Abstract
BACKGROUND
Continuous and intermittent bolus orogastric feedings are strategies used in infants unable to tolerate normal feeds.
METHODS
To determine the effects of feeding modality on protein synthesis in different tissues, neonatal pigs received a balanced formula by orogastric tube either as an intermittent bolus feed every 4 h or as a continuous infusion, or were fasted overnight.
RESULTS
Compared to fasting, protein synthesis in gastrocnemius, masseter, and soleus muscles, left ventricle, liver, pancreas, jejunum, and kidney increased in bolus and continuously fed pigs, but the greatest increase occurred after a bolus meal. Tuberous sclerosis complex (TSC2), the proline-rich AKT substrate of 40 kDa (PRAS40), eukaryotic initiation factor (eIF) 4E binding protein (4EBP1) and rp S6 kinase 1 (S6K1) phosphorylation in all tissues and the proportion of ribosomal protein S4 in liver polysomes were enhanced 90 minutes following the bolus meal, but not immediately before the meal or during continuous feeding. Eukaryotic elongation factor 2 (eEF2) and eIF2α phosphorylation were unaffected by feeding.
CONCLUSION
These results suggest that intermittent bolus feeding increases protein synthesis in muscles of different fiber types and visceral tissues to a greater extent than continuous feeding by stimulating translation initiation.
INTRODUCTION
More than 10% of newborns are of low birth-weight and many exhibit adverse long-term health problems (1, 2). There has been widely accepted recognition that adequate nutrition plays an important role in the survival and subsequent growth and development of low birth-weight infants (3, 4). Orogastric tube feeding by continuous infusion or intermittent bolus delivery is necessary for neonates who are unable to coordinate oral food ingestion. Bolus feeding compared to continuous feeding has been advocated to promote more normal feed-fast hormonal profiles and advance gastrointestinal development (5, 6). In some clinical studies, intermittent feeding has been reported to shorten the time required to reach full feeds, promote better feeding tolerance (7, 8), and produce a faster weight gain as compared to continuous feeding (8) although contrary results have also been published (9). Using the pig as a model for the human neonate, intermittent feeding as compared to continuous feeding has been shown to stimulate intestinal growth and development by increasing mucosal and intestinal protein mass (10). In spite of these data, whether intermittent bolus and continuous feeding affects the regulation of growth in different organ systems has not been determined.
Rates of growth and protein turnover are at their highest during the neonatal period (11-14). Using the neonatal pig as a model of the human neonate, we have shown that feeding stimulates protein synthesis in skeletal muscle and visceral organs (15, 16). The response in muscle is regulated independently by the postprandial rise in insulin and amino acids (17), whereas in liver and other visceral tissues only amino acids are effective (18). Feeding increases protein synthesis through activation of translation initiation. There are two regulatory processes controlling translation initiation (19, 20); the first is mediated by eukaryotic initiation factor (eIF)-2 and involves the binding of initiator met-tRNAi to the 40S ribosomal subunit to form the 43S pre-initiation complex. The second involves the activation of mammalian target of rapamycin (mTOR) which phosphorylates the eIF4E repressor protein, 4E binding protein 1 (4EBP1) and 70 kDa ribosomal protein S6 kinase 1 (S6K1), both of which regulate the binding of mRNA to the 43S ribosomal complex. Activation of the mTOR by protein kinase B (PKB) occurs in part through suppression of two inhibitors: tuberous sclerosis complex (TSC2) and the Proline-rich AKT substrate of 40 kDa (PRAS40) (21, 22). Insulin could also play a role in protein synthesis through dephosphorylation and deactivation of eukaryotic elongation factor 2 (eEF2) which controls peptide elongation (23).
Recently, we demonstrated that intermittent bolus feeding increases protein synthesis in fast-twitch glycolytic muscle to a greater extent than continuous feeding (24). However, no information is available on the impact of these different feeding modalities on the regulation of protein synthesis in muscles of different fiber types and vital organs. In the present study, the objectives were to determine whether intermittent bolus feeding compared to continuous feeding enhanced protein synthesis in muscle of different fiber types and in vital organs, and to identify how feeding modalities affect the mTOR signaling pathway in these tissues.
RESULTS
Plasma branched-chain amino acids and insulin
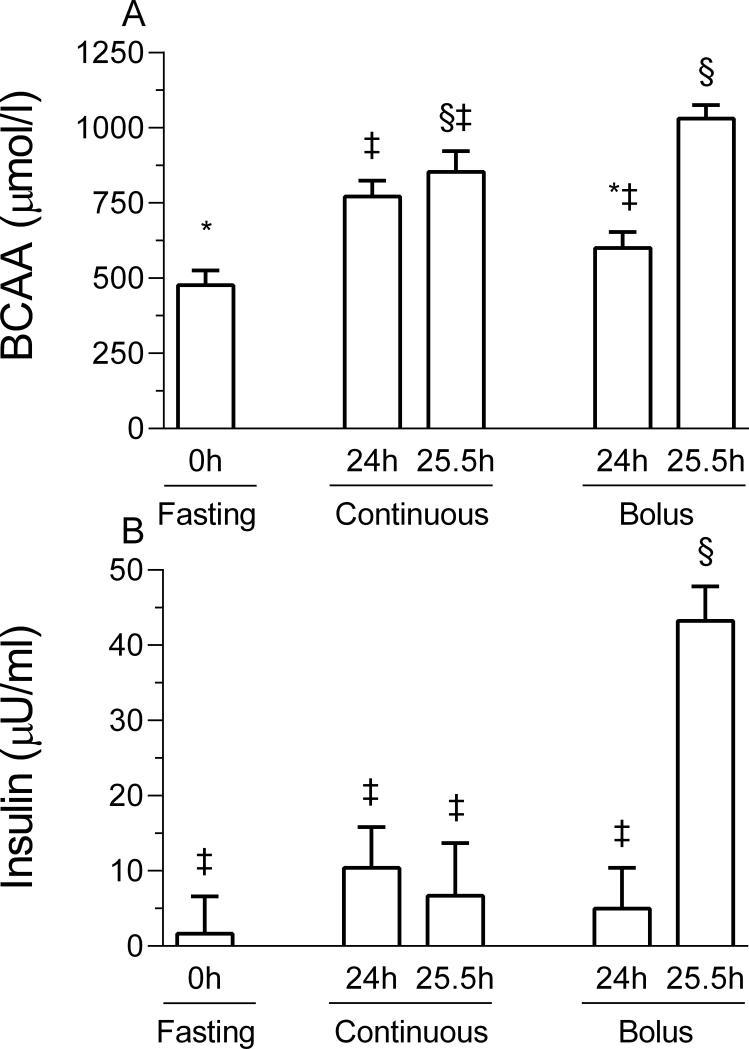
Circulating branched-chain amino acid (BCAA) and insulin concentrations over the whole feeding period were reported previously (24) and are presented in this article for reference. BCAA concentrations were highest in the 25.5 h bolus group (p < 0.05; Figure 1), whereas for the 24 h bolus group concentrations were similar to those in fasted pigs. Plasma BCAA levels for the 24 h and 25.5 h continuously fed pig were intermediate between those in the overnight fasted and those in the 25.5 h bolus fed groups. Plasma insulin concentration was highest for the 25.5 h bolus fed as compared to all other groups (p < 0.05).
Figure 1.
Plasma branched-chain amino acid (BCAA; A) and insulin (B) concentrations in pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h. Values are means ± SEM, n = 5-7. *, ‡, § Values not sharing common symbols differ significantly (p < 0.05).
Protein synthesis and mRNA abundance in liver polysomes
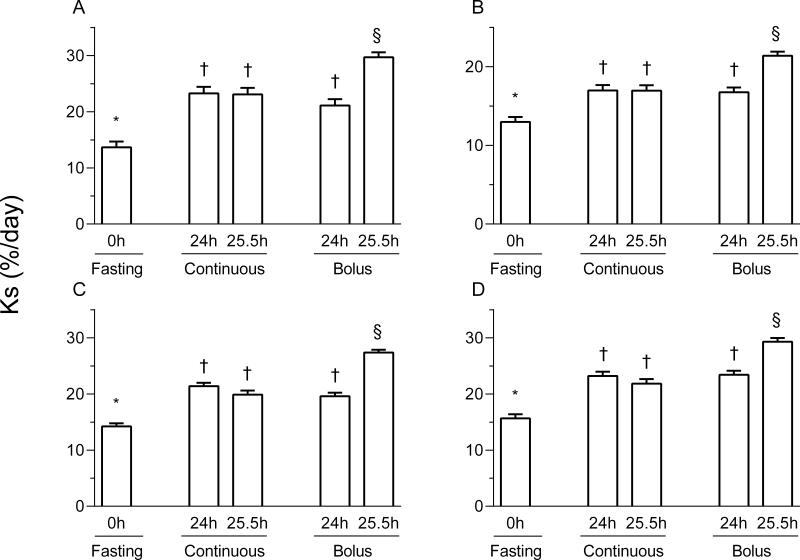
The rates of protein synthesis in the gastrocnemius, masseter, soleus, and cardiac muscles were significantly higher in the 24 h and 25.5 h continuously fed and 24 h bolus groups compared to the overnight fasted group (p < 0.05; Figure 2). After 25.5 h of bolus feeding (1.5 h after the last meal), protein synthesis rates in all muscles were greater compared to the fasted group (p < 0.05), and greater (p < 0.05) than after 24 h of intermittent bolus feeding (just before the last meal) as well as after 24 and 25.5 h of continuous feeding (p < 0.05).
Figure 2.
Protein synthesis in the gastrocnemius (A), masseter (B), soleus (C) and left ventricle (D) of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h. Ks is the fractional protein synthesis in %/d. Values are means ± SEM, n = 5-7. *, †, §Values not sharing common symbols differ significantly (p < 0.05).
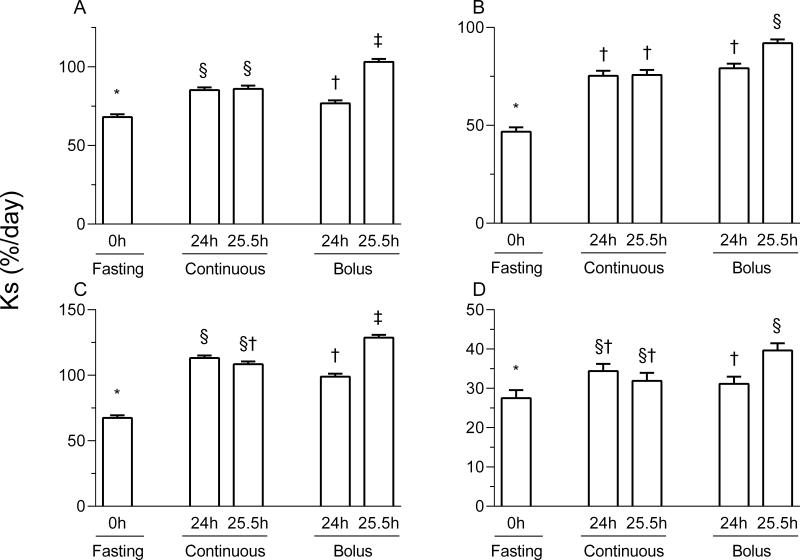
In the liver, jejunum, pancreas, and kidney, protein synthesis rates in all fed groups were higher than those in the fasted pigs (p < 0.05; Figure 3). In addition, protein synthesis rates in the bolus fed group at 25.5 h were significantly higher than those of the bolus fed group at 24 h(p < 0.05). In the jejunum and kidney, rates of synthesis for the continuously fed pigs were similar to the 24 h bolus group, however, for the liver and pancreas the synthesis rates for the continuously fed pigs were higher than those of the 24 h bolus group (p < 0.05). In the liver, jejunum, and pancreas, protein synthesis rates in the 25.5 h bolus feeding group were greater compared to other feeding groups.
Figure 3.
Protein synthesis in the liver (A), jejunum (B), pancreas (C) and kidney (D) of pigs either fasted overnight or fed continuously or intermittent bolus for 24 or 25.5 h. Ks is the fractional protein synthesis in %/d. Values are means ± SEM, n = 5-7. *, †, ‡, §Values not sharing common symbols differ significantly (p < 0.05).
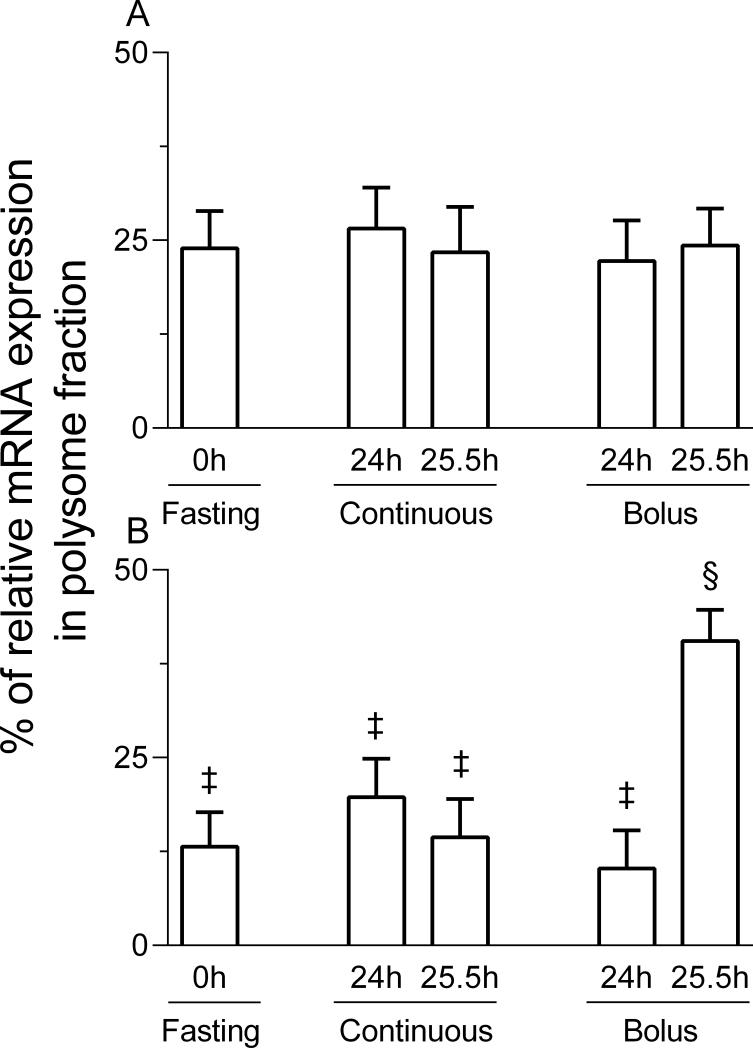
The proportion of rpS4 mRNA associated with polysomes in liver was greater in the 25.5 h bolus fed group compared with other groups (p < 0.05; Figure 4). There were no differences in rpS4 abundance among fasting, 24 and 25.5 h continuous feeding, and 24 h bolus feeding groups. The distribution of hepatic ornithine decarboxylase mRNA in sucrose density gradients, a negative control for the regulation of the terminal oligopyrimidine (TOP) mRNA, was unchanged by treatments.
Figure 4.
The proportion of ornithine decarboxylase (ODC; A) and ribosomal protein S4 (rpS4; B) mRNAs in the polysomal fraction in the liver of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h. Values are means ± SEM, n = 5-7. ‡, § Values not sharing common symbols differ significantly (p < 0.05).
Phosphorylation state of signaling components leading to protein synthesis
To ascertain the effect of feeding modality on the activation of upstream regulators of mTOR, the phosphorylation of TSC2 and PRAS40 was determined. TSC2 phosphorylation was greater in muscles and internal organs for the 25.5 h bolus group as compared to all other treatments (p < 0.05; Table 1). Similarly, phosphorylation of PRAS40 was greater in the 25.5 h bolus fed group compared with all other groups (p < 0.05; Table. 2). The phosphorylation of 4EBP1 and S6K1, downstream effectors of mTORC1 and major players in translation initiation, were determined in all tissues. In gastrocnemius, soleus, masseter, left heart, jejunum, and kidney the phosphorylation of 4EBP1 was greater for 25.5 h bolus group compared to all other groups (p < 0.05; Table 3). Phosphorylation of 4EBP1 did not differ between 24 and 25.5 h continuous fed, 24 h bolus fed, and the overnight fasted groups in all muscles, jejunum and kidney. However, 4EBP1 phosphorylation in liver and pancreas was greater for the 24 and 25.5 h continuous feeding and 24 h bolus feeding group than for the overnight fasted group. For both tissues 4EBP1 phosphorylation was greatest for the 25.5 h bolus group. In all tissues, phosphorylation of S6K1 was greater for the 25.5 h bolus group compared to all other groups (p < 0.05; Figures 5-6). To determine the activation of the other arm of translation initiation and peptide elongation, the phosphorylation of eIF2α and eEF2 were determined. In all tissues, neither eIF2α phosphorylation nor eEF2 phosphorylation were affected by treatments (Tables 4-5).
Table 1.
Phosphorylation of TSC2 in muscles and visceral tissues of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h
| Tissue | Fasting | Continuous | Bolus | ||
|---|---|---|---|---|---|
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Gastrocnemius | 0.37±0.08* | 0.47±0.07* | 0.57±0.08* | 0.45±0.06* | 0.88±0.06§ |
| Soleus | 0.24±0.11* | 0.33±0.11* | 0.34±0.12* | 0.26±0.11* | 0.85±0.09§ |
| Masseter | 0.28±0.11* | 0.28±0.10* | 0.24±0.11* | 0.35±0.09* | 0.87±0.08§ |
| Left Heart | 0.42±0.09* | 0.49±0.09* | 0.49±0.10* | 0.48±0.09* | 0.92±0.08§ |
| Liver | 0.30±0.08* | 0.39±0.08* | 0.47±0.09* | 0.38±0.08* | 0.87±0.07§ |
| Jejunum | 0.20±0.09* | 0.35±0.09* | 0.27±0.10* | 0.28±0.09* | 0.82±0.08§ |
| Pancreas | 0.35±0.07* | 0.32±0.07* | 0.38±0.08* | 0.36±0.07* | 0.87±0.06§ |
| Kidney | 0.27±0.10* | 0.32±0.10* | 0.29±0.11* | 0.33±0.10* | 0.85±0.09§ |
Values are means ± SEM in arbitrary units, n = 5-7 per group.
Values in a row not sharing common symbols differ significantly (p < 0.05).
Values in a row not sharing common symbols differ significantly (p < 0.05).
Table 2.
Phosphorylation of PRAS40 in muscles and visceral tissues of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h
| Tissue | Fasting | Continuous | Bolus | ||
|---|---|---|---|---|---|
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Gastrocnemius | 0.35±0.08* | 0.41±0.08* | 0.46±0.09* | 0.42±0.08* | 0.93±0.07§ |
| Soleus | 0.21±0.11* | 0.27±0.11* | 0.22±0.12* | 0.25±0.11* | 0.85±0.09§ |
| Masseter | 0.46±0.08* | 0.47±0.08* | 0.45±0.09* | 0.53±0.08* | 0.88±0.07§ |
| Left Heart | 0.22±0.09* | 0.23±0.09* | 0.24±0.10* | 0.32±0.09* | 0.87±0.07§ |
| Liver | 0.43±0.06* | 0.39±0.06* | 0.45±0.07* | 0.42±0.06* | 0.92±0.05§ |
| Jejunum | 0.35±0.08* | 0.38±0.08* | 0.40±0.09* | 0.38±0.08* | 0.91±0.07§ |
| Pancreas | 0.43±0.06* | 0.52±0.06* | 0.51±0.07* | 0.54±0.06* | 0.93±0.05§ |
| Kidney | 0.26±0.09* | 0.31±0.09* | 0.35±0.10* | 0.29±0.09* | 0.89±0.07§ |
Values are means ± SEM in arbitrary units, n = 5-7 per group.
Values in a row not sharing common symbols differ significantly (p< 0.05).
Values in a row not sharing common symbols differ significantly (p< 0.05).
Table 3.
Phosphorylation of 4EBP1 in muscles and visceral tissues of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h
| Tissue | Fasting | Continuous | Bolus | ||
|---|---|---|---|---|---|
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Gastrocnemius | 0.04±0.07* | 0.24±0.07* | 0.26±0.07* | 0.22±0.06* | 0.83±0.06§ |
| Soleus | 0.05±0.08* | 0.12±0.07* | 0.14±0.08* | 0.13±0.07* | 0.82±0.06§ |
| Masseter | 0.05±0.07* | 0.23±0.07* | 0.23±0.07* | 0.22±0.06* | 0.84±0.06§ |
| Left Heart | 0.12±0.11* | 0.46±0.09* | 0.47±0.11* | 0.36±0.09* | 0.90±0.08§ |
| Liver | 0.11±0.07‡ | 0.38±0.07*‡ | 0.45±0.07* | 0.46±0.06* | 0.88±0.06§ |
| Jejunum | 0.10±0.09* | 0.36±0.08* | 0.37±0.09* | 0.43±0.08* | 0.78±0.07§ |
| Pancreas | 0.16±0.09‡ | 0.59±0.08a* | 0.61±0.09a* | 0.60±0.08* | 0.93±0.08§ |
| Kidney | 0.06±0.09* | 0.26±0.08* | 0.23±0.09* | 0.23±0.08* | 0.81±0.07§ |
Values are means ± SEM in arbitrary units, n = 5-7 per group.
Values in a row not sharing common symbols differ significantly (p < 0.05).
Values in a row not sharing common symbols differ significantly (p < 0.05).
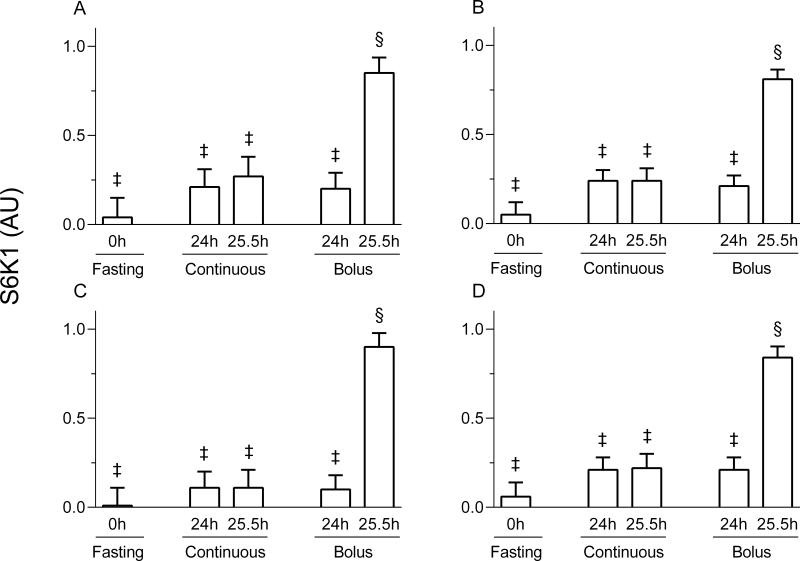
Figure 5.
Ribosomal protein S6 kinase 1 (S6K1) phosphorylation in the gastrocnemius (A), masseter (B), soleus (C) and left heart (D) of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25. 5h. AU = arbitrary units. Values are means ± SEM, n = 5-7. ‡, §Values not sharing common symbols differ significantly (p < 0.05).
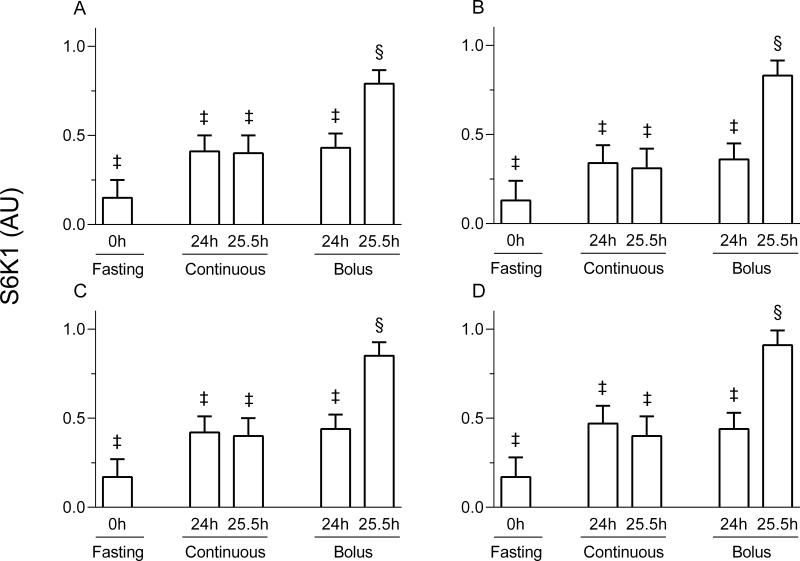
Figure 6.
Ribosomal protein S6 kinase 1 (S6K1) phosphorylation in the liver (A), jejunum (B), pancreas (C) and kidney (D) of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h. AU = arbitrary units. Values are means ± SEM, n = 5-7. ‡, §Values not sharing common symbols differ significantly (p < 0.05).
Table 4.
Phosphorylation of eIF2α in muscles and visceral tissues of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h
| Tissue | Fasting | Continuous | Bolus | ||
|---|---|---|---|---|---|
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Gastrocnemius | 0.79±0.14 | 0.88±0.13 | 0.73±0.14 | 0.90±0.12 | 0.83±0.11 |
| Soleus | 0.89±0.13 | 0.76±0.12 | 0.80±0.13 | 0.76±0.11 | 0.73±0.10 |
| Masseter | 0.74±0.15 | 0.73±0.14 | 0.76±0.15 | 0.70±0.13 | 0.71±0.12 |
| Left Heart | 0.80±0.15 | 0.66±0.14 | 0.85±0.15 | 0.69±0.13 | 0.66±0.12 |
| Liver | 0.81±0.15 | 0.87±0.14 | 0.66±0.15 | 0.65±0.13 | 0.74±0.12 |
| Jejunum | 0.71±0.20 | 0.70±0.18 | 0.76±0.20 | 0.85±0.16 | 0.80±0.15 |
| Pancreas | 0.77±0.20 | 0.84±0.18 | 0.76±0.20 | 0.86±0.16 | 0.81±0.15 |
| Kidney | 0.61±0.13 | 0.67±0.12 | 0.75±0.13 | 0.71±0.11 | 0.67±0.10 |
Values are means ± SEM in arbitrary units, n = 5-7 per group. No significant differences were detected.
Table 5.
Phosphorylation of eEF2 in muscles and visceral tissues of pigs either fasted overnight or fed continuously or by intermittent bolus for 24 or 25.5 h
| Tissue | Fasting | Continuous | Bolus | ||
|---|---|---|---|---|---|
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Gastrocnemius | 0.65±0.13 | 0.64±0.12 | 0.83±0.13 | 0.67±0.11 | 0.64±0.10 |
| Soleus | 0.80±0.20 | 0.89±0.17 | 0.62±0.17 | 0.90±0.16 | 0.86±0.15 |
| Masseter | 0.75±0.14 | 0.77±0.13 | 0.76±0.14 | 0.71±0.12 | 0.77±0.11 |
| Left Heart | 0.71±0.18 | 0.78±0.16 | 0.69±0.18 | 0.79±0.15 | 0.75±0.14 |
| Liver | 0.61±0.14 | 0.78±0.12 | 0.65±0.14 | 0.81±0.11 | 0.81±0.10 |
| Jejunum | 0.95±0.24 | 0.91±0.22 | 0.74±0.24 | 0.81±0.20 | 0.86±0.18 |
| Pancreas | 0.65±0.24 | 0.84±0.21 | 0.68±0.24 | 0.65±0.20 | 0.79±0.18 |
| Kidney | 0.76±0.16 | 0.84±0.15 | 0.82±0.16 | 0.71±0.13 | 0.78±0.12 |
Values are means ± SEM in arbitrary units, n = 5-7 per group. No significant differences were detected.
DISCUSSION
The adequacy of continuous vs. intermittent bolus feeding to sustain optimal growth rates remains controversial in spite of much research in this area (25). This is in part caused by confounders like disease state, duration of stay in the hospital and parenteral nutrient delivery which limit clinical studies (25). The piglet is used in nutritional studies as a model for the human neonate due to our ability to vary only one factor at a time and use invasive approaches to measure protein synthesis in vivo in the piglet. These studies are clinically relevant because little data are available to describe the mechanisms controlling protein synthesis under continuous and intermittent feeding. Previously, we have shown that intermittent feeding enhanced protein synthesis in the longissimus dorsi muscle compared to continuous feeding (26). Given that the protein synthesis response to nutritional stimuli may vary with muscle type (11) and in viscera (16), the aim of this study was to determine the effects of feeding modalities on protein synthesis in different muscle types and also in internal organs.
There is a paucity of data comparing feeding frequency and its effect on growth and protein synthesis in neonates. Results from one human study suggest that protein deposition was similar in infants fed intermittently or continuously (27). In that study, however, nitrogen deposition was determined by net balance, which may not be sufficiently sensitive to measure subtle changes in protein deposition over a short period (28, 29). In the current study, protein synthesis increased in all muscle types, ranging from those that contain primarily glycolytic to those with mainly oxidative fibers, and internal organs in response to feeding and the response was highest 1.5 h following a meal in those pigs that had been fed intermittently. Although we have previously reported differences in protein synthesis among different muscle types (26), it is likely that those differences represent only acute responses following 4 h of enteral feeding whereas in the current study, the differences in responses were diminished following more prolonged feeding of 24 h. The increased protein synthesis in the jejunum is consistent with the findings of a previous study in which intermittent feeding was shown to stimulate intestinal growth and development in newborn piglets by increasing mucosal and intestinal protein mass as compared to continuous feeding (10).
We have previously shown, using the pancreatic-substrate clamp, that insulin and amino acids independently stimulate protein synthesis in skeletal muscle (17) but that the response in visceral tissue is primarily driven by amino acids (16). Postprandial protein synthesis rates following a meal parallel the rapid rise in blood insulin and amino acid levels and returned to prefeeding rates by 4 h (26). In the current study, the greater increase in protein synthesis in the intermittent bolus compared to continuous fed pigs was associated with more profound changes in circulating insulin and amino acids. As the increase in protein synthesis with feeding occurred in visceral tissues as well as in muscle, it seems likely that the increase in protein synthesis is at least in part due to the feeding-induced elevation in amino acids in the current study. Our studies also have shown that meal feeding increases protein synthesis by enhancing the activation of translation initiation, a process regulated by mTOR (26, 30-32).
Protein synthesis is controlled by three regulatory mechanisms. The first involves eIF2 that facilitates the binding of methionyl-tRNA to the 40S ribosomal subunit, forming the 43S pre-initiation complex (19, 20). We have previously shown that the increased protein synthesis in response to feeding occurs independently of any changes in the phosphorylation of the α-subunit of eIF2 (24, 33), which is in agreement with the current findings in both continuously and intermittently fed pigs.
The second process involves the binding of the active mRNA to the 43S pre-initiation complex, facilitated by eIF4G•eIF4E complex, a downstream effector of mTOR. The activation of the mTOR signaling pathway following a meal occurs through phosphorylation of PKB which phosphorylates and inactivates the allosteric inhibitors of mTOR, TSC1 and PRAS40 (21, 22, 34). In this study, the phosphorylation of PKB target proteins, TSC2 and PRAS40, increased 1.5 h following a meal, likely due to the rise in insulin (33, 35). When activated, mTOR phosphorylates the downstream target proteins, S6K1 and 4EBP1, leading to the dissociation of eIF4E•4EBP1 and the formation of the active eIF4G•eIF4E complex which enhances translation initiation. In the current study, S6K1 and 4EBP1 phosphorylation increased 1.5 h following the meal in the intermittently fed pigs, but was not different from fasting either immediately before the meal, or when pigs had been fed continuously at both time points for all tissues measured. Thus, our data support our hypothesis that intermittent bolus meal feeding enhances protein synthesis through activation of translation initiation in all muscle types, including the heart, and in vital organs likely due to an increase in the activation of the insulin and/or amino acid signaling pathways. The modest elevation in tissue protein synthesis in continuous fed compared to fasting pigs that occurs despite the lack of stimulation of biomarkers of translation initiation may be due to an upregulation of reinitiation that is likely not mediated by this pathway (36).
The third process regulating protein synthesis is peptide elongation. Although phosphorylation of eEF2 can enhance peptide elongation in vitro (23), we have previously shown that under normal physiologic conditions in vivo, peptide elongation mediated by eEF2 phosphorylation does not change (24, 33). The results of the current study show no effect of feeding on eEF2 phosphorylation and support our previous findings that protein synthesis is mainly controlled by translation initiation rather than peptide elongation.
Transcripts of genes involved in the synthesis and control of ribosomal proteins contain a TOP tract at the 5’-end of the transcript (37). Ribosomal protein S4 is a TOP gene that encodes a component of the 40S ribosomal subunit (38, 39). Because only mRNAs associated with polysomes are translated (40, 41), the distribution of the TOP mRNA between polysomal and non-polysomal fractions correlates with active mRNA translation, and therefore protein synthesis. Previously, we showed that in response to meal feeding there was a temporal increase in the proportion of rpS4 mRNA in polysomes with a concurrent increase in protein synthesis (26). In the current study, the proportion of rpS4 mRNA associated with polysomes was higher in livers from pigs fed intermittently 1.5 h after a meal, than in those fed continuously or intermittently just before the meal. Although feeding stimulates protein synthesis in the liver, using hyperinsulinemic- euglycemic -amino acid clamps, we have shown that amino acids, but not insulin, can enhance protein synthesis in the liver (15). Likewise, oral administration of leucine simultaneously increased the proportion of hepatic rpS4 mRNA associated with polysomes and enhanced protein synthesis (42). Suppression of S6K1 phosphorylation by rapamycin inhibits 5’TOP mRNA translation (37). Thus, it is likely that the mechanism by which rpS4 translation is enhanced by intermittent feeding is through activation of the mTOR target, S6K1, and is mediated by amino acids.
The results of the current study showed that intermittent bolus compared to continuous feeding, increased protein synthesis in skeletal and cardiac muscles and in several vital organs of the neonatal pig. This increase occurred in association with activation of the mTOR signaling pathway and the subsequent enhanced phosphorylation of S6K1 and 4EBP1. In the liver, the enhanced protein synthesis occurred through greater translation of TOP mRNA encoding for ribosomal proteins possibly via activation of the mTOR pathway. These results suggest that intermittent bolus compared to continuous feeding enhances tissue protein synthesis through increased activation of amino acid and insulin-induced translation initiation and by biogenesis of ribosomal proteins. As the intermittent bolus pattern of feeding compared to continuous feeding enhanced protein synthesis similarly in all muscles and visceral tissues studied, the results suggest that intermittent bolus feeding may promote symmetric growth in neonates. The results of this study are of direct relevance for infants and children who are on parenteral nutrition and not tolerating full feeds, and/or those who are on continuously fed via orogastric or nasogastric tube due to feeding intolerance. Further studies are needed to determine whether the intermittent bolus pattern of feeding will enhance lean body mass and weight gain, compared to continuous feeding, in neonates.
MATERIALS AND METHODS
Animals and design
The protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. Sows and piglets were housed and managed as previously described (26). After birth, piglets resided with their sow and were not given supplemental creep feed. Piglets were studied at 5 to 7 d of age and weighing 2.0 ± 0.4 kg.
Catheters were inserted under general anesthesia using sterile techniques in an external jugular vein and common carotid artery 3 days before infusion, as described previously (26). After recovering from anesthesia, piglets were returned to their respective sows until the day of study.
Treatments and infusion
Treatments were randomly assigned to piglets using Complete Randomized Design. The five treatment groups (n = 5-7 • treatment−1) were: 1) overnight fasted, 2) intermittently bolus fed for 24 h, 3) intermittent bolus fed for 25.5 h, 4) continuously fed for 24 h, and 5) continuously fed for 25.5 h. Briefly, piglets assigned to the intermittent bolus fed groups were enterally fed by gavage (40 ml • kg body wt−1) a balanced enteral milk replacement every 4 h over a 15 min period and were killed at either 24 h (4 h after the last meal and just before a new meal) or 25.5 h (1.5 after the last meal). We have previously shown (26) that the postprandial rise in translation initiation signaling and protein synthesis is sustained from at least 0.5 to 2 h but falls to baseline by 4 h after a meal. Therefore, we opted to sample just before and 1.5 h after the last meal. The milk replacer contained 5% whey protein concentrate, 1% lactose, and 6.5% fat, providing 192 kcal, 10 g protein and 13 g fat per kg of body weight. The continuously fed groups received the same balanced enteral milk replacement at a rate of 10 ml • kg body wt−1 • h−1 maintained constant throughout the infusion period until the piglets were killed 24 h or 25.5 h later to allow for direct comparison in time with the bolus fed groups. The overnight food deprived piglets were killed at 0 h. Intermittent bolus and continuously fed pigs were provided the same amount of food over a 24 h period.
Tissue protein synthesis in vivo
Tissue protein synthesis was measured using a flooding dose of L-[4-3H] phenylalanine (43). Piglets received L-[4-3H] phenylalanine (1.5 mmol • kg body wt−1, 0.5 mCi • kg body wt−1, Amersham Bioscience, Piscataway, NJ) injected 30 min before they were killed. Samples were obtained from the gastrocnemius, masseter, and soleus muscles, left heart, liver, jejunum, pancreas, and kidney, immediately frozen in liquid nitrogen, and stored at −70°C until analyzed (43).
Reverse transcriptase and real-time quantitative PCR
The proportion of ribosomal protein S4 (rpS4) and ornithine decarboxylase (ODC) mRNAs in the polysomal fraction was determined in the liver as described previously (26).
Protein immunoblot analysis
Equal amounts of extracted protein from the muscle and visceral tissue homogenates were separated by electrophoresis on polyacrylamide gels (PAGE). For each assay, all samples were run at the same time on triple-wide gels (C.B.S. Scientific, Del Mar, CA) to minimize inter-assay variation. Proteins were electrophoretically transferred to polyvinlidene difluoride transfer membranes (Pall Corporation, Port Washington, NY), incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody (31).
Immunoblots that were probed with antiphospho-specific antibodies were normalized by stripping the blots in stripping buffer and then reprobed with corresponding nonphospho-specific antibodies (Pierce Biotechnology, Rockford, Illinois). Immunoblotting was performed using the following primary antibodies: TSC2 (total and Thr1462, Cell Signaling, Danvers, MA), PRAS40 (total and Thr246, Cell Signaling), 4EBP1 (total, Bethyl Laboratories, Montgomery, TX and Thr70, Cell Signaling), S6K1 (total and Thr398, Cell Signaling), eIF2 (total and Ser51, Cell Signaling), and eEF2 (total and Thr56, Cell Signaling). Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP, Upland, CA).
Calculations and statistics
The fractional rate of protein synthesis (Ks (%/day), percentage of protein mass synthesized in a day) was calculated as Ks = [(Sb/ Sa) × (1,440/t)] × 100, where Sb (dpm • nmol−1) is the specific radioactivity of the protein-bound phenylalanine, Sa (dpm • nmol−1) is the specific radioactivity of the tissue free phenylalanine at the time of tissue collection, corrected by linear regression with the blood specific radioactivity of the animal against time, t is the time of labeling in minutes, and 1,440 is for minutes-to-day conversion.
Statistical analysis was carried out using MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), using one-way ANOVA to determine main statistical differences between groups. When a significant effect was detected, all means were compared using Tukey-Kramer Multiple Comparison Test. Data are presented as least square means ± SEM and differences considered significant at p ≤ 0.05.
AKNOWLEDGEMENTS
We thank Robert Shulman and Douglas Burrin for helpful discussions, Rosemarie Almonaci and Sue Koo for technical assistance, Jerome Stubblefield for animal care, and E. O’Brian Smith for statistical assistance.
Statement of financial support: This work was supported by the National Institute of Health R01 AR44474 and the United States Department of Agriculture/Agricultural Research Service 6250-510000-055.
Footnotes
Disclosure: The authors have no conflicts of interest.
REFERENCES
- 1.Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55. doi: 10.1053/j.semperi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Saigal S, Stoskopf BL, Streiner DL, et al. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407–15. doi: 10.1542/peds.108.2.407. [DOI] [PubMed] [Google Scholar]
- 3.Marchand V. Enteral nutrition tube feedings. In: Baker S, Baker RD, Davis A, editors. Pediatric Nutrition Support. Jones and Bartlett Publishers; Sudbury, MA: 2007. pp. 249–60. [Google Scholar]
- 4.Kleinman RE, editor. Pediatric Nutrition Handbook. 5th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2003. Nutritional needs of the preterm infant. pp. 23–54. [Google Scholar]
- 5.Aynsley-Green A. The endocrinology of feeding in the newborn. Baillieres Clin Endocrinol Metab. 1989;3:837–68. doi: 10.1016/s0950-351x(89)80056-4. [DOI] [PubMed] [Google Scholar]
- 6.Mashako MN, Bernard C, Cezard JP, et al. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr. 1987;6:948–52. doi: 10.1097/00005176-198711000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Dollberg S, Kuint J, Mazkereth R, et al. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr. 2000;19:797–800. doi: 10.1080/07315724.2000.10718080. [DOI] [PubMed] [Google Scholar]
- 8.Schanler RJ, Shulman RJ, Lau C, et al. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics. 1999;103:434–9. doi: 10.1542/peds.103.2.434. [DOI] [PubMed] [Google Scholar]
- 9.Dsilna A, Christensson K, Alfredsson L, et al. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J Pediatr. 2005;147:43–9. doi: 10.1016/j.jpeds.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Shulman RJ, Redel CA, Stathos TH. Bolus versus continuous feedings stimulate small-intestinal growth and development in the newborn pig. J Pediatr Gastroenterol Nutr. 1994;18:350–4. doi: 10.1097/00005176-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Davis TA, Fiorotto ML, Nguyen HV, et al. Protein turnover in skeletal muscle of suckling rats. Am J Physiol - Regul Integr Comp Physiol. 1989;257:R1141–R46. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- 12.Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Ped Res. 1991;30:23–7. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Goldspink DF, Kelly FJ. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J. 1984;217:507–16. doi: 10.1042/bj2170507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis SE, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 1984;217:517–26. doi: 10.1042/bj2170517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis TA, Fiorotto ML, Beckett PR, et al. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol - Endocrinol Metab. 2001;280:E770–E79. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- 16.Suryawan A, O'Connor P, Bush J, et al. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2008;37:97–104. doi: 10.1007/s00726-008-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor PM, Kimball SR, Suryawan A, et al. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol - Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor PM, Kimball SR, Suryawan A, et al. Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. Am J Physiol - Endocrinol Metab. 2004;286:E994–E1003. doi: 10.1152/ajpendo.00391.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 20.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang Q, Wen Q, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Li W, Williams M, et al. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. Embo J. 2001;20:4370–9. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzaneo MC, Suryawan A, Orellana RA, et al. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr. 2011;141:2152–58. doi: 10.3945/jn.111.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev. 2011:CD001819. doi: 10.1002/14651858.CD001819.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzaneo MC, Orellana RA, Suryawan A, et al. Differential regulation of protein synthesis and mTOR signaling in skeletal muscle and visceral tissues of neonatal pigs after a meal. Pediatr Res. 2011;70:253–60. doi: 10.1203/PDR.0b013e3182276cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestre MA, Morbach CA, Brans YW, et al. A prospective randomized trial comparing continuous versus intermittent feeding methods in very low birth weight neonates. J Pediatr. 1996;128:748–52. doi: 10.1016/s0022-3476(96)70324-4. [DOI] [PubMed] [Google Scholar]
- 28.Bier DM. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabetes Metab Rev. 1989;5:111–32. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- 29.Tomé D, Bos C. Dietary protein and nitrogen utilization. J Nutr. 2000;130:1868S–73S. doi: 10.1093/jn/130.7.1868S. [DOI] [PubMed] [Google Scholar]
- 30.Frank JW, Escobar J, Suryawan A, et al. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J Nutr. 2005;135:1374–81. doi: 10.1093/jn/135.6.1374. [DOI] [PubMed] [Google Scholar]
- 31.Frank JW, Escobar J, Suryawan A, et al. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol - Endocrinol Metab. 2006;290:E225–E33. doi: 10.1152/ajpendo.00351.2005. [DOI] [PubMed] [Google Scholar]
- 32.Wilson FA, Suryawan A, Orellana RA, et al. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J Nutr. 2009;139:1873–80. doi: 10.3945/jn.109.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suryawan A, Orellana RA, Nguyen HV, et al. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol - Endocrinol Metab. 2007;293:E1597–E605. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;12:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol. 2010;109:1448–54. doi: 10.1152/japplphysiol.00428.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martineau Y, Derry MC, Wang X, et al. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008;28:6658–67. doi: 10.1128/MCB.00738-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferies HB, Fumagalli S, Dennis PB, et al. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. Embo J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherton CC, Wool IG. Determination of the number of proteins in liver ribosomes and ribosomal subunits by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1972;247:4460–67. [PubMed] [Google Scholar]
- 39.Wool IG, Chan Y-L, Paz V, et al. The primary structure of rat ribosomal proteins: The amino acid sequences of L27a and L28 and corrections in the sequences of S4 and S12. Biochim Biophys Acta - Gene Struct Expr. 1990;1050:69–73. doi: 10.1016/0167-4781(90)90143-p. [DOI] [PubMed] [Google Scholar]
- 40.Kimball SR, Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–7. doi: 10.1097/00075197-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Bioph Res Co. 2004;313:423–27. doi: 10.1016/j.bbrc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Anthony JC, Anthony TG, Kimball SR, et al. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J. Nutr. 2001;131:856S–60. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 43.Davis TA, Burrin DG, Fiorotto ML, et al. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol - Endocrinol Metab. 1996;270:E802–E09. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]