Abstract
Purpose of Review
Osteogenesis imperfecta (OI), or “brittle bone disease”, has mainly been considered a bone disorder caused by collagen mutations. Within the last decade, however, a surge of genetic discoveries has created a new paradigm for OI as a collagen-related disorder, where autosomal dominant type I collagen defects cause most cases, while rare, mostly recessive forms are due to defects in genes whose protein products interact with collagen protein. This review is both timely and relevant in outlining the genesis, development and future of this paradigm shift in the understanding of OI.
Recent Findings
BRIL and PEDF defects cause types V and VI OI via defective bone mineralization, while defects in CRTAP, P3H1 and CyPB cause types VII-IX via defective collagen post-translational modification. Hsp47 and FKBP65 defects cause types X and XI OI via aberrant collagen crosslinking, folding and chaperoning, while defects in SP7, WNT1, TRIC-B and OASIS disrupt osteoblast development. Finally, absence of the type I collagen C-propeptidase BMP1 causes type XII OI due to altered collagen maturation/processing.
Summary
Identification of these multiple causative defects has provided crucial information for accurate genetic counseling, inspired a recently proposed functional grouping of OI types by shared mechanism to simplify current nosology, and should prod investigations into common pathways in OI. Such investigations could yield critical information on cellular and bone tissue mechanisms and translate to new mechanistic insight into clinical therapies for patients.
Keywords: Osteogenesis Imperfecta, Bone Dysplasia, Recessive Osteogenesis Imperfecta, Collagen
Introduction
Osteogenesis imperfecta (OI), or “brittle bone disease”, is a heritable bone dysplasia resulting in fragile, deformed bones, short stature, and, usually, low bone mass. The current OI paradigm is that of a collagen-related bone dysplasia, with most dominant cases caused by defects in type I collagen itself, while rare recessive forms are caused by defects in genes whose products interact with collagen (1, 2). The classical OI types I–IV contain the majority of cases (Table 1); they are dominantly inherited and caused by mutations in COL1A1 or COL1A2, producing defects in type I collagen quantity or structure (3). Types V and VI OI (Table 1; Figure 1A) have defective bone mineralization. They were defined on clinical, histological and radiological features before their causative genes, IFITM5 and SERPINF1, were identified (4). Types VII-XII, with autosomal recessive inheritance, were delineated as their causative genes were identified. Types VII-IX OI have defects in the collagen prolyl 3-hydroxylation complex (Table 1; Figure 1B), which alter the post-translational modification of collagen (5, 6). Types X and XI OI affect collagen folding and chaperone functions and are caused by mutations in SERPINH1 and FKBP10 (2). Finally, absence of the type I collagen C-propeptide processing enzyme, BMP1, results in type XII OI (7). A functional grouping of OI types by shared collagen-related mechanism was recently proposed (8).
Table 1.
OI types caused by defects in genes and the collagen-related proteins which they encode. The mode of inheritance for the OI types is shown on the left, with the proportion of known OI cases on the right. The current nosology is shown only up to type XII, after which the OI types remain largely unclassified. The majority (85-90%) of OI cases, resulting from defects in collagen genes and subsequent defective quantitative and qualitative (structural) mechanisms, are listed as types I-IV. Defects in IFITM5 and SERPINH1 result in the mineralization defects seen in most OI types V and VI, respectively. Defects in CRTAP, LEPRE1, and PPIB result in the collagen 3-hydroxylation defects of most cases of OI types VII, VIII and IX, respectively. Defects in SERPINH1 and FKBP10 result in the collagen chaperoning and hydroxylation defects of OI types X and XI, respectively. Defects in BMP1 result in the collagen processing defects of OI type XII. Defects in the relatively novel OI genes SP7/OSX, WNT1, TMEM38B and CREB3L1 result in osteoblast differentiation defects of separate, currently unclassified OI cases/types.
| OI Type | Defective Gene | Defective Protein | Defective Mechanism | ||
|---|---|---|---|---|---|
| Autosomal Dominant | I | COL1A1 | α1(I) collagen | Col Quantity | 85-90% of OI cases |
| II | COL1A1 or COL1A2 | α1(I)/α2(I) collagen | Col Structure | ||
| III | COL1A1 or COL1A2 | α1(I)/α2(I) collagen | |||
| IV | COL1A1 or COL1A2 | α1(I)/α2(I) collagen | |||
| V | IFITM5 | BRIL | Matrix Mineralization | 10-15% of OI cases | |
| Autusomal Recessive | VI | SERPINF1 | PEDF | ||
| VII | CRTAP | CRTAP | Col 3-hydroxylation | ||
| VIII | LEPRE1 | P3H1 | |||
| IX | PPIB | CyPB | |||
| X | SERPINH1 | HSP47 | Col Chaperoning Telopeptide hydroxylation | ||
| XI | FKBP10 | FKBP65 | |||
| XII | BMP1 | BMP1/mTLD | Col Processing | ||
| Unclassified | SP7/OSX | SP7/OSTERIX | Osteoblast development | ||
| WNT1 | WNT1 | ||||
| TMEM38B | TRIC-B | ||||
| CREB3L1 | OASIS |
Figure 1.
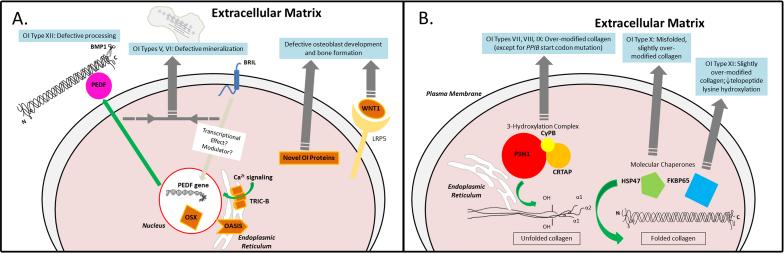
Abnormal molecular mechanisms in OI types caused by defects in collagen-related molecules. Dark grey arrows and the text in blue boxes show the aberrant mechanism in most OI cases linked to defects in the specific protein. A) Green arrow shows the normal secretion of PEDF (and subsequent collagen binding) after transcription in response to effects of BRIL protein and a possible modulator. The novel OI proteins all affect osteoblast development: specifically, nuclear OSTERIX affects osteoblast differentiation genes, the trimeric TRIC-B channel affects intracellular Ca2+ signaling, the ER-stress transducer OASIS modulates Col1 transcription when nuclear, and WNT1 binds LRP5 to affect bone formation. B) Green arrows show the normal effect of the proteins on collagen. The ER resident complex of CRTAP/P3H1/CyPB 3-hydroxylates specific prolines on the α1(I) and α2(I) chains, while chaperones contribute to normal folding of the collagen trimer.
Defects in collagen synthesis, structure or processing (COLI and BMP1 Mutations)
Type I collagen is a heterotrimer synthesized as a procollagen precursor containing two proα1(I) and one proα2(I) polypeptide chains, encoded by the COL1A1 and COL1A2 genes, respectively. Procollagen undergoes post-translational modifications in the endoplasmic reticulum (ER) during chain synthesis and helix formation. Short N- and C-propeptide domains flank the central helical domain, which contains uninterrupted Gly-Xaa-Yaa tripeptides, where X is often proline, and Y hydroxyproline. Procollagen maturation entails cleavage of the propeptides by specific N- and C-terminal propeptidases (9).
Defects in either structure or synthesis of type I collagen can cause OI. Dominantly inherited OI types include mild, non-deforming type I, perinatal lethal type II, progressively deforming type III and moderately deforming type IV. Mutations in either the COL1A1 or COL1A2 gene can cause the structural defects in collagen that underlie types II-IV OI. In contrast, a null COL1A1 allele causes type I OI, in which structurally normal type I collagen is synthesized at about half the normal amount. The collagen insufficiency of type I OI generally results from mutations causing premature termination, with nonsense mediated mRNA decay (NMD) of defective transcripts from the mutant allele, leaving only the structurally normal collagen from the normal allele (2, 3).
Over 80% of the mutations altering type I collagen structure are single base pair changes resulting in substitutions of glycine residues in either the α1(I) or α2(I) chain. The larger side chain of the substituting residue causes a delay in helix folding and subsequent post-translational overmodification of lysine and proline residues along the length of the helical region. Phenotypic severity varies with amino acid substitution, chain and position (1, 3). A set of fascinating mutations, causing an autosomal dominant high bone mass phenotype, alters the C-propeptide cleavage site (10). Interestingly, mutations in BMP1, encoding the C-propeptidase of type I procollagen, cause a recessive counterpart to High Bone Mass OI (type XII OI; Figure 1A) (7). Conversely, mutations in the N-propeptide cleavage site, the N-anchor domain of the helical region, or the N-propeptidase ADAMTS-2 cause EDS or OI/EDS phenotypes (11).
Defects in Collagen Modification- 3-Hydroxylation Complex Components (CRTAP, LEPRE1, PPIB) Mutations
Cartilage-associated protein (CRTAP), prolyl 3-hydroxylase 1 (P3H1), and cyclophilin B (CyPB) were the first proteins linked to recessive OI, specifically causing types VII, VIII and IX, respectively (12-16). These 3 proteins associate in 1:1:1 proportion to form the ER-localized collagen prolyl 3-hydroxlyation complex (Figure 1B). This complex modifies discrete collagen proline residues, specifically, α1(I)/α1(II) Proline-986 and α2(I) Proline-707, while the collagen chains are unfolded (1). It also has peptidyl prolyl cis-trans isomerase (PPIase) activity and is a collagen chaperone (17). It is not yet clear whether it is the absence of the complex or the collagen 3-hydroxylation modification that causes the bone dysplasia.
CRTAP, the ‘helper’ in the complex, is mutually protective with P3H1 in the ER, so that absence of either protein results in absence of both proteins (18). CRTAP was first identified in cartilage and is expressed in multiple tissues (16). Normally, up to 12% of the CRTAP produced by dermal fibroblasts is secreted, so it may have an additional role in extracellular matrix (ECM) (18). Loss of the matrix function of CRTAP, speculated to affect supramolecular assembly, may exacerbate the bone dysplasia in OI type VII, and cause renal and lung defects. Patients with CRTAP deficiency have no prolyl 3-hydroxylation complex and, consequently, absent α1(I) Proline-986 hydroxylation (14). Conversely, overmodification of the collagen helix in type VII proband cells by lysyl hydroxylase and prolyl 4-hydroxylase suggests delayed helix folding. Clinical presentations include severe to lethal osteochondrodysplasia with rhizomelia, neonatal fractures, broad undertubulated long bones, fragile ribs, severe growth defects, and “popcorn” epiphyseal calcifications in the occasional survivors (5, 19).
P3H1 (the enzyme in the complex), was first described as the matrix proteoglycan leprecan, and is encoded by the LEPRE1 (leucine and proline-enriched proteoglycan 1) gene. P3H1 may function in both the ECM and ER/Golgi compartments via its RGD cell adhesion domain and a KDEL ER-retention sequence, respectively, but the relative contribution of these roles to bone development is unknown (5). Type VIII OI, caused primarily by null LEPRE1 alleles, is clinically similar to type VII OI. The most common LEPRE1 allele is a founder mutation originating in West Africa that has also been reported in patients of African-American ancestry (20). Type I collagen from LEPRE1-null cells is overmodified, as in Types II-IV and VII OI, with an unanticipated 50% increase in collagen production versus controls (15).
CyPB, encoded by the PPIB (peptidyl- prolyl cis-trans isomerase B) gene, is the third protein in the 3-hydroxylation complex. Peptidyl-prolyl cis-trans isomerization is the rate limiting step in folding of the collagen helix, and CyPB was long considered the unique PPIase for this function. CyPB is ubiquitously expressed and its stability is independent of CRTAP/P3H1. PPIB-null cells, however, have moderately reduced CRTAP and P3H1 protein levels, suggesting CyPB provides some support to the complex (13, 18). Only 8 cases with PPIB defects have been reported, of which 4 have biochemical studies. Two lethal cases have 30% α1(I)Pro986 3-hydroxylation and overmodification of the collagen helix (1). Collagen overmodification in these cases may result from absence of the PPIase role of CyPB. Two moderately severe cases have normal α1(I) Pro986 3-hydroxylation, suggesting that the CRTAP/P3H1 complex can function in the total absence of CyPB. In addition, the case with a PPIB start codon mutation has normal levels of collagen helical modification, suggesting another PPIase can contribute to collagen folding (13). Collectively, the features of OI types VII, VIII and IX patients can be considered manifestations of defects in collagen modification.
Defects in Mineralization- Mutations in SERPINF1 and IFITM5
OI types V and VI (Figure 1A) have unique clinical phenotypes, characterized by distinct defects in bone mineralization (21, 22). Patients with type V OI have dominant inheritance of moderately severe bone dysplasia and fracture incidence, including vertebral compressions and, often, scoliosis. Features of type V OI include radial head dislocation, ossification of the forearm interosseous membrane, and hyperplastic callus. There is also a radiographically dense band prominent in the forearm metaphyses. The combination of features present in any given patient is variable, as is the timing of appearance. All patients with type V OI have a distinctive mesh-like lamellation pattern on bone histology (21, 23).
Patients with type VI OI have recessive inheritance of bone dysplasia, with clinical characteristics and bone histology distinct from type V OI. They do not have fractures at birth, but later have frequent fractures, progressive bone deformity, vertebral compressions and scoliosis. Growth deficiency in type VI is moderately severe, sclerae are white and teeth are normal. Children with type VI OI have elevated serum alkaline phosphatase levels; with a mean of 409 U/L (range 200-650 U/L). Bone histology is remarkable for broad bands of unmineralized osteoid and a fish-scale pattern under polarized light (22, 24).
Recently, the genes responsible for types V and VI OI have been delineated. All cases of type V OI are caused by a recurrent heterozygous mutation (c.-14C>T) in the 5’-UTR of IFITM5, which encodes BRIL (Bone Restricted Ifitm-Like protein, previously known as “Ifitm5”), a transmembrane protein enriched in osteoblasts during mineralization (25). The type V OI mutation putatively adds 5 amino acids to the N-terminus of BRIL and may have a gain-of-function mechanism (26, 27). The causative gene for type VI OI is SERPINF1, which encodes Pigment Epithelium-Derived Factor (PEDF) (28, 29). PEDF is a ubiquitously expressed secreted protein best known as a potent anti-angiogenic factor that inhibits tumor growth and metastasis (30). PEDF binds to two distinct sites on type I collagen, and this binding is critical to its anti-angiogenic function (31). A variety of recessive null mutations in SERPINF1 have been reported (28, 29, 32, 33). Serum PEDF is virtually absent in type VI OI patients, while normal PEDF values are reported in type V OI, as well as in OI caused by collagen defects (types I, III, and IV) (32).
The connection between BRIL and PEDF, and hence types V and VI OI, was revealed by a novel heterozygous IFITM5 mutation (p.S40L) in the intracellular domain of BRIL (34**-36). Interestingly, the patient with the BRIL S40L substitution had no features of type V OI, but instead had severe OI with bone histology typical of type VI OI. Her dermal fibroblasts and cultured osteoblasts displayed minimal secretion of PEDF, implying a connection between BRIL and PEDF functions in bone mineralization. Osteoblasts from a typical case of type V OI, with extended BRIL N-terminus, have increased SERPINF1 expression and PEDF secretion during osteoblast differentiation. Together, these data suggest that the type V OI mutation causes gain-of-function while p.S40L causes loss of BRIL function in bone development (34**).
Defects in Collagen Folding- Mutations in Collagen chaperones (SERPINH1, FKBP10)
Types X and XI OI (Figure 1A) are caused by mutations in the SERPINH1 and FKBP10 genes, respectively. The protein products of these genes, HSP47 and FKBP65, respectively, play crucial roles in the proper folding of triple helical procollagen molecules. FKBP65 is also a PPIase (37).
HSP47 (encoded by SERPINH1 gene) binds preferentially toward the N-terminus of triple helical procollagen, stabilizes the folded collagen in the ER, and assists shuttling of correctly folded collagen into the cis-Golgi. Only two SERPINH1 mutations causing bone dysplasia have been reported: one is a recessive SERPINH1 missense mutation (c.977T_C) in dachshunds (38); the second is a homozygous missense mutation (c.233T_C, p.Leu78Pro) in the only case of type X OI, whose clinical course was progressive and severe, with features both typical and atypical of OI (39). Mutant transcripts were stable, but HSP47 protein was substantially degraded. Residual HSP47 function likely enabled the survival of the child for 3 years, since Serpinh1-null mice are embryonic lethal (40). Knock-out mice have aggregation of collagen in the cell, delayed secretion and abnormal fibrillogenesis (41), while the collagen of the patient had near-normal collagen modification and secretion, despite increased site-specific susceptibility to proteolysis, again supporting residual function of the missense-containing HSP47.
Defects in FKBP10 (encoding FKBP65) have been particularly interesting, in that they encompass a spectrum of disorders formerly thought to be unrelated, i.e., OI, Bruck and Kuskokwim syndromes. FKBP10 mutations were first delineated in moderately severe OI (42). Shortly thereafter, Bruck syndrome I (BRKS1), a recessive disorder of severe OI with congenital contractures, was also shown to result from FKBP10 mutations (43). Since contractures are not always present in patients with the same FKBP10 mutation, even in siblings, it became clear that OI and BRKS1 were allelic conditions with variable contracture manifestation. Bruck syndrome can also be caused by mutations in PLOD2 (44**), which encodes LH2, the enzyme responsible for hydroxylation of collagen telopeptide lysine. Finally, Kuskokwim syndrome, a congenital contracture syndrome with minimal skeletal manifestations that occurs among the Yup'ik Eskimos of Alaska, was traced to an in-frame deletion of a conserved tyrosine in the third PPIase domain of FKBP65 (45**).
Collagen secreted from FKBP10-defective cells exhibited normal helical modification and α1(I) Pro986 3-hydroxylation. However, collagen C-telopeptide lysines from fibroblasts with null FKBP10 mutations showed less than 1% hydroxylation, versus 60% in normal cells (45**), a finding corroborated by data from bone tissue (46), while cells with the Kuskokwim mutation have 5-10% telopeptide hydroxylation (45**). The telopeptide lysine is critical to collagen crosslinking into matrix, likely underlying the reduced deposition of matrix collagen by mutant cells. It is not yet clear whether FKBP65 activates/stabilizes LH2, or whether deficiency of FKBP65 PPIase function limits LH2 access to the collagen telopeptide. (45**).
Unclassified/New genes
In the past few years, several genes found to cause OI appear to primarily affect osteoblast differentiation (Figure 1B). First, a homozygous deletion (c.1052delA) was identified in SP7/OSTERIX (OSX) in an Egyptian child with recessive OI (47). In mice, Osx is essential for bone formation. Osx-null mice show deficient osteoblast differentiation, reduced expression of osteoblast markers, including Col1a1, and bone bending deformities similar to those of OI patients (48).
Next, mutations in WNT1 were identified in multiple cases of OI (49, 50*, 51, 52). Heterozygous mutations lead to osteoporosis while several homozygous mutations cause severe OI (51). A number of WNT family members are key regulators of bone mass through β-catenin. WNT interacts with the cell surface low-density lipoprotein receptor–related protein 5 (LRP5) to activate bone formation. Homozygous mutations in LRP5 cause juvenile osteoporosis, resembling OI type IV in its skeletal features (53). The Wnt1 knockout mouse has severe abnormalities in brain development, but no reported skeletal phenotype. Conversely, only one of the OI patients reported with WNT1 mutations had a neurodevelopmental defect (54).
Third, a founder mutation (p.Gly152Alafs*5) in TMEM38B that deleted exon 4, causing premature termination, was identified in autosomal recessive OI among Bedouins from Saudi Arabia and Israel (55, 56). TMEM38B encodes TRIC-B, a monovalent cation-specific channel involved in intracellular Ca2+ release and active in cell differentiation. Tmem38b-knockout mice show neonatal lethality, thus, the skeletal phenotype was not determined (57). Alteration in TRIC-B might lead to autosomal recessive OI through defective intracellular Ca2+ signaling in bone cells.
Most recently, a homozygous genomic deletion of CREB3L1 gene, encoding OASIS (Old Astrocyte Specifically Induced Substance) was reported in a family with severe OI (58*). OASIS is an ER-stress transducer that regulates genes involved in developmental processes, differentiation, and maturation. Creb3l1- knockout mice have severe osteopenia and spontaneous fractures, and OASIS was shown to activate the transcription of Col1a1 (59).
Conclusions
Within the last decade, a virtual flood of genetic discoveries has generated a new paradigm for OI as a collagen-related disorder, in which autosomal dominant defects in the structure or synthesis of type I collagen are responsible for the great majority of cases, while rare, mostly recessive forms of OI are caused by defects in genes whose protein products interact with collagen post-translationally. In addition to the identification of causative defects providing the information essential for accurate genetic counseling, each of these discoveries has revealed proteins whose critical role in normal bone development had not been previously appreciated. The collagen-associated proteins are involved in collagen modification (members of the collagen prolyl 3-hydroxylation complex: CRTAP, P3H1 and CyPB), collagen mineralization (BRIL and PEDF), collagen folding, crosslinking and chaperoning (HSP47 and FKBP65), and osteoblast development (SP7, WNT1, TRIC-B and OASIS). Investigations of common pathways in dominant and recessive OI can be expected to yield critical insights into mechanism at cellular and bone tissue levels. The dynamism of OI research continues, with additional novel genes on the horizon and the potential to translate new understanding of mechanism into clinical therapies for affected individuals. Additionally, a novel approach to categorizing existing OI types based on connected metabolic mechanisms should prove effective in simplifying current nosology (8).
Key Points.
➢ The current OI paradigm is that of a collagen-related bone dysplasia, with most dominant cases caused by defects in type I collagen itself, and producing defects in type I collagen quantity or structure.
➢ Within the last decade, a flood of genetic discoveries has generated a new paradigm for OI as a collagen-related disorder, in which autosomal dominant defects in the structure or synthesis of type I collagen are responsible for the great majority of cases, while rare, mostly recessive, forms of OI are caused by defects in genes whose protein products interact with collagen post-translationally and whose critical role in normal bone development had not been previously appreciated.
➢ The collagen-related proteins are involved in collagen modification (members of the collagen prolyl 3-hydroxylation complex: CRTAP, P3H1 and CyPB), collagen mineralization (BRIL and PEDF), collagen folding, crosslinking and chaperoning (HSP47 and FKBP10), and osteoblast development (OSX, WNT1, TRICB and OASIS).
➢ Investigations of common pathways in dominant and recessive OI can be expected to yield critical insights into mechanism at cellular and bone tissue levels.
Acknowledgements
This work was supported by intramural funding (to JCM) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011 Sep;7(9):540–57. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marini JC, Blissett AR. New Genes in Bone Development: What's New in Osteogenesis Imperfecta. J Clin Endocrinol Metab. 2013 Aug;98(8):3095–103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007 Mar;28(3):209–21. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glorieux FH, Moffatt P. Osteogenesis imperfecta, an ever-expanding conundrum. J Bone Miner Res. 2013 Jul;28(7):1519–22. doi: 10.1002/jbmr.1982. [DOI] [PubMed] [Google Scholar]
- 5.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2010 Jan;339(1):59–70. doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007 Jul 15;6(14):1675–81. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Glez V, Valencia M, Caparros-Martin JA, Aglan M, Temtamy S, Tenorio J, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012 Feb;33(2):343–50. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forlino A, Marini JC. Osteogenesis Imperfecta: New forms reveal novel gene functions in bone development and unexpected protein-protein interactions. Lancet. 2014 in press. [Google Scholar]
- 9.Prockop DJ, Kivirikko KI. Collagens - Molecular-Biology, Diseases, and Potentials for Therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl K, Barnes AM, Fratzl-Zelman N, Whyte MP, Hefferan TE, Makareeva E, et al. COL1 C Propeptide Cleavage Site Mutations Cause High Bone Mass Osteogenesis Imperfecta. Hum Mutat. 2011 Jun;32(6):598–609. doi: 10.1002/humu.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabral WA, Makareeva E, Colige A, Letocha AD, Ty JM, Yeowell HN, et al. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem. 2005 May 13;280(19):19259–69. doi: 10.1074/jbc.M414698200. [DOI] [PubMed] [Google Scholar]
- 12.Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, Pace JM, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008 Dec;29(12):1435–42. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes AM, Carter EM, Cabral WA, Weis M, Chang W, Makareeva E, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010 Feb 11;362(6):521–8. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006 Dec 28;355(26):2757–64. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007 Mar;39(3):359–65. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006 Oct 20;127(2):291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa Y, Wirz J, Vranka JA, Nagata K, Bachinger HP. Biochemical characterization of the prolyl 3-hydroxylase 1.cartilage-associated protein.cyclophilin B complex. J Biol Chem. 2009 Jun 26;284(26):17641–7. doi: 10.1074/jbc.M109.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang W, Barnes AM, Cabral WA, Bodurtha JN, Marini JC. Prolyl 3-hydroxylase 1 and CRTAP are mutually stabilizing in the endoplasmic reticulum collagen prolyl 3-hydroxylation complex. Hum Mol Genet. 2010 Jan 15;19(2):223–34. doi: 10.1093/hmg/ddp481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obafemi AA, Bulas DI, Troendle J, Marini JC. Popcorn calcification in osteogenesis imperfecta: incidence, progression, and molecular correlation. Am J Med Genet A. 2008 Nov 1;146A(21):2725–32. doi: 10.1002/ajmg.a.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabral WA, Barnes AM, Adeyemo A, Cushing K, Chitayat D, Porter FD, et al. A founder mutation in LEPRE1 carried by 1.5% of West Africans and 0.4% of African Americans causes lethal recessive osteogenesis imperfecta. Genet Med. 2012 May;14(5):543–51. doi: 10.1038/gim.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000 Sep;15(9):1650–8. doi: 10.1359/jbmr.2000.15.9.1650. [DOI] [PubMed] [Google Scholar]
- 22.Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002 Jan;17(1):30–8. doi: 10.1359/jbmr.2002.17.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Cheung MS, Glorieux FH, Rauch F. Natural history of hyperplastic callus formation in osteogenesis imperfecta type V. J Bone Miner Res. 2007 Aug;22(8):1181–6. doi: 10.1359/jbmr.070418. [DOI] [PubMed] [Google Scholar]
- 24.Roughley PJ, Rauch F, Glorieux FH. Osteogenesis imperfecta--clinical and molecular diversity. Eur Cell Mater. 2003 Jun 30;5:41–7. doi: 10.22203/ecm.v005a04. discussion 7. [DOI] [PubMed] [Google Scholar]
- 25.Moffatt P, Gaumond MH, Salois P, Sellin K, Bessette MC, Godin E, et al. Bril: a novel bone-specific modulator of mineralization. J Bone Miner Res. 2008 Sep;23(9):1497–508. doi: 10.1359/jbmr.080412. [DOI] [PubMed] [Google Scholar]
- 26.Cho TJ, Lee KE, Lee SK, Song SJ, Kim KJ, Jeon D, et al. A single recurrent mutation in the 5'-UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet. 2012 Aug 10;91(2):343–8. doi: 10.1016/j.ajhg.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semler O, Garbes L, Keupp K, Swan D, Zimmermann K, Becker J, et al. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet. 2012 Aug 10;91(2):349–57. doi: 10.1016/j.ajhg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011 Mar 11;88(3):362–71. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homan EP, Rauch F, Grafe I, Lietman C, Doll JA, Dawson B, et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res. 2011 Dec;26(12):2798–803. doi: 10.1002/jbmr.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013 Apr;13(4):258–71. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiya A, Okano-Kosugi H, Yamazaki CM, Koide T. Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J Biol Chem. 2011 Jul 29;286(30):26364–74. doi: 10.1074/jbc.M111.252684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauch F, Husseini A, Roughley P, Glorieux FH, Moffatt P. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J Clin Endocrinol Metab. 2012 Aug;97(8):E1550–6. doi: 10.1210/jc.2012-1827. [DOI] [PubMed] [Google Scholar]
- 33.Venturi G, Gandini A, Monti E, Dalle Carbonare L, Corradi M, Vincenzi M, et al. Lack of expression of SERPINF1, the gene coding for pigment epithelium-derived factor, causes progressively deforming osteogenesis imperfecta with normal type I collagen. J Bone Miner Res. 2012 Mar;27(3):723–8. doi: 10.1002/jbmr.1480. [DOI] [PubMed] [Google Scholar]
- 34**.Farber CR, Reich A, Barnes AM, Becerra P, Rauch F, Cabral WA, et al. A Novel IFITM5 Mutation in Severe Atypical Osteogenesis Imperfecta Type VI Impairs Osteoblast Production of Pigment Epithelium-Derived Factor. J Bone Miner Res. 2014 Jan 13; doi: 10.1002/jbmr.2173. [First to describe novel mutation (S40L) in IFITM5 and suggest a relationship between BRIL and PEDF.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillen-Navarro E, Ballesta-Martinez MJ, Valencia M, Bueno AM, Martinez-Glez V, Lopez-Gonzalez V, et al. Two mutations in IFITM5 causing distinct forms of osteogenesis imperfecta. Am J Med Genet A. 2014 Jan 29; doi: 10.1002/ajmg.a.36409. [DOI] [PubMed] [Google Scholar]
- 36.Hoyer-Kuhn H, Semler O, Garbes L, Zimmermann K, Becker J, Wollnik B, et al. A Non-Classical IFITM5 Mutation Located in the Coding Region Causes Severe Osteogenesis Imperfecta with Prenatal Onset. J Bone Miner Res. 2013 Nov 30; doi: 10.1002/jbmr.2156. [DOI] [PubMed] [Google Scholar]
- 37.Zeng B, MacDonald JR, Bann JG, Beck K, Gambee JE, Boswell BA, et al. Chicken FK506-binding protein, FKBP65, a member of the FKBP family of peptidylprolyl cis-trans isomerases, is only partially inhibited by FK506. Biochem J. 1998 Feb 15;330(Pt 1):109–14. doi: 10.1042/bj3300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drogemuller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, et al. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009 Jul;5(7):e1000579. doi: 10.1371/journal.pgen.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, Alrasheed S, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010 Mar 12;86(3):389–98. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai N, Hosokawa M, Itohara S, Adachi E, Matsushita T, Hosokawa N, et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000 Sep 18;150(6):1499–506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bachinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell. 2006 May;17(5):2346–55. doi: 10.1091/mbc.E05-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alanay Y, Avaygan H, Camacho N, Utine GE, Boduroglu K, Aktas D, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010 Apr 9;86(4):551–9. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley BP, Malfait F, Bonafe L, Baldridge D, Homan E, Symoens S, et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res. 2011 Mar;26(3):666–72. doi: 10.1002/jbmr.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Puig-Hervas MT, Temtamy S, Aglan M, Valencia M, Martinez-Glez V, Ballesta-Martinez MJ, et al. Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome--osteogenesis imperfecta phenotypic spectrum. Hum Mutat. 2012 Oct;33(10):1444–9. doi: 10.1002/humu.22133. [Provides key insight into the connection between FKBP10 and PLOD2 defects in Bruck syndrome. It further showed that PLOD2, in addition to causing Bruck syndrome, is also associated with OI phenotypes of variable severity.] [DOI] [PubMed] [Google Scholar]
- 45**.Barnes AM, Duncan G, Weis M, Paton W, Cabral WA, Mertz EL, et al. Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum Mutat. 2013 Sep;34(9):1279–88. doi: 10.1002/humu.22362. [Pivotal in extending the characterization of FKBP10 defects as a spectrum of disorders ranging from OI to Bruck syndrome to Kuskokwim syndrome, all of which were previously considered unrelated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarze U, Cundy T, Pyott SM, Christiansen HE, Hegde MR, Bank RA, et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum Mol Genet. 2013 Jan 1;22(1):1–17. doi: 10.1093/hmg/dds371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapunzina P, Aglan M, Temtamy S, Caparros-Martin JA, Valencia M, Leton R, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet. 2010 Jul 9;87(1):110–4. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002 Jan 11;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 49.Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013 May;50(5):345–8. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 50*.Keupp K, Beleggia F, Kayserili H, Barnes AM, Steiner M, Semler O, et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet. 2013 Apr 4;92(4):565–74. doi: 10.1016/j.ajhg.2013.02.010. [First to describe that mutations in WNT1 cause different forms of bone fragility and affect WNT signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013 May 9;368(19):1809–16. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pyott SM, Tran TT, Leistritz DF, Pepin MG, Mendelsohn NJ, Temme RT, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am J Hum Genet. 2013 Apr 4;92(4):590–7. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012 Jan 15;492(1):1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faqeih E, Shaheen R, Alkuraya FS. WNT1 mutation with recessive osteogenesis imperfecta and profound neurological phenotype. J Med Genet. 2013 Jul;50(7):491–2. doi: 10.1136/jmedgenet-2013-101750. [DOI] [PubMed] [Google Scholar]
- 55.Shaheen R, Alazami AM, Alshammari MJ, Faqeih E, Alhashmi N, Mousa N, et al. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J Med Genet. 2012 Oct;49(10):630–5. doi: 10.1136/jmedgenet-2012-101142. [DOI] [PubMed] [Google Scholar]
- 56.Volodarsky M, Markus B, Cohen I, Staretz-Chacham O, Flusser H, Landau D, et al. A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum Mutat. 2013 Apr;34(4):582–6. doi: 10.1002/humu.22274. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X, Lin P, Yamazaki D, Park KH, Komazaki S, Chen SR, et al. Trimeric intracellular cation channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ Res. 2014 Feb 14;114(4):706–16. doi: 10.1161/CIRCRESAHA.114.301816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Symoens S, Malfait F, D'Hondt S, Callewaert B, Dheedene A, Steyaert W, et al. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J Rare Dis. 2013;8:154. doi: 10.1186/1750-1172-8-154. [First to describe a homozygous genomic deletion of CREB3L1 in a family with severe OI.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009 Oct;11(10):1205–11. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]