SUMMARY
1. We investigate here for the first time in primate brain the combinatorial expression of the three major functionally relevant proteins for catecholaminergic neurotrans-mission tyrosine hydroxylase (TH), aromatic acid acid decarboxylase (AADC), and the brain-specific isoform of the vesicular monoamine transporter, VMAT2, using highly specific antibodies and immunofluorescence with confocal microscopy to visualize combinatorial expression of these proteins.
2. In addition to classical TH, AADC, and VMAT2-copositive catecholaminergic neurons, two unique kinds of TH-positive neurons were identified based on co-expression of AADC and VMAT2.
3. TH and AADC co-positive, but VMAT2-negative neurons, are termed “nonexocytotic catecholaminergic TH neurons.” These were found in striatum, olfactory bulb, cerebral cortex, area postrema, nucleus tractus solitarius, and in the dorsal motor nucleus of the vagus.
4. TH-positive neurons expressing neither AADC nor VMAT2 are termed “dopaergic TH neurons.” We identified these neurons in supraoptic, paraventricular and periventricular hypothalamic nuclei, thalamic paraventicular nucleus, habenula, parabrachial nucleus, cerebral cortex and spinal cord. We were unable to identify any dopaergic (TH-positive, AADC-negative) neurons that expressed VMAT2, suggesting that regulatory mechanisms exist for shutting off VMAT2 expression in neurons that fail to biosynthesize its substrates.
5. In several cases, the corresponding TH phenotypes were identified in the adult rat, suggesting that this rodent is an appropriate experimental model for further investigation of these TH-positive neuronal cell groups in the adult central nervous system. Thus, no examples of TH and VMAT2 co-positive neurons lacking AADC expression were found in rodent adult nervous system.
6. In conclusion, the adult mammalian nervous system contains in addition to classical catecholaminergic neurons, cells that can synthesize dopamine, but cannot transport and store it in synaptic vesicles, and neurons that can synthesize only L-dopa and lack VMAT2 expression. The presence of these additional populations of TH-positive neurons in the adult primate CNS has implications for functional catecholamine neurotransmission, its derangement in disease and drug abuse, and its rescue by gene therapeutic maneuvers in neurodegenerative diseases such as Parkinson's disease.
Keywords: aromatic amino acid decarboxylase/dopa decarboxylase, CNS neuronal phenotypes, dopaergic, dopaminergic, tyrosine hydroxylase, vesicular monoamine transporter
INTRODUCTION
Tyrosine hydroxylase (TH) is a marker for dopamine, norepinephrine, and epinephrine-containing (catecholamine) neurons and endocrine cells. It is also expressed transiently, in development, in neurons and neuroendocrine cells that in adulthood no longer express TH or express it only at very low levels. Neurons that express TH transiently in the developing rat sympathetic nervous system include presumptive neuroblasts of the enteric nervous system of the gut (Cochard et al., 1978), cholinergic sympathetic projections to sweat glands (Landis et al., 1988), sympathetic innervation of the periosteum (Asmus et al., 2000), and intrinsic adrenergic cardiac cells in the fetal heart (Huang et al., 2005). Sensory and parasym-pathetic ganglia also express TH immunoreactivity during development (Jonakait et al., 1985) and in some cases into adulthood (Landis et al., 1987).
Two recent trends have prompted a closer examination of the presumptive monoaminergic phenotype during development and in the adult mammalian nervous system. First, it is now recognized that persistence of expression of monoamine-related gene products into adulthood may differ dramatically among mammalian species. TH is present in adult mouse sympathetic sudomotor innervation, for instance, but is absent in the rat (Rao et al., 1994). Second, the monoaminergic phenotype is increasingly viewed as a collection of individual monoaminergic traits that are not necessarily co-expressed in a concerted fashion in all neuronal subpopulations according to the classical understanding of monoaminergic neuro-transmission. Habecker et al. have noted that while TH expression is maintained in mouse sympathetic cholinergic sudomotor neurons into adulthood, there is a developmentally regulated down-regulation of GTP cyclohydrolase (GTPCH) expression, required for synthesis of the TH co-factor tetrahydrobiopterin in these neurons (Habecker et al., 2002). GTPCH may not be rate-limiting for catecholamine biosyn-thesis, especially in view of the wide range of its expression, from robust to immunohistochemically nondetectable, in neurons that nevertheless all synthesize and accumulate catecholamines (Hirayama and Kapatos, 1998). However, mouse sudo-motor neurons also fail to express the vesicular monoamine transporter VMAT2, and thus cannot package into secretory vesicles any TH-dependent catecholamines that might be synthesized in these neurons (Weihe et al., 2005). In contrast, adult primate, including human, sudomotor neurons express in addition to cholinergic markers a full complement of noradrenergic markers (TH, aromatic amino acid decarboxylase or AADC, dopamine ß-hydroxylase or DßH, and VMAT2), and thus are likely to be fully functionally noradrenergic, as well as cholinergic (Weihe et al., 2005). These observations may explain the primate-specific phenomenon of “nora-drenergic sweating” (Mack et al., 1986). A full complement of functional markers for cholinergic and noradrenergic transmission also occurs in vasomotor neurons of arteriovenous anastomoses in human skin (Weihe et al., 2005). The occurrence of the dual transmitter phenotype in sudomotor and neighboring vasomotor neurons in tandem may allow fine tuning of neurotransmitter control of thermoregulation in human skin.
While TH expression seems to be transient in intrinsic neurons of the developing rodent gastrointestinal (GI) tract, the adult human upper GI tract is endowed with a major intrinsic VMAT2 expressing dopaminergic innervation (Anlauf et al., 2003). In addition, neurons expressing TH, AADC, DßH, and VMAT2 are a unique novel feature of a major subpopulation of intrinsic parasympathetic cholinergic neurons of primate heart. In rodents, these neurons are not fully noradrenergic since, although they express TH, they lack VMAT2 expression (Weihe et al., 2005).
The findings listed above have deepened understanding of monoaminergic trait expression, and its role in regulation of chemical coding of neurotransmission in the peripheral and central nervous systems. In general, the combinatorial expression of biogenic amine neurotransmitter traits, rather than obligate en bloc expression, offers a complex set of possibilities for regulating the functional neurotransmitter properties of the neurons that express them. Nonclassical patterns of expression of monoaminergic traits may in particular be quite widespread in the brain, with expression patterns dictated by both species and developmental stage. Interest in them is fueled by the preponderance of monoaminergic systems as targets for psychoactive therapeutic drugs, and drugs of abuse. Almost three decades ago, the mapping of TH and AADC in the brain led to identification of olfactory bulb neurons referred to as dopaminergic because they expressed TH and AADC and lacked DßH (Halasz et al., 1977). Misu and colleagues have suggested, based on expression of TH, but not AADC, the existence in brain cardiovascular regulatory nuclei of neurons that elaborate dopa, but not dopamine, and hypothesized that these areas, including the nucleus tractus solitarius (NTS) and area postrema (AP), might function in interneuronal “dopaergic” signaling (Misu et al., 2002; Misu et al., 2003). Ugrumov and colleagues have identified in developing rodent hypothalamus, TH-positive, AADC-negative “monoenzymatic” neurons that function to supply L-dopa to TH-negative, AADC-positive cells capable of synthesizing dopamine from L-dopa, and apparently, of packaging it for exocytotic, regulated secretion (Balan et al., 2000; Ugrumov et al., 2002; Ugrumov et al., 2004). Mapping of monoamine plasma membrane and vesicular transporters in rodents during development has identified VMAT2- (Lebrand et al., 1998; Schütz et al., 1998a,b) and plasma membrane serotonin transporter (SERT)-expressing thalamocortical projection neurons (Lebrand et al., 1996). These are hypothesized to function by scavenging extracellular monoamines including serotonin for regulated secretion by neurons otherwise incapable of synthesizing serotonin on their own (Lebrand et al., 1996).
The purpose of the present study was to extend those observations, limited mainly to the developing, peripheral, or rodent nervous systems, into the adult primate central nervous system, and to include the co-expression of VMAT2. Despite reports in the literature indicating that TH-positive, AADC-negative neurons exist in CNS as do TH-negative, AADC-positive (nonserotonergic, nonhistaminergic) neurons (Komori et al., 1991; Kitahama et al., 1996; Ugrumov, 1997; Ikemoto et al., 1998a,b; Kitahama et al., 1998; Balan et al., 2000; Ershov et al., 2002a,b; Misu et al., 2002; Ugrumov et al., 2002; Misu et al., 2003; Ugrumov et al., 2004; Ershov et al., 2005), their functional status has not been fully assessed because the presence or absence of VMAT2 in these neurons was unknown.
Here, we asked first, what is the distribution of TH in the adult primate central nervous system in addition to the classical catecholaminergic pathways described almost a half-century ago and second, are there additional potential chemical phenotypes featuring TH expression in which chemical coding is not classically noradrenergic or dopaminergic. It is, we hope, an appropriate tribute to Julie Axelrod's leadership in the field of catecholaminergic chemical coding (Axelrod, 1986) that continued unbiased application of sensitive and specific assays for catecholamine biosynthetic enzymes (Saavedra et al., 1974), in this case immunohistochemical, has yielded a novel “postclassical” view of neurotransmission in the mammalian CNS. We suggest based on the results presented herein that there are two additional major types of TH-positive neurons in the adult central nervous system besides dopaminergic and noradrenergic neurons. One population is capable of dopamine synthesis but lacks a vesicular uptake system for its concentration and exocytotic release. A second is capable of L-dopa synthesis only, lacking both AADC for dopamine synthesis and VMAT for its vesicular uptake. The first (nonexocytotic dopaminergic) population may accumulate cytoplasmic dopamine and release it constitutively. The second (dopaergic) population may function, as postulated in the immature nervous system, to supply L-dopa to TH nonexpressing, AADC-expressing neurons capable of then synthesizing dopamine and perhaps releasing it via nonclassical secretory mechanisms.
MATERIALS AND METHODS
Tissue Processing for Immunocytochemistry
Rhesus monkey brain tissues were obtained from buffered saline- followed by buffered 4% formalin-perfused animals (three males and three females), and were immersed postfixed in Bouin Hollande. Experiments involving the use of rhesus macaques were approved by the Animal Care and Use Committee of Bioqual, Inc., an NIH-approved and Association for Assessment and Accreditation of Laboratory Animal Care-accredited research facility. All experiments were carried out using the ethical guidelines promulgated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rat and mouse brain tissues were obtained from animals which were perfused with buffered saline followed by perfusion with Bouin Hollande (Weihe et al., 2005) in accordance with the German Law for animal protection and approved by the Regierungspäsidium Giessen. All tissues were processed for double immunofluorescence confocal microscopy on deparaffinized sections. Adjacent sections (5–7 μm thick) were cut and deparaffinized. Antigen retrieval to increase the sensitivity of immunodetection was performed by heating the sections at 92–95°C for 15 min in 0.01 M citrate buffer (pH 6) according to the DAKO protocol (Hamburg, Germany). Nonspecific binding sites were blocked with 5% bovine serum albumin (BSA, Serva, Heidelberg, Germany) in PBS (pH 7.45) followed by an avidin-biotin blocking step (Avidin-biotin Blocking Kit, Boehringer Ingelheim, Germany).
Antibodies
Primary antibodies included rodent and primate-specific rabbit polyclonal antisera against the vesicular monoamine transporter types 1 and 2 (VMAT1, VMAT2) that recognize rat and primate VMAT2 (Weihe et al., 1994; Erickson et al., 1996; Weihe et al., 1998), mouse VMAT1 and VMAT2 (Weihe and Eiden, 2000) and rat, mouse, or human VMAT1 (Erickson et al., 1996; Weihe et al., 2005). These antibodies are described and referenced in detail in a previous publication (Weihe et al., 2005). Well-characterized commercially available rabbit and sheep polyclonal antibodies against tyrosine hydroxylase (TH) (Chemicon, Temecula, CA), aromatic amino acid decarboxylase (AADC) also referred to as dopa-decarboxylase (DDC) (Chemicon), and dopamine ß-hydroxylase (DßH) (Protos Biotech, New York) were also used as previously described (Weihe et al., 2005). Details about controls for the specificity of immunoreactions in central nervous system tissue (Saper and Sawchenko, 2003) for the antisera employed in this study have been summarized in detail in a recent publication from our laboratory (Weihe et al., 2005).
Confocal Double-Immunofluorescence Microscopy
Appropriate combinations of two primary antibodies raised in different donor species were coapplied in appropriate dilutions in 1% BSA/50 mM PBS at pH 7.4 and incubation was carried out overnight at 16°C followed by 2 h at 37°C as described (Weihe et al., 2005). After extensive washing with 1%BSA/PBS over 1 h, immunoreactions were visualized by appropriate species-specific secondary antibodies labeled with Alexa Fluor® 488, or Alexa Fluor® 647 (both from MoBiTec, Göttingen, Germany; dilution 1:200 in 1%BSA/PBS), and by species-specific biotinylated secondary antisera (Dianova, Hamburg, Germany; diluted 1:200 in 1%BSA/PBS) followed by streptavidin conjugated with Alexa Fluor® 488 or Alexa Fluor® 647 (diluted 1:200 in PBS). Biotinylated or fluorochrome-labeled secondary antibodies and streptavidin conjugated with flurorochromes were applied for 2 h at 37°C. Sections were extensively washed in PBS followed by distilled water for 1 h before they were coverslipped with FluorSaveTM reagent (Calbiochem, Merck Biosciences, Schwalbach, Germany). Immunofluorescence staining was documented as digitized false-color images obtained with an Olympus BX50WI laser scanning microscope (Olympus Optical, Hamburg, Germany).
RESULTS
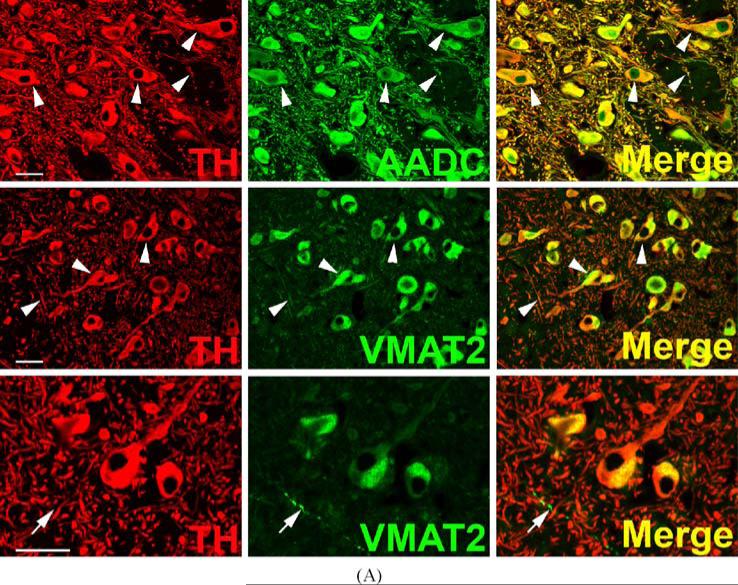
The classical staining pattern of dopaminergic (TH, AADC, and VMAT2-positive) and noradrenergic (TH, AADC, VMAT2, and DßH-positive) neurons of primate CNS is illustrated in Fig. 1. Staining allows complete visualization of the components of each phenotype, against which neurons deficient in the expression of any of these components can be compared. The intensities of cell body, dendrite, and nerve terminal staining for TH, AADC, DßH, and VMAT2 are quite equivalent in substantia nigra for dopaminergic (Fig. 1A) and locus coeruleus for noradrenergic (Fig. 1B) neurons. Although virtually all AADC and VMAT2-positive soma of substantia nigra are dopaminergic (i.e., also TH-positive), TH-negative, VMAT2-positive nerve terminals are observed in substantia nigra. These are more plentiful in the striatum, the target nucleus of the dopaminergic projection neurons of the nigra, and are most likely to be serotonergic. TH-negative neurons immunoreactive for AADC are observed in locus coeruleus. In the absence of triple-staining for these very rare neurons, it is as yet unclear whether AADC-positive, TH-negative neurons express VMAT2.
Fig. 1.
Coexpression of VMAT2 and catecholamine synthesizing enzymes in classical catecholaminergic cell groups of rhesus monkey brain. Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) Dopaminergic TH-positive neurons and processes of the substantia nigra pars compacta co-stain for AADC and VMAT2 (arrow heads). Note that VMAT2 staining in neuronal processes and fibers is comparatively weak and absent from some TH-positive fiber profiles. VMAT2-immunoreactivity appears granular and is asymmetrically concentrated in the perinuclear space while TH-positive staining comprises the entire cell bodies (lower panel). Note VMAT2-positive varicose fiber profiles lacking TH (arrows in lower panel). Bars are 30 μm. (B) Noradrenergic TH-positive neurons and processes of the locus coeruleus co-stain for AADC, DBH, and VMAT2 (arrow heads). Note two small AADC-positive cell profiles that lack TH (arrows). VMAT2-positive/TH-negative puncta are intermingled between TH-positive/VMAT2-positive neuronal cell bodies and processes (high magnification in lower panel). The VMAT2-positive/TH-negative puncta presumably represent serotonergic input. The upper three panels are adjacent sections showing the same blood vessel (asterisks). Note staining of portions or all of the region of the nucleus in some AADC-positive cells, which can be attributed to superimposition of positive staining in cytoplasm underlying or overlaying the nucleus in these 7–10 μm sections. Bars are 30 μm.
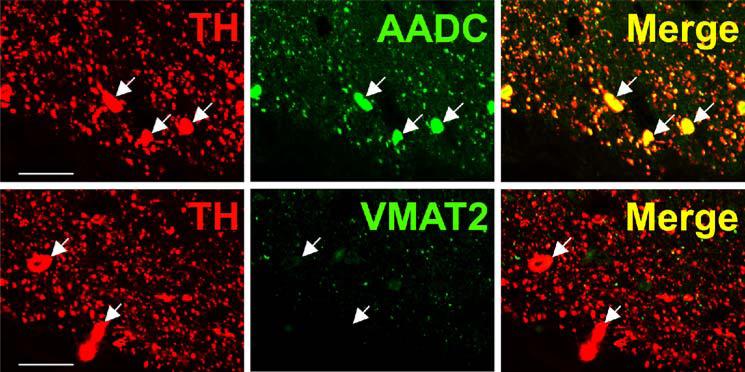
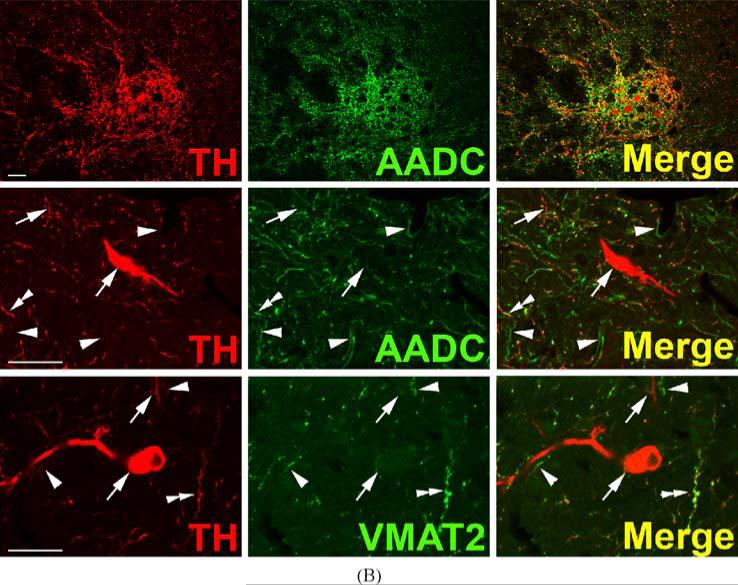
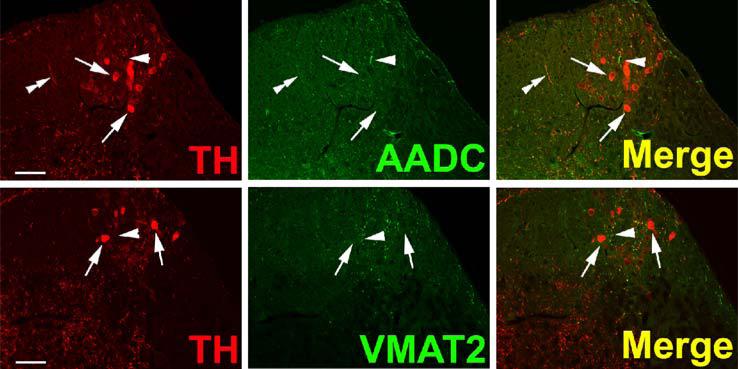
TH-positive, AADC-positive neurons of rhesus monkey olfactory bulb are depicted in Fig. 2. Sections are clearly and specifically positive for sparse VMAT2 nerve terminals, presumably originating from serotonergic, histaminergic, or non-classical TH-negative neurons, as well as occasional mast cells (not shown). However, neurons of the primate olfactory bulb itself are uniformly negative for VMAT2, illustrating the second category of TH-positive neurons in primate CNS (Table I), those which are apparently dopaminergic except for the absence of VMAT2. Staining of both cell bodies and abundant fibers for TH and AADC, but not VMAT2, can be seen in olfactory bulb. This same pattern of expression (TH and AADC-positive, VMAT2-negative) is seen in mouse and rat olfactory bulb (data not shown). TH-positive neurons of olfactory bulb are reported to be ablated along with peripheral (Guillemot et al., 1993) and central (Hirsch et al., 1998) noradrenergic neurons in MASH knock-out mice. This suggests the TH-expressing olfactory bulb neuronal population is more closely related, at least in terms of requirements for bHLH transactivating protein expression during development, to central nora-drenergic than to dopaminergic neurons.
Fig. 2.
Absence of VMAT2 expression from dopaminergic TH-positive neurons in rhesus monkey ol-factory bulb. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neuronal and cell fiber profiles co-express AADC, but lack VMAT2 (arrows). Note VMAT2-positive/TH-negative puncta which presumably represent serotonergic input. Bars are 30 μm.
Table I.
Types of TH-Positive CNS Neurons Based on Expression of TH, AADC, and VMAT2
| TH | AADC | VMAT2 | Examples |
|---|---|---|---|
| + | + | + | “classical catecholaminergic neurons” cell bodies in brain stem and hypothalamus |
| + | + | – | “nonexocytotic catecholaminergic neurons” striatum, olfactory bulb, cerebral cortex, area postrema, nucleus tractus solitarius, dorsal vagal motor nucleus |
| + | – | – | “dopaergic neurons” supraoptic and periventricular hypothalamus, paraventricular thalamic nucleus, habenula, parabrachial nucleus, spinal cord, cerebral cortex |
| + | – | + | none observed |
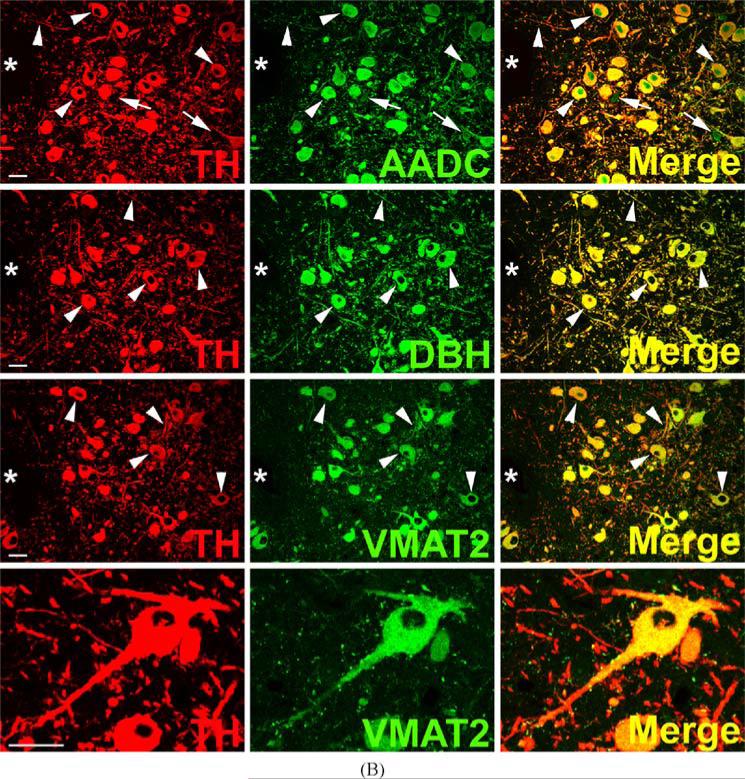
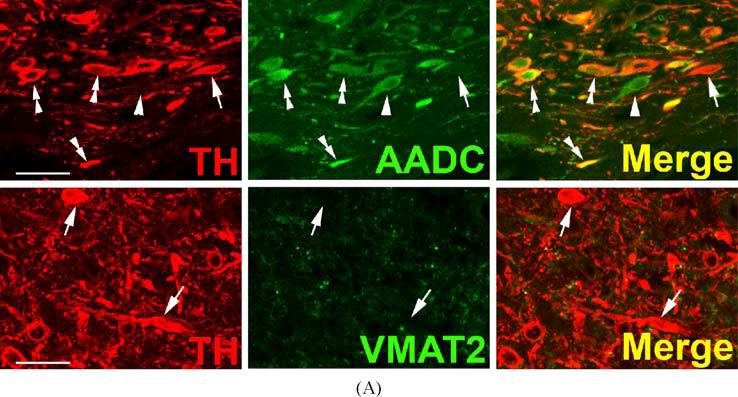
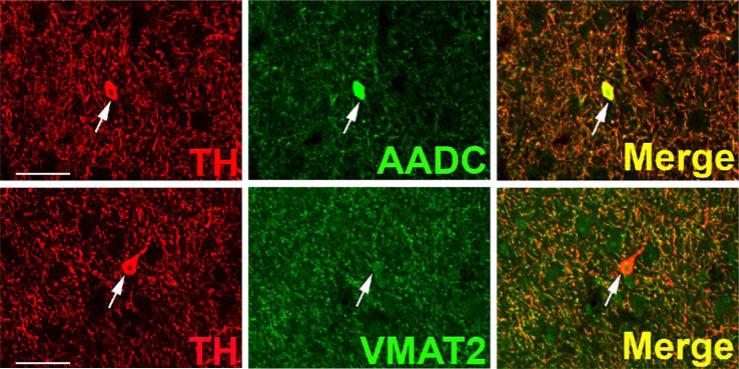
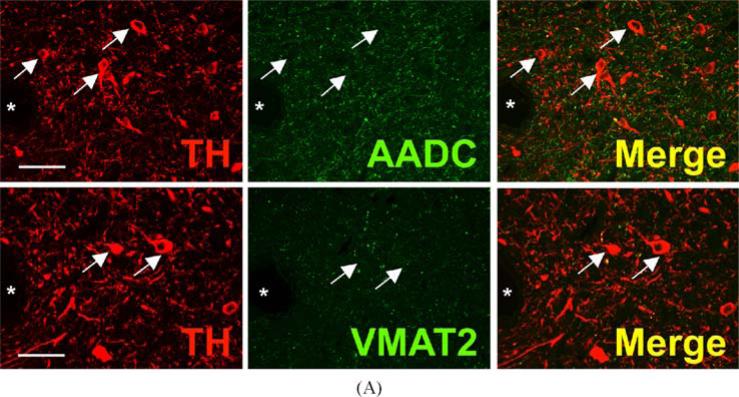
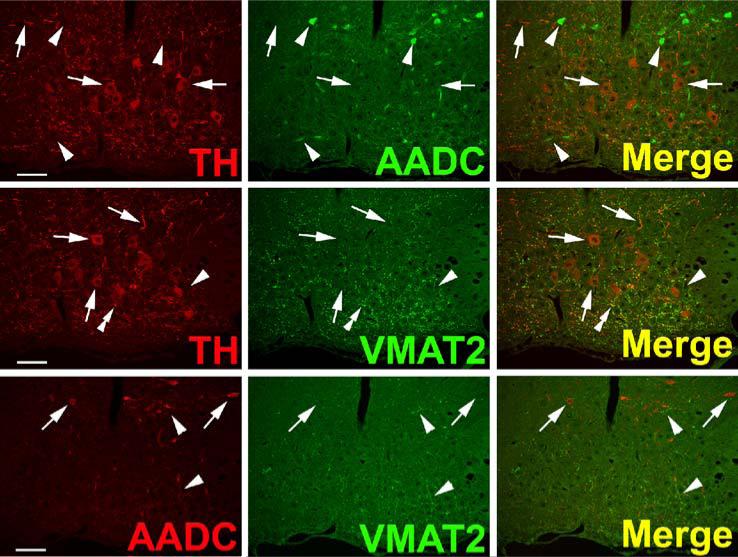
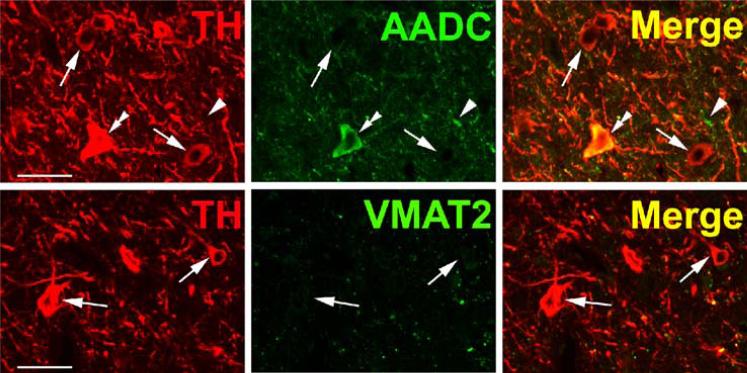
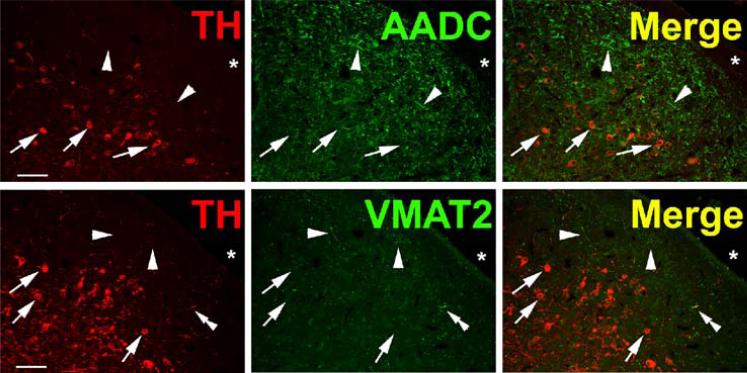
TH and AADC-positive neurons that do not express VMAT2 (“nonexocytotic catecholaminergic neurons”) are surprisingly abundant, and ubiquitous, in the adult primate central nervous system. In some cases, these neurons are contained within nuclei that are anatomically well defined, but highly heterogeneous with respect to their chemical neuroanatomy. Two examples are the area postrema (AP) and nucleus tractus solitarius (NTS) of the brain stem (Fig. 3). Both are important as integrative and relay nuclei in the central regulation of blood pressure (Andresen et al., 2001). Figure 3A depicts immunoreactive neurons and nerve terminals of the rhesus monkey AP stained for TH, AADC, and VMAT2. VMAT2 terminals innervating the AP are both TH-positive and TH-negative, and likely represent both peripheral noradrenergic and central serotonergic innervation of this nucleus. Interspersed TH and AADC co-positive and TH-positive, AADC-negative neurons of the AP are all devoid of VMAT2. Detailed analysis of the terminals of these soma will be required to determine their targets in the brain stem and possibly other projection areas. The NTS contains in addition to TH and AADC co-positive neurons lacking VMAT2, neurons without TH that express both AADC and VMAT2 and are likely serotonergic (Fig. 3B). TH-positive, AADC-positive, VMAT2-negative neurons are also seen in NTS of the rat (data not shown). Figure 4 depicts additional “nonexocytotic” TH and AADC co-positive neurons in the rhesus striatum. These neurons are scattered throughout the striatum, and are clearly VMAT2 negative, as seen against a background of VMAT2, TH co-positive fibers of the classical dopaminergic projection to striatum from the substantia nigra.
Fig. 3.
Heterogeneous phenotypes of presumed dopaminergic neurons in rhesus monkey area postrema (AP) and nucleus of the solitary tract (NTS). Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) The majority of TH-positive neurons of the AP coexpress AADC (double arrow heads in upper panel), but none of them coexpresses VMAT2 (arrows in lower panel). Note occurrence of both AADC-positive/TH-negative (arrow head) and TH-positive/AADC-negative (arrow) neurons (upper panel). VMAT2-positive/TH-positive and VMAT2-positive/TH-negative puncta are encountered. Bars are 30 μm. (B) TH-positive neurons in the NTS coexpress AADC (arrows in upper panel), but lack VMAT2 (arrows in lower panel). In addition, AADC-positive/TH-negative neurons (arrow heads in upper panel) and VMAT2-positive/TH-negative neurons and fibers (arrow heads in lower panel) are encountered. Upper and lower panels are from adjacent sections. Asterisks label the same blood vessel profile. As in Fig. 1, staining in the vicinity of the nucleus in some cells is attributable to overlaying or underlying positively stained cytoplasm for the soluble enzyme AADC. Bars are 100 μm.
Fig. 4.
Absence of VMAT2 from intrinsic TH-positive dopaminergic neurons of rhesus monkey striatum. Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. Adjacent sections show a TH-positive neuron costaining for AADC (upper panel), but lacking VMAT2 (lower panel) (arrows). Note that the vast majority of TH-positive dopaminergic input from the substantia nigra co-stains for AADC and VMAT2 (arrow heads). The AADC-positive/TH-negative and VMAT2-positive/TH-negative fibers/puncta are presumed to represent serotonergic input to the striatum (double arrow heads). Bars are 30 μm.
“Non-exocytotic”(minor) and “dopaergic”(major) TH-expressing neurons are also found scattered throughout primate (rhesus monkey) cortex, decreasing in frequency from frontal to occipital cortex (data not shown), and in the medial part of the dorsal motor nucleus of the vagus. TH-positive, AADC-positive neurons lacking VMAT2 extending from the lower medulla rostrally to the level of the area postrema are found (data not shown). While cortical “nonexocytotic” TH-positive neurons were not observed in the rodent, those of the dorsal motor nucleus of the vagus were seen in both rat and mouse (data not shown). Cortical “dopaergic” neurons were also found in rodent brain (data not shown).
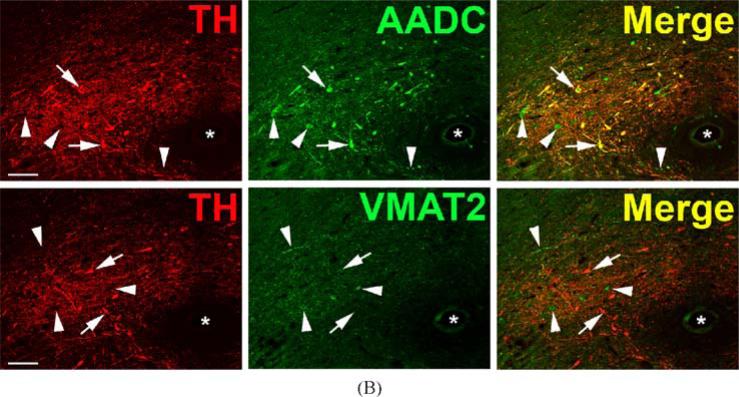
TH-positive “dopaergic” neurons (TH-positive, AADC-negative, VMAT2-negative) are found in several other locations in the rhesus monkey brain besides cortex (Fig. 5). In the lateral parabrachial nucleus these cells are seen within a neuropil that is both AADC and VMAT2 positive, possibly reflecting serotonergic input to this nucleus (Fig. 5A). Likewise, clusters of “dopaergic” TH-expressing neurons are seen in the intermediolateral cell column of the thoracic spinal cord (Fig. 5B) as well as in regions lateral and dorsolateral to the central canal (not shown). Note that “dopaergic” TH-positive neurons in both parabrachial nucleus and spinal cord are relatively small compared to soma of classical monoaminergic neurons and “nonexocytotic” TH-positive cells in the CNS. “Dopaergic” TH-positive neurons are also seen in rat CNS, in both habenula (Fig. 6) and supraoptic nucleus (Fig. 7). In hypothalamus, a population of AADC-positive neurons that lack TH was also observed (Fig. 7) as reported by Ugrumov and colleagues in the rat (Ugrumov 1997).
Fig. 5.
DOPAergic neurons in rhesus monkey lateral parabrachial nucleus and spinal cord. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. (A) TH-positive neurons in the lateral parabrachial nucleus are negative for AADC and VMAT2 (arrows). In addition to some TH-positive/AADC-positive and TH-positive/VMAT2-positive varicose fibers and puncta, AADC-positive/TH-negative and VMAT2-positive/TH-negative fibers and puncta are present (presumed serotonergic input). Asterisks label profiles of the same blood vessel. Bars are 30 μm. (B) TH-positive neurons in the intermediolateral cell column of the thoracic spinal cord lack AADC and VMAT2 (arrows). AADC-positive varicose fibers containing TH (double arrow heads in middle panel) and lacking TH (arrow heads in middle panel) are present. VMAT2-positive varicose fibers/puncta containing TH (double arrow heads in lower panel) and lacking TH (arrow heads in lower panel) are encountered. Note also TH-positive fibers lacking VMAT2 (uppermost arrow in lower panel). Bars are 30 μm.
Fig. 6.
DOPAergic neurons in the rat habenular nucleus. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neurons lack AADC (arrows in upper panel) and VMAT2 (arrows in lower panel). Some TH-positive varicose fibers costain for AADC (double arrow heads in upper panel). Presumed serotonergic AADC-positive fibers lack TH (arrow head in upper panel). Presumed serotonergic VMAT2-positive fibers lack TH (arrow head in lower panel). Bars are 30 μm.
Fig. 7.
Absence of AADC and VMAT2 from TH-positive neurons in rat supraoptic nucleus (SON). Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neurons in the SON lack AADC (arrows in upper panel) and VMAT2 (arrows in middle panel). AADC-positive neurons dorsal to the SON and fibers in the SON are TH-negative (arrow heads in upper panel). AADC-positive neurons dorsal to the SON also lack VMAT2 (arrows in lower panel). Note VMAT2-positive/TH-positive fibers (double arrow heads in middle panel) and VMAT2-positive/TH-negative fibers (arrow heads in middle panel) in the SON. Some VMAT2-positive fibers in and at the dorsal border of the SON are AADC- (arrow heads in lower panel) and TH-negative (arrow heads in middle panel) and are presumed to represent histaminergic input to the SON region. Bars are 100 μm.
The arcuate and periventricular nuclei of the rhesus hypothalamus is also populated by “dopaergic” TH-positive neurons (Fig. 8), and cells of this type are plentiful in the paraventricular thalamic nucleus (Fig. 9).
Fig. 8.
Heterogeneous phenotypes of TH + neurons in the rhesus monkey periventricular hypothalamus. Horizontal panels (rows) represent single co-stained sections analyzed by confocal microscopy. Upper panel: TH-positive neurons coexpressing AADC (double arrow heads) and TH-positive/AADC-negative neurons (arrows) are present. Note AADC-positive/TH-negative fiber (arrow head). Lower panel: TH-positive neurons lack VMAT2 (arrows). Note VMAT2-positive/TH-positive and VMAT2-positive/TH-negative puncta. Occasional immunopositivity in the region of the nucleus for the cytoplasmic antigen AADC is attributed to superimposition of cytoplasm above or below the nucleus in these sections. Bars are 30 μm.
Fig. 9.
Heterogeneous phenotypes of TH-positive neurons in the rhesus monkey paraventricular thalamic nucleus. Horizontal panels (rows) represent single co-stained section adjacent to each other analyzed by confocal microscopy. AADC and VMAT2 are absent from TH-positive neurons (arrows in upper and lower panels, respectively). Note AADC-positive/TH-negative fibers (arrow heads in upper panel) and VMAT2-positive/TH-negative fibers (arrow heads in lower panel) as well as VMAT2-positive/TH-positive fibers (double arrow heads in lower panel). Asterisks label third ventricle. Bars are 50 μm.
DISCUSSION
We have surveyed the adult primate and rodent central nervous systems for “nonclassical” TH-positive neurons that express AADC without VMAT2 (“nonexocytotic catecholaminergic neuronxs”) or neither AADC nor VMAT2 (“dopaergic” neurons). We have found nonexocytotic dopaminergic neurons in olfactory bulb, striatum, NTS and area postrema as well as cortex and the dorsal motor nucleus of the vagus. The neurotransmitter function of dopamine that would be synthesized by these cells is not clear, since accumulation of DA into secretory vesicles would presumably not occur. Constitutive dopamine release from these cells, and its potential physiological function, will require both in vivo and in vitro investigation of this novel cell population. Whether these cells are positive for the plasma membrane transporters, allowing carrier-mediated DA release, especially in the context of indirectly acting sympathomimetics such as amphetamine and phenylephrine, or even if they have a sufficient complement of GTP cyclohydrolase to synthesize DA in appreciable amounts, also remains to be investigated. Lack of storage of DA, if synthesized in these cells, might make them vulnerable to neurotoxic damage due to accumulation of DA autooxidation products. Braak et al., have identified populations of cells in dorsal vagal motor nucleus, olfactory bulb, reticular formation, and other sites, with similar neuropigmentation characteristics, that are vulnerable to Lewy body generation in early Parkinson's disease (Del Tredici et al., 2002). It is not known if these are TH positive or if the TH-positive neurons identified in this study represent a vulnerable population by virtue of intracellular, but not intravesicular accumulation of DA and its metabolites. Enhanced vulnerability to L-dopa toxicity is seen in classical DA neurons lacking one allele of VMAT2 (Kariya et al., 2005). One aspect of particular clinical importance is the possibility of these neurons accumulating an excess of cytosolic, and potentially toxic, dopamine in patients receiving L-dopa therapy for Parkinson's disease, potentially limiting the benefits of this treatment regimen (Carlsson, 2001).
Several laboratories have reported on the presence of AADC-negative, TH-positive neurons of the CNS. We show here that such cell groups exist in several locations in the primate CNS, in addition to the locations previously reported (Gaspar et al., 1987; Komori et al., 1991; Ikemoto et al., 1998b; Kitahama et al., 1998), and that they do not express VMAT2. These neurons have been referred to as “dopaergic” by several groups. Ugrumov and colleagues have discussed the possibility that in rodent hypothalamus, dopaergic neurons could supply L-dopa to the nerve terminals of other neurons that possess AADC, but lack TH, as a way of allowing coupling between two types of neurons that are each “monoenzymatic” for catecholamine biosynthesis. In fact, these workers have postulated that under conditions in which true dopaminergic neurons are decreased in number, there is an increase in dopamine synthesis in these “monoenzymatic” ones (Ershov et al., 2005). AADC-positive TH-negative as well as TH-positive, and AADC-negative neurons were indeed observed in primate hypothalamus in this study. However, both populations lack VMAT2, so that if the latter were to supply L-dopa to the former, produced DA would not be stored in secretory vesicles for regulated release. It should be noted that these neurons express neither VMAT2 nor the neuroendocrine iso-form of the vesicular monoamine transporter, VMAT1 (data not shown). Although VMAT1 mRNA has been reported to be expressed in both brain and neural crest during development in the rodent (Hansson et al., 1998a,b), we have not observed VMAT1 protein expression in adult or developing rodent or primate brain, using sensitive and specific C-terminally directed antibodies for both rodent and primate VMAT1 (Schütz et al., 1998b; Weihe and Eiden, 2000).
Lacking VMAT expression, TH-negative, AADC-positive neurons of the hypothalamus, at least those observed in this study, are unlikely to store the dopamine they produce upon accumulation of L-dopa from neighboring “dopaergic” monoenzymatic neurons. These neurons are, however, like “nonexocytotic catecholaminergic” cells, potential targets for generation of DA during high-dose L-dopa therapy in Parkinson's disease, and thus their altered function in the brains of PD patients treated with L-dopa, especially in vital physiological regulatory areas like hypothalamus, may need to be considered. Such neurons may also, even in the absence of VMAT2, function as depots for dopamine which may be released by indirectly acting sympathomimetic drugs of abuse including amphetamine. Indeed, amphetamine is capable of sustaining nonvesicular catecholamine release in VMAT2-deficient mice sufficient to prolong life through rescue of impaired feeding behavior (Fon et al., 1997).
Likewise, primate intrinsic striatal TH-expressing interneurons have been shown to be substantially increased in numbers after MPTP or lentiviral GDNF transfer treatment (Betarbet et al., 1997; Palfi et al., 2002). As we demonstrate lack of VMAT2 in these striatal neurons, they are, like the hypothalamic TH-positive, AADC-positive VMAT2-negative neurons, not fully functional with respect to vesicle-regulated release of dopamine.
In addition to the possibility that TH-positive, AADC-negative, VMAT2-negative neurons are in fact functionally dopaergic, both types of TH-positive non-classical neurons may represent developmentally immature neurons, even in the adult CNS. TH-positive, AADC-positive neurons that do not express VMAT2 may secrete dopamine in a nonvesicular manner, or represent a pool of stem cells for maturation to a different and/or DAergic DA-secreting phenotype. As mentioned above, the presence of nonclassical TH-expressing neurons in primate CNS also raises the possibility that these neurons may be sites of action of drugs of abuse or therapeutic drugs that act on plasma membrane transporters, or increased brain concentrations of transmitters such as serotonin that could become “neurotransmitters of opportunity” in these neurons, as suggested in rodent developing thalamo-cortical neurons by Gaspar and colleagues (Cases et al., 1998).
The existence of nonclassical TH-positive neurons in the adult primate central nervous system, in nuclei of manifest importance for endocrine and cardiovascular regulation and in areas in which adult pluripotent neuronal cells are generated or migrate to, make further examination of the properties and roles in transmission and disease of these neurons of considerable interest. In this regard, Sanchez-Gonzalez and co-workers have recently identified a putative dopaminergic neuronal circuit, i.e., neurons expressing DA, the dopamine transporter DAT, and TH, and converging on the thalamus in the primate brain (Sanchez-Gonzalez et al., 2005). It remains to be seen if this circuit also expresses VMAT2, and releases DA classically through exocytosis from secretory granules, or lacks VMAT2, suggesting that this pathway and other neuronal cell groups already identified as “nonexocytotic” dopaminergic neurons in this report, might release DA through nonclassical modes. The origins of the TH neurons projecting to the thalamus was quite diverse, and TH neurons of diverse origins differed in their dopamine content and endowment with DAT. Interestingly, parabrachial TH neurons with projections to the thalamus lacked DAT (Sanchez-Gonzales et al., 2005) and apparently also lack VMAT2 (this study). Amphetamine is able to rescue dopamine release from midbrain dopaminergic neurons of VMAT2-deficient mice (Fon et al., 1997), and releases neurotransmitter from reserpine-treated synaptosomes via reversal of the plasma membrane transporter (Liang and Rutledge, 1982), suggesting catecholamine release via efflux through plasma membrane transporter as a potential mechanism for nonexocytotic release from TH-positive, AADC-positive, VMAT2-negative (and TH-negative, AADC-positive “monoenzymatic”) neurons that express DAT.
In summary, TH neurons in the CNS exhibit trait expression patterns beyond those associated with classical dopaminergic, noradrenergic, and adrenergic neurons. Specifically, the presence or absence of AADC and VMAT2, determines the TH-positive neuronal phenotypes described here, that may be dopaminergic, but without regulated vesicular secretion, and dopaergic, respectively. These neurons of primate CNS need now to be characterized with respect to expression of DAT, GTPCH, and other biogenic amine plasma membrane transporters (Hoffman et al., 1998), for a full appreciation of their role in primate brain function.
ACKNOWLEDGMENTS
We thank Petra Lattermann, Elke Rodenberg-Frank, Romy Weber and Marion Zibuschka, for technical assistance and Uw Schneider and Heidemarie Schneiter for preparing digital images. This work was supported in part by a grant from the Volkswagen-Stiftung to EW and LEE, and by the National Institute of Mental Health Intramural Research Program.
REFERENCES
- Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann. N.Y. Acad. Sci. 2001;940:132–141. doi: 10.1111/j.1749-6632.2001.tb03672.x. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Schäfer MK-H, Eiden LE, Weihe E. Chemical coding of the human gastrointestinal nervous system: Cholinergic, VIPergic and catecholaminergic phenotypes. J. Comp. Neurol. 2003;459:90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J. Neurosci. 2000;20:1495–1504. doi: 10.1523/JNEUROSCI.20-04-01495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. Doing research in the Intramural Program of the National Institutes of Health. Perspect. Biol. Med. 1986;29:S131–S137. doi: 10.1353/pbm.1986.0077. [DOI] [PubMed] [Google Scholar]
- Balan IS, Ugrumov MV, Calas A, Mailly P, Kreiger M, Thibault J. Tyrosine hydroxylase-expressing and/or aromatic L-amino acid decarboxylase-expressing neurons in the mediobasal hypothalamus of perinatal rats: differentiation and sexual dimorphism. J. Comp. Neurol. 2000;425:167–176. doi: 10.1002/1096-9861(20000918)425:2<167::aid-cne1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT. Dopaminergic neurons intrinsic to the primate striatum. J. Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. A paradigm shift in brain research. Science. 2001;294:1021–1024. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase. A knock-outs. J. Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard P, Goldstein M, Black IB. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc. Natl. Acad. Sci. U.S.A. 1978;75:2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schäfer MK-H, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershov PV, Ugrumov MV, Calas A, Krieger M, Thibault J. Differentiation of tyro-sine hydroxylase-synthesizing and/or aromatic L-amino acid decarboxylase-synthesizing neurons in the rat mediobasal hypothalamus: Quantitative double-immunofluorescence study. J. Comp. Neurol. 2002a;446:114–22. doi: 10.1002/cne.10173. [DOI] [PubMed] [Google Scholar]
- Ershov PV, Ugrumov MV, Calas A, Krieger M, Thibault J. Degeneration of dopaminergic neurons triggers an expression of individual enzymes of dopamine synthesis in nondopaminergic neurons of the arcuate nucleus in adult rats. J. Chem. Neuroanat. 2005;30:27–33. doi: 10.1016/j.jchemneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ershov PV, Ugrumov MV, Calas A, Makarenko IG, Krieger M, Thibault J. Neurons possessing enzymes of dopamine synthesis in the mediobasal hypothalamus of rats. Topographic relations and axonal projections to the median eminence in ontogenesis. J. Chem. Neuroanat. 2002b;24:95–107. doi: 10.1016/s0891-0618(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun B-C, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Volta Horni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catechol aminergic group? Neurosci. Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo L-C, Hohnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Klein MG, Sundgren NC, Li W, Woodward WR. Developmental regulation of neurotransmitter phenotype through tetrahydrobiopterin. J. Neurosci. 2002;22:9445–9452. doi: 10.1523/JNEUROSCI.22-21-09445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz N, Ljungdahl A, Hokfelt T, Johansson O, Goldstein M, Park D, Biberfeld P. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 1977;126:455–474. doi: 10.1016/0006-8993(77)90597-2. [DOI] [PubMed] [Google Scholar]
- Hansson S, Mezey E, Hoffman BJ. Ontogeny of vesicular monoamine transporter mRNAs VMAT1 and VMAT2. II. Expression in neural crest derivatives and their target sites in the rat. Dev. Brain Res. 1998a;110:159–174. doi: 10.1016/s0165-3806(98)00103-5. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Hoffman BJ, Mezey E. Ontogeny of vesicular monoamine transporter mRNAs VMAT1 and VMAT2. I. The developing rat central nervous system. Dev. Brain Res. 1998b;110:135–158. doi: 10.1016/s0165-3806(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Kapatos G. Nigrostriatal dopamine neurons express low levels of GTP cyclohydrolase I protein. J. Neurochem. 1998;70:164–170. doi: 10.1046/j.1471-4159.1998.70010164.x. [DOI] [PubMed] [Google Scholar]
- Hirsch M-R, Tiveron M-C, Guillemot F, Brunet J-F, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Palkovits M, Pacak K, Hansson SR, Mezey E. Regulation of dopamine transporter mRNA levels in the central nervous system. Adv. Pharmacol. 1998;42:202–206. doi: 10.1016/s1054-3589(08)60728-0. [DOI] [PubMed] [Google Scholar]
- Huang MH, Bahl JJ, Wu Y, Hu F, Larson DF, Roeske WR, Ewy GA. Neuroendocrine properties of intrinsic cardiac adrenergic cells in fetal rat heart. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H497–H503. doi: 10.1152/ajpheart.00591.2004. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Nagatsu I, Kitahama K, Jouvet A, Nishimura A, Nishi K, Maeda T, Arai R. A dopamine-synthesizing cell group demonstrated in the human basal forebrain by dual labeling immunohistochemical technique of tyrosine hydroxlase and aromatic l-amino acid decarboxylase. Neurosci. Lett. 1998a;243:129–132. doi: 10.1016/s0304-3940(98)00103-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Nagatsu I, Nishimura A, Nishi K, Arai R. Do all of human midbrain tyrosine hydroxylase neurons synthesize dopamine? Brain Res. 1998b;805:255–258. doi: 10.1016/s0006-8993(98)00661-1. [DOI] [PubMed] [Google Scholar]
- Jonakait GM, Markey KA, Goldstein M, Dreyfus CF, Black IB. Selective expression of high-affinity uptake of catecholamines by transiently catecholaminergic cells of the rat embryo: studies in vivo and in vitro. Dev. Biol. 1985;108:6–17. doi: 10.1016/0012-1606(85)90003-x. [DOI] [PubMed] [Google Scholar]
- Kariya S, Takahaski N, Hirano. M, Ueno S. Increased vulnerability to L-Dopa toxicity in dopaminergic neurons from VMAT2 heterozygote Knockout mice. J. Mol. Neurosci. 2005;27:277–279. doi: 10.1385/JMN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Ikemoto K, Jouvet A, Nagatsu I, Geffard M, Okamura H, Pearson J. Dopamine synthesizing enzymes in paraventricular hypothalamic neurons of the human and monkey (Macaca fuscata). Neurosci. Lett. 1998;243:1–4. doi: 10.1016/s0304-3940(98)00057-3. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Sakamoto N, Jouvet A, Nagatsu I, Pearson J. Dopamine-beta-hydroxylase and tyrosine hydroxylase immunoreactive neurons in the human brainstem. J. Chem. Neuroanat. 1996;10:137–146. doi: 10.1016/0891-0618(96)00111-1. [DOI] [PubMed] [Google Scholar]
- Komori K, Fujii T, Nagatsu I. Do some tyrosine hydroxylase-immunoreactive neurons in the human ventrolateral arcuate nucleus and globus pallidus produce only L-dopa? Neurosci. Lett. 1991;133:203–206. doi: 10.1016/0304-3940(91)90570-j. [DOI] [PubMed] [Google Scholar]
- Landis SC, Jackson PC, Fredieu JR, Thibault J. Catecholaminergic properties of cholinergic neurons and synapses in adult rat ciliary ganglion. J. Neurosci. 1987;7:3574–3587. doi: 10.1523/JNEUROSCI.07-11-03574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC, Siegel RE, Schwab M. Evidence for neurotransmitter plasticity in vivo. II. Immunocytochemical studies of rat sweat gland innervation during development. Dev. Biol. 1988;126:129–140. doi: 10.1016/0012-1606(88)90246-1. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrlé R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Liang NY, Rutledge CO. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem. Pharmacol. 1982;31:2479–2484. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- Mack GW, Shannon LM, Nadel ER. Influence of beta-adrenergic blockade on the control of sweating in humans. J. Appl. Physiol. 1986;61:1701–1705. doi: 10.1152/jappl.1986.61.5.1701. [DOI] [PubMed] [Google Scholar]
- Misu Y, Goshima Y, Miyamae T. Is DOPA a neurotransmitter? Trends Pharmacol. Sci. 2002;23:262–268. doi: 10.1016/s0165-6147(02)02013-8. [DOI] [PubMed] [Google Scholar]
- Misu Y, Kitahama K, Goshima Y. l-3,4-Dihydroxyphenylalanine as a neurotransmitter candidate in the central nervous system. Pharmacol. Ther. 2003;97:117–137. doi: 10.1016/s0163-7258(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Palfi S, Leventhal L, Chu Y, Ma SY, Emborg M, Bakay R, Deglon N, Hantraye P, Aebischer P, Kordower JH. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J. Neurosci. 2002;22:4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Jaszczak E, Landis SC. Innervation of footpads of normal and mutant mice lacking sweat glands. J. Comp. Neurol. 1994;346:613–625. doi: 10.1002/cne.903460412. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Brownstein M, Palkovits M, Kizer S, Axelrod J. Tyrosine hydroxylase and dopamine-beta-hydroxylase: distribution in the individual rat hypothalamic nuclei. J. Neurochem. 1974;23:869–871. doi: 10.1111/j.1471-4159.1974.tb04416.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J. Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J. Comp. Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schütz B, Schäfer MK-H, Eiden LE, Weihe E. Ontogeny of vesicular amine transporter expression in the rat: new perspectives on aminergic neuronal and neuroendocrine differentiation. Adv. Pharmacol. 1998a;42:903–908. doi: 10.1016/s1054-3589(08)60893-5. [DOI] [PubMed] [Google Scholar]
- Schütz B, Schäfer MK-H, Eiden LE, Weihe E. Vesicular amine transporter expression and isoform selection in developing brain, peripheral nervous system and gut. Dev. Brain Res. 1998b;106:181–204. doi: 10.1016/s0165-3806(97)00196-x. [DOI] [PubMed] [Google Scholar]
- Ugrumov M, Melnikova V, Ershov P, Balan I, Calas A. Tyrosine hydroxylase- and/or aromatic L-amino acid decarboxylase-expressing neurons in the rat arcuate nucleus: ontogenesis and functional significance. Psychoneuroendocrinology. 2002;27:533–548. doi: 10.1016/s0306-4530(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV. Hypothalamic monoaminergic systems in ontogenesis: Development and functional significance. Int. J. Dev. Biol. 1997;41:809–816. [PubMed] [Google Scholar]
- Ugrumov MV, Melnikova VI, Lavrentyeva AV, Kudrin VS, Rayevsky KS. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience. 2004;124:629–635. doi: 10.1016/j.neuroscience.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Weihe E, Anlauf M, Schäfer M-KH, Hartschuh W, Eiden LE. VMAT2 is the transporter mediating sequestration of monoamines in rat and human platelets, mast cells, and cutaneous dendritic cells. (abstract). Soc. Neurosci. Abstr. 1998 Nov.:7–12. No. 301.1. [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schäfer MK-H, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J. Mol. Neurosci. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schutz B, Hartschuh W, Anlauf M, Schafer MK, Eiden LE. Co-expression of cholinergic and noradrenergic phenotypes in human and non-human autonomic nervous system. J. Comp. Neurol. 2005;492:370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]