Abstract
The rodent cerebellum is richly supplied with PACAPergic innervation. Exogenous pituitary adenylate cyclase-activating polypeptide (PACAP) increases cerebellar granule cell survival and differentiation in culture, and enhances the number of neuroblasts in the molecular and internal granule cell layers (IGL) when injected postnatally into the cerebellum in vivo. Here, we have investigated the role of endogenous PACAP during cerebellar development by comparing the morphology of normal and PACAP-deficient mouse cerebellum, and the response of cerebellar granule cells from normal and PACAP-deficient mice subjected to neurotoxic insult in culture. There was no difference in cerebellar volume or granule cell number, in 11-day-old wild type versus PACAP-deficient mice. Cultured cerebellar neurons from PACAP-deficient and wild type mice also showed no apparent differences in survival and differentiation either under depolarizing conditions, or non-depolarizing conditions in the presence or absence of either dibutyryl cAMP or 100 nM PACAP. However, cultured cerebellar neurons from PACAP-deficient mice were significantly more sensitive than wild type neurons to ethanol- or hydrogen peroxide-induced toxicity. Differential ethanol toxicity was reversed by addition of 100 nM exogenous PACAP, suggesting that endogenous PACAP has neuroprotective activity in the context of cellular insult or stress. The neuroprotective action of PACAP was mimicked by dibutryl cAMP, indicating that it occurred via activation of adenylate cyclase. These results indicate that PACAP might act to protect the brain from paraphysiological insult, including exposure to toxins or hypoxia.
Keywords: Knock-out, Granule neurons, Development
1. Introduction
The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors are highly expressed in the rodent cerebellum during post-natal development [2,3,14,17]. PACAP receptors are abundant on neurons within the external granule cell layer (EGL) between post-natal days 4 (P4) and P20, a period of intense neurogenesis [1]. When cerebellar granule cells are cultured under conditions that promote apoptosis, treatment with PACAP significantly retards cell death [4,6,20,26]. PACAP also counteracts the neurotoxic effect of ethanol or oxidative stress in cultured granule cells, by blocking caspase-3 activation [24,25].
During the first 2 weeks of rodent life, the cerebellar cortex undergoes intense development and maturation. In particular, immature neurons generated in the EGL migrate along radial processes of glial cells through the molecular layer to reach their final destination within the internal granule cell layer (IGL) [19]. In vivo administration of PACAP at the surface of the cerebellum of 8-day-old rats increased cerebellar cortical volume [21], mainly through an increase in the number of granule cells in the IGL. All these effects of exogenous PACAP and the presence of PACAP and its receptors in the developing cerebellum, point towards a role of PACAP either as a trophic factor or neuroprotectant during post-natal cerebellar granule cell development. Accordingly we examined both normal cerebellar granule cell development, and granule cell survival after oxidative stress, in wild type and in PACAP-deficient mouse cerebellum, and in granule cell cultures derived from each.
2. Materials and methods
2.1. Animals
Transgenic animals used in this study were wild type and PACAP knock-out C57BL/6 mice generated as previously reported [10]. PACAP-deficient mice were obtained from mixed strain C57BL/6 × 129/SvJ mice backcrossed onto the C57BL/6 background for seven generations. Pups from matched homozygous wild type and PACAP-deficient breeding pairs were used for cultured cell experiments, and pups from within litters generated from heterozygous breeding pairs were used for in situ detailed morphological comparisons. Genotyping was performed by PCR of tail DNA as previously described [10]. All experimental procedures were approved by the National Institute of Mental Health Intramural Research Program's Animal Care and Use Committee.
2.2. Morphometric studies
Eleven-day-old mice were killed by decapitation. Frozen cerebella were cut in the frontal plane into 10-μm thick serial sections. One section every 100 μm was stained with Cresyl violet and the areas of the cerebellar cortex and medulla were measured by means of a computer-assisted image analysis station BIO500 (Biocom, Les Ulis, France). The volumes of the cortex and medulla were calculated by integrating the areas of each structure.
The thickness of the cerebellar cortical layers, and the density of granule and Purkinje cells within each layer, were measured in the simplex lobule [21] using a confocal laser scanning microscope (Noran Instruments, Middleton, WI, USA). Quantification was systematically performed at the level of the caudal extremity of the colliculi and the rostral extremity of lobule 1 as previously described [21]. Morphological analysis was carried out using the Intervision software (Noran).
2.3. Autoradiographic analysis of PACAP binding sites
The 27-amino-acid form of PACAP ([125I]-PACAP27; Phoenix Pharmaceuticals Inc., Belmont, CA, USA) was radiolabeled by means of the lactoperoxidase technique. The radioligand was purified by reversed phase high pressure liquid chromatography on a Vydac C-18 column (25 cm × 0.46 cm, Sigma, St. Louis, MO, USA) using a gradient of acetonitrile/water containing trifluoroacetic acid (0.1%, pH 2.4). Radioiodinated PACAP27 eluted at 36% acetonitrile and the specific radioactivity of the radioligand was approximately 800 Ci/mmol.
After slicing on a cryostat, unfixed cerebellar tissue sections were preincubated for 30 min at 20 °C in 50 mM Tris–buffer (pH 7.4) containing 1% bovine serum albumin (BSA), 32 mM sucrose, 5 mM MgCl2 and 0.5 μg/ml bacitracin. Sections were incubated with [125I]-PACAP27 (70 pM) for 1 h at 20 °C in the same buffer supplemented with 2% BSA. For competition studies, the sections were incubated in the presence of various concentrations of unlabeled PACAP38 or vasoactive intestinal polypeptide (VIP) (10−10 to 10−6 M). The slices were then washed three times for 5-min periods at 4 °C in 50 mM Tris–buffer containing 0.1% BSA, 5 mM MgCl2 and 0.5 μg/ml bacitracin. Finally, the sections were dried under a cold air stream and apposed onto Amersham 3H-Hyperfilms (Amersham, Orsay, France) for 5 days. After exposure for autoradiography, the slices were stained by Cresyl violet for histological examination. Autoradiograms were quantified by means of a computer-assisted image analysis system (Samba Technologies, Meylan, France). The PACAP binding sites were measured in the external granule cell layer of the cerebella and quantified with Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA).
2.4. Cell culture
Granule cell suspensions were prepared from cerebella of 6-day-old mice. Dispersed cells were seeded in multiwell plates (Costar, Cambridge, MA, USA) coated with poly-l-lysine at a density of 5 × 105 cells per well. The cells were cultured in a medium consisting of 75% Dulbecco's Eagle's medium and 25% Ham's F12 supplemented with 10% fetal bovine serum, 25 mM potassium and 1% of an antibiotic–antimycotic solution (10,000 U/ml penicillin, 10 mg/ml streptomycin, 25 μg/ml fungizone, Biowhittaker, Wakersville, MD, USA). After 3–4 days of culture, the cells were shifted to a chemically defined medium containing 5 or 25 mM potassium chloride, where serum was replaced with 2 mM glutamine, 1 mM sodium pyruvate and 1% N-2 supplement (×100; Life Technologies, Grand Island, NY, USA). Cells were grown at 37 °C in a humidified incubator in an atmosphere of 5% CO2/95% air.
2.5. Cell survival
After serum removal, cells were treated with ethanol (0–400 mM) or hydrogen peroxide (0–4000 nM) and cultured for another 12 h in the absence or presence of PACAP38 (10−7 M; Phoenix Pharmaceuticals Inc., Belmont, CA, USA). Twelve hours after serum removal, sister cultures that had not been exposed to neurotoxic substances were also treated with PACAP38 (10−10 to 10−6 M), potassium (25 mM) or dbcAMP (10−3 M) and cultured for an additional 24 h. For quantification of surviving neurons, cells were incubated for 8 min with 15 μg/ml fluorescein diacetate (Sigma), rinsed with phosphate-buffered saline and lysed with a Tris–HCl solution. Fluorescence intensity was measured with a Victor2 microplate reader (Perkin-Elmer, Boston, MA, USA). Pilot experiments have shown that the fluorescence intensity is proportional to the cell number (in the range 5 × 104 to 1 × 106 cells/ml).
2.6. Quantitative RT-PCR
For measurement of PACAP expression, total RNA from cultured cerebellar granule cells plated at 700,000/ml, 2 ml per well, in six-well culture plates, was purified as previously reported [10] 6 h after the initiation of the treatment. Real-time quantitative PCR was performed on cDNA from 0.5 μg RNA (Taq-Man 7700 sequence detection system, Applied Biosystems, Foster City, CA, USA) by using 90 nM primers and 150 nM probe. RNA levels were corrected for variations in amount of input mRNA with GAPDH cDNA signal. GAPDH primers and probes were from Applied Biosystems. PACAP primers and probe used were as follows: mPACAPf (forward primer), CTTCACAGATAGCTACAGCCGC; mPACAPr (reverse primer), GGCAGCTGATCTGCTACAAGTATG; mPACAPp (probe), 6FAM-CGCCAAGTATTTCTTGACAGCCATT TGTTTT-TAMRA. 6FAM represents 6-carboxyfluorescein and TAMRA represents N,N,N′,N′-tetramethyl-6-carboxyrhodamine.
Cerebella from post-natal days 6 (P6) and 12 (P12) wild type and PACAP-deficient pups were also harvested for quantitative PCR. Pups were anesthetized by CO2 inhalation for 10–15 s followed by surgical pneumothorax. The cerebellum was removed and separated from the brainstem for PCR preparation as above. Complementary DNA was prepared from 1 μg total RNA. Q-PCR was performed using SYBR Green quantification with 100 nM primers using the BioRad iCycler (Hercules, California, USA). PACAP primers and probes were as previously reported [10]. VIP primers and probes were mVIPf1, GCCCTGCCTGAAGGAAACA; mVIPr1, CTGAGAGAACAGCACACTGAAGAGT; and mVIPp1, 6FAM-ATGGAAGCCAGAAGCAAGCCTCAGTTC-TAMRA. GAPDH primers from IDT (Coralville, IA, USA) were used with Bio-Rad SYBR Green Supermix (BioRad Laboratories, Hercules, CA) mastermix; mGAPDHf, GGCCTTCCGTGTTCCTACC; mGAPDHr, CCTGCTTCACCACCGTCTTGA.
2.7. Statistical analysis
Statistical analyzes were conducted by two-way ANOVA, followed by Bonferroni post-tests using the computer software PRISM (GraphPad Software, San Diego, CA, USA).
3. Results
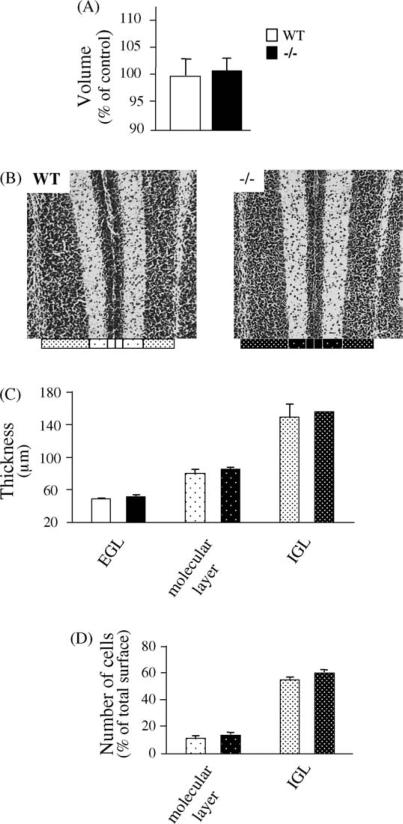
Central nervous system morphology was grossly unperturbed by PACAP deficiency. In particular, whole cerebellum weights were not different in wild type compared to PACAP-deficient mice, and were (in milligrams) 21.67 ± 0.88 and 28.00 ± 0.57 for wild type mice at P6 and P12, compared to 21.67 ± 1.20 and 29.00 ± 0.57 for PACAP-deficient mice at P6 and P12 (n = 3, S.E.M.; no significant differences at corresponding ages). Cerebellar volume and cortical thickness was measured in P11 wild type and PACAP-deficient mouse pups. Cerebellar volume was unaffected by the absence of PACAP at this time point during neonatal development (Fig. 1A). At a higher level of magnification, measurements of the thickness of the EGL, molecular layer and IGL confirmed that the lack of PACAP does not change the development of the cerebellar cortex (Fig. 1B and C) and does not modify the number of granule cells in either the molecular layer or the IGL (Fig. 1D).
Fig. 1.
Comparison of PACAP wild type versus PACAP-deficient cerebellar development of 11-day-old mice. (A) Quantification of the volume of the cerebellar cortex, as percentage of control (wild type); (B) illustration of the EGL, molecular layer and IGL structure at the level of the Sim lobule of 11-day-old (P11) mice; (C) quantification of the thickness of the EGL, molecular layer and IGL from the Sim of P11 mice; (D) quantification of the density of granule cells in the molecular layer and IGL at the level of the Sim of P11 mice. Each value represents the mean ± S.E.M. of the quantification of seven animals.
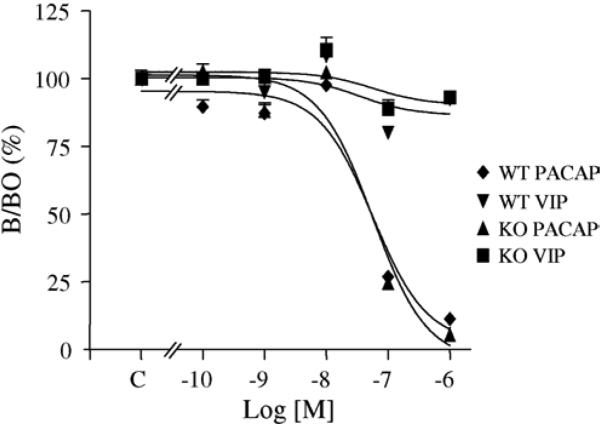
At P11, a high expression level of PACAP binding sites was detected in the EGL and medulla. The density of PACAP binding sites was similar in both wild type and PACAP-deficient animals as measured by total [125I]-PACAP27 binding. The effect of graded concentrations of PACAP38 or VIP (10−10 to 10−6 M) on binding of [125I]-PACAP27 on P11 mice cerebellar slices are illustrated in Fig. 2. There was a dose-dependent inhibition of [125I]-PACAP27 binding by PACAP38 in EGL of both wild type and PACAP-deficient animals. Competition curves revealed an IC50 of 5.6 × 10−8 M and 5.7 × 10−8 M in wild type and PACAP-deficient animals, respectively. Autoradiographic labelling was totally abolished in the presence of 10−6 M PACAP38 and only slightly affected with the same concentration of VIP (Fig. 2).
Fig. 2.
Displacement of [125I]-PACAP27 binding sites by synthetic PACAP38 and VIP on 11-day-old mice cerebellar tissue slices. Competition curves were established after quantification of autoradiograms in the external granule cell layer of both wild type and knock-out animals.
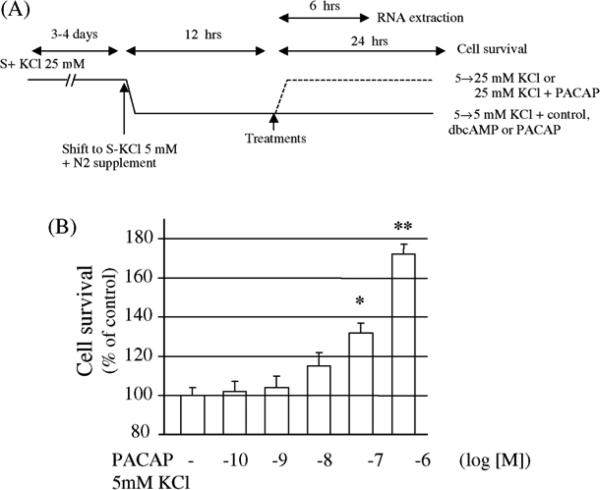
Elevated extracellular potassium, dibutyryl cAMP, and PACAP itself promoted granule cell survival in cultures from both wild type and PACAP-deficient animals (Table 1) according to the protocol illustrated in Fig. 3A. Dibutyryl cAMP (1 mM) and potassium (25 mM), previously shown to enhance cultured rat granule cell survival, exerted neurotrophic activity on cultured mouse cerebellar neurons, which was equivalent in wild type and PACAP-deficient cultures. In control conditions, neuronal differentiation and cell survival from both wild type and PACAP-deficient animals were identical. As previously reported in rat [4,6] and mouse [20], treatment with graded concentrations of PACAP38 induced a dose-related increase of granule cell survival (Fig. 3B). The amplitude of the effect of PACAP was identical for both wild type and PACAP-deficient granule cell cultures. For all subsequent experiments, the lower concentration of PACAP38 that gave a significant increase in cell survival (10−7 M) was used. It is noteworthy that the Kd for PAC1 receptor binding is higher (above 10 nM) than efficacy in stimulating cAMP (generally 0.5 nM or so) (see T. Mustafa and L. Eiden, Handbook of Neurochemistry, vol. 9, in press). EC50 values for neuroprotection by PACAP38 for cerebellar granule cell survival in normal medium, or to reverse cell death upon exposure to ethanol are close to those for receptor binding, in both rat and mouse [20,25]. Since neuroprotection is clearly cAMP-dependent in this system [22], we favor the explanation that the EC50 for cyclic AMP generation in a 15–30 min experiment may be different than that required for prolonged cAMP generation and neuronal survival.
Table 1.
Effect of neurotrophic factors on cultured cerebellar granule cells from wild type and PACAP-deficient mice
| Treatment | Wild type | PACAP-deficient |
|---|---|---|
| PACAP | 140 ± 7** | 131 ± 6# |
| Potassium | 197 ± 12** | 169 ± 10### |
| dbcAMP | 129 ± 4** | 119 ± 4# |
After culturing the cells for 3–4 days with serum and 25 mM potassium, serum was removed and potassium was shifted to 5 mM. After 12 h of culture in serum free medium and 5 mM potassium, cells were treated with control medium (serum free and 5 mM potassium), PACAP (10–7M), potassium (25 mM) or dbcAMP (10–3 M). Cell survival was measured 24 h after treatment and reported as percentage ofcontrol. Each value represents the mean (±S.E.M.) of three independent experiments performed in quadruplicate.
P < 0.01 vs. wild type control.
P < 0.05 vs. PACAP-deficient control.
P < 0.001 vs. PACAP-deficient control.
Fig. 3.
Effect of PACAP on cerebellar granule cell survival. (A) Diagram of the protocol used for the culture and treatment with KCl, dbcAMP, and/or PACAP in the absence of toxic agents, S+ (with serum), S (without serum) (Tables 1 and 2); (B) effect of graded concentrations of−PACAP on granule cell survival from wild type animals. Each value represents the mean (±S.E.M.) of three independent experiments performed in quadruplicate. *P < 0.05 vs. wild type control; **P < 0.01 vs. wild type control.
PACAP mRNA was expressed in granule cells cultured from wild type but not from PACAP-deficient mice (Table 2). After 6 h of treatment the expression of PACAP mRNA was increased 2-fold by PACAP itself, and 2.8-fold by raising the potassium concentration from 5 to 25 mM. When both PACAP and 25 mM potassium were added to the cells, no additive effect was observed.
Table 2.
Quantification of PACAP mRNA expression in cultured cerebellar granule cells treated with elevated potassium or PACAP
| Treatment | ng PACAP mRNA/sample |
|---|---|
| K5 | 1.01 ± 0.29 |
| K5 + PACAP | 2.41 ± 0.44* |
| K25 | 3.56 ± 0.72* |
| K25 + PACAP | 3.47 ± 0.88* |
Values for PACAP mRNA are expressed as ng per sample (1,400,000 cells) using a VIP cDNA standard curve as measured by quantitative RT-PCR assay. K5, potassium (5 mM); K25, potassium (25 mM); PACAP (10–7 M); RT–, negative control for reverse transcription is subtracted for each set of values and represented 0.41 ± 0.22 ng/sample for the wild type and ±0.001 ng/sample for PACAP-deficient cultures. Water (no input sample) controls routinely represented a value of ±0.001 ng PACAP/sample in all cases and was not subtracted from the values above. Corresponding values for neuronal cultures from PACAP-deficient mice treated, harvested, and assayed for PACAP mRNA exactly as for wild type samples all yielded values ±0.1 ng/sample. Each value represents the mean (±S.E.M.) of three independent experiments performed in triplicate.
P <0.05 vs. wild type K5.
Whole cerebella were removed free of brainstem from P6 and P12 WT and PACAP-deficient pups. As shown in Table 3, PACAP mRNA was found in P6 WT mouse cerebella and declined by P12. PACAP levels in cerebella of P12 pups were found to be about 16-fold less (4 CTs) than those seen in the adult mouse hypothalamus [10]. Furthermore, VIP mRNA levels, low in cerebellum of the mouse, did not increase in PACAP-deficient mice compared to controls at P6 or P12 (data not shown).
Table 3.
Quantification of relative PACAP mRNA expression in mouse cerebellum at post-natal days 6 and 12
| Post-natal day | CT PACAP | CT GAPDH | % adult HT |
|---|---|---|---|
| 6 | 29.23 ± 0.29 | 20.42 ± 0.03 | 11.66 |
| 12 | 29.54 ± 0.05 | 19.83 ± 0.04 | 6.25 |
PACAP mRNA was measured in total cerebellum from post-natal days 6 and 12 wild type and PACAP-deficient mice as described in Section 2. The threshold cycle number (CT) ± S.E.M. for PACAP and GAPDH transcripts was measured (n = 3), and used to calculate the concentration of PACAP mRNA in developing cerebellum as a function of its concentration in a PACAP-rich mouse reference tissue, the hypothalamus (HT).
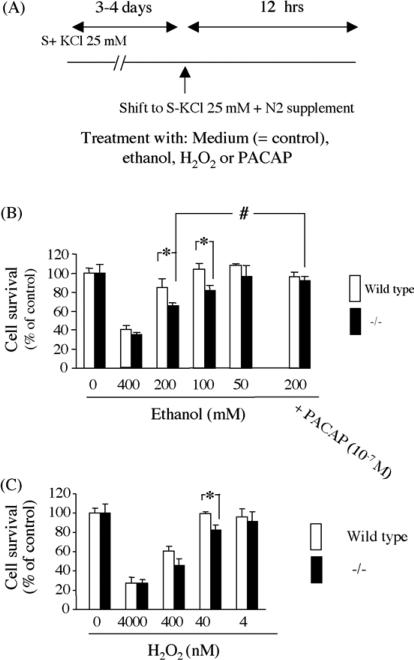
Incubation of granule cells with graded concentrations of ethanol, according to the protocol shown in Fig. 4A, resulted in a dose-dependent cell death with cells from PACAP-deficient pups more sensitive to 100 and 200 mM ethanol toxicity than those from wild type animals (Fig. 4B). Similarly, incubation of granule cells with hydrogen peroxide revealed a dose-dependent cell death with a higher toxicity in the presence of 40 nM H2O2 for cells cultured from PACAP-deficient pups (Fig. 4C). The neurotoxic effect of ethanol on both wild type and PACAP-deficient granule cell cultures could be blocked by addition of exogenous PACAP38 (10−7 M; Fig. 4B).
Fig. 4.
Survival of cerebellar granule cells cultured with neurotoxic agents. (A) Diagram of the protocol used for the culture and treatment with ethanol, H2O2 or PACAP; (B) effect of graded concentrations of ethanol on granule neurons from wild type and PACAP−/− animals; (C) effect of graded concentrations of H2O2 on granule neurons from wild type and PACAP−/− animals. Each value represents the mean (±S.E.M.) of three independent experiments performed in quadruplicate. *P< 0.05 vs. wild type; #P < 0.05 vs. no PACAP.
4. Discussion
PACAP has been reported to exert neurotrophic activities on cultured granule neurons or when injected in vivo to P8 rats [21,23] and PACAP-deficient animals exhibit a locomotor phenotype suggestive of changes in brain architecture [11]. These observations led to the hypothesis that PACAP should affect brain development. However, we did not observe any morphological aberrations in cerebellar cortex of PACAP knock-out mice, compared to normal littermates, suggesting that the developmental effects of PACAP leading to a distinct behavioral phenotype must occur in brain regions other than cerebellum, or lead to subtler synaptic effects rather than gross changes in cerebellar granule cell number. The lack of modifications of cerebellar cortex architecture in PACAP-deficient animals is consistent with similar observations made in PAC1 knock-out mice [12].
Since PACAP deficiency had no effect on cerebellar development in vivo, we have examined the ability of PACAP to promote mouse cerebellar granule cell survival in vitro. The amplitude of the neuroprotective activity of PACAP observed in the present study is consistent with the effect previously observed with mouse and rat granule cells [6,20]. As cultured granule cells from wild type mice express PACAP [20], we investigated possible differences between wild type and PACAP-deficient cells in culture. In particular, cells were oxidatively stressed with either ethanol or hydrogen peroxide. We have previously demonstrated that alcohol-induced hippocampal granule cell neurodegeneration was ameliorated by antioxidant treatment [9], and that PACAP exerts neuroprotective effects against ethanol in rat cerebellar granule cells in culture [25].
The fact that cultured cerebellar granule cells from wild type animals are more resistant to neurotoxic insult by both ethanol and hydrogen peroxide than PACAP-deficient neurons is consistent with the hypotheses that ethanol-induced neurotoxicity proceeds largely through an oxidative mechanism [9] and that endogenous PACAP released into the culture medium can reduce apoptotic cell death, as has been reported to occur via a caspase-3-related mechanism in rat cerebellar granule cells [23]. Concerning the mechanisms involved, it has been shown that ethanol and PACAP exert an opposite effect on the delayed outward rectifier potassium current which in turn modulate caspase-3 activity that finally contributes to the control of granule cell survival [15]. The antiapoptotic effect of PACAP after oxidative stress may also play a major role in the protection of the brain during several forms of neuronal insult due to a decrease of blood flow and/or oxidative damage [18].
The ability of PACAP to stimulate its own gene expression could at least in part explain why a short term exposure of granule cells to PACAP is sufficient to get its full neuro-protective effect on cultured neurons [22]. An additive effect of PACAP treatment with elevated extracellular potassium has already been reported on cerebellar granule cell survival [6]. An increase in PACAP expression induced by potassium depolarization may explain a greater neurotrophic effect of potassium in cells from wild type animals that express PACAP, compared to cells cultured from PACAP-deficient mice.
Under normal conditions, PACAP-deficient animals do not exhibit morphologically obvious neurodevelopmental abnormalities. In fact, most of the phenotypes reported so far in PACAP- or PAC1-deficient mice have been observed under paraphysiological conditions, such as metabolic stress after insulin injection [10,11] thermoregulatory stress [7] and septic shock following LPS injection [13]. These observations suggest that PACAP mainly acts as an emergency response factor to reinforce and sustain normal homeostatic mechanisms during prolonged periods of challenge to them.
It is possible, that PACAP does play a significant role in normal cerebellar development that is masked, in PACAP-deficient mice, by compensatory alterations in VIP, or the VIP/PACAP receptors VPAC1 and VPAC2. However, binding experiments revealed that the level of expression of the PAC1 or the VPAC receptors was not different between wild type and PACAP-deficient mice. Although binding experiments revealed that PAC1 receptors are predominant, the VPAC1 receptor has been shown to be slightly expressed by granule neurons in vitro [16]. While it is possible that VIP, which increases granule cell survival in mice [5] and exhibits neurotrophic activities during development [8], may at least in part compensate for the lack of PACAP, this seems unlikely here, since we were unable to detect any compensatory increase in VIP mRNA in cerebella of PACAP-deficient, compared to wild type mice. The fact that PACAP-deficient neurons are more sensitive to neurotoxic insult suggests that PACAP mainly acts as an emergency response peptide supporting neuronal survival under sustained pathophysiological conditions, which might suggest some useful applications for PACAP-like ligands in these conditions.
Abbreviations
- EGL
external granule cell layer
- IGL
internal granule cell layer
- P4
post-natal day 4
- PACAP
pituitary adenylate cyclase-activating polypeptide
- VIP
vasoactive intestinal polypeptide
References
- 1.Altman J. Postnatal development of the cerebellar cortex in the rat 3. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 3.Basille M, Gonzalez BJ, Leroux P, Jeandel L, Fournier A, Vaudry H. Localization and characterization of PACAP receptors in the rat cerebellum during development: evidence for a stimulatory effect of PACAP on immature cerebellar granule cells. Neuroscience. 1993;57:329–38. doi: 10.1016/0306-4522(93)90066-o. [DOI] [PubMed] [Google Scholar]
- 4.Cavallaro S, Copani A, D'Agata V, Musco S, Petralia S, Ventra C, et al. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol Pharmacol. 1996;50:60–6. [PubMed] [Google Scholar]
- 5.Fukuchi M, Sakuragawa S, Tabuchi A, Tsuda M. Calcium signal-mediated expression of the vasoactive intestinal polypeptide gene and its small contribution to activity-dependent survival of mouse cerebellar granule cells. J Neurosci Res. 2004;77:26–34. doi: 10.1002/jnr.20132. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–30. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- 7.Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–54. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- 8.Gressens P, Hill JM, Paindaveine B, Gozes I, Fridkin M, Brenneman DE. Severe microcephaly induced by blockade of vasoactive intestinal peptide function in the primitive neuroepithelium of the mouse. J Clin Invest. 1994;94:2020–7. doi: 10.1172/JCI117555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamelink C, Hampson A, Wink D, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005:314. doi: 10.1124/jpet.105.085779. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee H-W, et al. Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–6. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci USA. 2001;98:13355–60. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–15. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99:1053–8. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuo Y, Tokito F, Matsumoto Y, Shimamoto N, Fujino M. Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) and its binding sites in the rat brain. Neurosci Lett. 1994;170:43–6. doi: 10.1016/0304-3940(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 15.Mei YA, Vaudry D, Basille M, Castel H, Fournier A, Vaudry H, et al. PACAP inhibits delayed rectifier potassium current via a cAMP/PKA transduction pathway: evidence for the involvement of Ik in the anti-apoptotic action of PACAP. Eur J Neurosci. 2004;19:1446–58. doi: 10.1111/j.1460-9568.2004.03227.x. [DOI] [PubMed] [Google Scholar]
- 16.Nicot A, Lelievre V, Tam J, Waschek JA, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. J Neurosci. 2002;22:9244–54. doi: 10.1523/JNEUROSCI.22-21-09244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen HS, Hannibal J, Fahrenkrug J. Expression of pituitary adeny-late cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. NeuroReport. 1998;9:2639–42. doi: 10.1097/00001756-199808030-00039. [DOI] [PubMed] [Google Scholar]
- 18.Reglodi D, Somogyvari-Vigh A, Vigh J, Li M, Lengvari I, Arimura A. Pituitary adenylate cyclase activating polypeptide is highly abundant in the nervous system of anoxia-tolerant turtle, Pseudemys scripta elegans. Peptides. 2001;22:873–8. doi: 10.1016/s0196-9781(01)00412-0. [DOI] [PubMed] [Google Scholar]
- 19.Ryder EF, Cepko CL. Migration patterns of clonally related granule cells and their progenitors in the developing chick cerebellum. Neuron. 1994;12:1011–28. doi: 10.1016/0896-6273(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 20.Tabuchi A, Koizumi M, Nakatsubo J, Yaguchi T, Tsuda M. Involvement of endogenous PACAP expression in the activity-dependent survival of mouse cerebellar granule cells. Neurosci Res. 2001;39:85–93. doi: 10.1016/s0168-0102(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 21.Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H. Neurotrophic activity of pituitary adenylate cyclase-activating polypep-tide on rat cerebellar cortex during development. Proc Natl Acad Sci USA. 1999;96:9415–20. doi: 10.1073/pnas.96.16.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide stimulates both c-Fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998;84:801–12. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- 23.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 24.Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, et al. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002;15:1451–60. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- 25.Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci USA. 2002;99:6398–403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villalaba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]