Abstract
Background
Much of the recent increase in hospital admission rates and mortality associated with hepatitis C in Canada is believed to be because of a higher prevalence of hepatitis C virus infection among those born between 1945 and 1965 (the baby boomer generation). We explored the effects of birth cohort on the rates of and projected trends in hospital admissions associated with hepatitis C.
Methods
The hospital records of 17 344 inpatients with a diagnosis of chronic hepatitis C and liver disease, including liver cancer, were extracted from the Canadian Discharge Abstract Database for April 2004 to March 2011. For each 5-year birth cohort from 1915 to 1984, regression analysis was used to estimate the temporal trends associated with the average age of the cohort during the study period. Future hospital admissions were predicted based on the assumption that past trends would continue.
Results
Hospital admissions associated with hepatitis C and liver disease increased an average of 6.0% (95% confidence interval [CI] 4.4%–7.7%) a year over the study period. As of 2010, hospital admission rates were highest for the 1950–1954 and 1955–1959 birth cohorts, at 17.6 (95% CI 13.2–23.5) and 13.7 (95% CI 10.3–18.2) times the rate for the 1970–1974 birth cohort. The corresponding same-age rate ratios predicted under a status quo scenario were 3.6 (95% CI 2.3–4.9) and 3.4 (95% CI 2.1–4.7). Same-age rate ratios were significantly higher for the four 5-year birth cohorts between 1950 and 1969 compared with other birth cohorts.
Interpretation
Hospital admissions associated with chronic hepatitis C and liver disease were significantly higher for the 1950–1954 and 1955–1959 birth cohorts than for most other birth cohorts. Without further interventions, the disease burden associated with hepatitis C will continue to increase for most birth cohorts, likely peaking after age 70 years. The substantial disease burden emerging in younger birth cohorts should be monitored.
In August 2012, the US Centers for Disease Control and Prevention released recommendations to expand screening for hepatitis C virus (HCV) infection to include a 1-time blood test for anyone born between 1945 and 1965 (i.e., the baby boomer generation). This recommendation was based in part on estimates that this cohort accounts for three-quarters of all hepatitis C cases in the United States.1 Furthermore, of the estimated 4.3% of the population born in the 1950s who were infected, 50% were unaware of their status.2,3 Currently in Canada, screening recommendations for HCV are based on an individual assessment of risk rather than the patient’s age or year of birth.4 The Canadian Health Measures Survey, a nationally representative household survey, estimated the seroprevalence of HCV for 2007–2011 to be 0.5% (95% confidence interval [CI] 0.3%–0.9%), with only 30% of those people (95% CI 16%–51%) aware of their infection. Prevalence was elevated among those aged 50–79 years compared with those aged 14–49 years.5 Similar to other household surveys, the Canadian Health Measures Survey does not include the homeless or prison populations, in which the prevalence of HCV infection is considerably higher.
Hepatitis C has resulted in a considerable morbidity and mortality burden in Canada.6–8 Based on health-adjusted life-years, a composite measure of premature mortality and reduced functioning because of disease, hepatitis C accounts for the largest proportion of disease burden among 51 infectious diseases in Ontario.9 A study in British Columbia found significantly elevated standardized mortality ratios for liver- and drug-related causes of death as well as for all-cause mortality among people who had tested positive for HCV compared with those who had tested negative.10
The natural history of hepatitis C is only partly understood, and the progression to liver cirrhosis is variable.11–13 A small portion of people, estimated at 15% by the American National Health and Nutrition Examination Survey,14 clear their infection. For others, symptoms of chronic infection often emerge 20 years or more after the initial infection. Disease progression from fibrosis to cirrhosis and hepatocellular carcinoma is not linear over time; rather, the rate of progression is related to many factors, including time since infection, age and alcohol consumption.11–13
Substantial increases in liver-related hospital admissions and mortality rates in Canada6 have been attributed to hepatitis C; however, trends by birth cohort have not been described previously. We explored the effect of birth cohort on trends in hospital admissions associated with hepatitis C and liver disease, predicted future lifetime hospital admissions by 5-year birth cohort and compared hospital admission rates for different birth cohorts.
Methods
Sources of data
Hospital discharge records for patients admitted to an acute care hospital with a diagnosis of chronic hepatitis C from Apr. 1, 2004, to Mar. 31, 2011 were extracted from the Canadian Institute for Health Information patient-specific Discharge Abstract Database.8 We chose this timeframe because it was a period when all provinces participating in the database used the Canadian version of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10-CA) for diagnostic coding. Because the province of Quebec does not participate in the Discharge Abstract Database, the database includes about 75% of all acute care hospital discharges in Canada.
We stratified the 38 901 hospital admissions by 5-year birth cohort from 1915 to 1984, by fiscal year of discharge (from Apr. 1 to Mar. 31 of the following calendar year), by discharge status (alive or dead) and by the presence of a co-diagnosis of liver disease (ICD-10-CA codes K70–K77, R18), including hepatic carcinoma (ICD-10-CA code C22). Hospital admissions associated with chronic hepatitis C were identified by the ICD-10-CA code B18.2. Population denominators for rate calculations were obtained from Statistics Canada’s population projections, medium growth scenario M1.15The M1 projection is the standard growth scenario that best reflects past trends.
Statistical analysis
We used a regression model to estimate trends in the number of hospital admissions associated with hepatitis C and liver disease for each 5-year birth cohort from 1915–1919 to 1980–1984 over the 7-year study period (Apr. 1, 2004, to Mar. 31, 2011), and then associated these trends with the average age of the cohort. Although many factors likely influenced these trends, we assumed that the age-related effects of an HCV infection were the main factor over the study period. The estimated age-specific trends were interpolated to each single year of age and were used to project the burden of hospital admissions for each cohort until age 90. Because reducing the near-term burden is an urgent public health concern, we calculated rate ratios for the last year of data (2010/11) and for predictions over the next 5, 10 and 20 years to identify the birth cohorts with the highest burden of hepatitis C–related hospital admissions. The same-age rate ratios (ratio of the projected hospital admission rate for 2 birth cohorts at age 75) calculated from this status quo projection provide a measure of the relative lifetime burden likely associated with underlying differences in prevalence. The future lifetime hospital admission rates provide another point of view and are a component used in cost-effectiveness analyses, although this study design only provides a status quo estimate. Confidence intervals were derived from the regression model. Additional methodologic details are available in Appendix 1.
Results
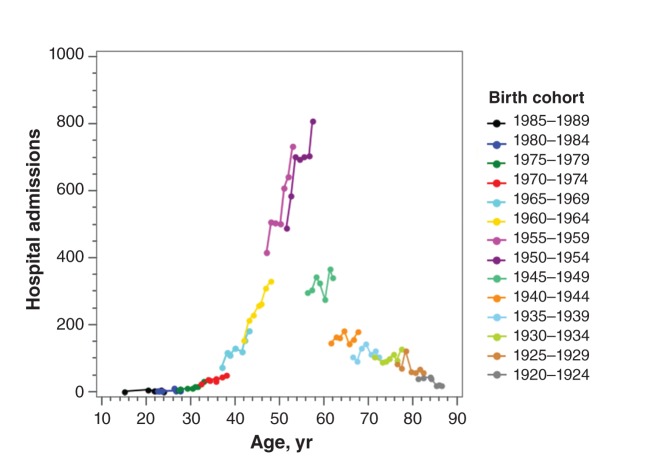
The number of hospital admissions associated with chronic hepatitis C increased from 4700 in 2004/05 to 6400 in 2010/11, a rate of 4.1% (95% CI 2.8%–5.3%) per year. Of the 38 901 admissions associated with chronic hepatitis C, 44.6% (17 344) had a codiagnosis of liver disease; this proportion increased with increasing age, from 4.6% among patients under 30 years of age to 60.6% among patients 65 years of age or older. Hospital admissions associated with hepatitis C and liver disease increased at a rate of 6.0% (95% CI 4.4%–7.7%) per year. Although not specific to hepatitis C, alcoholic liver disease (ICD-10-CA code K70) accounted for 24.3% of the admissions and liver cancer another 10.7%. The fatality rate increased with increasing age, from 10.8% among patients under 30 years of age to 19.4% among patients 65 years of age or older. The baby boom cohorts (1945–1964) accounted for 74.1% of all hospital admissions in 2010/11. Although hospital admission rates were lower in the younger cohorts, by the end of the study period, the number of hospital admissions among the 1965–1969 birth cohort had already increased to levels seen 5 years earlier in the 1960–1964 birth cohort (Figure 1).
Figure 1:
Annual hospital admissions associated with chronic hepatitis C and liver disease by 5-year birth cohort in Canada, excluding Quebec, from 2004/05 to 2010/11. For each cohort, the 7 data points (for each year of the study), representing average age at time of admission, have been joined to create curves showing the number of admissions by age for a single birth cohort. Combined, the cohort curves indicate the trend in hospital admissions by age (i.e., increasing from age 25 to 55 and decreasing from age 80 onward). For successive birth cohorts, there is an overlap of 2 data points. Vertical spaces between the cohort-specific series at the same age indicate a cohort effect attributed to different levels of exposure to hepatitis C virus as well as differences in the availability of treatments.
Trends
We found statistically significant increases in the number of hospital admissions associated with hepatitis C and liver disease over the study period for the six 5-year birth cohorts corresponding to people born from 1950 to 1979, an approximate age range of 25–60 years (Table 1). For the 1920–1924 and 1925–1929 birth cohorts, the number of hospital admissions declined significantly. The rate of increase was highest at about 30 years of age (Table 1).
Table 1: Rates of hospital admission associated with chronic hepatitis C and liver disease and average annual percentage change from 2004/05 to 2010/11 by birth cohort in Canada, excluding Quebec.
| Birth cohort | Average age, yr | No. of hospital admissions in 2010/11 | 2010 population, thousands | Admission rate in 2010/11, no. per 100 000 (95% CI)* |
Average annual percentage change from 2004/05 to 2010/11, % (95% CI)* | Trend p value |
|---|---|---|---|---|---|---|
| 1915–19 |
89 |
8 |
100 |
8.9 (4.8 to 16.6) |
−6.8 (−20.9 to 9.4) |
0.4 |
| 1920–24 |
83 |
19 |
288 |
6.7 (4.5 to 10.1) |
−13.9 (−22.0 to −5.1) |
0.003 |
| 1925–29 |
79 |
58 |
481 |
12.4 (9.8 to 15.8) |
−6.6 (−12.3 to −0.6) |
0.03 |
| 1930–34 |
74 |
126 |
657 |
17.1 (14.2 to 20.6) |
3.5 (−1.9 to 9.1) |
0.2 |
| 1935–39 |
69 |
103 |
796 |
14.9 (12.5 to 17.8) |
1.1 (−3.9 to 6.2) |
0.7 |
| 1940–44 |
64 |
178 |
1 030 |
16.4 (14.1 to 19.1) |
1.6 (−2.6 to 5.9) |
0.5 |
| 1945–49 |
59 |
340 |
1 403 |
24.4 (21.9 to 27.1) |
2.2 (−0.8 to 5.3) |
0.2 |
| 1950–54 |
54 |
808 |
1 666 |
48.2 (45.0 to 51.8) |
6.6 (4.4 to 8.9) |
< 0.001 |
| 1955–59 |
50 |
733 |
1 948 |
36.5 (33.9 to 39.4) |
8.9 (6.5 to 11.4) |
< 0.001 |
| 1960–64 |
45 |
331 |
2 142 |
15.8 (14.2 to 17.7) |
11.5 (7.7 to 15.4) |
< 0.001 |
| 1965–69 |
40 |
183 |
1 933 |
9.1 (7.8 to 10.6) |
12.7 (7.4 to 18.4) |
< 0.001 |
| 1970–74 |
35 |
50 |
1 817 |
2.6 (1.9 to 3.5) |
10.1 (0.7 to 20.6) |
0.04 |
| 1975–79 |
30 |
32 |
1 753 |
1.6 (1.0 to 2.4) |
33.6 (14.3 to 57.8) |
< 0.001 |
| 1980–84 |
25 |
7 |
1 835 |
0.4 (0.2 to 0.9) |
25.0 (−5.1 to 69.3) |
0.1 |
| All | 55 | 2 976 | 17 848 | 16.5 (15.9 to 17.1) | 6.0 (4.1 to 7.9) | < 0.001 |
Note: CI = confidence interval. *Results are from the regression model.
Projections
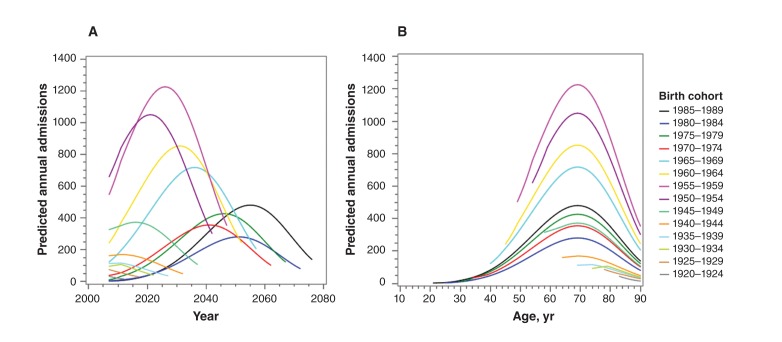
Applying these estimated trends to the historical hospital admission data produced projections based on the status quo that suggest the 1950–1954 and 1955–1959 birth cohorts will continue to experience the highest number of annual hospital admissions well past 2020 (Figure 2A). The number of annual hospital admissions is projected to peak around age 70–80 (Figure 2B). The 6.0% (95% CI 4.1%–7.9%) annual increase observed over the study period for all birth cohorts combined (1915–1984) is projected to decline to 2.5% per year by 2020 and eventually plateau about 10 years later.
Figure 2:
Projection of annual hospital admissions associated with chronic hepatitis C and liver disease based on current trends. The 1970–1974 birth cohort (red line) was used as reference for the calculation of rate ratios. (A) Projections are plotted against calendar year. (B) Projections are plotted against the average age of the birth cohort. Rate ratios calculated for the same age (age 75 was used for the calculations) are a measure of the relative burden and are closely related to the relative hepatitis C prevalence. Future lifetime hospital admissions were calculated as the area under the curve from 2011/12 to age 90.
Rate ratios
Over the next 10 years, the rate of admission to hospital for the 1950–1954 and 1955–1959 cohorts is predicted to remain high at 10.7 (95% CI 6.7–14.7) and 9.1 (95% CI 5.8–12.4) times that of the 1970–1974 cohort (Table 2). The model suggests that the rates associated with hepatitis C and liver disease for the 1950–1954 cohort will be about 3.6 (95% CI 2.3–4.9) times higher than the rate for the 1970–1974 cohort at the same age, whereas the estimated ratio for the future lifetime hospital admission burden is slightly lower at 2.4 (95% CI 1.5–3.4) times the 1970–1974 cohort rate. Although the disease burden for both 1960s cohorts is currently lower than that of the two 1950s cohorts, the future lifetime burden is predicted to be similar for all four birth cohorts. It is uncertain whether the decline in same-age rate ratios seen in successive 5-year birth cohorts from 1955–1959 to 1970–1974 will continue with younger cohorts, although the future lifetime burden for people born after 1970 is possibly elevated compared with those born before 1944 (Table 2, same-age rate ratios and confidence intervals).
Table 2: Average projected rates of hospital admission associated with chronic hepatitis C and liver disease by birth cohort in Canada, excluding Quebec, compared with the 1970–1974 cohort*.
| Birth cohort | Average projected admission rate as a ratio of the 1970–1974 cohort rate (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| End of study period (2010/11) | 1-yr projection (2011/12) | 5-yr projection (2011/12–2015/16) |
2015/16 | 10-yr projection (2011/12–2020/21) |
2020/21 | Same-age† | Until age 90 (from 2011/12) |
|
| 1915–1919 |
2.9 (1.4–6.1) |
2.7 (0.9–4.5) |
– |
– |
– |
– |
– |
– |
| 1920–1924 |
2.4 (1.4–4.1) |
2.1 (1.5–2.8) |
1.6 (0.5–2.7) |
1.2 (0–2.3) |
– |
– |
– |
– |
| 1925–1929 |
4.4 (3.0–6.4) |
3.7 (2.3–5.2) |
2.4 (1.3–3.6) |
1.6 (0.6–2.7) |
1.6 (0.5–2.6) |
0.8 (0–2.1) |
– |
– |
| 1930–1934 |
7.0 (5.0–9.7) |
6.1 (3.8–8.3) |
3.5 (2.0–5.0) |
2.2 (1.0–3.4) |
2.0 (1.0–3.0) |
0.8 (0.2–1.4) |
– |
0.2 (0.1–0.3) |
| 1935–1939 |
4.7 (3.4–6.6) |
5.8 (3.4–8.2) |
4.1 (2.6–5.7) |
3.1 (1.8–4.5) |
2.6 (1.5–3.7) |
1.1 (0.4–1.8) |
0.8 (0.4–1.2) |
0.3 (0.1–0.4) |
| 1940–1944 |
6.3 (4.6–8.6) |
6.0 (4.3–7.8) |
4.7 (3.0–6.4) |
3.9 (2.3–5.6) |
3.5 (2.1–5.0) |
2.3 (1.1–3.4) |
1.0 (0.5–1.5) |
0.5 (0.2–0.7) |
| 1945–1949 |
8.8 (6.5–11.8) |
9.3 (6.2–12.4) |
6.9 (4.5–9.4) |
5.7 (3.5–8.0) |
5.3 (3.2–7.4) |
3.8 (2.0–5.6) |
1.6 (0.9–2.3) |
0.9 (0.5–1.4) |
| 1950–1954 |
17.6 (13.2–23.5) |
17.8 (10.9–24.7) |
14.0 (9.3–18.6) |
11.7 (7.3–16.0) |
10.7 (6.7–14.7) |
7.5 (4.0–10.9) |
3.6 (2.3–4.9) |
2.4 (1.5–3.4) |
| 1955–1959 |
13.7 (10.3–18.2) |
13.9 (9.7–18.1) |
11.5 (7.7–15.3) |
10.0 (6.3–13.6) |
9.1 (5.8–12.4) |
6.6 (3.7–9.4) |
3.4 (2.1–4.7) |
2.6 (1.6–3.6) |
| 1960–1964 |
5.6 (4.2–7.6) |
6.2 (4.5–7.9) |
5.6 (3.7–7.5) |
5.2 (3.2–7.1) |
4.8 (3.0–6.6) |
3.8 (2.2–5.5) |
2.2 (1.4–3.1) |
1.9 (1.2–2.6) |
| 1965–1969 |
3.4 (2.5–4.7) |
3.7 (2.5–5.0) |
3.4 (2.2–4.6) |
3.3 (2.1–4.6) |
3.2 (2.0–4.4) |
3.0 (1.8–4.1) |
2.1 (1.3–2.8) |
1.9 (1.2–2.6) |
| 1970–1974* |
– |
– |
– |
– |
– |
– |
– |
– |
| 1975–1979 |
0.7 (0.4–1.0) |
0.7 (0.4–1.0) |
0.6 (0.3–1.0) |
0.6 (0.2–1.0) |
0.6 (0.2–1.0) |
0.6 (0.2–1.1) |
1.1 (0.4–1.8) |
1.1 (0.4–1.8) |
| 1980–1984 | 0.1 (0.1–0.3) |
0.1 (0–0.3) |
0.3 (0–0.6) |
0.3 (0–0.8) |
0.3 (0–0.7) |
0.3 (0–0.8) |
0.7 (0–1.5) |
1.0 (0–2.5) |
Note: CI = confidence interval. *The number of annual hospital admissions was too small to use either the oldest or youngest birth cohort as a reference. The 1970–1974 birth cohort was selected to be the reference group and to allow comparison of prevalence ratios with the same age group used in the development of the US Centers for Disease Control and Prevention guidelines. †Same-age rate ratios were calculated at age 75.
Interpretation
Hospital admissions associated with chronic hepatitis C and liver disease were significantly elevated for the 1950–1954 and 1955–1959 birth cohorts compared with most other birth cohorts. Without further interventions, we expect hospital admissions for these cohorts to continue to increase and peak at about 1.5 times the 2010/11 rate in about 2025–2030. Although the projected hospital admission rates among those born in the 1960s are lower than the projected rates for those born in the 1950s at similar ages, the potential to reduce the disease burden over a longer life span in younger cohorts suggests that the 1960s cohorts could also benefit from earlier detection and treatment to stop disease progression. It is too early to assess fully the relative hepatitis C burden for those born after 1974, because the number of hospital admissions associated with liver disease was still small as of 2010/11. However, this analysis suggests that continued monitoring of these younger birth cohorts would be appropriate.
The rate of annual increase in hospital admissions over the study period is considerably less than earlier estimates of 15% to 30%.6,16 The higher rates of increase found in the earlier studies are similar to those estimated in this study for people aged 30–45 (Table 1), an age range that corresponds to the age of people born in the 1950s and 1960s in the earlier studies. In contrast to the increase in hospital admission rates, the number of new hepatitis C diagnoses has been declining in recent years for most birth cohorts over the age of 30.17,18
Our estimates of age-specific rates of increase in hospital admissions are in close agreement with trends in the number of hepatitis C patients with advanced liver disease estimated in a large study of Medicare claims that used birth cohorts to assess age-specific trends.19 In other studies of smaller cohorts, disease progression rates varied depending on the characteristics of the cohorts studied.11–13,20 One difference is noted: we did not detect a statistically significant decline in the number of hospital admissions until age 80, whereas Zalesak and colleagues19 found that the number of patients with hepatitis C and severe liver disease who were born before 1945 had already started to decline in 2008 (at age 64 and older).
Because Canadian estimates of hepatitis C prevalence are not yet available for specific birth cohorts, same-age rate ratios were calculated to compare the likely cohort effect in Canada with the situation in the US. Estimates from the American National Health and Nutrition Examination Survey found that people born in the 1950s had the highest anti-HCV prevalence at 4.3 times that of people born in the 1970s.2 In comparison, our model estimating same-age rate ratios suggests that the hepatitis C disease burden among Canadians born in the 1950s is likely 3.5 times that of Canadians born from 1970 to 1974. However, the Canadian estimates of anti-HCV household prevalence from the Canadian Health Measures Survey are lower than the US estimate by a factor of 3 (0.5% v. 1.6%).2,5 If we use our same-age rate ratios to prorate the Canadian Health Measures Survey estimate,5 the results suggest an anti-HCV Canadian household prevalence of about 1.1% for people born in the 1950s and 0.5% and 0.3% for Canadians born in 1945–1949 and 1970–1974, respectively. However, household prevalence is considered an underestimate of the national prevalence, because groups such as the homeless and prison inmates, who are at high risk of hepatitis C, are not included in the household surveys.
Limitations
The primary limitation of this study is that we assumed the main driver of the birth-cohort–specific trends was age; we could not account for the effects of other factors, such as time since infection, lifestyles associated with various routes of transmission and immigration. Age may be a good indicator of time since infection in younger birth cohorts for whom injection drug use is considered the primary risk factor for HCV infection. However, the relation between age and time since infection may be quite different for older birth cohorts for whom blood transfusion, blood products or organ transplant before 1992 are recognized sources of infection in Canada.4 Later age at acquisition of hepatitis C has been associated with a greater prevalence of cirrhosis 20 years later and with faster rates of progression to cirrhosis.12,21 Injection drug use is also associated with a higher risk of death from other causes.10 As a result, the disease burden for people born after 1950 may peak somewhat earlier and decline faster than suggested by our data (Figure 2).
Improvements in treatment options over the study period likely influenced the historical trends, and further improvement in this area or the development of drug resistance could alter the future disease burden substantially. Patients in whom hepatitis C was diagnosed over the study period contributed to the historical trends if they were admitted to hospital for a liver disease, and screening for hepatitis C may lead to diagnosis at an earlier stage of the disease. These additional sources of uncertainty were not included in our estimated confidence intervals; better treatments should reduce hospital admission rates to levels below our status quo estimates, perhaps as soon as 2015.
Our study design was limited to assessing the hospital burden and not the full morbidity of people with advanced liver disease. Because we did not attempt to correct for data quality or potential misclassifications (e.g., alcoholic liver disease or liver cancer in people with hepatitis C that was not due to hepatitis, or the omission of a hepatitis C diagnosis in the electronic record), the number of admissions associated with hepatitis C and liver disease has been interpreted as a measure of the relative disease burden. Because data for Quebec were not available for the full study period owing to the province’s late conversion to ICD-10-CA coding, this study is not national in scope.
Despite these limitations, by following hospital admission records from an administrative database by birth cohort, we have provided insight into the birth cohort effects of hepatitis C not currently available from other sources in Canada, and our results appear to be in reasonable agreement with estimates from other studies. Information from this study is only one component leading to the development of screening recommendations. Other information, such as other sources of epidemiologic data on hepatitis C, patient and provider values and preferences, acceptability of the intervention and cost-effectiveness estimates must also be considered.
Conclusion
Our analysis showed an increasing hospital admission burden arising from liver disease associated with hepatitis C between 2004/05 and 2010/11. The disease burden for the 1950–1954 and 1955–1959 birth cohorts is especially high and will continue to be noticeably elevated over many years without additional intervention. The potential to reduce the disease burden over a longer life span in younger cohorts suggests that earlier detection and treatment should be considered for younger birth cohorts as well.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/2/3/E139/suppl/DC1
Supplementary Material
Acknowledgements
The authors acknowledge the support of the Canadian Institute for Health Information and all those involved in the collection and compilation of the Discharge Abstract Database. As well, the authors thank the Data Coordination and Access Program of the Public Health Agency of Canada for providing access to this database. The cooperation of all those involved in these activities is gratefully acknowledged. Special thanks to the reviewers for their probing questions and helpful and insightful comments.
References
- 1.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012;61:1-32 [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144:705-14 [DOI] [PubMed] [Google Scholar]
- 3.Denniston MM, Klevens RM, McQuillan GM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 2012;55:1652-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinette GD, Cox JJ, Heathcote J, et al. Primary care management of chronic hepatitis C: professional desk reference 2009. Ottawa: College of Family Physicians, Public Health Agency of Canada; 2009. Available: www.catie.ca/sites/default/files/Primary-Care-Management-of-Chronic-Hepatitis-C-Professional-Desk-Reference.pdf (accessed 2013 Oct 24).
- 5.Rotermann M, Langlois K, Andonov A, et al. Seroprevalence of hepatitis B and C virus infections: results from the 2007 to 2009 and 2009 to 2011 Canadian Health Measures Survey. Health Rep 2013;24:3-13 [PubMed] [Google Scholar]
- 6.Myers RP, Liu M, Shaheen AA. The burden of hepatitis C virus infection is growing: a Canadian population-based study of hospitalizations from 1994 to 2004. Can J Gastroenterol 2008;22:381-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian vital statistics — death database. Ottawa: Statistics Canada; 2008. [Google Scholar]
- 8.Discharge abstract database. Ottawa: Canadian Institute for Health Information; 1994–2010.
- 9.Kwong JC, Ratnasingham S, Campitelli MA, et al. The impact of infection on population health: results of the Ontario burden of infectious diseases study. PLoS ONE 2012;7:e44103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu A, Spinelli JJ, Cook DA, et al. Mortality among British Columbians testing for hepatitis C antibody. BMC Public Health 2013;13:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascione A, Tartaglione MT, Di Costanzo GG. Natural history of chronic hepatitis C virus infection. Dig Liver Dis 2007;39(Suppl 1):S4-7 [DOI] [PubMed] [Google Scholar]
- 12.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809-16 [DOI] [PubMed] [Google Scholar]
- 13.Dusheiko GM. The natural course of chronic hepatitis C: implications for clinical practice. J Viral Hepat 1998;5(Suppl 1):9-12 [DOI] [PubMed] [Google Scholar]
- 14.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160:293-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Table 052-0005: Projected population, by projection scenario, sex and age group as of July 1, Canada, provinces and territories. In: CANSIM (database). Ottawa: Statistics Canada; 2010. Available: www5.statcan.gc.ca/cansim/pick-choisir?lang=eng&id=520005&pattern=520005&searchTypeByValue=1&p2=42 (accessed 2013 Nov. 21).
- 16.Grant WC, Jhaveri RR, McHutchison JG, et al. Trends in health care resource use for hepatitis C virus infection in the United States. Hepatology 2005;42:1406-13 [DOI] [PubMed] [Google Scholar]
- 17.O’Brien SF, Fan W, Xi G, et al. Declining hepatitis C rates in first-time blood donors: insight from surveillance and case–control risk factor studies. Transfusion 2008;48:902-9 [DOI] [PubMed] [Google Scholar]
- 18.Hepatitis C in Canada: 2005–2010 surveillance report Ottawa: Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada; 2011. [Google Scholar]
- 19.Zalesak M, Francis K, Gedeon A, et al. Current and future disease progression of the chronic HCV population in the United States. PLoS ONE 2013;8:e63959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeting MJ, De Angelis D, Neal KR, et al. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. J Clin Epidemiol 2006;59:144-52 [DOI] [PubMed] [Google Scholar]
- 21.Thein HH, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.