Scientific Abstract
When attention is directed to one information stream over another, the brain can be configured in advance to selectively process the relevant stream and suppress potentially distracting inputs. One key mechanism of suppression is through the deployment of anticipatory alpha-band (~10Hz) oscillatory activity, with greater alpha-band power observed in cortical regions that will ultimately process the distracting stream. Atypical attention has been implicated in autism spectrum disorder (ASD), including greater interference by distracting task-irrelevant inputs. Here we tested the integrity of these alpha-band mechanisms in ASD using an intersensory attention task. EEG was recorded while participants were cued on a trial-by-trial basis to selectively deploy attention to the visual or auditory modality in anticipation of a target within the cued modality. Whereas typically developing children showed the predicted alpha-band modulation, with increased alpha-band power over parieto-occipital scalp when attention was deployed to the auditory compared to the visual modality, this differential pattern was entirely absent at the group level in the ASD cohort. Further, only the ASD group showed impaired performance due to the presence of task-irrelevant sensory information. These data suggest that impaired modulation of alpha-band activity plays a role in increased distraction from extraneous sensory inputs in ASD.
Keywords: Autism, EEG, Oscillations, Attention
INTRODUCTION
In a crowded noisy restaurant, one might employ selective attention to focus on the menu while ignoring the discussions at neighboring tables. In this situation, both visual (the text printed on the menu) and auditory (the surrounding conversations) inputs compete for limited neural resources. Selective attention serves to bias competition between multiple inputs toward the input that immediately serves the behavioral goals of the organism (e.g., choosing an entree at the restaurant) (Desimone and Duncan 1995), both by enhancing processing of relevant sensory inputs and by suppressing processing of those that are irrelevant. Canonical features of Autism Spectrum Disorders (ASD), particularly those falling within the diagnostic category of rigid and repetitive behaviors, have been hypothesized to result in part from atypical selective attention (Ciesielski, Courchesne et al. 1990; Townsend and Courchesne 1994; Ciesielski, Knight et al. 1995; Teder-Salejarvi, Pierce et al. 2005; Remington, Swettenham et al. 2012). Previous investigations have suggested 'overselective' attention in ASD (Lovaas, Schreibman et al. 1971), referring to a tendency to attend intensely to one stimulus while completely disregarding other sources of information, as well as impairments in the orienting and subsequent reorienting of attention (Wainwright-Sharp and Bryson 1993; Burack 1994; Courchesne, Townsend et al. 1994; Iarocci and Burack 2004; but see Iarocci and Burack 2004). More recently, however, there is mounting evidence that individuals with an ASD also have a specific deficit in filtering out, or inhibiting, distracting task-irrelevant information (Christ, Holt et al. 2007; Christ, Kester et al. 2011; Adams and Jarrold 2012). For example, in variations on the flanker visual filtering task (Eriksen and Eriksen 1974), in which participants must detect a visual target that is surrounded by varying degrees of distracting information, individuals with an ASD are more impaired by the presence of distractors than are TD individuals (Christ, Holt et al. 2007; Christ, Kester et al. 2011; Adams and Jarrold 2012). Further support comes from an fMRI study on selective attention by Ohta, Yamada et al. (2012) in which there was reduced suppression of distractor information in visual cortex in ASD compared to TD adults. Unfortunately, with ceiling performance for both groups, there was no behavioral correlate to this reduced suppression of neural activity and thus it is not clear if this reflected impaired visual suppressive mechanisms in ASD, or alternatively that such suppression was simply not necessary to perform the task. Yet additional evidence for suboptimal biasing of the brain's neural resources in ASD comes from electrophysiological investigations of selective attention, which have reported poorer discrimination performance and more false alarms to non-target stimuli, and reduced selective neural processing of information that is to be attended versus ignored in ASD (Ciesielski, Courchesne et al. 1990; Ciesielski, Knight et al. 1995; Teder-Salejarvi, Pierce et al. 2005).
Non-invasive high-density recordings of the brain's electrical activity have demonstrated that spectral power in the alpha rhythm (~8–14 Hz) modulates in accord with the distribution of attention. This has been shown under spatial (Worden, Foxe et al. 2000; Kelly, Lalor et al. 2006; Rihs, Michel et al. 2007; Banerjee, Snyder et al. 2011), feature-based (Snyder and Foxe 2010), and intersensory (Foxe, Simpson et al. 1998; Fu, Foxe et al. 2001; Gomez-Ramirez, Kelly et al. 2011) manipulations of attention. Alpha is typically greater over task-irrelevant cortical areas, and it is thought that alpha activity acts to filter out irrelevant sensory input (Foxe, Simpson et al. 1998; Fries 2001; Kelly, Lalor et al. 2006; Thut, Nietzel et al. 2006; Rihs, Michel et al. 2007; Capotosto, Babiloni et al. 2009; Romei, Gross et al. 2010; Bollimunta, Mo et al. 2011; Foxe and Snyder 2011; Buschman, Denovellis et al. 2012). Since individuals with an ASD show abnormalities in the suppression of task-irrelevant information, here we sought to evaluate, for the first time to the best of our knowledge, the integrity of these alpha suppression mechanisms.
We recorded high-density EEG while children and adolescents with an ASD and age and IQ matched typically developing controls performed a cued intersensory selective attention task. In this paradigm, on each trial participants receive a cue followed by a unisensory or bisensory stimulus. The cue informs them whether to perform a visual or an auditory target detection task, thus biasing their attention toward one sensory modality and away from the other. Previous work from our laboratory has shown this to induce robust suppression of information in the task irrelevant sensory modality, as indexed by increases in alpha power in the interval between the cue and the imperative stimulus over parieto-occipital regions when an individual is attending the auditory modality and must ignore distracting information in the visual modality (Foxe, Simpson et al. 1998; Fu, Foxe et al. 2001; Gomez-Ramirez, Kelly et al. 2011). This has been interpreted as a 'full-field' suppression of visual inputs, which are wholly irrelevant when the participant has been cued to attend only to the auditory stimuli. Here we assessed the degree to which both ASD and TD children deploy alpha strategically, and related this to behavioral indices of distractibility by task-irrelevant sensory information.
METHODS
Participants
We chose to restrict the participant age-range to between 9 and 16 years, a range within which participants were expected to be able to follow task instructions. Twenty ASD children and adolescents (4 female) and 20 age and nonverbal IQ matched TD children and adolescents (4 female) participated in the experiment (see Table 1A for participant descriptives). An additional 7 participants (4 ASD) were excluded from the study due to an inability to successfully perform the task. Five (3 ASD) had chance-level behavior during a preliminary psychophysical titration session, and an additional 2 (one ASD) passed this stage of the study, but performed below chance during the experimental session (see Table 1B for excluded participant descriptives).
Table 1.
Means and standard deviations (in parentheses) for the demographic data of the participants (A) and excluded participants (B).
| A. Participant demographics | ||
|---|---|---|
| TD | ASD | |
| Age | 12.20(1.93) | 12.22(1.71) |
| PIQ | 108.65(13.37) | 108.25(15.78) |
| VIQ | 119.15(13.70) | 107.50(13.26) |
| FSIQ | 115.70(13.01) | 109.10(13.91) |
| N | 20 | 20 |
| No. of females | 4 | 4 |
| B. Excluded participant demographics | ||
| TD | ASD | |
| Age | 11.87(2.97) | 12.27(2.82) |
| PIQ | 111.33(12.86) | 105.25(18.86) |
| VIQ | 111.67(7.77) | 88.25(27.32) |
| FSIQ | 113.00(10.58) | 96.50(23.44) |
| N | 3 | 4 |
| No. of females | 2 | 0 |
For the ASD group, diagnoses of ASD were made using both the Autism Diagnostic Interview-Revised (ADI; Lord, Rutter et al. 1994) and the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter et al. 1999) and confirmed by judgment of an experienced clinician. All participants passed the algorithmic thresholds for diagnosis of ASD on both the ADI and ADOS. Of the 20 children in the ASD group, 9 had a diagnosis of autistic disorder and 11 of Asperger’s disorder. Parents were asked to refrain from giving their children (n=4) stimulant medication in the 24 hour period prior to the testing session. Five children were taking other psychoactive medications (aripiprazole, sertraline, gabapentin, atomoxetine) at the time of participation.
Exclusionary criteria for both groups included a nonverbal IQ below 80, and a history of head trauma, epilepsy, or premature birth. Nonverbal IQ was measured with the Wechsler Abbreviated Scale of Intelligence (Weschler 1999), the Wechsler Abbreviated Scale of Intelligence-Second Edition (Weschler 2011), or the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; Weschler 2003). All participants were screened for normal or corrected-to-normal vision as well as normal hearing. Exclusion criteria for the TD group included use of psychoactive medications or a history of developmental, psychiatric, learning, or attention difficulties as assessed by a parent history questionnaire. TD children were also excluded if they had a biological first degree relative with a known developmental disorder.
Participants were matched in a pair-wise fashion, such that no TD-ASD pairing exceeded a threshold of ± 1 SD with respect to performance IQ (PIQ) or ~1 year of age. An analysis of variance (ANOVA) indicated that there were no significant differences between participant groups in age (F(1,38) = 0.003, p = 0.95), PIQ (F(1,38) = 0.007, p = 0.93), or full-scale IQ (FSIQ) (F(1,38) = 2.40, p = 0.13). A between groups effect, however, did reach significance on the measure of verbal IQ (VIQ) (F(1,38) = 7.46, p = 0.009), reflecting that the ASD group tended to have lower (though within normal range) VIQ scores than their TD counterparts.
Before participation, a parent or legal guardian of each child provided written informed consent, and written or verbal assent was obtained from each child. All procedures were approved by the Institutional Review Board of the Albert Einstein College of Medicine, where the experiments were conducted, and conformed to the tenets for the responsible conduct of human research as laid out in the Declaration of Helsinki. Participants received a modest fee ($12/hour) for their efforts.
Stimuli and Task
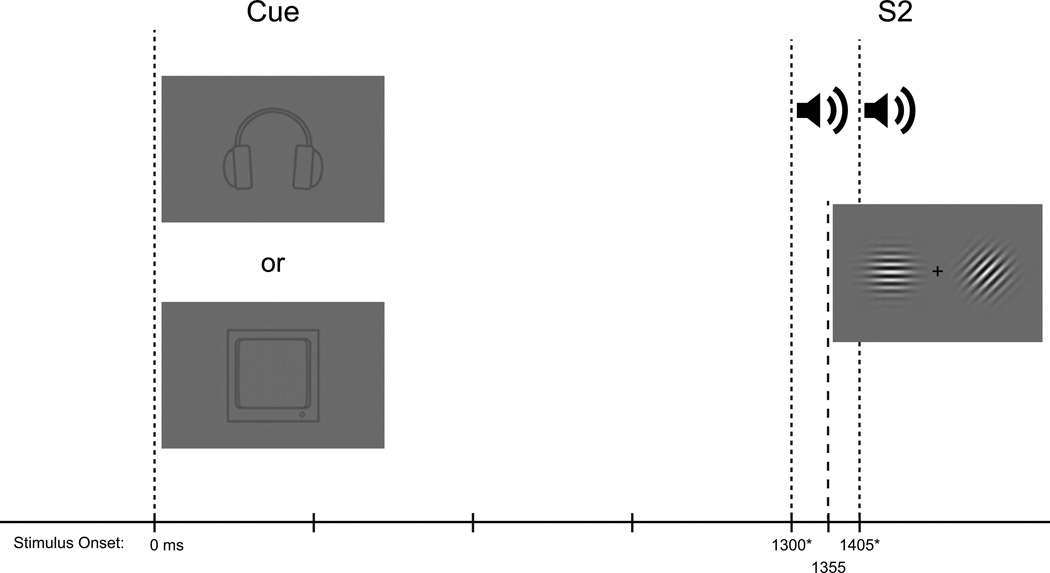
A cued intersensory attention task was employed in which each trial consisted of an instructional cue, an intervening blank preparatory period, followed by a task-relevant second stimulus (S2) (see FIG 1). Instructional cues were used such that participants were directed only to respond to targets within the cued sensory modality (auditory or visual) and to ignore any stimuli in the uncued sensory modality.
Fig 1.
Schematic of the experimental paradigm. At time 0 participants received a pictorial cue (200 ms in duration) indicating which stimulus modality to attend. Next came a blank interval during which only the fixation cross was presented. This was followed by presentation of the “S2” stimulus. For trials including auditory tone pairs, the first tone onset at 1300ms (*or 1350ms: for half of the participants the auditory stimuli were unintentionally delayed by 50 ms), and the onset of the second tone was at 1405 ms (*or 1455 ms for half the participants, again due to the delay in the auditory stimuli). For trials including the visual stimulus, visual stimulation always onset at 1355 ms.
Visual stimuli were presented on a gray background. The cue stimulus consisted of a simple gray line-drawing depicting either a pair of headphones(~3° square visual angle, Weber contrast = −0.14) or a computer monitor (~3° square visual angle, Weber contrast = −0.10). These cue stimuli instructed the participant as to which sensory modality (auditory or visual) was to be attended when the S2 arrived. The S2 stimuli took the form of either a unisensory stimulus in the cued modality or a compound bisensory auditory-visual stimulus. For both cue conditions, the likelihood of receiving a bisensory S2 was 63% and the likelihood of receiving a unisensory S2 was 37%. Participants performed a go/no-go detection task on the S2 within the cued modality, responding with a button click on a computer mouse using the index finger of the right hand. Participants were cued pseudorandomly on a trial-by-trial basis to attend to either the visual or auditory components of the upcoming S2 event. The likelihood of a task switch or repeat (i.e. attend to the same modality as the previous trial or switch to the other modality) was manipulated such that the probability of a given trial being a repeat rather than a switch trial was 70%.
The auditory S2 stimulus consisted of two sequentially presented sinusoidal tones (100 ms duration; 60 dB SPL; 10 ms rise/fall) with a 5 ms interval between presentations. On non-target trials, the two tones were of identical frequency and participants were asked to withhold responses when no difference between the tones was detected. On target trials, the two tones presented were of different frequency. One of the two tones was 2000 Hz, whereas the frequency of the other tone was psychophysically titrated based on each participant’s performance using a staircase procedure administered prior to the main task (see Procedure below). When subjects detected a frequency difference between the pair of tones, they were instructed to respond with a fast, accurate button push.
The visual S2 stimulus consisted of a pair of gabor patches (100 ms duration, 4.8° in diameter, 0.25 cycles per degree) centered 5.2° to the left and right of the fixation cross. On target and non-target trials the two gabors were of different and identical orientation, respectively. As with the auditory stimuli, the orientation difference between the gabors was psychophysically titrated for each participant (see Procedure below), and participants were instructed to respond to targets with a button push. The likelihood of receiving a target stimulus within the cued sensory modality was set at 20%.
The stimulus onset asynchrony (SOA) between the cue and target (i.e. the Cue-S2 period) was fixed at 1300 or 1350 ms1 similar to previous applications of this paradigm from our laboratory. A black fixation cross (subtending 0.3° vertically and horizontally) was presented in the center of the monitor throughout testing. The inter-trial interval (i.e., the S2–Cue period) was randomized (2000 to 3000 ms, square distribution) during which the fixation cross remained on the screen (see FIG 1 for a schematic of the stimulation paradigm).
Procedure
Participants were seated in a double-walled, darkened, sound-attenuated, electrically shielded booth (International Acoustics Company, Bronx, New York). Visual stimuli were presented on a LCD monitor positioned 100 cm from the participant. Auditory stimuli were presented on a single speaker centered directly behind the monitor. Stimuli were delivered using Presentation software (Neurobehavioral Systems, Albany, CA). All participants underwent a staircase procedure at the beginning of testing for each of the two tasks. This procedure, known as the Up-Down Transformed Rule (UDTR) was used to rapidly equate performance across the two tasks and across participants (Wetherill and Levitt 1965) before the beginning of the formal experimental sessions. The UDTR procedure employs different rules that converge on specific levels of accuracy. We used a 3-up, 1-down rule, meaning that, when a participant made three consecutive correct responses, we adjusted the stimulus one step harder and for any incorrect response, we adjusted the stimulus one step easier. This rule necessarily converges on an accuracy level of 79.4%. Importantly, the UDTR procedure employed only unisensory S2s. Thus, the acquired thresholds used for the remainder of the experimental session reflected performance on the unisensory target detection task only (i.e., without a task irrelevant stimulus in the uncued modality), and as such, left open the possibility of either task facilitation or interference with the addition of the second task-irrelevant stimulus.
During the experimental session, participants were instructed to respond as quickly and accurately as possible to targets within the cued modality and to withhold responses otherwise. Each participant completed approximately 20 blocks of 27 trials each, resulting in the collection of ~270 trials per cue condition.
Behavioral Measurements
To obtain measures of behavioral performance d-prime and reaction time (RT) measures were calculated. Only correct RTs (i.e., hits) within the latency window of 200 to 2000 ms following the onset of the second tone in the cue auditory condition, and following the onset of the gabors in the cue visual condition, were included.
The d' measure is widely used to assess the detectability of an imperative stimulus in a manner independent of a given individual's response criteria, or fluctuations thereof. d' is computed by taking into account the probability of correctly responding to targets when a target is present (termed a 'hit') and the probability of incorrectly initiating a response in the absence of a target (a 'false alarm')(Green and Swets 1966). For the estimation of d', hits were calculated using the same 95% threshold time window as in the case of the RTs. Correct responses to targets outside this window were labeled as misses. Inspection of the behavioral data (d') on a block-by-block basis, indicated that several participants had temporarily waned in task performance, or even ceased to perform the task, for certain blocks. In order to restrict our analyses to periods in which participants were clearly performing the task, we discarded any blocks in which the average d' value in either the cue-visual or cue-auditory conditions fell to zero or below. d' values of zero indicate that the probability of a false-alarm is equal to the probability of a hit, and thus detection can be said to be at chance. This threshold is quite liberal but it ensured that participants were performing the task above chance for all analyzed blocks.
Prior to the exclusion of blocks based on these criteria, the TD group completed a mean 21.15 (SD = 2.98) blocks, and the ASD group completed a mean 21.15 (SD = 3.25) blocks. Of these blocks, 9.42% (SD = 12.68) were rejected in the TD group and 14.16% (SD = 17.80) were rejected in the ASD group. An Independent samples t-test indicated that rates of block rejection were not statistically different between diagnostic groups (p>0.3). Across diagnostic groups the rate of block rejection bore no statistically reliable relationship to the age of the participant (r = 0.07, p > 0.6). Block rejection showed a negative trend as a function of PIQ, but did not reach statistical significance (r = −0.26, p = 0.09), and breaking this analysis out among the two diagnostic groups did not reveal a significant relationship for either group (TD: r = −0.18, p > 0.4; ASD: r = −0.32, p > 0.2). Furthermore, across the two groups, the relationship between block rejection and VIQ was not significantly different (r = −0.21, p > 0.2), nor was this relationship significant within either of the two groups (TD: r = −0.03, p > 0.8; ASD: r = −0.27, p > 0.3).
EEG Acquisition and Preprocessing
Continuous EEG was recorded, with a band-pass of DC to 134 Hz, from 72 scalp electrodes (Biosemi Active Two System: Amsterdam, Netherlands) at an analog-to-digital sampling rate of 512 Hz. Biosemi replaces the ground electrodes that are used in conventional EEG systems with two separate electrodes: Common Mode Sense (CMS) and Driven Right Leg (DRL) passive electrode. These two electrodes create a feedback loop, thus rendering them as references. EEG data were processed using MATLAB (The MathWorks Inc., Natick, Massachusetts). Scripts from the FieldTrip toolbox (Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, the Netherlands. See http://www.ru.nl/neuroimaging/fieldtrip) as well as the EEGLAB toolbox (Delorme and Makeig 2004) were applied for the analysis of the data.
The offline analysis of the EEG data proceeded as follows. First, the recorded data were low-pass filtered at 40 Hz (Butterworth IIR, 23 db/octave, zero-phase), high-pass filtered at 0.5 Hz (Butterworth IIR, 20 db/octave, zero-phase), and re-referenced to FPz, a central fronto-polar site. Next, in order to retain as many trials as possible while minimizing artifactual contributions from blinks and eye movements, we employed the following artifact correction procedure. For each participant, an independent component analysis (ICA) was performed on the data, concatenated over all data blocks, using the infomax algorithm (Bell and Sejnowski 1995) as implemented in the EEGLAB toolbox. Following the ICA decomposition, we used a two-step procedure to identify components reflecting occulomotor activity. First, we computed the mutual information (MI) shared between the time-courses of EOG channels (one vertical EOG channel, and a bipolar horizontal EOG channel) and the component time-courses. Any component that exceeded a threshold of 3 standard deviations beyond the median MI was marked as artifactual. Second, the component topographies were manually inspected to ensure that the components automatically identified as EOG-related also presented close correspondence to topographies representing horizontal or vertical EOG-activity. All remaining components identified as EOG were removed, and the data were transformed back to sensor space.
Following the ICA procedure, data were epoched from −1000 to 2500 ms around the onset of the cue stimulus. Errant electrodes were identified on a trial-by-trial basis, such that if an electrode exceeded a z score of 3 in 1) its variance, 2) its range, or 3) its mean, then it was considered bad. If a given trial contained more than 3 bad electrodes across the array of 72 channels, then it was discarded. Otherwise, bad electrodes were interpolated using 3 to 4 nearest neighbors. Finally, over all scalp electrodes, a trial rejection threshold of ± 120 µV was used.
Frequency Analysis
To measure changes in oscillatory power in the preparatory period, the data were analyzed using a short-term Fourier transform (STFT) approach (as implemented in the EEGLAB function newtimef), with fixed data segments of 250 ms multiplied by a hanning window, and 5 ms steps. Only bisensory S2 stimuli, which accounted for 67% of the total trials, were submitted to this analysis. This resulted in physically identical stimuli (within participant) across the two cued attention conditions. Since the STFT technique employed a fixed window size of 250 ms for all frequencies examined, a given time point in the STFT time-course reflects the spectral decomposition of the original data over this entire window. Although the hanning window employed in the analysis emphasizes data in the center of the window relative to the edges, care must still be taken when interpreting the output of the STFT. To avoid spectral input from the post-stimulus period, we used a causal STFT technique. Specifically, rather than centering the window around a data point of interest for the STFT, the window incorporated data from −250-0 ms for a given time point in the decomposition. Although this temporally smears the data forward in time to an extent, it nevertheless insures that a given data point in the STFT only reflects activity up to that point, and not after it. The power spectra were then baselined by subtracting the mean power spectra from −750 to 0 ms prior to cue onset, and dividing by the standard deviation in this period. This method produces baseline-adjusted z score values (Roach and Mathalon 2008), thus normalizing across possible inter-subject variability in raw power. All alpha power indices are in these baseline-adjusted z-scores unless otherwise noted.
RESULTS
Behavioral data
A UDTR procedure was performed to equate performance among participants on the unisensory S2 conditions as described above. The mean frequency difference between the 2000 Hz standard and the deviant tone, as estimated by the UDTR, was 98.00 Hz (SD = 65.54) for the ASD participants, and 77.75 Hz (SD = 69.25) for the TD participants. Likewise, for the visual target, the mean polar angle of the deviant gabor relative to the horizontally oriented standard was 14.00° (SD = 10.66) for the ASD participants, and 13.40° (SD = 10.02) for the TD participants. The threshold estimates between the diagnostic groups were not statistically different for the auditory (t(38) = .93, p > 0.3) or the visual (t(38) = 0.18, p > 0.8) tasks.
Detection (d-prime) analysis
D-prime data for each condition is presented in Table 2A. Within the cue-visual task, the TD exhibited a slight increase in detection on bisensory relative to unisensory S2 conditions (Vbi = 2.14(.83) versus Vuni = 2.06(.85)), whereas the ASD group exhibited a decrease on bisensory relative to unisensory trials (Vbi = 1.89(1.00) versus Vuni = 2.10(.93)). Within the cue-auditory task, both groups showed a decrease in detection on bisensory trials relative to unisensory trials. This difference was numerically greater in the ASD group (ASD: Abi = 1.45(.95) versus Auni = 1.90(.70), TD: Abi = 1.62(.83) versus Auni = 1.86(.68)).
Table 2.
Behavior means and standard deviations (in parentheses). (A) d-prime and (B) RT data for all conditions and the two diagnostic groups.
| A. d-prime | Cue Auditory | Cue Visual | ||||
|---|---|---|---|---|---|---|
| ASD | Unisensory | Bisensory | Mean | Unisensory | Bisensory | Mean |
| Repeat | 1.80(0.89) | 1.44(0.96) | 1.62 | 2.31(0.97) | 1.87(0.98) | 2.10 |
| Switch | 2.00(0.75) | 1.45(0.94) | 1.72 | 1.88(1.03) | 1.91(1.03) | 1.89 |
| Mean | 1.90 | 1.45 | 1.67 | 2.10 | 1.89 | 1.99 |
| TD | Unisensory | Bisensory | Mean | Unisensory | Bisensory | Mean |
| Repeat | 1.83(0.67) | 1.59(0.85) | 1.71 | 2.23(0.90) | 2.17(0.83) | 2.20 |
| Switch | 1.89(0.79) | 1.66(0.82) | 1.77 | 1.89(0.97) | 2.10(0.85) | 2.00 |
| Mean | 1.86 | 1.62 | 1.74 | 2.06 | 2.14 | 2.10 |
| B. RT (ms) | Cue Auditory | Cue Visual | ||||
| ASD | Unisensory | Bisensory | Mean | Unisensory | Bisensory | Mean |
| Repeat | 959(204) | 927(195) | 943 | 837(216) | 912(227) | 875 |
| Switch | 968(254) | 919(216) | 923 | 831(267) | 912(225) | 871 |
| Mean | 963(209) | 923(204) | 943 | 834(227) | 912(226) | 873 |
| TD | Unisensory | Bisensory | Mean | Unisensory | Bisensory | Mean |
| Repeat | 843(183) | 889(183) | 866 | 829(139) | 838(166) | 834 |
| Switch | 830(310) | 899(196) | 864 | 802(186) | 829(154) | 815 |
| Mean | 836(224) | 894(189) | 865 | 816(141) | 834(160) | 825 |
The d-prime data were statistically analyzed using a mixed model ANOVA with diagnostic group (ASD, TD) as the between-groups factor, and Cue (cue to visual, cue to auditory), S2 (unisensory S2, bisensory S2), and Trial (switch trial, repeat trial) as within-groups factors. A main effect of Cue (F(1,38) = 5.953, p = 0.019) reflected that detection was better for the cue visual (M = 2.05, SD = 0.85) compared to the cue auditory trials (M = 1.71, SD = 0.74). A main effect of S2 (F(1,38) = 13.26, p = 0.001) further supported that target detection was better on unisensory (mean = 1.98, SD = 0.65) than bisensory trials (M = 1.77, SD = 0.75). Interpretation of these main effects is modulated by several interactions.
Interference effects
A Cue × S2 interaction (F(1,38) = 8.48, p = 0.006) was followed-up with paired t-tests comparing unisensory to bisensory S2 conditions within each cue condition (collapsed across Diagnostic Group). This revealed a significant effect of S2 in the cue auditory condition (t(39) = 4.49, p <0.001), but not in the cue visual condition (t(39) = 0.87, p > 0.4). On cue auditory trials, detection was better on unisensory (M = 1.88, SD = 0.68) compared to bisensory trials (M = 1.54, SD = 0.88), whereas on cue visual trials this relationship did not holdup statistically.
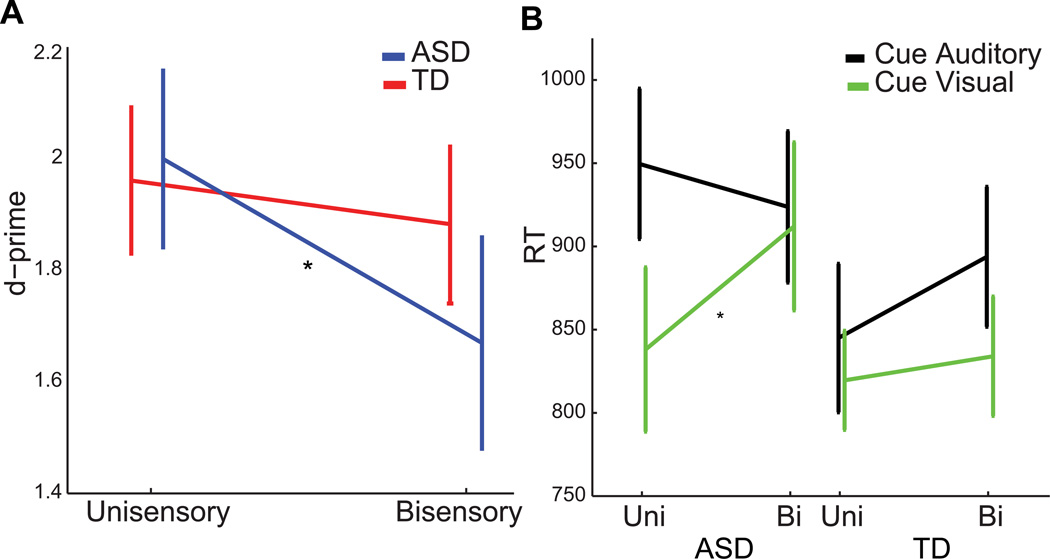
A Diagnostic Group × S2 interaction (F(1,38) = 5.05, p = 0.030) was followed up with separate paired t-tests comparing unisensory to bisensory S2 conditions (collapsed across Cue conditions) within each of the diagnostic groups. There was no significant effect of S2 for the TD group (t(19) = 1.15, p > 0.30). Within the ASD group, there was a significant effect of S2 (t(19) = 3.690, p = 0.002) that was driven by a decrease in d-prime on bisensory trials (M = 1.67, SD = 0.86) relative to unisensory trials (M = 2.00, SD = 0.72). These results indicate that task irrelevant sensory information in the bisensory trials interfered with performance in the ASD but not the TD group (FIG 2A).
Fig 2.
(A) Unisensory and bisensory d-prime data for the two diagnostic groups, collapsed across auditory and visual trials. (B) RT data for unisensory and bisensory S2s within the cue auditory and visual conditions. Asterisks indicate significant differences at α < 0.05. The error bars indicate ±1 SE (standard error).
Switch effects
A Cue × Trial interaction (F(1,38) = 8.90, p = 0.005) as well as a Cue × S2 × Trial interaction (F(1,38) = 6.07, p = 0.018) also reached significance. In order to disentangle these, follow-up two-way ANOVAs with factors S2 and Trial were performed, for each cue type. The ANOVA on the cue-auditory data revealed only a main effect of S2 (F(1,38) = 20.17, p<0.001), that was driven by better overall detection in the unisensory trials (M = 1.88, SD = 0.68) compared to the bisensory trials (M = 1.54, SD = 0.88). The ANOVA on the cue-visual data showed a main effect of S2 (F(1,38) = 9.85, p = 0.003) as well as an interaction of S2 × Trial (F(1,38) = 9.50, p = 0.004). Follow-up paired t-tests revealed a significant reduction in target detection for unisensory switch trials compared to the unisensory repeat trials (t(39) = 3.23, p = 0.003), whereas the comparison of cue-visual bisensory repeat trials to their switch counterparts did not reach statistical significance (t(39) = .57, p > 0.6).
Thus, within the cue-visual task, a cost of switching was observed in the unisensory (switch: M = 1.88, SD = 0.99, repeat: M = 2.27, SD = 0.93) but not the bisensory S2 trials (switch: M = 2.00, SD = 0.94, repeat: M = 2.02, SD = 0.90). Notably, the lack of an interaction between Trial and Diagnostic Group in the main ANOVA indicates that this switch cost did not differ statistically between ASD and TD groups.
Reaction-time analysis
RT data for each condition is presented in Table 2B. A 2 × 2 × 2 × 2 mixed model ANOVA was conducted on the RT data with the within group factors of Modality (auditory, visual), Trial (repeat, switch), and S2 (unisensory, bisensory), and the between group factor Diagnosis (ASD, TD).
Across the diagnostic groups, participants were faster to respond to visual targets (M = 848.95 ms, SD = 184.41) compared to auditory targets (M = 904.30 ms, SD = 193.63) as indicated by a main effect of Modality (F(1,38) = 5.33, p = 0.027). Participants were also marginally faster to respond to unisensory targets (M = 862.46 ms, SD = 177.34) compared to bisensory targets (M = 890.80 ms, SD = 180.65)(F(1,38) = 4.06, p = 0.051).
Interference effects
The main effects of Modality and S2 were mediated by a three-way interaction of Modality × S2 × Diagnosis (F(1,38) = 6.77, p = 0.013). In order to further investigate this interaction, we performed paired t-tests within each diagnostic group and modality comparing unisensory and bisensory targets. Of these, only the comparison of visual unisensory to visual bisensory targets within the ASD group reached significance (t(19) = −2.91, p = 0.009)(FIG 2B). This indicates that the three-way interaction of Modality × S2 × Diagnosis was driven by a modality specific (visual) difference between RTs to unisensory and bisensory targets within the ASD group, such that, in this group, unisensory visual targets (M = 834.31, SD = 227.25) were responded to faster than bisensory visual targets (M = 912.06, SD = 225.81)(FIG 2B).
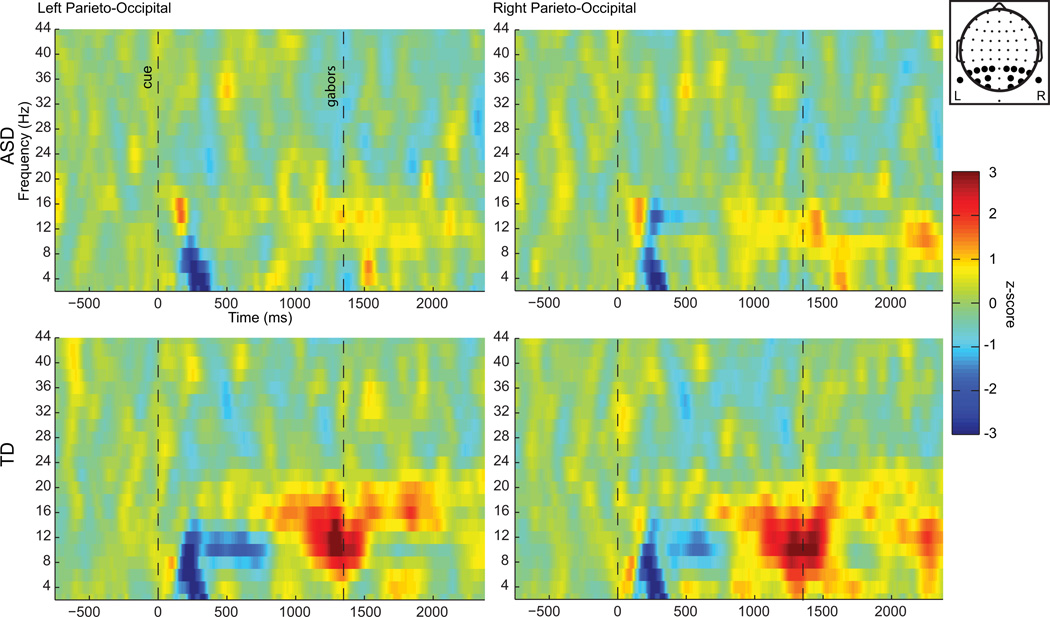
Electrophysiological data
Observation of the spectral activity in the alpha band (8–14 Hz) in FIGs 3 and 4 reveals clear task-dependent alpha power modulation in the expected direction in the TD group starting at about 1000 ms after the presentation of the cue stimulus. In contrast, in the ASD group there is very little indication of task-based modulation. Statistical analyses were focused on the last 200 ms prior to the onset of the S2 stimulus since previous work has shown that the strongest task-dependent modulations in the alpha-band occur in this timeframe (Foxe, Simpson et al. 1998; Worden, Foxe et al. 2000; Rihs, Michel et al. 2007; Gomez-Ramirez, Kelly et al. 2009). Within this latency window, electrodes over parieto-occipital scalp, where intersensory selective-attention alpha modulations are typically observed (Foxe, Simpson et al. 1998; Fu, Foxe et al. 2001; Gomez-Ramirez, Higgins et al. 2007), were selected (P1,P3, P5, P7, P9, PO7, PO3 and O1 on the left, and P2, P4, P6, P8, P10, PO8, PO4, and O2 on the right). These data were subjected to a 2 × 2 × 2 mixed model ANOVA with factors Cue, Hemisphere, and Diagnostic Group.
Fig 3.
Spectrograms of the subtraction of the cue-visual condition from cue-auditory condition, averaged over the left or right parieto-occipital electrodes used in the statistical analysis. Time zero indicates cue onset. The head map at upper right indicates the electrode positions. Units are baseline normalized z-scores.
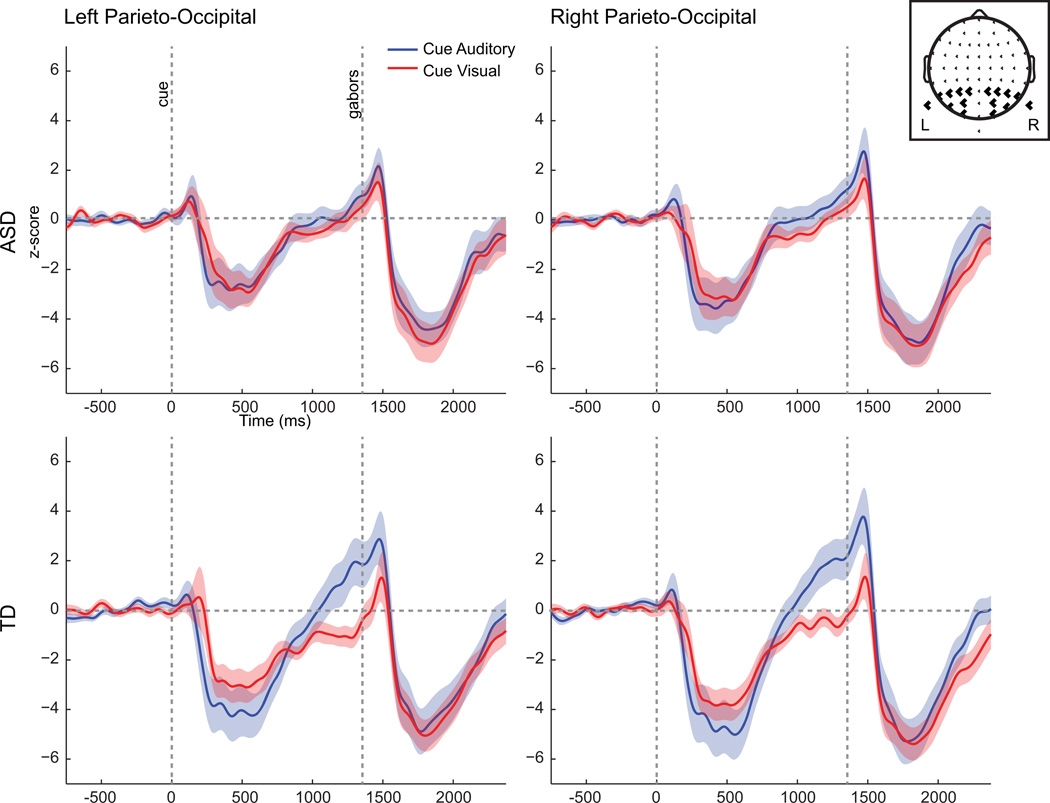
Fig 4.
Alpha waveforms (8–14 Hz) for cue-auditory and cue-visual conditions, averaged across left or right parieto-occipital electrodes used in the statistical analyses. Head map at upper right indicates the electrode positions. Units are baseline normalized z-scores. The semi-transparent color represents ± 1 SE.
A main effect of Cue (F(1,38) = 10.08, p = 0.003), and a Cue × Diagnostic Group interaction (F(1,38) = 4.67, p = 0.037) reflected that TD participants exhibited greater task-dependent alpha power modulations (cue auditory: Mean = 1.42, SD = 3.48; cue visual: M = −0.81, SD = 1.51) than the ASD participants (cue auditory: M = 0.40, SD = 1.89; cue visual: M = −0.02, SD = 1.19). Additionally, a main effect of hemisphere (F(1,38) = 4.27, p = 0.046) indicated that alpha power in this time window was greater over the right hemisphere (M = 0.41, SD = 1.79) than over the left (M = 0.08, SD = 1.83) across conditions and diagnostic groups.
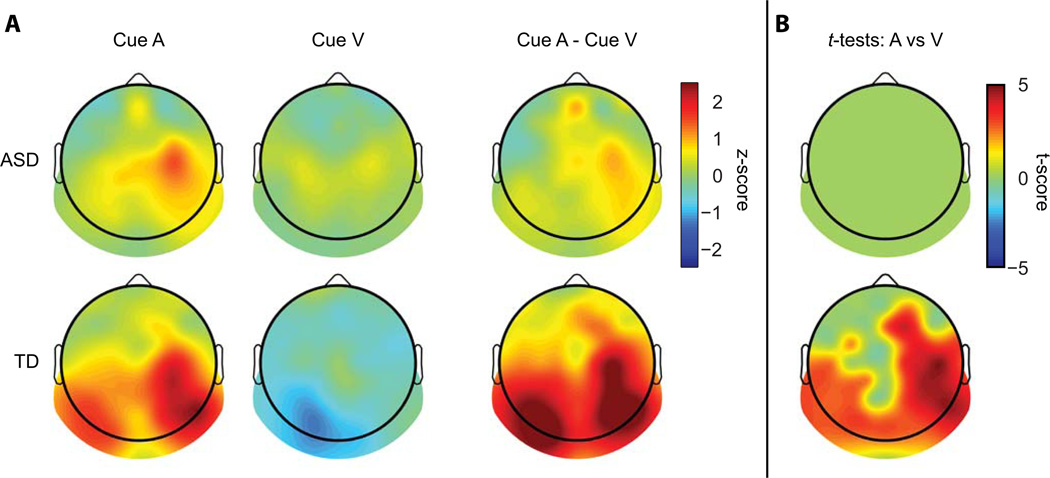
Follow-up paired t-tests within each diagnostic group comparing cue-auditory alpha to cue-visual alpha (collapsed across right and left hemisphere) were run to unpackage the Cue × Diagnostic Group interaction. The t-test on the TD group revealed a significant difference between cue conditions (t(19) = 3.32, p = 0.004) due to greater alpha power in the cue-auditory condition. The analysis of the ASD group showed no significant difference between cue conditions (t(19) = 0.85, p > 0.4).. FIG 5A depicts the topographic distribution of alpha in the two cueing conditions as well as their difference. It is evident that the task-related alpha modulation is largest over the posterior scalp in the TD participants. To explore whether the apparent differences in alpha modulation between the ASD and TD groups were the result of the regions on the scalp that were selected for analysis, paired t-tests were performed within each diagnostic group comparing alpha power in the two cueing conditions over all scalp electrodes. As before the average alpha power in the 200 ms leading up to the onset of the S2 stimuli was used for the analysis. The False Discover Rate (FDR) was used to correct for multiple comparisons (Benjamini and Yekutieli 2001). In the TD group, a pattern of significant difference between cueing conditions distributed over posterior scalp regions was again evident (FIG 5B bottom). Comparisons in the ASD group yielded no significant electrodes (FIG 5B top). Of note, in the ASD group, even prior to FDR correction no comparisons reached significance.
Fig 5.
(A) Topographic representation of alpha power for the two cued attention conditions and their subtraction, averaged over the 200 ms before S2 onset. Units are baseline normalized z-scores. (B) Topographies representing t-scores of significant electrodes resulting from paired t-tests of cue-auditory versus cue-visual alpha power in the 200 ms window prior to S2 onset across all electrodes, FDR corrected for multiple comparisons.
Exploring the relationship between task-based modulation of alpha power and task performance
An exploratory correlation analysis was performed to test the relationship between modulations in alpha power and behavior. If increases in alpha power over parieto-occipital cortices reflect active suppression of visual throughput when performing a demanding auditory task, then greater alpha power increases during the auditory task relative to the visual task should be positively related to performance on the auditory task. Generally, previous work has used one of two approaches in relating alpha indices and behavior, either by (1) comparing these metrics within participants by sorting individual trials (Thut, Nietzel et al. 2006; Kelly, Gomez-Ramirez et al. 2009), or (2) by comparing these metrics across individuals (Dockree, Kelly et al. 2007; Hanslmayr, Aslan et al. 2007; Yamagishi, Callan et al. 2008). Within participant approaches are arguably more sensitive to alpha-behavior relationships, as they exploit the fact that the attentional system - and the nervous system as a whole - is not time-invariant, and as such these measures can exhibit high variance throughout an experimental session. On the other hand, to the degree that this mechanism is successfully deployed in all neurologically typical individuals, one might predict a weak between participant relationship for alpha and behavior. Nevertheless, here we were more interested in inter-individual relationships between alpha and behavior, under the assertion that the reduced task-dependent alpha modulation in the ASD group reflects the atypical functioning of a mechanistic process with behavioral consequences. We were further motivated to take a between participant approach due to the relatively low trial numbers within conditions for each participant (i.e., binning alpha power into quintiles as a function of performance, as is sometimes done, would produce extremely noisy estimates).
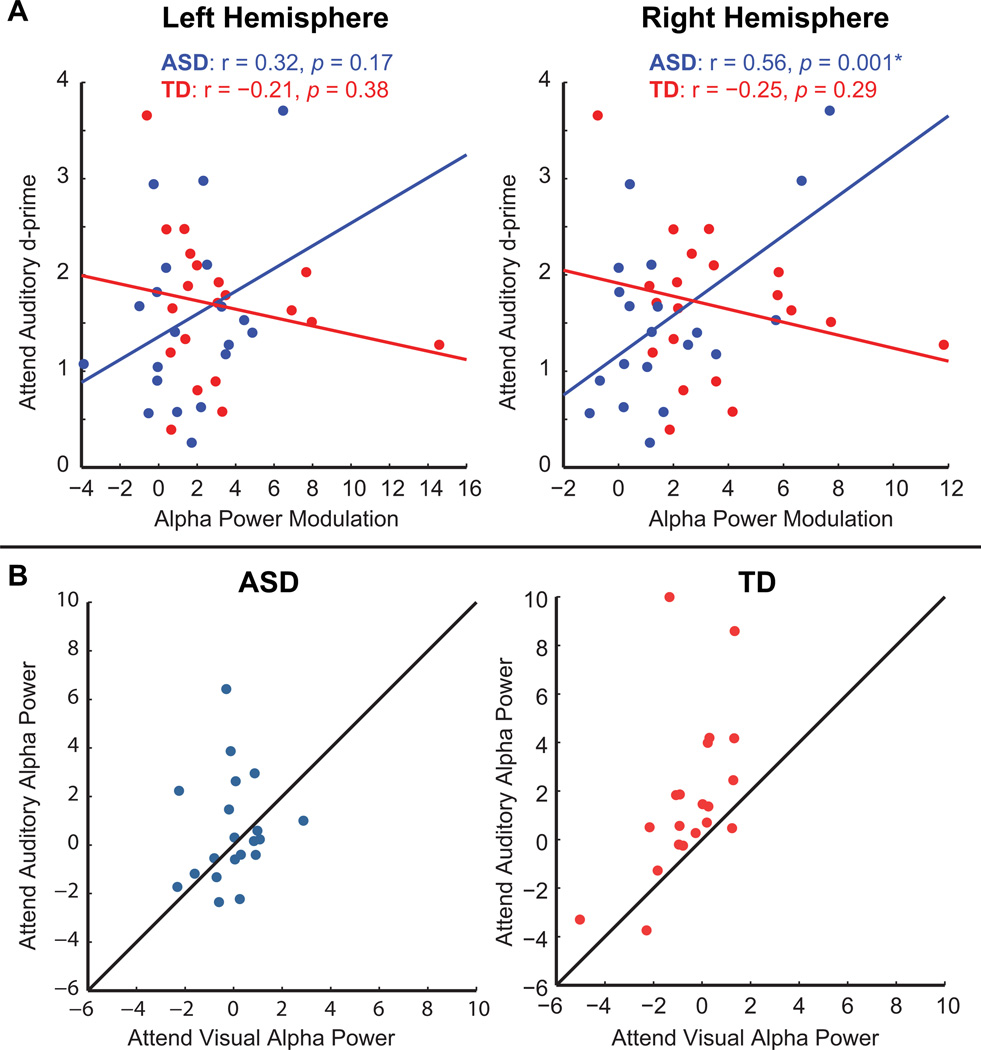
For each participant the data point within the original 200 ms window of analysis for which the subtraction of cue visual alpha from cue auditory alpha yielded the highest value was used. We reasoned that this alpha modulation index between conditions ought to more faithfully index strategic deployment of alpha, compared to absolute alpha power on one or the other cueing conditions. We further focused on alpha activity over the right hemisphere where it tends to be largest (Foxe, Simpson et al. 1998; Gomez-Ramirez, Higgins et al. 2007; and the present data), using the same right hemisphere electrodes as in our original analysis. For the performance metric, we took the d-prime value for the cue-auditory condition, averaged for uni- and bi-sensory targets.
For the ASD group the correlation was significant at r = 0.56, p = 0.01, whereas for the TD group it was not (r = −0.25, p = 0.29)(FIG 6A). For completeness we performed the same analyses for the corresponding left hemisphere electrodes, which revealed no significant relationships between the two measures (ASD: r = 0.32, p = 0.17; TD: r = −0.21, p = 0.38). When the above analyses were performed using only d-prime values from bisensory cue auditory trials the same pattern of relationships were obtained.
Fig 6.
(A) Scatter plots depict the relationship between alpha power modulation (cue auditory minus cue visual) and behavioral performance (d-prime) on the auditory task. Solid lines represent the least squares fit of the data. (B) Scatter plots depict the relationship of average alpha power on auditory and visual trials for each participant in the ASD group (left) and the TD group (right). Solid lines delineate equality between conditions.
As suggested by a reviewer, we additionally explored the correlation between VIQ and task-related alpha power modulation, as well as the relationship between VIQ and behavioral performance. The two participant groups, while matched for age, sex, and PIQ, nevertheless had different mean VIQ scores (ASD: M = 107.50, SD = 13.26; TD: M = 119.15, SD = 13.70).
The alpha modulation index demonstrated a significant positive relationship to VIQ among the ASD participants, over the right hemisphere (r = 0.45, p = 0.05). This relationship was not statistically significant over the left hemisphere for the ASD participants (r = 0.03, p > 0.8), nor was it for either hemisphere in the TD participants (Left: r = −0.2, p > 0.4; Right: r = −0.1 p > 0.5). Performance on the visual task was positively correlated with VIQ in the TD participants (r = 0.5, p = 0.02), but not the ASD participants (r = 0.4, p > 0.09). VIQ was not significantly related to d-prime on the auditory task in TD participants (r = −0.01, p > 0.8) or in ASD participants, although this exhibited a trend toward significance (r = 0.4, p = 0.08).
DISCUSSION
Recent evidence points to impaired inhibition of irrelevant sensory information in autism. Here we tested a key mechanism by which the processing of irrelevant sensory information is thought to be suppressed, task-dependent modulation of oscillatory power in the alpha band. Whereas the TD group showed alpha modulation as would be predicted based on highly replicated findings in adults, in the ASD group there was no evidence at the group level for task-based modulation of preparatory alpha power. The behavioral data were well aligned with these neurophysiological findings. That is, task irrelevant sensory information interfered with performance in the ASD but not the TD group. Specifically, the ASD group showed significant reductions in target detection for the bisensory versus unisensory S2 stimuli, and was slower to respond to visual targets that were accompanied by irrelevant auditory information. In contrast, TD group performance was not significantly affected by the extraneous sensory information. These behavioral data suggest a higher degree of interference in ASD participants within contexts involving distracting information in task-irrelevant modalities. Together these findings point toward reduced suppression of task-irrelevant distracting information in ASD, and altered functioning of neural oscillatory mechanisms employed in top-down selective attention.
Previous findings on the integrity of alpha oscillatory activity in ASD
Previous investigations examining alpha band activity in individuals with an ASD have employed either resting-state paradigms, in which the participant sits inactive while EEG is recorded (Chan and Leung 2006; Murias, Webb et al. 2007; Coben, Clarke et al. 2008; Mathewson, Jetha et al. 2012) or recorded during passive visual stimulation (Isler, Martien et al. 2010; Milne 2011). Findings regarding alpha power over posterior parieto-occipital areas in ASD individuals relative to controls are highly ambiguous, and often contradictory. Alpha power at rest has been reported to be greater (Chan and Leung 2006), reduced (Murias, Webb et al. 2007), and no different (Coben, Clarke et al. 2008). Mathewson, Jetha et al. (2012) proposed that a degree of variability in the findings may be due to whether the participants were at rest with their eyes open or closed. This is of particular interest as it has been known since the early EEG recordings by Berger (Berger 1929) that alpha power is greater over posterior scalp when the eyes are closed and that it reduces substantially when the eyes are opened. Mathewson, Jetha et al. (2012) reported that alpha power was similar between groups during an eyes-closed resting condition, but ASD individuals exhibited greater alpha power in an eyes-open resting condition. This was interpreted as greater alpha modulation as a function of cue condition in the TD group relative to the ASD group, similar to what we observe in the current findings. Investigation of alpha oscillatory activity during visual stimulation has suggested reduced desynchronization during periods of stimulation in ASD children compared to TD controls (Isler, Martien et al. 2010), although without a pre-stimulus measurement of alpha power it remains unclear whether this was a reflection of differential modulation of alpha power with visual stimulation or an overall increase in alpha power in the ASD group. Further, it has also been reported that inter-trial phase locking in ASD adolescents is reduced relative to controls (Milne 2011). Thus, there is some evidence in the literature of decreased alpha modulation and increased variability of phase in the alpha band with visual stimulation.
Correlations between task-dependent deployment of alpha and performance in ASD
In our data, exploratory analysis revealed that greater task-related modulation of alpha power predicted better performance on the auditory selective attention task in the ASD group. It is important to note however that the ASD group exhibited a unique pattern of task-modulated alpha power in which half of the participants had either no alpha modulation or showed alpha modulation in the opposite of the predicted direction (greater alpha on cue-visual than cue-auditory trials; FIG 6B). Significantly, it is the participants who had this opposite pattern of modulation who performed worst on the auditory task. These alpha 'misfires' likely help power the relationship found in the ASD group. The specific relationship between performance on the auditory task and right hemisphere alpha in the ASD participants suggests that when these mechanisms are effectively deployed, they engage right-hemisphere biased posterior top-down attentional control mechanisms. A right hemisphere bias for posterior attentional processes is a highly replicated finding in the literature (Mesulam, 1981; Corbetta et al., 1993; Szczepanski et al., 2010), and alpha modulation on selective attention tasks has been shown to parallel this right hemisphere bias (Fu et al., 2001; Gomez-Ramirez et al., 2007; Banerjee et al., 2011). In contrast, the TD group did not reveal a significant relationship between alpha modulation and performance. This may be considered surprising in the face of a number of reports showing alpha power modulation to be predictive of performance on visual spatial selective-attention tasks (Thut, Nietzel et al. 2006; Yamagishi, Callan et al. 2008; Kelly, Gomez-Ramirez et al. 2009), a detection task (Hanslmayr, Aslan et al. 2007), and a sustained attention task (Dockree, Kelly et al. 2007). As noted earlier, we were constrained in our approach to investigating the relationship between alpha modulation and behavior. A likely explanation for the failure to observe a significant relationship in the TD group is that without the negative alpha values that were present in the ASD group we were simply underpowered to observe such a relationship (see results section).
When we probed the relationship between verbal IQ (VIQ) and our dependent measures we found that in the ASD group task-based alpha modulation correlated with VIQ, whereas this was not the case in the TD group. As for the behavioral data, only the TD group demonstrated a significant relationship between VIQ and performance, and only for the visual task, but there were trends toward significant correlation between behavior-VIQ in the ASD group as well. Together these findings hint at a role for language in the effective deployment of cued attention. Indeed, the disruption of inner speech has been shown to affect performance on a cued attention task where the cue required a degree of decoding, such as in the present study (i.e., retrieving the association between a symbolic cue and the appropriate task)(Miyake et al., 2004), and inner speech has been hypothesized to be reduced in ASD participants (Williams and Jarrold, 2010; Lidstone et al., 2009; Wallace et al., 2009; Whitehouse et al., 2006). Alternately, the nature of the observed correlation between VIQ and alpha modulations in ASD could be mediational in nature insofar as ASD individuals with high verbal ability could use inner speech to compensate for dysfunction elsewhere in the cortical networks of executive function and selective attention. While these propositions are appealing in that they tie together the language dysfunction and attentional abnormalities observed in ASD individuals, these interpretations are nevertheless highly speculative, and a relationship between VIQ and cued attention is only modestly supported by our current post hoc analyses. Further work is clearly needed to adequately explore the complex relationship of language to neurophysiological and behavioral indices of selective attention, and the interplay of cue decoding, in both TD and ASD individuals.
Alpha oscillations, top-down attention, and the neural dysconnectivity hypothesis of ASD
A distributed network of top-down attention is theorized to direct alpha-band attentional mechanisms in sensory cortices (Klimesch, Sauseng et al. 2007; Foxe and Snyder 2011). This is necessarily subserved by long-range white matter tracts that allow for communication between dorso-lateral prefrontal cortex, the frontal eye fields, parietal cortex, and sensory specific areas. The current data as it pertains to ASD individuals could thus reflect inefficient communication between spatially separated regions of the dorsal network of top-down attention.
There is compelling multimodal evidence for disordered neural connectivity in ASD (Courchesne and Pierce 2005; Uhlhaas and Singer 2006; Murias, Webb et al. 2007; Casanova and Trippe 2009; Lazarev, Pontes et al. 2010; Just, Keller et al. 2012). Some of the more consistent evidence comes from diffusion tensor imaging (DTI) studies, which have reported reduced integrity of several white matter tracts in this group (see Müller, Shih et al. 2011 for review). Importantly, differences in white matter integrity do not appear to reflect a global reduction in ASD individuals but rather evidence is emerging in support of a pattern of sparing of certain tracts (and even increased integrity in some tracts relative to controls, for instance see Thomas, Humphreys et al. 2011) and reduced integrity of others.
Among the investigated tracts, the superior longitudinal fasciculus (SLF) has been associated with reduced indices of integrity (Sahyoun, Belliveau et al. 2010; Shukla, Keehn et al. 2011). The SLF is the primary candidate tract for top-down attentional signals originating in the frontal cortices and traveling to the posterior parietal cortex. Damage to the SLF produces visual neglect (Doricchi and Tomaiuolo 2003) and direct electrical stimulation of this tract in the right hemisphere results in a profound rightward bias on a line bisection task (Thiebaut de Schotten, Urbanski et al. 2005). Our findings indicate reduced modulation of preparatory alpha power during top-down selective attention. Given the compelling case for long range dysconnectivity in ASD, this dysfunction may well indicate reduced long-range communication between cortical regions that play an interactive role in top-down selective attention.
A recent functional imaging study from Ohta, Yamada et al. (2012) lends support to both dysconnectivity among brain regions in ASD as well as reduced suppression of irrelevant sensory information. In a visual spatial-selective attention fMRI design, as previously mentioned, these authors found that suppression of distracting information in visual cortex was reduced in adult ASD participants, and that while functional connectivity between the intraparietal sulcus (IPS) and visual cortices increased with the demands of the task (and thus presumably the need to suppress the unattended stimuli) in the TD participants, it did not in the ASD participants. Reduced top-down suppression of task-irrelevant information via connectivity between the parietal lobe and visual cortices may thus be central to deficiencies of selective attention in ASD.
Evidence for typical task switching in ASD
In addition to investigating alpha suppressive mechanisms in ASD, our design was also sensitive to whether intersensory switching was compromised in ASD, as would be suggested by clinical observations as well as by some experimental findings (Courchesne, Townsend et al. 1994; Reed and McCarthy 2012). Contrary to what one might predict based upon the literature, there was not an increase in the cost of switching tasks in ASD under the current conditions. Both diagnostic groups exhibited similar, albeit delimited, switch costs (i.e., performance decrements following task switches compared to repetitions of the same task). Specifically, d-prime values were poorer for trials in which participants switched to the visual task after previously performing the auditory task as compared to repeating the visual task. This switch cost was only present for the unisensory target stimuli (i.e., a visual stimulus alone with no auditory distractors). Thus within the visual modality there was an advantage to repeating the task on unisensory trials, but this advantage was lost on bisensory trials. It is thus possible that the presence of distracting stimuli in the unattended modality offset the behavioral benefit conferred by a repetition of the task. To summarize, a rather specific switch cost was observed in our measure of detection, and this did not differ between the ASD and TD groups.
These findings add to a body of research regarding task switching in ASD (see Geurts, Corbett et al. 2009 for review). The rigid and repetitive behaviors often observed in ASD individuals have led to the reasonable proposition that cognitive mechanisms associated with task switching are impaired in this group. As yet, there is no consensus on the severity (or presence) of task switching deficits in ASD. One study that was similar to the present did identify such deficits (Courchesne, Townsend et al. 1994). In this study participants switched between a visual and an auditory task, both of which required the detection of a rare oddball stimulus. ASD participants demonstrated poorer accuracy relative to controls when a target occurred between 400 and 2500 ms after a task switch, but their performance was very similar to the TD group at latencies beyond this, suggesting a switching deficit in the ASD group only at short preparatory intervals. Importantly, the detection of a target in the attended modality served as the cue to switch attention to the alternate modality. In the present study, on every trial, a visual cue explicitly cued one of the two attention conditions, and this onset 1250 ms prior to the arrival of the to-be-attended stimuli. This might be considered a more overt and effective cue than the one used by Courchesne, Townsend et al. (1994).
The current findings indicate that ASD individuals are able to switch between simple auditory and visual tasks in a manner much like that of their neurotypical counterparts. This combined with null findings from several other studies gives grounds for caution surrounding assertions of a global deficit in task switching in ASD individuals (Pascualvaca, Fantie et al. 1998; Poljac, Simon et al. 2010; Stoet and Lopez 2011; de Vries and Geurts 2012).
Conclusions
While we are presented with many instances in which the integration of information from multiple sensory modalities confers greater insight into our environment (e.g., face-to-face conversation in a noisy conference hall)(Ross, Saint-Amour et al. 2007; Ross, Molholm et al. 2011), there are other instances in which sensory information from one modality can interfere with performance of a task requiring sensory input from another modality. Here we find evidence that mechanisms of selective attention are not as effectively instantiated in ASD as they are in TD. Namely, the typical modulation of preparatory alpha band activity, which is associated with the suppression of the processing of task-irrelevant sensory information, was not observed in the ASD group. Further, behavioral data revealed that task-irrelevant sensory inputs interfered with performance in the ASD but not the TD group, indicating that "irrelevant" information is not typically dampened in ASD. This finding provides a potential explanation for the delimiting of the environment that is commonly observed in ASD.
ACKNOWLEDGMENTS
We thank Frantzy Acluche and Sarah Ruberman for their tremendous help with data collection. Foremost, we are exceedingly appreciative of the time and effort of the individuals who participated in this study and their families.
FUNDING
Primary funding for this work was provided by a grant from the U.S. National Institute of Mental Health (MH085322 to S.M. and J.J.F). The Human Clinical Phenotyping Core, where the children enrolled in this study were clinically evaluated, is a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (IDDRC) which is funded through a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593).
Footnotes
Participants were run on one of two “identical” testing rooms. After data collection it became apparent that the interaction of the stimulus presentation software with the operating system installed on one of the systems resulted in a delay of the onset of the auditory S2 stimulus by 50 ms, as verified by a two channel oscilloscope (Tektronix TDS2012C, Beaverton, Oregon). In total, of the 20 participants in each diagnostic group, 11 ASD and 9 TD individuals were run on the experimental setup with the 50 ms delayed S2 auditory stimuli (see Figure 1). Diagnostic groups were similarly represented in each testing booth and there were no significant differences in participant characteristics as a function of ‘Booth’. We performed analyses to determine how 'Booth' might impact any of the dependent measures (RT, d-prime, and alpha power). We analyzed the data in precisely the same manner as reported below, but used Booth as the grouping variable in place of Diagnosis. Neither main effects of Booth or interactions with Booth approached significance for any of these analyses, suggesting that the small timing difference did not significantly influence any of the results reported below.
References
- Adams NC, Jarrold C. Inhibition in autism: children with autism have difficulty inhibiting irrelevant distractors but not prepotent responses. J Autism Dev Disord. 2012;42(6):1052–1063. doi: 10.1007/s10803-011-1345-3. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, et al. Oscillatory Alpha-Band Mechanisms and the Deployment of Spatial Attention to Anticipated Auditory and Visual Target Locations: Supramodal or Sensory-Specific Control Mechanisms? Journal of Neuroscience. 2011;31(27):9923–9932. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics. 2001:1165–1188. [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. European Archives of Psychiatry and Clinical Neuroscience. 1929;87(1):527–570. [Google Scholar]
- Bollimunta A, Mo J, et al. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31(13):4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack JA. Selective attention deficits in persons with autism: preliminary evidence of an inefficient attentional lens. J Abnorm Psychol. 1994;103(3):535–543. doi: 10.1037//0021-843x.103.3.535. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, et al. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76(4):838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, et al. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29(18):5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1522):1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Leung WWM. Differentiating autistic children with quantitative encephalography: A 3-month longitudinal study. J Child Neurol. 2006;21(5):391–399. doi: 10.1177/08830738060210050501. [DOI] [PubMed] [Google Scholar]
- Christ SE, Holt DD, et al. Inhibitory control in children with autism spectrum disorder. J Autism Dev Disord. 2007;37(6):1155–1165. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- Christ SE, Kester LE, et al. Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology. 2011;25(6):690–701. doi: 10.1037/a0024256. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Courchesne E, et al. Effects of focused selective attention tasks on event-related potentials in autistic and normal individuals. Electroencephalogr Clin Neurophysiol. 1990;75(3):207–220. doi: 10.1016/0013-4694(90)90174-i. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE, et al. Event-related potentials in cross-modal divided attention in autism. Neuropsychologia. 1995;33(2):225–246. doi: 10.1016/0028-3932(94)00094-6. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, et al. EEG power and coherence in autistic spectrum disorder. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2008;119(5):1002. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, et al. A PET study of visuospatial attention. The Journal of Neuroscience. 1993;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current opinion in neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, et al. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994;108(5):848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- de Vries M, Geurts HM. Cognitive Flexibility in ASD; Task Switching with Emotional Faces. J Autism Dev Disord. 2012:1–11. doi: 10.1007/s10803-012-1512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dockree PM, Kelly SP, et al. Optimal sustained attention is linked to the spectral content of background EEG activity: greater ongoing tonic alpha (similar to 10 Hz) power supports successful phasic goal activation. European Journal of Neuroscience. 2007;25(3):900–907. doi: 10.1111/j.1460-9568.2007.05324.x. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14(17):2239–2243. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. [Google Scholar]
- Foxe JJ, Simpson GV, et al. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9(17):3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fu KMG, Foxe JJ, et al. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Cognitive Brain Research. 2001;12(1):145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, et al. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Higgins BA, et al. The deployment of intersensory selective attention: a high-density electrical mapping study of the effects of theanine. Clinical neuropharmacology. 2007;30(1):25. doi: 10.1097/01.WNF.0000240940.13876.17. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Kelly SP, et al. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. The Journal of Neuroscience. 2011;31(50):18556–18567. doi: 10.1523/JNEUROSCI.2164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Kelly SP, et al. The effects of L-theanine on alpha-band oscillatory brain activity during a visuo-spatial attention task. Brain Topogr. 2009;22(1):44–51. doi: 10.1007/s10548-008-0068-z. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Hanslmayr S, Aslan A, et al. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37(4):1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Iarocci G, Burack JA. Intact covert orienting to peripheral cues among children with autism. J Autism Dev Disord. 2004;34(3):257–264. doi: 10.1023/b:jadd.0000029548.84041.69. [DOI] [PubMed] [Google Scholar]
- Isler J, Martien K, et al. Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clinical Neurophysiology. 2010;121(12):2035–2043. doi: 10.1016/j.clinph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, et al. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity.”. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, et al. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. European Journal of Neuroscience. 2009;30(11):2224–2234. doi: 10.1111/j.1460-9568.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, et al. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95(6):3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, et al. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lazarev V, Pontes A, et al. Interhemispheric asymmetry in EEG photic driving coherence in childhood autism. Clinical Neurophysiology. 2010;121(2):145–152. doi: 10.1016/j.clinph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Lidstone JS, Fernyhough C, et al. Brief report: Inner speech impairment in children with autism is associated with greater nonverbal than verbal skills. Journal of autism and developmental disorders. 2009;39(8):1222–1225. doi: 10.1007/s10803-009-0731-6. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, et al. Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, et al. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lovaas OI, Schreibman L, et al. Selective responding by autistic children to multiple sensory input. J Abnorm Psychol. 1971;77(3):211–222. doi: 10.1037/h0031015. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Jetha MK, et al. Regional EEG alpha power, coherence, and behavioral symptomatology in autism spectrum disorder. Clinical Neurophysiology. 2012 doi: 10.1016/j.clinph.2012.02.061. [DOI] [PubMed] [Google Scholar]
- Mesulam M. A cortical network for directed attention and unilateral neglect. Annals of neurology. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front Psychol. 2011:2. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, et al. Inner speech as a retrieval aid for task goals: The effects of cue type and articulatory suppression in the random task cuing paradigm. Acta psychologica. 2004;115(2):123–142. doi: 10.1016/j.actpsy.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Müller RA, Shih P, et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, et al. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yamada T, et al. An fMRI study of reduced perceptual load-dependent modulation of task-irrelevant activity in adults with autism spectrum conditions. Neuroimage. 2012;61(4):1176–1187. doi: 10.1016/j.neuroimage.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Pascualvaca DM, Fantie BD, et al. Attentional capacities in children with autism: Is there a general deficit in shifting focus? J Autism Dev Disord. 1998;28(6):467–478. doi: 10.1023/a:1026091809650. [DOI] [PubMed] [Google Scholar]
- Poljac E, Simon S, et al. Impaired task switching performance in children with dyslexia but not in children with autism. The Quarterly Journal of Experimental Psychology. 2010;63(2):401–416. doi: 10.1080/17470210902990803. [DOI] [PubMed] [Google Scholar]
- Reed P, McCarthy J. Cross-modal attention-switching is impaired in autism spectrum disorders. J Autism Dev Disord. 2012;42(6):947–953. doi: 10.1007/s10803-011-1324-8. [DOI] [PubMed] [Google Scholar]
- Remington AM, Swettenham JG, et al. Lightening the load: perceptual load impairs visual detection in typical adults but not in autism. J Abnorm Psychol. 2012;121(2):544–551. doi: 10.1037/a0027670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, et al. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25(2):603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia bulletin. 2008;34(5):907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Gross J, et al. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30(25):8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Molholm S, et al. The development of multisensory speech perception continues into the late childhood years. European Journal of Neuroscience. 2011;33(12):2329–2337. doi: 10.1111/j.1460-9568.2011.07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Saint-Amour D, et al. Do you see what I am saying? Exploring visual enhancement of speech comprehension in noisy environments. Cerebral Cortex. 2007;17(5):1147–1153. doi: 10.1093/cercor/bhl024. [DOI] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, et al. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain and cognition. 2010;73(3):180. doi: 10.1016/j.bandc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, et al. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory Attentional Suppression of Visual Features Indexed by Oscillatory Alpha-Band Power Increases: A High-Density Electrical Mapping Study. Journal of Neuroscience. 2010;30(11):4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoet G, Lopez B. Task-switching abilities in children with autism spectrum disorder. European Journal of Developmental Psychology. 2011;8(2):244–260. [Google Scholar]
- Szczepanski SM, Konen CS, et al. Mechanisms of spatial attention control in frontal and parietal cortex. The Journal of Neuroscience. 2010;30(1):148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teder-Salejarvi WA, Pierce KL, et al. Auditory spatial localization and attention deficits in autistic adults. Brain Res Cogn Brain Res. 2005;23(2–3):221–234. doi: 10.1016/j.cogbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309(5744):2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, et al. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex. 2011;47(7):863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, et al. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26(37):9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E. Parietal damage and narrow “spotlight” spatial attention. Journal of Cognitive Neuroscience. 1994;6(3):220–232. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. J Autism Dev Disord. 1993;23(1):1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Silvers JA, et al. Brief report: Further evidence for inner speech deficits in autism spectrum disorders. Journal of autism and developmental disorders. 2009;39(12):1735–1739. doi: 10.1007/s10803-009-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weschler D. Wechsler intelligence scale for children. Fourth edition. Bloomington, MN: Pearson, Inc.; 2003. [Google Scholar]
- Weschler D. Wechsler abbreviated scale of intelligence. Second edition. San Antonio, TX: The Psychological Corporation; 2011. [Google Scholar]
- Wetherill GB, Levitt H. Sequential Estimation of Points on a Psychometric Function. Br J Math Stat Psychol. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Maybery MT, et al. Inner speech impairments in autism. Journal of Child Psychology and Psychiatry. 2006;47(8):857–865. doi: 10.1111/j.1469-7610.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- Williams DM, Jarrold C. Brief report: Predicting inner speech use amongst children with autism spectrum disorder (ASD): The roles of verbal ability and cognitive profile. Journal of autism and developmental disorders. 2010;40(7):907–913. doi: 10.1007/s10803-010-0936-8. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, et al. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. Journal of Neuroscience. 2000;20(6) doi: 10.1523/JNEUROSCI.20-06-j0002.2000. RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, et al. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]