Abstract
Background and Aims
Enteral nutrient deprivation via total parenteral nutrition (TPN) in a mouse model leads to a local mucosal inflammatory response. This pro-inflammatory response leads to a loss of epithelial barrier function and atrophy of the intestine. Although, the underlying mechanisms are unknown, a potential contributing factor is the impact TPN has on the intestinal microbiome. We recently identified a shift in the intestinal microbial community in mice given TPN; however, it is unknown whether such changes occur in humans. We hypothesized that similar microbial changes occur in humans during periods of enteral nutrient deprivation.
Methods
A series of small bowel specimens were obtained from pediatric and adult patients undergoing small intestinal resection. Mucosally-associated bacteria were harvested, and analyzed using 454 pyrosequencing techniques. Statistical analysis of microbial diversity and differences in microbial characteristics were assessed between enterally-fed and enterally-deprived portions of the intestine. Occurrence of post-operative infectious and anastomotic complications was also examined.
Results
Pyrosequencing demonstrated a wide variability in microbial diversity within all groups.. Principal coordinate analysis demonstrated only a partial stratification of microbial communities between fed and enterally deprived groups. Interestingly, a tight correlation was identified in patients who had a low level of enteric microbial diversity and those who developed post-operative enteric-derived infections or intestinal anastomotic disruption.
Conclusions
Loss of enteral nutrients and systemic antibiotic therapy in humans is associated with a significant loss of microbial biodiversity within the small bowel mucosa. These changes were associated with a number of enteric-derived intestinal infections and intestinal anastomotic disruptions.
Keywords: Intestine, Microbiota, Microflora, Parenteral Nutrition
INTRODUCTION
Parenteral nutrition (PN) is an alternative form of nutrition for those patients with short-term gastrointestinal dysfunction1, as well as a life-saving nutritional replacement for patients with intestinal failure requiring long-term support2,3. While essential and clearly beneficial for many, PN use is associated with numerous complications ranging from an increase in systemic infections to a loss of immune reactivity4–8. Previous studies have shown distinct physical and immunologic differences in the intestinal immunology of mice maintained on total PN (TPN)9–15. These changes include increased inflammatory cytokines and decreased regulatory cytokines within the bowel wall leading to a pro-inflammatory state in the gastrointestinal tract. There is also a decrease in intestinal epithelial cell proliferation, an increase in apoptosis with an associated atrophy of the small bowel mucosa16–18. The underlying mechanisms for the above changes are unknown, but the immunologic disorder may lead to a loss of epithelial barrier function (EBF)18,19 and is theorized to increase bacterial translocation through a more permeable intestinal mucosa20–22.
Enteral deprivation may help explain the dramatic observations seen in our TPN mouse model. TPN provides sufficient energy and nutrient needs, but puts the intestinal microbiota in an abrupt state of nutrient withdrawal. The intestinal microbial population is highly sensitive to local environmental alterations, and may rapidly change its composition in response to such dramatic nutritional deviation from baseline23,24. In mice, administration of TPN leads to profound changes in the small intestinal microbiota; moving from a gram-positive Firmicutes-flora to a gram-negative Proteobacteria-dominated community25.
While the abovementioned small bowel and microbial changes are well documented in rodent models of TPN administration, it is less clear whether such changes occur in humans26–30. A major limitation of previous studies is that each is based on very limited numbers of patients, and only a very superficial evaluation of mucosal changes has been investigated. Importantly, no previous study has investigated whether the administration of TPN results in changes in the microbial population of the gastrointestinal tract. Alteration in types of nutrient feeding has long been thought to impact the composition of the human intestinal microbiome31. Such fluctuations have been implicated in the development of a number of pathologic conditions including necrotizing enterocolitis, inflammatory bowel disease32, obesity33 and food allergies34. A deep understanding of the microbial shifts associated with each disease process has been difficult in humans as each person has a unique microbiome making concrete correlations challenging35. Whether the complete removal of enteral nutrition from a portion of the intestine impacts the composition of the microbial population has yet to be tested in humans, yet this extreme modification in nutrient delivery has the potential to best address the implications of nutrients on the alteration of the intestinal microbial communities. To address this, we used a series of surgical biopsies from small bowel of patients and compared the microbiota with relation to the degree of enteral nutrient deprivation. Secondarily, we followed the clinical course of each patient from which a sample was taken.
METHODS
Handling of Human Tissue
All experiments were done in accordance with University of Michigan IRB # HUM00024263. In all cases the degree to which the small bowel segment was in contact with enteral nutrients was recorded. All specimens were sent fresh to Pathology from the operating room. Adhering to sterile technique, a pathologist performed gross examination of the specimen. A fresh portion of the specimen was cut and placed into sterile RPMI 1640 with glutamine (Invitrogen, Carlsbad, CA) and taken to the laboratory for tissue processing (see later paragraphs). All samples were de-identified; however, the following data was recorded: patient age, diagnosis, sex, location of bowel, whether the bowel was exposed to enteral nutrients or not and duration of enteral nutrient deprivation. Definition of enteral nutrient deprivation included the following: isolated intestinal segments or defunctionalized limbs of bowel, without exposure to enteral nutrients. As well, nutrient deprivation included patients without any enteral nutrients. Patients with active inflammatory conditions (e.g., Crohn disease, active necrotizing enterocolitis) were excluded.
Bacterial pyrosequencing)
From each segment, tissue was opened, adherent stool rinsed off in sterile media consisting of RPMI 1640 with glutamine (Invitrogen, Carlsbad, CA) and 5% fetal bovine serum (FBS) (Invitrogen). The tissue was then scraped to obtain the mucosally associated bacteria and snap-frozen in liquid nitrogen until analyzed. The bacterial tag-encoded FLX-Titanium amplicon pyrosequencing method targeting the V1–V3 variable regions of 16S rRNA was used to create amplicon libraries36. V1–V3 primer sets corresponded to 27F (5′-GAGTTTGATCNTGGCTCAG-3′) and 519R (5′-GTNTTACNGCGGCKGCTG-3′), along with appropriate sample nucleotide bar codes and the Roche A&B primers. Pyrosequencing was performed following established protocols 37 at Research and Testing Laboratories (Lubbock, TX).
Identified sequences were then classified using the Michigan State Ribosomal Database Project (RDP) classifier. Analysis of sequenced data was performed using Mothur, an open-source, community-supported software for describing and comparing microbial communities38, following the example of Costello Stool Analysis with default software settings. The metrics we examined were alpha and Unifrac principal coordinate analyses.
Another metric to evaluate the microbiota is the inverse Simpson index, which is an example of an alpha-diversity measure that describes how much variety exists within a given community. The Simpson index always falls between 0 and 1, with 0 meaning infinite variety, and 1 meaning no variety. The inverse Simpson index is more intuitive in that higher numbers indicate higher alpha-diversity (or variety, and thus the greater the score, the higher the diversity).
Statistical Analysis
Alpha diversity and Unifrac principal coordinate analysis was used to study the microbial communities. The inverse Simpson index was further used to define the variability in diversity depending on degree of enteral nutrient deprivation. An arbitrary setting of 10 for the inverse Simpson index was used to stratify between low (<10) and high diversity (≥10).
RESULTS
Demographics
Fifteen samples from 12 different patients were collected for analysis of their microbiota between January 2009 to October 2010 (Table 1). Loop enterostomy takedown resulted in more samples than the number of patients. In these cases, one limb of bowel had exposure to nutrients and the other was isolated from nutrition (i.e. in these cases there was no re-feeding of the distal limb). There were 6 males and 6 females with a mean age of 9.2±8.4 years (range 2 days to 22 years). Additional items recorded included the disease process, and location of the bowel. Intestinal samples were ileal (n=13) or jejunal (n=2). The operative indications for resection were most commonly enterostomy takedown (n=6). Others indications included EC fistula takedown (n=2), small bowel obstruction (n=2, from adhesive disease and anastomotic stricture) and one case each for ileal atresia and parastomal hernia. Underlying pathology included previous cases of necrotizing enterocolitis (n=3), previous diagnosis of ulcerative colitis (n=3), intestinal atresia, anastomotic stricture, cloacal exstrophy, total colonic Hirschsprung disease, enterocutaneous fistula, and trauma.
Table 1.
Patient demographics and clinical information of patients from which small bowel was collected and studied.
| ID | Additional History | Feed Status | Antibiotics: (other than Pre op) | Immune-modulator | Prior Infections | Complication |
|---|---|---|---|---|---|---|
|
Newborn1* 2 day F |
Ileal atresia | NPO | ||||
|
Newborn2
* 2 day F |
Ileal atresia | NPO | ||||
|
Chronic NPO 16 year F |
IBD | NPO x2 mo, TPN | Amox/clavulanate and Metro (H) | Alpha strep, line infection | EC Fistula from anastomosis | |
|
MF1‡ 3 month M |
Previous NEC | MF not fed | Gentamycin (P) | Klebsiella UTI | ||
|
MF2† 17 year M |
Previous Blunt Trauma | MF not fed | ||||
|
Partial1‡ 3 month M |
Previous NEC | TPN and Feeds | Gentamycin (P) | Klebsiella UTI | ||
|
Partial2 2 month M |
Previous NEC | TPN and Feeds | Pip/Tazo (P) | Klebsiella bacteremia | Central venous line infection(Klebsiella) | |
|
Partial3 22 year F |
Cloacal exstrophy, parastomal hernia | TPN and Feeds | Metro, Nystatin, aztreonam (A) | E. Coli UTI | ||
|
Full feeds1 3 year F |
History of malrotation, jejunal stricture | Full Feeds, clear liquid diet 5 days | Anastomotic ulcer and stricture | |||
|
Full feeds2† 17 year M |
Previous Blunt Trauma | Full Feeds | ||||
|
Full feeds3 17 yo M |
IBD | Full Feeds | Steroids (H) | Wound infection (MSSA) | ||
|
Full feeds4 8 month M |
Hirschsprung Disease | Full Feeds | Multiple Abx regimen (P) | Recurrent Enterocolitis | ||
|
Full feeds5 22 year M |
IBD with small bowel obstruction | Full Feeds | Pip/Tazo and Flagyl (P) | Steroids (A) | Unclear etiology | Wound Infection (Klebsiella, Candida) |
|
Full feeds6 9 year F |
EC Fistula sp jejunal-tube | Full Feeds | ||||
|
Full feeds7 7 year F |
Previous NEC | Full Feeds | Pip/Tazo (P) | Enterococcus, Pseudomonas | anastomotic leak |
denote samples from the same patient.
Abbreviations: ID. Identifier; IBD, inflammatory bowel disease; MSSA Methicillin Sensitive Staph Aureus; NEC, Necrotizing Enterocolitis; Pip/Tazo, Piperacillin and Tazobactam; Amox, amoxicillin; H, Historic-refers to prior treatment that ended two weeks or greater before operation; P, previous-refers to therapy continuing up to within one week of operation; A, active-denotes current therapy.
Seven samples were from fully fed segments of bowel and 3 were partially fed. The partially fed patients received the majority of nutrients parenterally (>80%), as only trophic feedings were tolerated in these patients. All samples in the partially fed group were chronically (over 2 weeks) on PN support. Five samples were unfed, three of which had no enteral nutrition for at least 6 weeks (65, 47, and 42 days). Two of these came from patients with mucus fistulae out of continuity of enteric flow but receiving either full enteral feeds or partial feeds. The other patient was TPN dependent with no enteral nutrition for over 2 months. Two samples were from neonates that never received enteral feedings.
454 pyrosequencing, biodiversity and correlation to clinical outcomes
Figure 1 shows the intestinal mirobiota sorted by phylum. Quite similar to most human data, there was marked heterogeneity among the samples39. Three of these samples, however, stood out above all others. These were the two segments from the same 2 day old infant, and the sample from a patient who was without enteral nutrients for over 2 months. These samples were distinct in that there was a marked loss of diversity in these patients who had little to no nutrient exposure. As it is known that neonatal fecal microbes are quite different from adults, the latter patient’s microbiome (NPO for >2 months), which was comprised virtually all of Proteobacteria is more relevant to this study. The data suggests that prolonged periods of enteral deprivation can led to a marked change in intestinal mucosal microbiota with a decline in its diversity.
Figure 1.
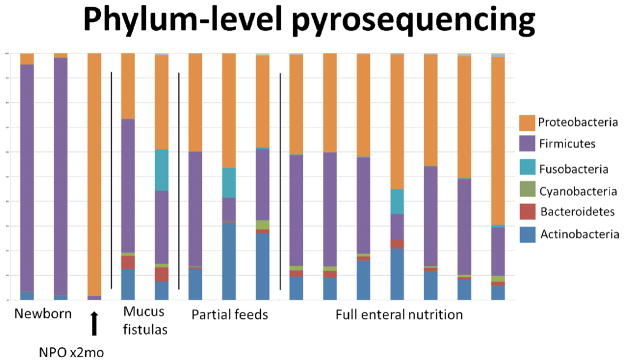
Phylum level analysis after Ribosomal Database Project (RDP) classification of pyrosequenced small bowel mucosa-associated bacteria samples. Groups of patients are broken down by degree of enteral nutrition, as well as by separating the two neonatal samples. Mucous fistula denotes bowel completely unexposed to nutrients and partial feeding meant intestine where <20% of nutrients entered the gastrointestinal tract.
A further breakdown of the bacterial genus is shown in Table 2. In the Table, three representative non-fed and fed microbial populations are shown. Characteristic of human microbial populations, each patient had a unique distribution, however, some important distinctions are found. Although there is a large overlap in speciation and no statistically significant differences found, some groups were expanded in the fed group (Staphylococcus, Pseudomonas, Campylobacter, Propionibacterium, Chryseomonas) and others in the enterally-deprived group (Enterobacter, Shigella, Klebsiela and Fusobacterium).
Table 2.
Representative 454 pyrosequencing results at the genus level from 3 non-fed (excluding neonatal specimens) and 3 enterally-fed portions of bowel. Note the broader representation of bacteria from multiple bacterial genra. As well, note certain predominant gram negative groups in the non-fed patients including Enterococcus, Shigella, Klebsiela and Fusobacterium.
| Bacterial Genus | NPO | NPO | NPO | Enteral | Enteral | Enteral |
|---|---|---|---|---|---|---|
| Staphylococcus | 22 | 1274 | 523 | 962 | 1646 | 1597 |
| Enterococcus | 33 | 1835 | 210 | 1385 | 57 | 199 |
| Klebsiella | 11876 | 0 | 231 | 218 | 43 | 571 |
| Pseudomonas | 1 | 466 | 538 | 1085 | 1351 | 1955 |
| Shigella | 1475 | 43 | 186 | 210 | 82 | 197 |
| Bifidobacterium | 0 | 0 | 37 | 0 | 54 | 12 |
| Corynebacterium | 7 | 806 | 247 | 532 | 621 | 434 |
| Campylobacter | 9 | 1 | 131 | 681 | 35 | 289 |
| Fusobacterium | 1 | 1 | 1508 | 0 | 3 | 47 |
| Enterobacter | 656 | 284 | 227 | 326 | 105 | 202 |
| Anaerococcus | 6 | 263 | 88 | 259 | 152 | 211 |
| Citrobacter | 456 | 1 | 282 | 56 | 21 | 447 |
| Streptococcus | 2 | 199 | 334 | 167 | 285 | 215 |
| Propionibacterium | 1 | 58 | 19 | 291 | 284 | 166 |
| Proteus | 2 | 240 | 42 | 133 | 87 | 168 |
| Finegoldia | 10 | 527 | 61 | 171 | 149 | 149 |
| Clostridium | 68 | 0 | 45 | 69 | 2 | 96 |
| Delftia | 0 | 0 | 198 | 1304 | 1 | 8 |
| Prevotella | 0 | 384 | 322 | 248 | 55 | 25 |
| Chryseomonas | 4 | 9 | 40 | 142 | 176 | 98 |
| Allobaculum | 0 | 0 | 172 | 975 | 3 | 0 |
| Veillonella | 13 | 113 | 68 | 0 | 38 | 18 |
| Minor Genera (<1000) | 110 | 1178 | 2335 | 2455 | 1115 | 2226 |
| Total Called | 14752 | 7682 | 7844 | 11669 | 6365 | 9330 |
| Total Sequences | 16921 | 8542 | 9137 | 13773 | 7344 | 12237 |
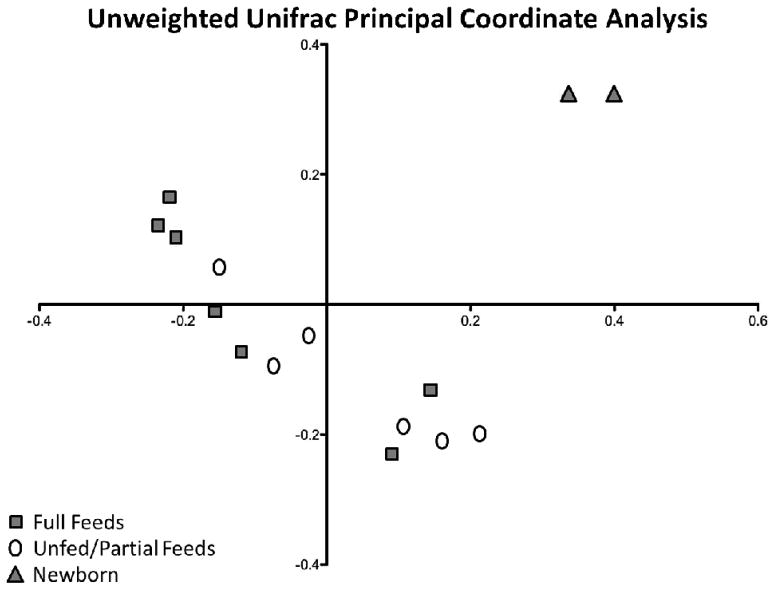
To better characterize and quantify differences in the intestinal flora between individuals, Unifrac Principal Coordinate analysis (PCoA) was applied to the samples. In PCoA differences between microbial communities are first divided into multiple weighted components based on their genetic sequences, or operational taxonomic units. Figure 2 shows the components that accounted for the largest percentage of the differences between communities and is expressed as an X-Y plot. This unweighted PCoA plot of the first two axes accounted for 20.5% of the total differences between microbial communities in fed versus un-fed bowel. In an unweighted analysis, only presence or absence of a bacterium is counted. Because of this, the neonatal samples were distinctly different from the other samples. A clear separation of groups based on feeding status in not seen. There is a trend of unfed or partially fed samples clustering towards the bottom right and fully fed samples clustering to the top left. This indicated an underlying similarity among the unfed versus fed intestinal samples. But, as with Table two, there was no clear distinction of these groups based solely on feeding status.
Figure 2.
Unweighted Unifrac principal coordinates analysis of control and TPN small bowel samples. Axis-1 (X) and axis-2 (Y) account for 11.9% and 8.6% of overall differences, respectively.
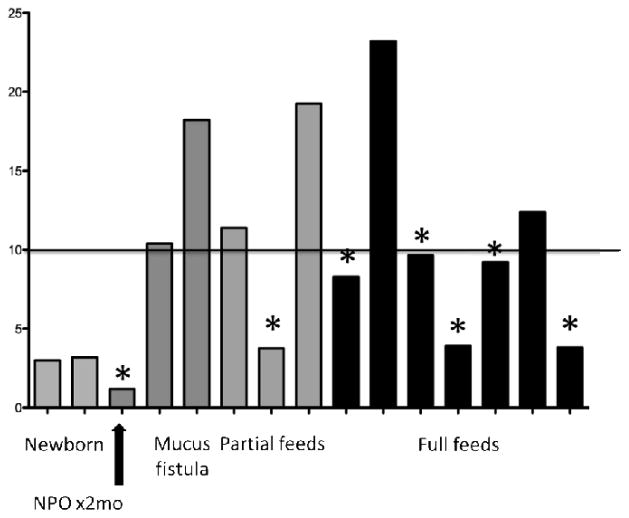
To further analyze the microbiome data, an analysis of biodiversity was next performed. Figure 3 shows an analysis of the samples using inverse Simpson indices. At initial analyses, there appeared to be little correlation between alpha diversity and feeding status of the patients. However, a further analysis was performed examining those patients who had a post-operative infectious complication. Seven such post-operative complications were found which included 3 anastomotic problems (2 with complete disruption), 2 abdominal wound infections (Klebsiella pneumioniae, Candida albicans in one patient and methicillin sensitive Staphylococcus aureus [MSSA] in the other), 1 central venous line infection(Klebsiella pneumioniae) and 1 case of recurrent enterocolitis.
Figure 3.
Inverse Simpson index and enteral nutrition. All patients outside of the newborn period with a sample that scored less than 10, signifying a less diverse microbiota, were complicated with an infectious or anastomotic complication(*).
Interestingly, the proportion of samples with a lower alpha-diversity (or a lower inverse Simpson index, <10) was significantly (P<0.01 using Chi Square analysis) greater (all but one patient) in the group of patients who had the above listed postoperative complications. Whereas those having a higher inverse Simpson index fell into the group that was far less prone to infectious complications. In fact no patient suffered infectious complications with an inverse Simpson index >10. This suggested that those patients with lower levels of biodiversity had an increased susceptibility to infections, or potentially had a more virulent type of bacteria which predisposed to these complications.
DISCUSSION
While the causality is not fully established, it was interesting that a significant increase in infectious and anastomotic complications was associated in those patients who had loss of microbial diversity. Such an increase in infectious complications has been well-established in patients receiving TPN5,40,41 and the results suggest that lower diversity in the intestinal microbiome may impact these infectious complications. The lower diversity was not clearly defined in the fed and enterally deprived groups as hypothesized, but rather seems multifactorial. Importantly, the one sample from which enteral nutrients was withheld for greater than two months did have the lowest diversity. An in depth investigation of more completely NPO individuals is needed to further substantiate this finding. It is also important to note that other confounding factors which could influence microbial diversity would include repetitive use of antimicrobial agents as was given in many of our patients in this study.
In both humans and rodents, the dominant intestinal phyla of bacteria are Firmicutes and Bacteroides; comprised of mostly Gram positive bacteria42. While the general trend of more Firmicutes and Bacteroides is present, the specific sub-phylum composition of the microbiota is quite variable. Thus, it was not surprising to see this overall diversity between the patients we examined. Our laboratory’s mouse model of enteral nutrient deprivation with TPN administration demonstrates a marked shift of the intestinal microbiota from that of a Firmicute dominant flora to that of a Proteobacteria dominant population. While there was a definite trend toward this shift in the human samples, a significant loss of diversity was not shown in all unfed portions of small intestine. However, this trend is best seen in the individual who was enterally-deprived for 2 months had a nearly complete shift to a Proteobacteria profile.
When specifically looking at the unfed samples, one could argue based on the RDP data (figure 1) that two of the five unfed specimen are not dissimilar to the partially fed and fed samples. However, when considering the unfed specimens it is critical to consider the patient history. The neonates, never having received food, had low diversity as expected43. The third of the five patients who was totally NPO for 65 days showed a dramatic loss in microbial diversity. The other two samples were from mucus fistulae from patients with loop enterostomies. One may expect these samples to look more like the patient NPO for 65 days as they were out of intestinal continuity for similar duration. Both of the patients with mucus fistulae were fed in their proximal limb. This is an important consideration as the bacterial contents from the proximal (fed limb) may have colonized the distal limb resulting in greater diversity in this group. This could be the case as the stoma drains from the same stomal skin site into the same stool collection apparatus.
It was striking to note that the patients who developed infectious and anastomotic complications were those who had a significantly lower microbial diversity using the inverse Simpson index regardless of feeding status. Other factors may influence the heterogeneity of microbes. One example could be patient specific antimicrobial use that could disrupt the intestinal microflora44. In this study, four of the six fed or partially fed patients that experienced a complication were on an antibiotic regimen either at the time of the operation or recently completed antibiotic treatment prior to surgery (Table 1). There may be other contributing factors leading to loss of microbial diversity that were not uncovered in the current study, including exposures to other patients or pharmacologics which could influence the gastrointestinal tract. Nevertheless, this data suggests a strong correlation between loss of microbial diversity and an aberration of underlying physiology of the bowel wall leading to higher rates of infectious complications.
One of the limitations of this study is the relatively low sample numbers, and a highly heterogeneous patient population. We also acknowledge that the cutoff of 10 for the inverse Simpson index is somewhat arbitrary, and further validation of this value is required to increase the robustness of this study. Further, we have used pyrosequencing data to define the microbiota. While a widely accepted method for analysis of the microbiome, currently there is little application of this technique in a clinical setting. This data provides a much more diverse set of microbes alluding to the presence of unculturable intestinal bacteria, yet clinically we must rely on culture data. Our analysis of the 454 data was also limited. Potentially, a much more complex examination could have been done. However the authors felt that the data presented demonstrates the changes in microbial diversity and fits the needs of this study. Despite these limitations, we feel that our study provides valuable insight into the complex interaction between the host and its intestinal microbiota.
In conclusion, this study we showed A loss in microbial diversity is associated with an increase in post-operative microbial infections and other major GI surgical complications. It is possible that enteral nutrient deprivation, among other factors, leads to a shift in the microbiome, and thus a loss of microbial diversity. This may be due to an aberration in host response to microbial infection. This is evident by the increase in infectious and anastomotic complications in those with decreased diversity. With further investigation, this knowledge could lead to therapies aimed at improving the interaction between the microbiota and the host inflammatory signaling cascade in order to decrease perioperative complications.
Clinical Relevancy Statement.
Parenteral nutrition is widely used in the pediatric and adult population for patients with short and long term intestinal failure. The benefits of this therapy are clear, however, there are many detrimental effects associated with its use. There is little understanding of the underlying mechanisms for many of the known complications described with parenteral nutrition. This study represents the first report to investigate the intestinal microbiome in those patients on TPN. Regardless of cause, low intestinal microbial diversity correlates with increased incidence of anastomotic and infectious complications. An improved understanding of the interaction between loss of enteral nutrition, the microbiome and the host response may lead to therapy to decrease complications in an already high risk population.
Acknowledgments
Grant Support: T-32HD007505 (to EAM), NIH-R01 AI-44076-14 (to DHT).
Footnotes
Disclosures: The authors have no relevant disclosures.
References
- 1.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378–86. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47 (Suppl 1):S33–6. doi: 10.1097/MPG.0b013e3181819007. [DOI] [PubMed] [Google Scholar]
- 3.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–9. doi: 10.1097/01.sla.0000179647.24046.03. discussion 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition. 1995;11:339–44. [PubMed] [Google Scholar]
- 5.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525–32. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 6.Minard G, Kudsk KA. Effect of route of feeding on the incidence of septic complications in critically ill patients. Semin Respir Infect. 1994;9:228–31. [PubMed] [Google Scholar]
- 7.Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–8. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47:109–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langekamp-Henken B. Effect of parenteral and enteral nutrition on gut-associated lymphoid tissue. Journal of Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G833–41. doi: 10.1152/ajpgi.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Yang H, Nose K, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–47. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G629–G37. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Sun X, Yang H, Teitelbaum D. Dissociation of E-Cadherin and beta-catenin in a mouse model of total parenteral nutrition: A mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol (London) 2009;587:641–54. doi: 10.1113/jphysiol.2008.162719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukatsu K, Kudsk KA. Nutrition and Gut Immunity. Surgical Clinics of North America. 2011;91:755. doi: 10.1016/j.suc.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonker MA, Hermsen JL, Sano Y, Heneghan AF, Lan J, Kudsk KA. Small intestine mucosal immune system response to injury and the impact of parenteral nutrition. Surgery. 2012;151:278–86. doi: 10.1016/j.surg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukatsu K, Kudsk KA, Zarzaur BL, Wu Y, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15:318–22. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Feng Y, Sun X, Teitelbaum DH. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:338–46. doi: 10.1111/j.1749-6632.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Yang H, Nose K, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–47. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003;31:1118–25. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 20.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–11. doi: 10.1097/00000658-199205000-00013. discussion 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiristioglu I, Antony P, Fan Y, et al. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Digestive Diseases and Sciences. 2002;47:1147–57. doi: 10.1023/a:1015066813675. [DOI] [PubMed] [Google Scholar]
- 22.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res. 1998;79:91–6. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- 23.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–85. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 24.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 25.Miyasaka EA, Erb-Downward JR, Falkowski NR, Gillilland M, Huffnagle GB, Teitelbaum DH. Total Parenteral Nutrition (TPN) in a Mouse Model Leads to Major Population Shifts in the Intestinal Microbiome. Gastroenterology. 2011;140:S103–S. [Google Scholar]
- 26.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–60. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds JV, Kanwar S, Welsh FK, et al. 1997 Harry M. Vars Research Award. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery? Jpen: Journal of Parenteral & Enteral Nutrition. 1997;21:196–201. doi: 10.1177/0148607197021004196. [DOI] [PubMed] [Google Scholar]
- 28.Santos AA, Rodrick ML, Jacobs DO, et al. Does the route of feeding modify the inflammatory response? [see comments] Annals of Surgery. 1994;220:155–63. doi: 10.1097/00000658-199408000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchman A, Mestecky J, Moukarzel A, Ament M. Intestinal immune function is unaffected by parenteral nutrition in man. J Amer Coll Nutr. 1995;14:656–1. doi: 10.1080/07315724.1995.10718556. [DOI] [PubMed] [Google Scholar]
- 30.Sedman PC, MacFie J, Palmer MD, Mitchell CJ, Sagar PM. Preoperative total parenteral nutrition is not associated with mucosal atrophy or bacterial translocation in humans [see comments] British Journal of Surgery. 1995;82:1663–7. doi: 10.1002/bjs.1800821226. [DOI] [PubMed] [Google Scholar]
- 31.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlisle EM, Morowitz MJ. Pediatric surgery and the human microbiome. J Pediatr Surg. 2011;46:577–84. doi: 10.1016/j.jpedsurg.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–32. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willyard C. Microbiome Gut Reaction. Nature. 2011;479:S5–S7. [Google Scholar]
- 35.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowd SE, Callaway TR, Wolcott RD, et al. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) BMC Microbiol. 2008;8:125. doi: 10.1186/1471-2180-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 78:1509–19. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224–44. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–11. doi: 10.1097/00000658-199205000-00013. discussion 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–88. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 44.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]