Abstract
Sexual reproduction shuffles genetic variation, potentially enhancing the evolutionary response to environmental change. Many asexual organisms utilize facultative sexual reproduction as a means to escape the trap of low genetic diversity under stress. Self-fertilizing organisms are subject to similar genetic limitations: the consistent loss of genetic diversity within lineages restricts the production of variation through recombination. Selfing organisms may therefore benefit from a similar shift in mating strategy during periods of stress. We determined the effects of environmental stress via starvation and exposure to the stress-resistant dauer stage on mating system dynamics of Caenorhabditis elegans, which reproduces predominantly through self-fertilization but is capable of outcrossing in the presence of males. Starvation elevated male frequencies in a strain-specific manner through differential male survival during dauer exposure and increased outcrossing rates after dauer exposure. In the most responsive strain, the mating system changed from predominantly selfing to almost exclusively outcrossing. Like facultative sex in asexual organisms, facultative outcrossing in C. elegans may periodically facilitate adaptation under stress. Such a shift in reproductive strategy should have a major impact on evolutionary change within these populations and may be a previously unrecognized feature of other highly selfing organisms.
Keywords: dauer, male maintenance, recombination
Sex, although widespread, is theoretically disadvantageous as a consistent reproductive strategy (Maynard Smith 1978). However, facultative sexual reproduction during periods of compromised fitness is predicted to be an evolutionarily stable strategy that could potentially invade both sexual and asexual populations (Hadany and Otto 2007). Environmental stress is known to initiate sexual reproduction in a broad range of species that normally undergo asexual reproduction (Bell 1982; Dacks and Roger 1999; Dubnau 1991; Gemmill et al. 1997; Harris 1989; Kleiven 1992; Mai 2000). Asexual species are subject to deleterious mutation accumulation through Muller’s Ratchet as well as a decline in genetic variation due to a lack of recombination (Gabriel et al. 1993; Lynch et al. 1993; Muller 1964). Sex in predominantly asexual organisms is thought to enhance fitness through the infusion of genetic variation and removal of deleterious mutations, thereby promoting survival and facilitating adaptation under stressful conditions (Bell 1982; Colegrave et al. 2002; Hoffman 1997; Kaltz and Bell 2002; Muller 1964; Peck et al. 1999).
The long-term genetic consequences of obligate self-fertilization closely resemble many of the genetic hazards associated with asexual reproduction, largely because of the systematic loss of genetic variation within lineages due to continuous inbreeding (Charlesworth and Charlesworth 1995; Charlesworth et al. 1993; Heller and Maynard Smith 1972; Kondrashov 1985; Lande and Schemske 1985; Lynch et al. 1995; Stebbins 1957). Extended periods of obligate self-fertilization result in the production of offspring harboring predominantly homozygous loci, which limits the effectiveness of recombination within any given lineage (Heller and Maynard Smith 1972). Extreme inbreeding and the lack of recombination coupled with natural selection and genetic drift result in the consistent loss of population-level genetic diversity in selfing populations. Further, these characteristics of obligate self-fertilization are predicted to reduce the mean time to extinction for selfing populations relative to outcrossing populations (Lynch et al. 1995; Schultz and Lynch 1997). Although currently unexplored, facultative outcrossing may enhance the adaptive response of high selfing populations in the face of environmental stress. Here we explore this possibility using the nematode Caenorhabditis elegans as a model system.
A useful study system for examining the potential of stress-induced outcrossing is one that is predominantly self-fertilizing (but is capable of outcrossing), that provides a means of consistently and accurately determining the outcrossing rates within a population, and that displays a distinct response to environmental stress. The mostly selfing soil nematode C. elegans is an ideal system for addressing these questions. C. elegans populations are composed of self-fertile hermaphrodites that harbor two copies of the X-chromosome and males with a single X-chromosome as their only sex chromosome (Brenner 1974). Hermaphrodites cannot mate with other hermaphrodites and so outcrossing can only occur via mating with males. Although males facilitate outcrossing, they are rare and tend to be quickly driven out of laboratory populations by their hermaphrodite counterparts (Cutter 2005; Stewart and Phillips 2002; Teotónio et al. 2006). Males and outcrossing also appear to be rare within natural populations (Barriere and Felix 2005; Sivasundar and Hey 2005). Therefore, C. elegans populations seem to be predominantly self-fertilizing, but capable of outcrossing in the presence of males. Outcrossing rates within C. elegans populations are relatively straightforward to measure. Outcrossing events, the fertilization of eggs (X) by male sperm (X or Ø), result in the production of 50% male offspring and 50% hermaphroditic offspring (Nigon 1949). Self-fertilization produces 99.9% hermaphrodites, with rare X-chromosome nondisjunction events resulting in the production of males (0.1%) (Ward and Carrel 1979). After correcting for the number of males produced through X-chromosome nondisjunction, male frequency thus functions as an indicator of the outcrossing rate (Stewart and Phillips 2002).
Early in development and prior to sexual maturation, C. elegans larvae that encounter environmental stress (starvation, overcrowding, desiccation, high temperatures) enter a stage of developmental arrest, known as the dauer stage (Cassada and Russell 1975). This is a migratory non-feeding stage that is common in natural populations and centrally important across most nematode groups (Barriere and Felix 2005). Once the worms reach a new food source in the absence of the dauer pheromone (an indicator of overcrowding), they resume normal development. C. elegans strains exhibit natural variation for sensitivity to dauer-inducing conditions (Viney et al. 2003), and the genetic basis of the signaling pathway is well characterized (Gottlieb 1994; Kenyon et al. 1993; Thomas 1993; Vowels 1992). Larva can survive in the dauer stage for greater than twice their regular lifespan under normal conditions (Kenyon et al. 1993). The dauer stage therefore provides a rich ecological and functional context within which to explore the influence of environmental stress on mating system dynamics in populations of C. elegans.
Here, we test for stress induced facultative outcrossing directly by repeatedly passing several different natural isolates of C. elegans through the dauer stage and observing the subsequent strain specific increases in male frequency. We determine that male frequency can increase both during dauer exposure and in the generation following dauer. We find that an enhanced male presence after dauer coupled with greater outcrossing rates results in a facultative shift in C. elegans reproductive strategy from predominantly selfing to primarily outcrossing.
Methods
POPULATION MAINTENANCE AND DAUER INDUCTION
C. elegans strains are stock populations originally derived from a single individual isolated from a natural population. Two of these isolates, N2 and CB4856 (originally from Bristol, England and Hawaii, USA, respectively), were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN) and one, JU440 (originally obtained from Beauchene, France), was obtained from the laboratory of H. Teotónio (Instituto Gulbenkian de Ciencia, Portugal). The N2 strain maintains males at very low frequencies and has been in a laboratory setting for thousands of the generations (Brenner 1974; Teotónio et al. 2006), whereas the JU440 strain is a more recent natural isolate but maintains males a similarly low levels (Teotónio et al. 2006). Like JU440, CB4856 is a relatively recent natural isolate, but maintains males at much higher rates than either N2 or JU440 (Teotónio et al. 2006). These strains were chosen because they are some of the most distinct genotypes that have yet been collected (Haber 2005; M. Rockman, pers. comm.), thus allowing a good sampling of available genetic and phenotypic diversity. All strains were inbred for 10 generations before use to minimize within strain genetic variation. Replicate populations were maintained at 20°C on 10cm agar plates seeded with OP50 Escherichia coli to serve as their bacterial food source. Populations were chunk transferred (approximately five hundred individuals), predominantly as young (L1 or L2) larva to freshly seeded plates each generation (Stiernagle 2006).
Upon transfer a starting density of approximately 500 nematodes per plate permitted the populations to initially experience standard laboratory conditions but subjected the next generation to dauer-inducing conditions, via starvation and overcrowding. The starvation status of populations was determined by assessing the ratio of dauer larvae (measured phenotypically; Cassada and Russell 1975) to adults in a plate transect representing approximately 20% of the total plate area. When the ratio of dauers to adults (L1 and L2 larvae were not counted) was at least 19:1 the population was determined to be “starved.” The dauer ratio of each population was measured daily after initial transfer to a new food source until the population was determined to be starved. Most populations were deemed starved approximately one week after transfer. The populations remained on the same depleted plate for twenty-one days after being identified as starved, and chunk transferred to a fresh food source allowing them to resume development and reproduce.
EFFECTS OF STARVATION STRESS ON MALE FREQUENCY
Populations of each strain (N2, CB4856, and JU440) were subjected to two different starting conditions (no initial males and 10% initial males) and three different treatments (control, single dauer exposure, and successive dauer exposure) within each starting condition. The two starting conditions were chosen to test the response in male frequency based on the initial presence or absence of males. The single dauer exposure treatment was utilized to determine the immediate and long-term effects of a single dauer exposure on male frequency, whereas the successive dauer exposure treatment was used to investigate the compounded effects of multiple dauer exposures. Four replicate populations were run for each combination of strain, starting condition, and treatment. All replicate populations were maintained separately throughout the experiment. Each population was maintained for ten generations.
Populations in the “no initial male” starting condition were composed of approximately five hundred hermaphrodites. Populations that started in the “10% male” starting condition were composed of approximately fifty males and four hundred and fifty hermaphrodites. The control treatment consisted of populations that were maintained under standard laboratory conditions (not starved). “Single dauer exposure” populations were initially starved for a single generation and then continually maintained under standard laboratory conditions for the duration of the experiment. The “successive dauer exposure” populations were alternately starved one generation then maintained under standard laboratory conditions the next generation for eight generations, and then maintained under standard laboratory conditions for the final two generations. The first dauer exposure was imposed upon individuals in the second generation of the experiment (Figure 1), allowing us to measure male frequency prior to starvation.
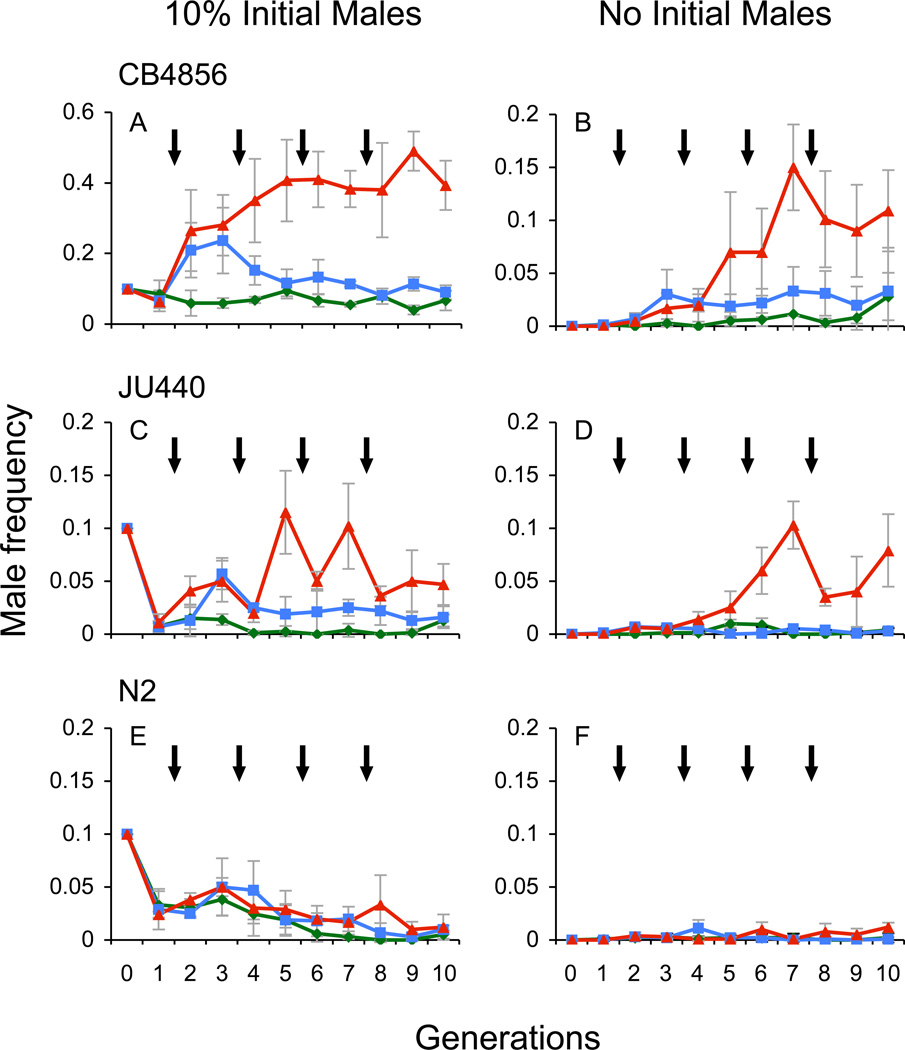
Figure 1.
Dauer exposure generates strain-specific increases in male frequency. Nematodes were either not exposed to dauer inducing starvation conditions (green lines), were exposed to a single episode of dauer (blue lines), or were repeatedly exposed to dauer (red lines). Arrows represent periods of dauer exposure (single dauer exposure occurred at the first arrow). The first point after the arrow represents the frequency of males in populations that have directly experienced dauer. The second point after the arrow represents the frequency of males in the offspring of the individuals that have gone through dauer. (a) CB4856 10% initial male populations, (b) CB4856 no initial male populations, (c) JU440 10% initial male populations, (d) JU440 no initial male populations, (e) N2 10% initial male populations, (f) N2 no initial male populations. The C. elegans strains CB4856 and JU440 both exhibit increases in male frequency over time following starvation-induced dauer exposure (P < 0.0001). The CB4856 strain approaches a mean male frequency of 50%, which is the theoretical maximum male frequency for C. elegans populations. Male frequencies were elevated in these strains regardless of the presence or absence of males upon first dauer exposure. The N2 strain, however, exhibited no male frequency response after dauer exposure (P = 0.6508). Data points indicated the mean male frequency (± 2 s.e.) of replicate populations measured over 10 generations.
Male frequency was assessed each generation for ten generations by sexing worms across a transect representing ~20% of the total plate area and dividing the total number of males counted by the total number of individuals counted (Stewart and Phillips 2002). Male frequency counts were taken three days after transfer to a fresh plate. Only adult and L4 (the latest larval stage) worms were assayed, as only these life-stages exhibit phenotypic sexual differentiation.
The data was analyzed using both a repeated measures categorical data analysis (CATMOD procedure in SAS 9.1, Cary, NC) and a repeated measures MANOVA (JMP-IN 5.1, Cary, NC) testing the effects of strain, initial male presence, dauer exposure, and replicate. The results of both approaches were consistent with one another, so we only report the MANOVA results.
MALE FREQUENCY INCREASES DURING DAUER
To examine any possible increases in male frequency while in dauer, we tested male vs. hermaphrodite survival during dauer and hermaphrodite to male sexual conversion during dauer. To assess relative male survival during dauer replicate populations were chunk transferred to two different freshly seeded plates and were allowed to reproduce and populate the plates. One population was subsequently chunk transferred to a freshly seeded plate and scored for male frequency, while the other population was subjected to dauer exposure for a specific period of time (0, 1, 21, or 42 days), and then chunked to a freshly seeded plate and scored for male frequency. Five replicate population pairs were maintained for each period of time. Changes in relative survival were tested by performing a one-way ANOVA on the log transformed differences in frequency between the treatment and control plates.
Sexual conversion
The JK2735 strain, derived from an N2 background, possesses a constitutively expressed GFP-marker on its X-chromosome that is inherited by only hermaphrodite progeny in a cross between a male that carries the marker and an unmarked hermaphrodite. This pattern of inheritance and subsequent expression can serve as an early indicator of sex in the F1 progeny, thus permitting a test of sexual conversion during the dauer stage (Prahlad et al. 2003). The JK2735 GFP-marker was backcrossed into the CB4856 background for 5 generations and inbred for 10 generations to produce the PX360 strain. Ten individual PX360 males, harboring a single green fluorescent protein (GFP)-marked X-chromosome, were mated with approximately two hundred CB4856 hermaphrodites apiece on 35mm agar plates. The large number of hermaphrodite mates ensured that the F1 individuals would encounter dauer-inducing conditions. Once in dauer, GFP-expressing and nonGFP-expressing offspring were separated, maintained in the dauer stage for approximately twenty-one days, and monitored for the loss or gain of GFP expression. The dauer worms were then moved to freshly seeded plates to resume development. Upon reaching sexual maturity they were sexed and monitored for the loss or gain of GFP expression. As a control PX360 hermaphrodites were crossed with CB4856 males demonstrating that PX360 x CB4856 crosses can produce males that express GFP.
MALE FREQUENCY INCREASES AFTER DAUER
To test for possible increases in male frequency caused by changes in X-chromosome nondisjunction, two hundred L4 hermaphrodites were transferred to twenty replicate populations apiece for each strain (JU440, CB4856, and N2). By starting populations with only hermaphrodites, any male individuals present in the next generation must be the result of X-chromosome nondisjunction (Rose and Baillie 1979). The replicate populations were split evenly into two groups, one exposed to dauer, the other maintained under standard laboratory conditions. Dauer exposure was approximately 21 days. Male frequency was measured in the populations after one generation under their respective rearing conditions. Possible effects of dauer exposure were analyzed using logistic regression in the CATMOD procedure of SAS.
Outcrossing rates
We compared outcrossing rates between group matings in which the males and hermaphrodites were subject to either dauer or lab conditions prior to mating. The dauer group was starved and allowed to remain in dauer for three weeks then chunked to fresh plates to resume development and mature to the L4 stage, whereas the control group was maintained under standard laboratory conditions. Four L4 hermaphrodites and one L4 male were picked to a single 35mm agar plate seeded with OP50, allowed to mate, and the proportion of male offspring scored. Within each strain four crosses were conducted: dauer male x dauer hermaphrodites, dauer male x fed hermaphrodites, fed male x dauer hermaphrodite, fed male x fed hermaphrodite. Each cross was replicated thirteen times within each strain, and the entire assay was replicated twice. The male frequency of each cross was determined by sexing a sample of the progeny across a transect representing ~20% of the total plate area (Stewart and Phillips 2002). Outcrossing rates were determined by 2(m – µ), where m is the frequency of the male offspring and µ is the rate of X chromosome nondisjunction (modified from Eq. 3 in Stewart and Phillips 2002). The strain-specific X chromosome nondisjunction rates were used as estimates of µ (Figure 2). The data was analyzed using an ANOVA performed in JMP-IN 5.1 to test for possible effects of dauer treatment, strain, sex, and dauer status of mate on outcrossing rate. A Tukey’s HSD test, testing the effect of having one or both mates experience dauer versus no dauer exposure for either mate, was conducted post-hoc.
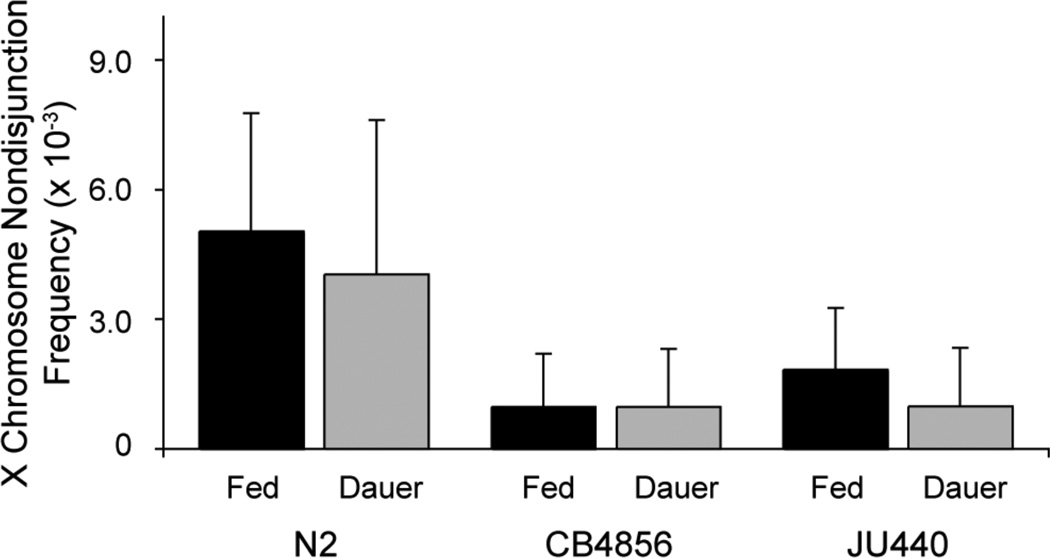
Figure 2.
X-chromosome nondisjunction events are not responsible for the increase in male frequency after dauer exposure. Exposure to dauer does not increase the X-chromosome nondisjunction rate (P = 0.8759). Nondisjunction frequencies were measured in all-hermaphrodite populations after undergoing twenty-one days in dauer. Each bar represents the mean nondisjunction rate (± 2 s.e.) of replicate populations.
EFFECTS OF DAUER ON HERMAPHRODITE SELF FECUNDITY
To determine if elevated outcrossing rates after dauer exposure were the product of sperm-limitation in hermaphrodites or greater mating success by males, we compared the total fecundity of hermaphrodites that experienced dauer exposure to that of hermaphrodites maintained under standard laboratory conditions. Six replicate populations were established by chunking from a single source population. Three of those replicate populations were exposed to dauer for approximately 21 days, while three were maintained under standard laboratory conditions. Approximately thirty L4 hermaphrodites were sampled from each population (after the dauer populations had resumed development after dauer exposure) and total fecundity calculated for each hermaphrodite. The data was analyzed using a one-way ANOVA.
EFFECTS OF MALE FREQUENCY ON OUTCROSSING RATES
We compared the outcrossing rates in populations started with a broad range of male frequencies. Populations were established by picking twenty L4 worms to a single 5cm agar plate seeded with OP50. Specific numbers of male and hermaphrodites were placed on each plate to generate the desired initial male frequencies (0%, 10%, 20%, 30%, 40%, and 50%). Three replicate plates were established at each initial male frequency. The worms were allowed to mate (hermaphrodites were permitted to self-fertilize in addition to outcrossing with males) and reproduce. Then, the offspring were transferred to a seeded 10cm plate, allowed to reach sexual maturation, and sexed. The outcrossing rates were determined as previously stated. The data was analyzed using regression analysis in JMP-IN 5.1. The outcrossing rate was regressed with the initial male frequency and a stepwise polynomial regression used to assess the best fitting model.
Results
EFFECTS OF STARVATION STRESS ON MALE FREQUENCY
Successive exposures to the dauer stage permitted males to sweep into populations of the CB4856 and JU440 natural isolates, even into populations in which males were initially absent (Figure 1; F1,51 = 124.42, P < 0.0001). Repeated exposure to dauer-inducing conditions was especially effective at generating prolonged maintenance of high male frequencies, with the overall increase depending on whether males were initially present in the population or not (CB4856 F1,51 = 219.48, P < 0.0001; JU440 F1,51 = 13.47, P = 0.0006). In the most extreme case, replicates of CB4856 moved from 10% males to close to the theoretical maximum of 50% after just two or three exposures to dauer (Figure 1a). These increases were sustained as long as the populations continued to experience periodic starvation. In contrast, a single exposure to the dauer stage raised levels of male frequency in the 10% initial male treatments in both the CB4856 and JU440 strains (CB4856 F2,50 = 74.44, P < 0.0001; JU440 F2,50 = 3.44, P = 0.0398), but failed to exhibit prolonged male maintenance (Figure 1a,c). The male frequency in all treatments of the N2 strain was unaffected by exposure to the dauer stage (F1,51 = 0.21, P = 0.6508; Figure 1e,f). All populations maintained under standard laboratory conditions failed to exhibit a significant increase in male frequency (F9,43 = 1.80, P = 0.0965), while the JU440 and N2 populations rapidly lost males in the 10% initial male treatment (Figure 1c,e).
CAUSES OF ELEVATED MALE FREQUENCY
The elevated male frequencies resulting from dauer exposure can be generated from two possible sources: a change in the male to hermaphrodite ratio during dauer and/or a shift in the reproductive dynamics after exposure to the dauer stage. In the first case, any increase in male frequency immediately following dauer exposure is generated by factors acting directly on individuals experiencing dauer, since reproduction has yet to occur. In the second case, changes in male frequency occur in the generation following dauer exposure and therefore result directly from mating and/or reproduction. We will examine each possible cause of the increase in male frequency in turn.
INCREASE IN MALE FREQUENCY DURING DAUER
The frequency of males within CB4856 populations steadily increases over time while in dauer, indicating either the addition of males or the loss of hermaphrodites (F3,26 = 6.09, P = 0.0028; Figure 3). The other strains do not exhibit this effect (instead they tend to lose males during dauer), and so we focus our initial analysis on the CB4856 strain.
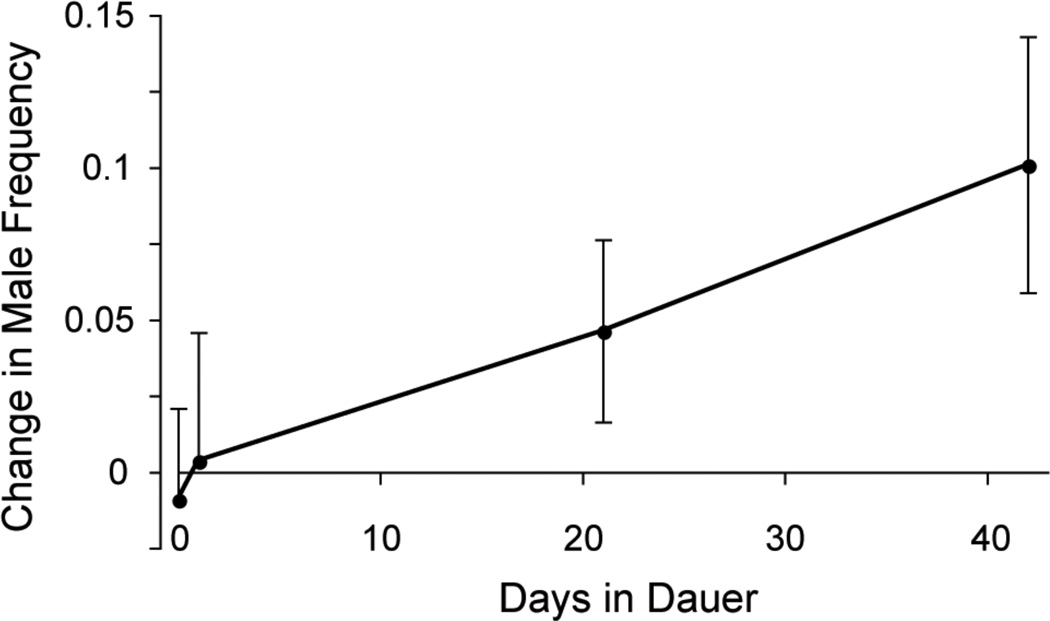
Figure 3.
CB4856 males survive dauer at rates greater than hermaphrodites. Male frequency increases with time spent in dauer (P = 0.0028). The increase in male frequency is a direct of result of a greater proportion of males living through dauer, as compared to the proportion of hermaphrodites that survive dauer exposure. The data points represent the change in mean male frequency (± 2 s.e.) of replicate populations exposed to dauer for varying lengths of time.
Sexual conversion
Dauer-induced sexual conversion, the transformation of hermaphrodites into sexually functional males, is a possible source for the increase in male frequency during dauer exposure (Prahlad et al. 2003). Using a GFP-marker to determine sex prior to dauer exposure we found no instances of dauer-induced hermaphrodite to male sexual conversion (0% sexual conversion, power = 80% to determine a 1% conversion rate). A sexual conversion rate of approximately 20% would be required to solely account for the increase in male frequency during dauer. Therefore the increase in male frequency during dauer is not due to sexual conversion.
Differential survival during dauer
If hermaphrodites are not sexually converting into males, the increase in male frequency during dauer must be the result of male survival and hermaphrodite mortality while in the dauer stage. We observe a 10% increase in male frequency over a period of 42 days in dauer (Figure 3). Male survival coupled with hermaphrodite mortality therefore accounts for the increase in male frequency exhibited in populations that have directly experienced dauer (Figure 1).
INCREASE IN MALE FREQUENCY FOLLOWING DAUER
Differential survival of males and hermaphrodites cannot explain the observed subsequent increase in males that occurs in the generation following dauer exposure (Figure 1). This delayed response is especially clear in JU440, but is also present in CB4856 as well. An increase in either the X-chromosome nondisjunction rate or the outcrossing rate is required to explain the increase in male frequency in the generation following exposure to dauer because the individuals do not directly experience the dauer stage.
X-chromosome nondisjunction
Dauer-induced increases in X-chromosome nondisjunction rates could elevate male frequencies in the offspring of dauer-exposed hermaphrodites by increasing the number of spontaneously produced males. However, passage through the dauer stage did not increase the rate of X-chromosome nondisjunction in hermaphrodites (F1,2 = 0.26, P = 0.8759; Figure 2). Therefore the elevated male frequencies must be the product of altered mating dynamics after dauer exposure.
Facultative outcrossing
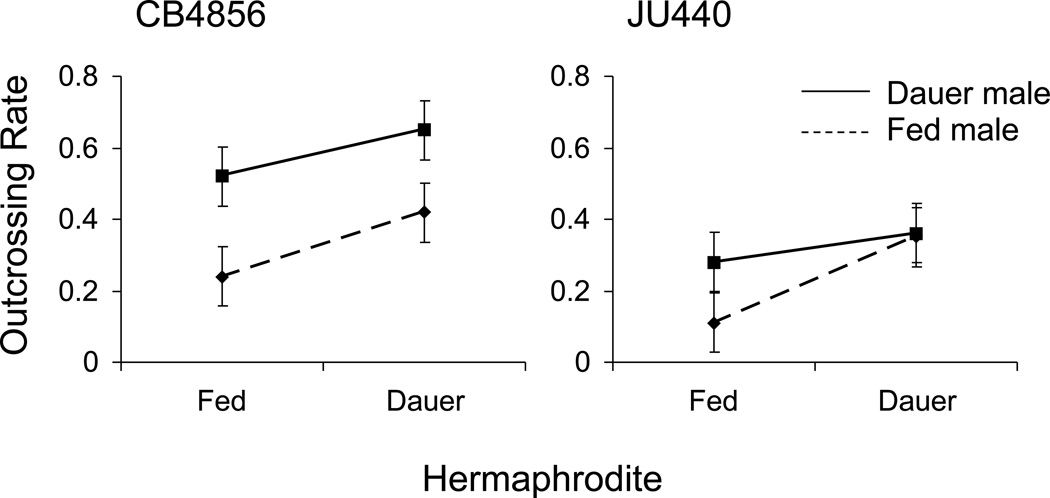
The outcrossing rate in both the CB4856 and JU440 strains increases dramatically following dauer (F1,187 = 58.22, P < 0.001; Figure 4). Thus, the environmental stress generated by starvation leads directly to an increase in outcrossing within these two strains. Indeed, outcrossing rates are elevated when at least one of the partners has experienced dauer (Figure 4). The increased outcrossing rates are not a consequence of sperm-limitation in dauer-exposed hermaphrodites, as hermaphrodite self-fecundity is not reduced by dauer exposure (CB4856: control mean = 198.8, dauer mean = 211.3; F1,174 = 3.93, P = 0.049; JU440: control mean = 205.1, dauer mean = 216.9; F1,86 = 1.24, P = 0.261). Therefore the increase in outcrossing rate is the product of more frequent fertilization by males. The effects are nearly additive for the CB4856 strain (i.e., outcrossing rates increase significantly when both sexes have gone through dauer), but saturate in the JU440 strain. These results clearly show that the elevated male frequencies following dauer are the result of an increase in outcrossing.
Figure 4.
Dauer exposure induces facultative outcrossing. Outcrossing rates are elevated when individuals mate with others that have been previously exposed to dauer (P < 0.001). The increase in outcrossing occurs whether the male or the hermaphrodite is the partner exposed to dauer. Exposure of both partners further increases outcrossing in CB4856 but yields the same outcrossing rate as single partner exposure in JU440. Data points represent mean outcrossing rates (± 2 s.e.) of replicates for each category of mating.
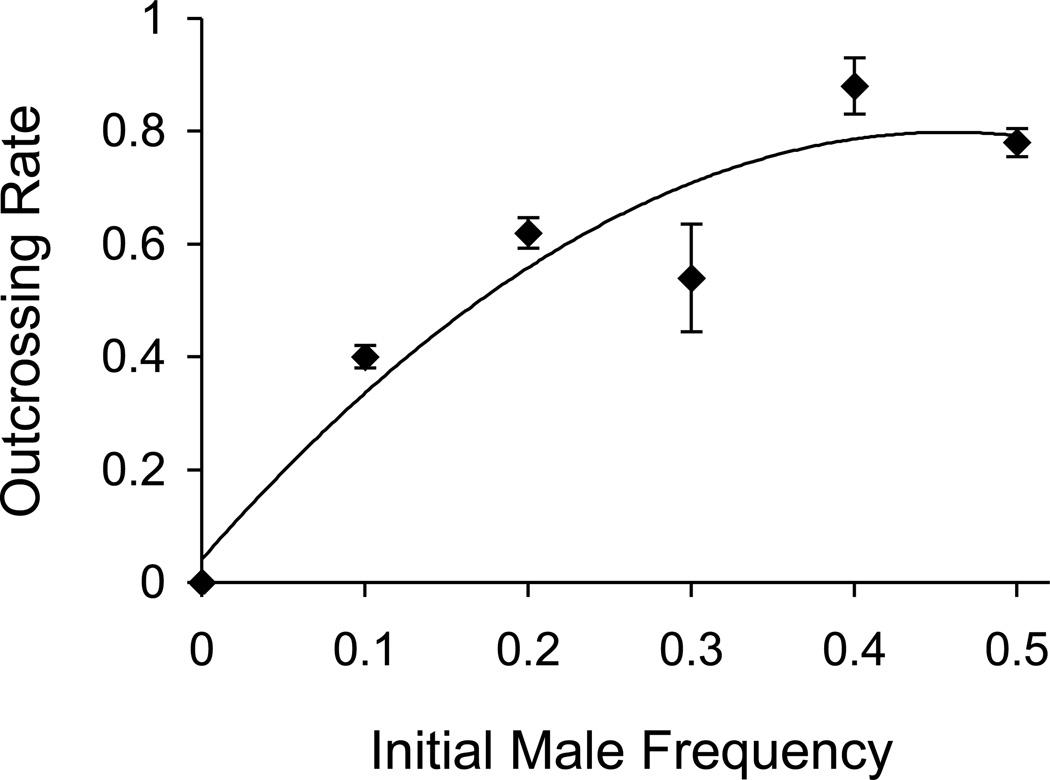
For the CB4856 strain, greater male survivorship through dauer and the effect of dauer on mating interact synergistically, as the positive correlation between male frequency and outcrossing (r2 = 0.91; Figure 5) demonstrates that increases in male survivorship translate directly into heightened outcrossing rates. Therefore, the elevated male frequencies generated through environmental stress via exposure to the dauer stage are the combined result of differential male survival while in the dauer stage and increased outcrossing rates after dauer exposure.
Figure 5.
Male maintenance function. Population-level outcrossing rates rapidly increase with increasing initial male frequency in CB4856 (r2 = 0.91). Each point shows the outcrossing rate (± 2 s.e.) of replicate populations started at different initial male frequencies. The line shows the best quadratic fit to the data, given by the line outcrossing rate = 0.13 + 0.79 m – 2.04 (m – 0.24)2, where m is the initial male frequency. The overall model is highly significant (F2,13 = 70.80, P < 0.0001), as is each individual coefficient (P < 0.001).
Discussion
Laboratory populations of C. elegans exhibit little or no outcrossing and therefore maintain males poorly (Cutter 2005; Stewart and Phillips 2002; Teotónio et al. 2006). This observation is the basis for the view that C. elegans males are evolutionary relics and not functional genetic contributors (Chasnov and Chow 2002). Here, however, we demonstrate that exposure to the dauer stage not only increases male frequency but also elevates outcrossing rates independent of the initial male frequency in two natural isolates of C. elegans (Figure 1 and Figure 5). This shift in mating system dynamics, from predominantly selfing to at least partially outcrossing, is ultimately induced by environmental stress.
CAUSES OF FACULTATIVE OUTCROSSING
The increase in outcrossing rates following exposure to dauer can be generated by sex-specific differences in survival during dauer and by dauer-induced changes in mating patterns following dauer exposure. Ailion and Thomas (2000) found that males are more sensitive to the dauer pheromone, entering the dauer stage more readily than hermaphrodites, which should further contribute to the increase in male frequency resulting from dauer exposure apart from male survival. Here, we show that this sex difference is amplified by greater male survival through dauer (Figure 3). Although sex-specific, the disparity between the male and hermaphroditic response and survival in dauer occurs before sexual maturation.
The CB4856 and JU440 strains also exhibited stress-induced increases in outcrossing rates (Figure 4). Interestingly, elevated outcrossing rates were not sex-specific, indicating altered mating dynamics in both hermaphrodites and males after dauer exposure (Figure 4). The increases in hermaphrodite outcrossing rates are not due to sperm limitation, but rather are driven by interactions between hermaphrodites and males. Srinivasan et al. (2008) established a link between the dauer stage and mating dynamics by demonstrating that a C. elegans male attractant was composed of a blend of several dauer-inducing glycolipids. Dauer may induce changes in hermaphrodite mate signaling, receptivity to mating, or sperm preference that enables males to sire a greater proportion of offspring.
The presence of males is required for facultative outcrossing, because males are required for outcrossing. Populations initiated with males experienced a rapid increase in outcrossing rates, exhibiting facultative outcrossing and the subsequent increase in male frequency even after a single exposure to dauer (Figure 1). Populations that were established without males were originally dependent upon male production through nondisjunction, requiring more time to generate facultative outcrossing (Figure 1). These populations required successive exposures to the dauer stage before males could become established and thereby enhance outcrossing rates. A large proportion of C. elegans natural isolates are hermaphrodites, therefore multiple exposures to dauer may be required for most natural populations to experience high levels of outcrossing in response to stress (Barriere and Felix 2005; Sivasundar and Hey 2005).
OUTCROSSING WITHIN C. ELEGANS
The strains that displayed a reproductive response to dauer, CB4856 and JU440, are more recent natural isolates than the N2 strain, which failed to exhibit facultative outcrossing (Figure 1) and is known to suffer developmental defects resulting from dauer exposure (Kim and Paik 2008). An overwhelming proportion of soil natural isolates are found in the dauer stage, indicating that dauer inducing conditions are a consistent selective pressure in natural populations (Barriere and Felix 2005).
Recent studies investigating natural C. elegans populations have concluded that outcrossing is usually, but not always, rare (Barriere and Felix 2005; Haber et al. 2005; Sivasundar and Hey 2003; 2005). Natural isolates have been recovered with signatures of periodic outcrossing (Haber et al. 2005; Sivasundar and Hey 2005), leading to speculation that outcrossing may occur intermittently as conditions dictate (Fitch 2005). Populations sampled in California (USA) appear to exhibit relatively high levels of outcrossing (Sivasundar and Hey 2005) as compared to populations sampled in France (Barriere and Felix 2005). However, outcrossing will leave a genetic signal only if there is sufficient genetic variation present within a population to be shuffled by segregation and recombination. Outcrossing in highly inbred populations that are isolated from other such populations will have no effect on the pattern of genetic variation within that population. Thus, stress-induced outcrossing under natural conditions will not necessarily leave a genetic signature unless there is sufficient genetic variation in the immediate local population. Recent analysis of a genetic incompatibility system within this species (Seidel et al. 2008) indicates that there has been extensive recombination among strains, even in genomic regions very close to the incompatibility loci. Given the observed strain differences and the temporal nature of dauer-induced facultative outcrossing (Figure 1), it would be expected that some natural populations would exhibit signs of outcrossing while others may appear as obligate selfers.
EVOLUTIONARY CONSEQUENCES OF FACULTATIVE OUTCROSSING
Through stress-induced facultative sex, normally asexual species utilize sexual reproduction as a novel reproductive strategy to overcome the genetic limitations of asexual reproduction. Theoretical models have demonstrated that alleles that modify the rate of recombination in response to stress can readily invade sexual (Agrawal et al. 2005) and asexual (Hadany and Otto 2007) populations. Selection on induced recombination in diploid populations is weaker because heterozygosity decreases the association between the modifier response and the effects on recombination (Agrawal et al. 2005). Although more theory on this is needed, close inbreeding generates the needed cis-coupling between the recombination modifiers and the affected loci, which functionally mimics the asexual situation, and should therefore generate strong selection on stress-induced recombination via outcrossing. Obligate selfers share the same genetic predicament as asexual individuals: a lack of genetic variation within lineages and the potential to accumulate slightly deleterious mutations, due to perpetual and excessive inbreeding (Charlesworth et al. 1993; Heller and Maynard Smith 1972; Kondrashov 1985; Lande and Schemske 1985). Outcrossing has the potential to introduce genetic variation and allow for the production of offspring harboring fewer deleterious mutations than the parental generation (Bell 1982; Charlesworth et al. 1993; Heller and Maynard Smith 1972; Peck et al. 1999). In this way the genetic consequences of self-fertilization parallel those of asexual reproduction (with the additional complication of homozygosity), and therefore self-fertilizing organisms should also benefit from stress-induced facultative outcrossing.
The long-term evolutionary stability of obligate self-fertilization as a reproductive strategy has long been suspect (Stebbins 1957). In addition to a large body of theoretical work, the phylogenetic positioning of several obligatory selfing species indicates that selfing may be an evolutionary dead-end (Takebayashi and Morrell 2001). Ultimately, all obligate self-fertilizing populations may be at risk of mutation accumulation due to the systematic loss of lineage-specific genetic variation through perpetual inbreeding (Charlesworth et al. 1993; Lynch et al. 1995). The threat of extinction is likely elevated under stressful conditions, as the potential lack of genetic variation at the population level may inhibit adaptation to novel environments. Continued self-fertilization under stress will perpetuate these risks, but timely outcrossing may increase the efficacy of recombination thus providing relief from mutation accumulation and facilitate rapid adaptation. Many plant species once thought to rely solely upon obligate self-fertilization as a reproductive strategy have been found to utilize a broad range of mixed mating strategies by incorporating differing degrees of outcrossing with self-fertilization (Goodwillie et al. 2005). We would therefore predict that stress-induced facultative outcrossing might be a common, but currently unexplored, feature that many partial selfers utilize to periodically generate genetic variation under stressful conditions.
ACKNOWLEDGEMENTS
We thank Z. May for his perspective and friendship, S. McNamara and J. Chiem for logistical help, and H. Teotonio and D. Manoel for providing the JU440 strain. We would also like to thank S. Otto, L. Hadany, C. Lively, the members of the Phillips and Cresko labs, and two anonymous reviews for helpful comments and discussion pertaining to this work. Funding was provided by NSF Grants DEB-0236180, DEB- 0710386, and DEB-0641066 and REU supplement DEB-0425301. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). LTM and PCP conceived and designed the experiments. LTM, BJC, and JLA performed the experiments. LTM and PCP analyzed the data. LTM, JLA and PCP wrote the paper.
LITERATURE CITED
- Agrawal AF, Hadany L, Otto SP. The evolution of plastic recombination. Genetics. 2005;171:803–812. doi: 10.1534/genetics.105.041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Current Biology. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Berkley, CA: University of California Press; 1982. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a postembryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Quantitative genetics in plants: the effect of breeding system on genetic variability. Evolution. 1995;49:911–920. doi: 10.1111/j.1558-5646.1995.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Morgan MT, Charlesworth B. Mutation accumulation in finite, outbreeding, and inbreeding populations. Genetical Research. 1993;61:39–56. [Google Scholar]
- Chasnov JR, Chow KL. Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics. 2002;160:983–994. doi: 10.1093/genetics/160.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrave N, Kaltz O, Bell G. The ecology and genetics of fitness in Chlamydomonas. VIII. The dynamics of adaption to novel environments after a single episode of sex. Evolution. 2002;56:14–21. doi: 10.1111/j.0014-3820.2002.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Cutter AD. Mutation and the experimental evolution of outcrossing in Caenorhabditis elegans. J Evol Biol. 2005;18:27–34. doi: 10.1111/j.1420-9101.2004.00804.x. [DOI] [PubMed] [Google Scholar]
- Dacks J, Roger AJ. The first sexual lineage and the relevance of facultative sex. J. Mol. Evol. 1999;48:779–783. doi: 10.1007/pl00013156. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991;55:395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch DH. Evolution: an ecological context for C. elegans. Curr Biol. 2005;15:R655–R658. doi: 10.1016/j.cub.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Gabriel W, Lynch M, Burger R. Mullers Ratchet and mutational meltdowns. Evolution. 1993;47:1744–1757. doi: 10.1111/j.1558-5646.1993.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Gemmill AW, Viney ME, Read AF. Host immune status determines sexuality in a parasitic nematode. Evolution. 1997;51:393–401. doi: 10.1111/j.1558-5646.1997.tb02426.x. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Ekert C. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology and Systematics. 2005;36:47–79. [Google Scholar]
- Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Schungel M, Putz A, Muller S, Hasert B, Schulenburg H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Mol Biol Evol. 2005;22:160–173. doi: 10.1093/molbev/msh264. [DOI] [PubMed] [Google Scholar]
- Haber M, Schungel M, Putz A, Muller S, Hasert B, Schulenburg H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Molecular Biology and Evolution. 2005;22:160–173. doi: 10.1093/molbev/msh264. [DOI] [PubMed] [Google Scholar]
- Hadany L, Otto SP. The evolution of condition-dependent sex in the face of high costs. Genetics. 2007;176:1713–1727. doi: 10.1534/genetics.107.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. New York: Academic Press; 1989. [Google Scholar]
- Heller J, Maynard Smith J. Does Muller's Ratchet work with selfing? Genetical Research. 1972;8:269–294. [Google Scholar]
- Hoffman AA, Parsons PA. Extreme environmental change and evolution. Cambridge, UK: Cambridge Univ. Press; 1997. [Google Scholar]
- Kaltz O, Bell G. The ecology and genetics of fitness in Chlamydomonas. XII. Repeated sexual episodes increase rates of adaption to novel environments. Evolution. 2002;56:1743–1753. doi: 10.1111/j.0014-3820.2002.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim S, Paik YK. Developmental and reproductive consequences of prolonged non-aging dauer in Caenorhabditis elegans. Biochemical and Biophysical Research Communications. 2008;368:588–592. doi: 10.1016/j.bbrc.2008.01.131. [DOI] [PubMed] [Google Scholar]
- Kleiven OT, Larsson P, Hobaek A. Sexual reproduction in Daphnia magna requires three stimuli. Oikos. 1992;65:197–206. [Google Scholar]
- Kondrashov AS. Deleterious mutations as an evolutionary factor. II. Facultative apomixis and selfing. Genetics. 1985;111:635–653. doi: 10.1093/genetics/111.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants.1. genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lynch M, Burger R, Butcher D, Gabriel W. The mutational meltdown in asexual populations. Journal of Heredity. 1993;84:339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery J, Burger R. Mutational meltdowns in sexual populations. Evolution. 1995;47:1744–1757. doi: 10.1111/j.1558-5646.1995.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Mai B, Breeden L. CLN1 and its repression by Xbp1 are important for efficient sporulation in budding yeast. Mol. Cell. Biol. 2000;20:478–487. doi: 10.1128/mcb.20.2.478-487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. The Evolution of Sex. Cambridge, UK: Cambridge University Press; 1978. [Google Scholar]
- Muller HJ. The relation of recombination to mutational advance. Mutation Research. 1964;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Nigon V. Les modalités de la réproduction et le déterminisme de sexe chez quelques Nématodes libres. Ann. Sci. Nat., Zool. 1949;11:1–132. [Google Scholar]
- Peck JR, Yearsley J, Barreau G. The maintenance of sexual reproduction in a structured population. Proc. R. Soc. Lond. B. Biol. Sci. 1999:266. [Google Scholar]
- Prahlad V, Pilgrim D, Goodwin EB. Roles for mating and environment in C. elegans sex determination. Science. 2003;302:1046–1049. doi: 10.1126/science.1087946. [DOI] [PubMed] [Google Scholar]
- Rose AM, Baillie DL. The effect of temperature and parental age on recombination and nondisjunction in C elegans. Genetics. 1979;92:409–418. doi: 10.1093/genetics/92.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz ST, Lynch M. Mutation and extinction: The role of variable mutational effects, synergistic epistasis, beneficial mutations, and degree of outcrossing. Evolution. 1997;51:1363–1371. doi: 10.1111/j.1558-5646.1997.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Seidel HS, Rockman MV, Kruglyak L. Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science. 2008;319:589–594. doi: 10.1126/science.1151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasundar A, Hey J. Population genetics of Caenorhabditis elegans: The paradox of low polymorphism in a widespread species. Genetics. 2003;163:147–157. doi: 10.1093/genetics/163.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasundar A, Hey J. Sampling from natural populations with RNAi reveals high outcrossing and population structure in Caenorhabditis elegans. Current Biology. 2005;15:1598–1602. doi: 10.1016/j.cub.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008 doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Self-fertilization and population variation in higher plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Stewart AD, Phillips PC. Selection and maintenance of androdioecy in Caenorhabditis elegans. Genetics. 2002;160:975–982. doi: 10.1093/genetics/160.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. In: Maintenance of C. elegans. Community R, editor. Wormbook; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi N, Morrell PL. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am J Bot. 2001;88:1143–1150. [PubMed] [Google Scholar]
- Teotónio H, Manoel D, Phillips PC. Genetic variation for outcrossing among Caenorhabditis elegans isolates. Evolution. 2006;60:1300–1305. [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney ME, Gardner MP, Jackson JA. Variation in Caenorhabditis elegans dauer larva formation. Dev Growth Differ. 2003;45:389–396. doi: 10.1046/j.1440-169x.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev. Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]