maternal T-cell engraftment29 was documented in 51% of 88 children evaluated (Fig. S1 and Table S2 in the Supplementary Appendix).
Table 1.
Characteristics of the Infants.*
| Characteristic | All Infants (N = 240) |
|---|---|
| Demographic characteristics | |
| Age — no. (%) | |
| ≤3.5 mo | 68 (28) |
| >3.5 mo | 172 (72) |
| Sex — no. (%) | |
| Male | 173 (72) |
| Female | 67 (28) |
| Race or ethnic group — no. (%)† | |
| Non-Hispanic white | 118 (49) |
| Black | 25 (10) |
| Hispanic | 67 (28) |
| Asian or Native Pacific Islander | 9 (4) |
| Native American | 8 (3) |
| Other | 13 (5) |
| Immunologic characteristics‡ | |
| CD3+ T-cell count — per mm3 | |
| Median | 20 |
| Range | 0–9708 |
| T-cell proliferation in response to PHA — no. (%) | |
| <10% of lower limit of normal range | 180 (75) |
| 10–30% of lower limit of normal range | 0 |
| >30% of lower limit of normal range | 1 (<1) |
| Missing data | 59 (25) |
| CD19+ or CD20+ B-cell count — per mm3 | |
| Median | 582 |
| Range | 0–5453 |
| B-cell phenotype — no. (%)§ | |
| B− | 64 (27) |
| Blow | 32 (13) |
| B+ | 129 (54) |
| Missing data | 15 (6) |
| CD3-CD56+ or CD16+CD56+ NK-cell count — per mm3 | |
| Median | 76 |
| Range | 0–2890 |
| NK-cell phenotype — no. (%)¶ | |
| NK− | 84 (35) |
| NKlow | 38 (16) |
| NK+ | 92 (38) |
| Missing data | 26 (11) |
| Infection|| | |
| Ever had infection — no. (%); no. with active infection at time of transplantation (%) | |
| Yes | 171 (71); 106 (62) |
| No | 69 (29) |
| Type of infection — no. (%); no. with active infection at time of transplantation (%) | |
| Bacterial infection | 104 (43); 29 (28) |
| Mycobacterial infection | 2 (1); 2 (100) |
| Pneumocystis jirovecii infection | 61 (25); 13 (21) |
| Respiratory viral infection | 50 (21); 37 (74) |
| RSV infection | 12 (5); 9 (75) |
| Parainfluenza virus infection | 23 (10); 20 (87) |
| Influenza | 8 (3); 4 (50) |
| Rhinovirus infection | 12 (5); 5 (42) |
| DNA viral infection** | 26 (11); 22 (85) |
| CMV infection | 17 (7); 15 (88) |
| EBV infection | 1 (0); 1 (100) |
| Adenovirus infection | 6 (2); 3 (50) |
| HHV-6 infection | 2 (1); 2 (100) |
| Varicella–zoster virus infection | 2 (1); 2 (100) |
| Systemic fungal infection | 20 (8); 9 (45) |
CMV denotes cytomegalovirus, EBV Epstein–Barr virus, HHV-6 human herpesvirus 6, NK natural killer, PHA phytohemagglutinin, and RSV respiratory syncytial virus.
Race or ethnic group was determined from the medical record when available.
The CD3+ T-cell count was measured in 240 infants, the CD19+ or CD20+ B-cell count in 225 infants, and the CD3-CD56+ NK-cell count in 214 infants.
B− was defined as a B-cell count of less than 50 per cubic millimeter, Blow as a count of 50 to 400 per cubic millimeter, and B+ as a count of more than 400 per cubic millimeter.
NK− was defined as an NK-cell count of less than 40 per cubic millimeter, NKlow as a count of 40 to 100 per cubic millimeter, and NK+ as a count of more than 100 per cubic millimeter.
For the percentages of infants with active infection at the time of transplantation, the denominator is the number of infants who ever had infection or ever had that type of infection.
Some infants had more than one DNA viral infection.
Infections were documented before transplantation in 171 of the 240 infants (71%), of whom 106 (62%) remained infected at the time of transplantation (Table 1). Although bacteria and Pneumocystis jirovecii were the most common causes of infection, infections with DNA viruses or respiratory viruses were more likely to be active rather than resolved at the time of transplantation (Table 1).
The median age at diagnosis and at transplantation was 138.5 days and 180.0 days, respectively; the distribution was bimodal, with inflection at approximately 3.5 months for age at transplantation (Table 1, and Fig. S2 in the Supplementary Appendix). As compared with infants older than 3.5 months of age, younger infants were more likely to have a family history of SCID and were less likely to have prior infection, active infection at the time of transplantation, or failure to thrive (Table S3 in the Supplementary Appendix).
TRANSPLANTATION
sCharacteristics of transplants and donors and methods of pretransplantation conditioning and GVHD prophylaxis are shown in Table 2. Most grafts from HLA-matched siblings and phenotypically HLA-matched related donors, as well as transplants of marrow or peripheral-blood stem cells from unrelated donors and cord blood from HLA-mismatched unrelated donors, were unmodified and administered with immunosuppressive drugs as prophylaxis against post-transplantation GVHD. The majority of grafts from haploidentical, HLA-mismatched related donors were T-cell depleted, by means of soybean agglutinin and E-rosette depletion,4–7 CD34 selection,30 or other methods, and administered without additional prophylaxis against GVHD.
Table 2.
Donor and Transplant Characteristics.*
| Characteristic | All Donors (N = 240) | Matched Sibling Donors (N = 32) | Mismatched Related Donors (N = 138) | Other Related Donors (N = 8) | Unrelated Donors (N = 62) |
|---|---|---|---|---|---|
| number (percent) | |||||
| Graft type | |||||
| Bone marrow | 139 (58) | 31 (97) | 85 (62) | 8 (100) | 15 (24) |
| Mobilized peripheral blood | 58 (24) | 1 (3) | 53 (38) | 0 | 4 (6) |
| Umbilical-cord blood | 43 (18) | 0 | 0 | 0 | 43 (69) |
| GVHD prophylaxis | |||||
| None | 15 (6) | 11 (34) | 1 (1) | 1 (12) | 2 (3) |
| T-cell depletion of graft before transplantation | 137 (57) | 3 (9) | 132 (96) | 0 | 2 (3) |
| Soybean agglutination and E-rosette depletion | 71(30) | 1 (3) | 70 (51) | 0 | 0 |
| CD34 selection | 50 (21) | 0 | 50 (36) | 0 | 0 |
| Other T-cell depletion | 16 (7) | 2 (6) | 12 (9) | 0 | 2 (3) |
| Medications given after transplantation | 88 (37) | 18 (56) | 5 (4) | 7 (88) | 58 (94) |
| CNI-based medications | 86 (36) | 18 (56) | 5 (4) | 6 (75) | 57 (92) |
| Other medications | 2 (1) | 0 | 0 | 1 (12) | 1 (2) |
| Conditioning regimen | |||||
| None | 120 (50) | 21 (66) | 87 (63) | 6 (75) | 6 (10) |
| Immunosuppression | 39 (16) | 7 (22) | 16 (12) | 1 (12) | 15 (24) |
| Reduced intensity | 35 (15) | 2 (6) | 10 (7) | 1 (12) | 22 (35) |
| Myeloablative | 46 (19) | 2 (6) | 25 (18) | 0 | 19 (31) |
CNI denotes calcineurin inhibitor, and GVHD graft-versus-host disease.
Although most infants receiving grafts from matched sibling donors (66%) or mismatched related donors (63%) did not undergo conditioning, 90% of infants receiving grafts from unrelated donors or cord-blood grafts underwent immunosuppression, reduced-intensity conditioning, or myeloablative conditioning. The median time from diagnosis to transplantation was longer for recipients of bone marrow or peripheral-blood stem-cell transplants from unrelated donors than for recipients of transplants from other donor types (88.0 days vs. 28.5 to 39.0 days, P<0.001) (Table S2 in the Supplementary Appendix).
T-CELL ENGRAFTMENT AND GRAFT FAILURE
Of the 240 infants, 172 (72%) had engraftment and development of donor T cells with or without donor B cells after receiving a single transplant; the rest received a boost, a second transplant, or both (Table 3). The risk of graft failure was lowest among recipients of grafts from matched sibling donors (Fig. 1A). Donor type (Fig. 1A, and Table S4 in the Supplementary Appendix), genotype (Table S4 in the Supplementary Appendix), and use or type of conditioning regimen were not significantly associated with graft failure requiring a second transplant (P = 0.15, P = 0.23, and P=0.44, respectively).
Table 3.
Transplantation Complications and Outcomes.*
| Complication or Outcome | No. of Infants Who Could Be Evaluated | Value |
|---|---|---|
| % (95% CI)† | ||
| Transplant-related event | ||
| Boost or second transplant at 5 yr‡ | 240 | 18 (13–23) |
| Acute GVHD of grade 2–4 at 100 days | 236 | 20 (17–28) |
| Acute GVHD of grade 3–4 at 100 days | 236 | 8 (5–12) |
| Chronic GVHD at 2 yr | 233 | 15 (10–20) |
| Overall survival at 5 yr | 240 | 74 (68–79) |
| no. of infants (%) | ||
| Immune reconstitution at 2–5 yr | ||
| T-cell immunity | ||
| CD3+ count >1000/mm3 | 125 | 88 (70) |
| CD4+ count >500/mm3 | 125 | 49 (39) |
| T-cell proliferation in response to PHA >30% of lower limit of normal range | 111 | 102 (92) |
| B-cell immunity | ||
| CD19+ or CD20+ count >400/mm3 | 126 | 65 (52) |
| Normal IgA level | 117 | 66 (56) |
| Independence from IVIG therapy | 136 | 74 (54) |
| Response to vaccine among children with independence from IVIG therapy | ||
| Protective response | 74 | 39 (53) |
| Detectable response | 74 | 8 (11) |
| No response | 74 | 1 (1) |
| Unknown response | 74 | 26 (35) |
CI denotes confidence interval, and IVIG intravenous immune globulin.
Percentages for transplant-related events are cumulative incidences.
A total of 23 children received a boost only, 34 received a second transplant only, and 11 received both a boost and a second transplant. A boost was defined as an additional transplant from the same donor without conditioning. A second transplant was defined as an additional transplant from a different donor (with or without conditioning) or from the same donor with conditioning.
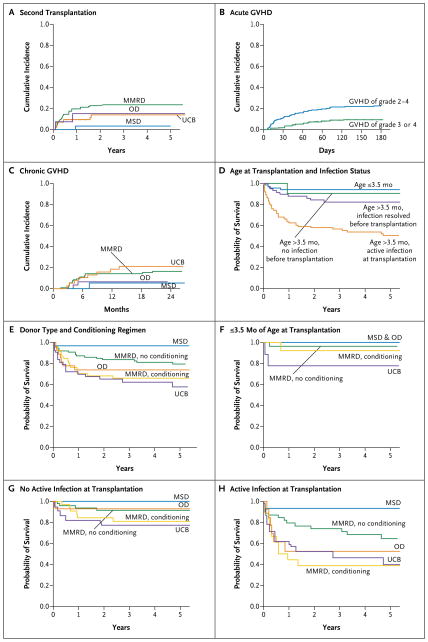
Figure 1. Cumulative Incidence of a Second Transplantation, Cumulative Incidence of Graft-versus-Host Disease (GVHD), and Survival in 240 Infants with Severe Combined Immunodeficiency.
The cumulative incidence of a second transplantation at 5 years in recipients of grafts from HLA-matched sibling donors (MSD), recipients of umbilical-cord blood (UCB), recipients of bone marrow or peripheral blood from other matched related or unrelated donors (OD), and recipients of grafts from mismatched related donors (MMRD) was 3%, 14%, 15%, and 24%, respectively (Panel A). The cumulative incidence of acute GVHD of grade 2 to 4 at 100 days, acute GVHD of grade 3 or 4 at 100 days, and chronic GVHD at 2 years was 20%, 8%, and 15%, respectively (Panels B and C). For acute GVHD, there were no significant differences according to donor type (Table S5 in the Supplementary Appendix). For chronic GVHD, a four-group comparison of donor types (Panel C) and a comparison including only transplants with rigorous T-cell depletion (Table S5 in the Supplementary Appendix) showed no significant differences. Factors that significantly affected survival in multivariate analyses include age at the time of transplantation and infection status, donor type, and conditioning regimen (Panels D through H). The survival rate at 5 years was higher among infants who received transplants at 3.5 months of age or younger (94%) and among older infants without prior infection (90%) than among older infants with resolved infection (82%) or with active infection (50%) at the time of transplantation (Panel D). Survival among children receiving transplants from alternative donors (MMRD, OD, or UCB) was inferior to that among children receiving MSD transplants; in addition, survival was reduced among recipients of MMRD transplants who underwent a conditioning regimen of immunosuppression, reduced-intensity conditioning, or myeloablative conditioning (Panel E). Infants 3.5 months of age or younger at the time of transplantation had high survival rates regardless of donor type or conditioning (Panel F). The effect of donor type and conditioning on the survival rate was not significant among infants of any age who did not have active infection at the time of transplantation (MSD, 100% among 17 infants; OD, 93% among 14 infants; MMRD with no conditioning, 91% among 48 infants; MMRD with conditioning, 81% among 33 infants; UCB, 77% among 22 infants; P = 0.16) (Panel G) but was significant among infants with active infection at the time of transplantation (MSD, 93% among 15 infants; MMRD with no conditioning, 65% among 39 infants; OD, 53% among 13 infants; UCB, 40% among 21 infants; MMRD with conditioning, 39% among 18 infants; P = 0.006) (Panel H).
GVHD
The cumulative incidence of acute GVHD of grade 2 to 4 at 100 days was 20%, the cumulative incidence of acute GVHD of grade 3 or 4 at 100 days was 8%, and the cumulative incidence of chronic GVHD at 2 years was 15% (Fig. 1B and 1C). The incidence did not differ significantly among recipients of grafts from matched sibling donors, recipients of T-cell–depleted grafts from mismatched related donors, and recipients of grafts from other donors (unrelated donors and phenotypically matched related donors considered together) (Fig. 1C, and Table S5 in the Supplementary Appendix).
SURVIVAL
The overall survival rate at 5 years was 74%, according to the Kaplan–Meier estimate (178 of 240 children) (Table 3). Among children who received a second transplant, the rate was 56% (26 of 45 children). Most deaths occurred in the first year after transplantation, and most deaths were due to infections (24 of 62 deaths [39%]) or pulmonary complications (23 of 62 deaths [37%]) (Table S6 in the Supplementary Appendix). Deaths due to pulmonary complications were more common among children who underwent myeloablative conditioning than among those who underwent reduced-intensity or immunosuppressive conditioning or did not undergo conditioning.
Older age and active infection at the time of transplantation were strongly associated with a lower survival rate. Infants who received transplants at 3.5 months of age or younger had the highest 5-year survival rate (94% [64 of 68 children surviving]). This rate was similar to that among infants older than 3.5 months of age at the time of transplantation and with no history of infection (90% [21 of 23 children surviving]) or whose infection had resolved by the time of transplantation (82% [48 of 58]). The survival rate was lowest for children who were older than 3.5 months of age and had active infection at the time of transplantation (50% [45 of 91 children surviving]) (Table 4 and Fig. 1D).
Table 4.
Results of Multivariate Analysis of Outcomes and Contributing Factors.
| Outcome and Contributing Factors | Percent with Outcome (95% CI) | Relative Effect (Hazard Ratio for Death) | P Value |
|---|---|---|---|
| Survival at 5 yr | |||
| Age at transplantation and infection status | <0.001 | ||
| 0–3.5 mo | 94 (85–98) | 1.00 | |
| >3.5 mo, active infection | 50 (39–61) | 10.88 | <0.001 |
| >3.5 mo, infection resolved | 82 (70–90) | 2.88 | 0.07 |
| >3.5 mo, no infection | 90 (67–98) | 1.03 | 0.97 |
| Donor type and conditioning regimen | 0.008 | ||
| Matched sibling donor | 97 (79–100) | 1.00 | |
| Mismatched related donor, no conditioning | 79 (69–87) | 6.27 | 0.07 |
| Mismatched related donor, with conditioning | 66 (51–77) | 15.70 | 0.008 |
| Cord-blood donor | 58 (40–72) | 13.10 | 0.01 |
| Other unrelated or related donor | 74 (53–87) | 14.20 | 0.01 |
| (Odds Ratio) | |||
| CD3+ T-cell count >1000/mm3 at 2–5 yr | |||
| Donor type | 0.04 | ||
| Matched sibling | 76 (55–91) | 1.00 | |
| Mismatched related | 66 (53–77) | 0.18 | 0.01 |
| Other related or unrelated | 76 (58–89) | 0.15 | 0.04 |
| Mismatched related vs. other related or unrelated | 1.17 | 0.82 | |
| Conditioning regimen | |||
| None or immunosuppression | 62 (51–73) | 1.00 | |
| Reduced-intensity or myeloablative conditioning | 89 (75–97) | 8.84 | 0.007 |
| Lymphocyte phenotype | |||
| B+ (vs. B− or Blow) | 87 (76–94) | 7.82 | <0.001 |
| NK+ (vs. NK− or NKlow) | 57 (42–71) | 0.23 | 0.02 |
| Independence from IVIG therapy at 2–5 yr | |||
| Donor type | <0.001 | ||
| Matched sibling | 81 (61–93) | 1.00 | |
| Mismatched related | 37 (26–49) | 0.10 | <0.001 |
| Other related or unrelated | 70 (53–84) | 0.21 | 0.02 |
| Mismatched related vs. other related or unrelated | 0.47 | 0.13 | |
| Conditioning regimen | |||
| None or immunosuppression | 41 (31–52) | 1.00 | |
| Reduced-intensity or myeloablative conditioning | 84 (69–93) | 8.87 | <0.001 |
Donor type was a significant predictor of survival; the survival rate was highest among recipients of grafts from matched sibling donors (97%). Children who received T-cell–depleted grafts from mismatched related donors and did not undergo conditioning had the next highest survival rate (79%, P = 0.07) (Table 4 and Fig. 1E). The survival rate was similar among children who received grafts from mismatched related donors after undergoing any type of conditioning (66%), cord-blood recipients (58%), and recipients of other grafts (74%).
Survival rates among children 3.5 months of age or younger at the time of transplantation were high for all transplant types (78 to 100%) (Fig. 1F) and ranged from 77 to 100% among children of any age without active infection at the time of transplantation (Fig. 1G). However, among children of any age with active infection at the time of transplantation, survival rates were inferior to that of children who received grafts from matched sibling donors for all alternative donor types except children who received T-cell–depleted grafts from mismatched related donors without any conditioning (Fig. 1H). Among children with active infection who received grafts from mismatched related donors, those who did not undergo conditioning had a higher survival rate than those who did (65% vs. 39%, P = 0.006). Survival rates among cord-blood recipients (40%) and recipients of grafts from other unrelated donors or matched related non-sibling donors (53%) were similar to the rate among children who received grafts from mis-matched related donors after undergoing conditioning (Fig. 1H). In contrast to the findings in prior studies, genotype and B− phenotype did not significantly affect survival.
IMMUNE RECONSTITUTION
Reconstitution of T lymphocytes, B lymphocytes, and immune function at 2 to 5 years after transplantation was analyzed among 149 children who survived to 2 years after receiving a single transplant. Of 111 children tested, 102 (92%) had restoration of T-cell responses to phytohemagglutinin. However, only 88 of 125 children tested (70%) had CD3+ T-cell counts of more than 1000 per cubic millimeter. In multivariate analyses (Table 4), recipients of grafts from matched sibling donors consistently had CD3+ T-cell counts that met this threshold, whereas recipients of grafts from mismatched related donors or grafts from other donors were likely to have lower CD3+ T-cell counts (P = 0.01 and P = 0.04, respectively). Conditioning with myeloablative or reduced-intensity regimens, as compared with immunosuppression or no conditioning, enhanced the probability of recovery of CD3+ T-cells to this level (P = 0.007). Phenotype influenced T-cell recovery. Children with B+ SCID were more likely to have recovery of normal levels of CD3+ T cells than were children with the B− or Blow phenotype (P<0.001), and children with NK+ SCID had poor recovery of T-cell populations as compared with those with the NK− or NKlow phenotype (P=0.02). RAG1, RAG2, and DCLRE1C variants were associated with poor CD3+ T-cell recovery in a univariate analysis (4 of 14 children vs. 49 of 57 children with IL2RG variants, P<0.001), a finding that is consistent with the phenotype results. Finally, active infection at the time of transplantation was significantly associated with poor CD3+ T-cell recovery. In univariate analyses, 34 of 39 infants (87%) who had clearance of infection by the time of transplantation and 26 of 37 infants (70%) with no history of infection had a CD3+ T-cell count of more than 1000 per cubic millimeter, as compared with 28 of 49 infants (57%) with active infection at the time of transplantation (P=0.009).
Total CD4+ T-cell recovery was more likely among recipients of grafts from matched sibling donors and other matched related or unrelated donors than among recipients of grafts from mismatched related donors (Table S7 in the Supplementary Appendix). Total CD4+ T-cell recovery was not influenced by conditioning. However, 23 children who underwent reduced-intensity or myeloablative conditioning had evidence of improved thymic output, as indicated by a higher median CD4+CD45RA+ naive T-cell count (1094 per cubic millimeter; range, 98 to 3131) than that in 62 children who underwent immunosuppression or did not undergo conditioning (192 per cubic millimeter; range, 0 to 2870; P<0.001) (Fig. S3 in the Supplementary Appendix).
Among children with B− SCID, univariate analysis showed that B-cell reconstitution (CD19+ or CD20+ B-cell count >400 per cubic millimeter) was associated with reduced-intensity or myeloablative conditioning (9 of 11 children [82%] with reduced-intensity or myeloablative conditioning vs. 9 of 37 children [24%] with immunosuppression or no conditioning, P<0.001).
Of 67 children tested, only 13 of 33 recipients of grafts from mismatched related donors (39%) had full or mixed donor B-cell chimerism, as compared with 8 of 10 recipients of grafts from matched sibling donors (80%) and 17 of 24 recipients of grafts from other donors (71%) (P<0.001). B-cell chimerism was more common in children who underwent reduced-intensity or myeloablative conditioning (23 of 26 children [88%]) than in those who underwent immunosuppression or did not undergo conditioning (7 of 31 children [23%], P<0.001) (Table S8 in the Supplementary Appendix).
Among survivors evaluated at 2 years after transplantation, recipients of grafts from matched sibling donors were significantly more likely to have independence from IVIG therapy and to have normal IgA levels than recipients of grafts from mismatched related donors or other donors (Table 4, and Table S7 in the Supplementary Appendix). Furthermore, among children who received grafts from mismatched related donors or other donors, those who underwent reduced-intensity or myeloablative conditioning were more likely to have normal CD3+ T-cell counts, B-cell chimerism, normal IgA levels, and independence from IVIG therapy than those who underwent immunosuppression or did not undergo conditioning (P = 0.005, P<0.001, P<0.001, and P<0.001, respectively). Moreover, full donor chimerism in whole blood and full or mixed chimerism in B cells or myeloid cells were associated with independence from IVIG therapy (P = 0.002 for whole blood, P<0.001 for B cells, and P = 0.003 for myeloid cells) (Table S8 in the Supplementary Appendix). Neither phenotype nor genotype was significantly associated with independence from IVIG therapy.
DISCUSSION
Our results confirm previously reported excellent outcomes for children with classic SCID who received transplants from matched sibling donors without undergoing conditioning2,7,14,15,17 and significantly better outcomes for children who received transplants in early infancy (≤3.5 months of age vs. >3.5 months of age) (Fig. 1).22,24 Even after transplantation of grafts from alternative donor types, these very young infants had excellent outcomes that were similar to those of recipients of grafts from matched sibling donors (Fig. 1F). Because earlier transplantation is more successful, our findings suggest that newborn screening for SCID and early transplantation may improve survival.31
Clinicians in regions that have not implemented newborn screening commonly face the dilemma of how to perform a transplantation in an infant with SCID who presents with infection. Controversies include what type of donor should be selected if a matched sibling donor is not available, whether treatment of infection should be attempted before transplantation, and whether to use a conditioning regimen.13,32–34 In our cohort, survival among infants who had never had infection or had infection that resolved was similar to that among infants 3.5 months of age or younger, irrespective of donor type, a finding that suggests that prevention and successful treatment of infection are predominant determinants of a good transplantation outcome. Furthermore, among children with active infection at the time of transplantation, the use of any conditioning regimen, including immunosuppression, was associated with an adverse effect on survival among recipients of grafts from mismatched related donors (and possibly among cord-blood recipients and recipients of grafts from unrelated donors, the large majority of whom underwent conditioning). In addition to the acute toxicity of reduced-intensity and myeloablative conditioning regimens (Table S6 in the Supplementary Appendix), these regimens have long-term complications, including infertility, poor growth, and neurocognitive effects.35–37
For survivors who received grafts from donors other than matched siblings, however, reduced-intensity or myeloablative conditioning, as compared with immunosuppression or no conditioning, was associated with improved T-cell counts and more consistent B-cell function, findings that are consistent with previous reports.13,32,34 For selected patients (e.g., uninfected infants with specific genotypes), the high probability of survival and the advantages of complete immune reconstitution may outweigh the risks of conditioning-associated toxicity. However, for patients with active infection, the risks appear to outweigh the benefits. For patients with active infection, transplantation performed without conditioning followed by administration of donor-derived, pathogen-specific T cells, or sequential transplantations (the first performed without conditioning to correct T-cell deficiencies and the second performed with a conditioning regimen that secures B-cell engraftment), might improve overall results while reducing both mortality soon after transplantation and morbidity in the long term.
In summary, our data indicate that children with classic SCID diagnosed at birth or before the onset of infection who receive transplants from mismatched related donors, transplants from unrelated donors, or cord-blood transplants soon after diagnosis have more than a 90% probability of survival with T-cell and variable B-cell immune reconstitution. We found that mortality was increased for patients who had active infection at the time of transplantation. For such patients who did not receive transplants from matched donor siblings, the survival rate was highest among those who received T-cell–depleted grafts from mismatched, related haploidentical donors without undergoing conditioning.
Supplementary Material
Acknowledgments
The opinions expressed in this article are those of the authors and do not represent the position of the National Institute of Allergy and Infectious Diseases (NIAID), the National Human Genome Research Institute, the Office of Rare Diseases Research, the National Center for Advancing Translational Sciences, the National Institutes of Health (NIH), or the U.S. government.
Supported by grants from the NIAID (1U54AI082973) and the NIH Office of Rare Diseases Research, National Center for Advancing Translational Sciences (R13AI094943), a Translational Investigator Service Award (to Dr. Pai), and grants from the National Cancer Institute (CA23766, to Dr. O’Reilly), the David Center, Texas Children’s Hospital (to Dr. Shearer), and the National Heart, Lung, and Blood Institute (NHLBI) (HL085288 and HL36444, to Dr. Burroughs). Activities of the Pediatric Blood and Marrow Transplant Consortium, a core group representing 19 centers participating in this study, were also partially supported by grants from the NHLBI (2U01HL069254) and the St. Baldrick’s Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM. org.
We thank the data management coordinating center (University of South Florida), including Dr. Jeffrey Krischer and Ms. Holly Ruhlig, for data collection, management, and database implementation; Ms. Jessica Carlson and Ms. Elizabeth Dunn for project management and assistance in data cleanup and verification; the study coordinators and research nurses for collection of clinical data from the Primary Immune Deficiency Treatment Consortium clinical sites; and Mr. Ramzi S. Khalaf, Dr. Robert Krance, Dr. Caridad Martinez, and Ms. Stephanie Edwards for their contributions to the data collection and regulatory process.
APPENDIX
The authors’ affiliations are as follows: Boston Children’s Hospital, Division of Pediatric Hematology–Oncology (S.-Y.P.), Division of Immunology, Manton Center for Orphan Disease Research, Harvard Stem Cell Institute (L.D.N.), and the Department of Pediatric Oncology, Dana–Farber Cancer Institute (S.-Y.P.), Boston; Division of Biostatistics, Medical College of Wisconsin, Milwaukee (B.R.L., Q.X.); Bone Marrow Transplant Program, Memorial Sloan Kettering Cancer Center, New York (T.N.S., R.J.O.); Division of Allergy, Immunology, and Transplantation (L.M.G.), and Clinical Center, Department of Laboratory Medicine (T.A.F.), National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Departments of Pediatrics and Immunology, Duke University Medical Center, Durham, NC (R.H.B., R.E.P.); Benioff Children’s Hospital, Division of Allergy–Immunology–Blood and Marrow Transplantation, University of California, San Francisco, San Francisco (C.C.D., J.M.P., M.J.C.); Department of Pediatrics, Children’s Hospital Los Angeles, Los Angeles (N.K.); Texas Children’s Hospital, Section of Pediatric Immunology, Allergy, and Rheumatology, Baylor College of Medicine, Houston (I.C.H., W.T.S.); Bone Marrow Transplantation and Immunodeficiency, Cincinnati Children’s Hospital Medical Center, Cincinnati (A.H.F.); Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia (S.J., K.E.S.); Seattle Children’s Hospital, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle (L.B., S.S.-S.); Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta (A.E.H., A. Grizzle), and Emory University School of Medicine (A.H.) — both in Atlanta; Primary Children’s Hospital, Division of Hematology, University of Utah–Huntsman Cancer Institute, Salt Lake City (M.A.P.); Texas Transplant Institute and Methodist Children’s Hospital of South Texas, San Antonio (K.W.C.); Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago (R.L.F.); Centre Hospitalier Universitaire Sainte-Justine, University of Montreal, Montreal (E.H.); Division of Blood and Marrow Transplantation and Allergy and Immunology, Children’s National Medical Center, Washington, DC (B.L.); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas (V.M.A.); Pediatric Blood and Marrow Transplant Program, Hackensack University Medical Center, Hackensack, NJ (A. Gillio); Department of Pediatrics, BC Children’s Hospital, Vancouver, BC (J.D.), and Department of Pediatrics and Child Health, University of Manitoba, Winnipeg (M.L.S.) — both in Canada; Division of Pediatric Allergy and Immunology, Cardinal Glennon Children’s Medical Center, Saint Louis University, St. Louis (A.K.); Division of Pediatric Blood and Marrow Transplantation, University of Minnesota, Minneapolis (A.R.S.); Mattel Children’s Hospital, University of California, Los Angeles, Los Angeles (T.B.M., D.B.K.); Department of Pediatrics, Children’s of Alabama, University of Alabama at Birmingham, Birmingham (F.D.G.); Department of Pediatric Hematology–Oncology, University of Michigan, Ann Arbor (J.A.C.); and Division of Hematology–Oncology–Stem Cell Transplantation, Stanford University, Stanford, CA (M.H.P.).
References
- 1.Al-Herz W, Bousfiha A, Casanova J-L, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–9. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly RJ, Dupont B, Pahwa S, et al. Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med. 1977;297:1311–8. doi: 10.1056/NEJM197712152972403. [DOI] [PubMed] [Google Scholar]
- 4.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–8. [PubMed] [Google Scholar]
- 5.Cowan MJ, Wara DW, Weintrub PS, Pabst H, Ammann AJ. Haploidentical bone marrow transplantation for severe combined immunodeficiency disease using soybean agglutinin-negative, T-depleted marrow cells. J Clin Immunol. 1985;5:370–6. doi: 10.1007/BF00915333. [DOI] [PubMed] [Google Scholar]
- 6.O’Reilly RJ, Brochstein J, Collins N, et al. Evaluation of HLA-haplotype disparate parental marrow grafts depleted of T lymphocytes by differential agglutination with a soybean lectin and E-rosette depletion for the treatment of severe combined immunodeficiency. Vox Sang. 1986;51(Suppl 2):81–6. doi: 10.1111/j.1423-0410.1986.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 7.Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya A, Slatter MA, Chapman CE, et al. Single centre experience of umbilical cord stem cell transplantation for primary immunodeficiency. Bone Marrow Transplant. 2005;36:295–9. doi: 10.1038/sj.bmt.1705054. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji Y, Imai K, Kajiwara M, et al. Hematopoietic stem cell transplantation for 30 patients with primary immunodeficiency diseases: 20 years experience of a single team. Bone Marrow Transplant. 2006;37:469–77. doi: 10.1038/sj.bmt.1705273. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes JF, Rocha V, Labopin M, et al. Transplanting patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119:2949–55. doi: 10.1182/blood-2011-06-363572. [DOI] [PubMed] [Google Scholar]
- 11.Sarzotti-Kelsoe M, Win CM, Parrott RE, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114:1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley RH, Win CM, Moser BK, Parrott RE, Sajaroff E, Sarzotti-Kelsoe M. Post-transplantation B cell function in different molecular types of SCID. J Clin Immunol. 2013;33:96–110. doi: 10.1007/s10875-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad E, Landais P, Friedrich W, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91:3646–53. [PubMed] [Google Scholar]
- 14.Antoine C, Müller S, Cant A, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 15.Gennery AR, Slatter MA, Grandin L, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126:602–10. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Neven B, Leroy S, Decaluwe H, et al. Long-term outcome after haematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency: long-term outcome of HSCT in SCID. Blood. 2009;113:4114–24. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 17.Railey MD, Lokhnygina Y, Buckley RH. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J Pediatr. 2009;155(6):834.e1–840.e1. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puck JM SCID Newborn Screening Working Group. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120:760–8. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Routes JM, Grossman WJ, Verbsky J, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302:2465–70. doi: 10.1001/jama.2009.1806. [DOI] [PubMed] [Google Scholar]
- 20.Comeau AM, Hale JE, Pai S-Y, et al. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. J Inherit Metab Dis. 2010;33(Suppl 2):S273–S281. doi: 10.1007/s10545-010-9103-9. [DOI] [PubMed] [Google Scholar]
- 21.Kwan A, Church JA, Cowan MJ, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–50. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 23.Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown L, Xu-Bayford J, Allwood Z, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117:3243–6. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- 25.Griffith LM, Cowan MJ, Kohn DB, et al. Allogeneic hematopoietic cell transplantation for primary immune deficiency diseases: current status and critical needs. J Allergy Clin Immunol. 2008;122:1087–96. doi: 10.1016/j.jaci.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2013;133:1092–8. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood: reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–93. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 28.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Müller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98:1847–51. doi: 10.1182/blood.v98.6.1847. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak CC, Hung G-Y, Horn B, Dunn E, Oon C-Y, Cowan MJ. Megadose CD34(+) cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplantation. 2008;14:1125–33. doi: 10.1016/j.bbmt.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak CC, Cowan MJ, Logan BR, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the Primary Immune Deficiency Treatment Consortium prospective study 6901. J Clin Immunol. 2013;33:1156–64. doi: 10.1007/s10875-013-9917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109:4575–81. doi: 10.1182/blood-2006-07-029090. [DOI] [PubMed] [Google Scholar]
- 33.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131:994–1000. doi: 10.1016/j.jaci.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavazzana-Calvo M, André-Schmutz I, Fischer A. Haematopoietic stem cell transplantation for SCID patients: where do we stand? Br J Haematol. 2013;160:146–52. doi: 10.1111/bjh.12119. [DOI] [PubMed] [Google Scholar]
- 35.Sanders JE. Endocrine complications of high-dose therapy with stem cell transplantation. Pediatr Transplant. 2004;8(Suppl 5):39–50. doi: 10.1111/j.1398-2265.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 36.Borgmann-Staudt A, Rendtorff R, Reinmuth S, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant. 2012;47:271–6. doi: 10.1038/bmt.2011.78. [DOI] [PubMed] [Google Scholar]
- 37.Titman P, Pink E, Skucek E, et al. Cognitive and behavioral abnormalities in children after hematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–13. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.